Abstract
The COVID-19 pandemic continues to significantly impact the treatment of people living with aortic stenosis, and access to transcatheter aortic valve implantation. Transcatheter aortic valve implantation (TAVI) programmes require unique coordinated processes that are currently experiencing multiple disruptions and are guided by rapidly evolving protocols. We present a series of recommendations for TAVI programmes to adapt to the new demands, based on recent evidence and the international expertise of nurse leaders and collaborators in this field. Although recommended in most guidelines, the uptake of the role of the TAVI programme nurse is uneven across international regions. COVID-19 is further highlighting why a nurse-led central point of coordination and communication is a vital asset for patients and programmes. We propose an alternative streamlined evaluation pathway to minimize patients’ pre-procedure exposure to the hospital environment while ensuring appropriate treatment decision and shared decision-making. The competing demands created by COVID-19 require vigilant wait list management, with risk stratification, telephone surveillance and optimized triage and prioritization. A minimalist approach with close scrutiny of all parts of the procedure has become an imperative to avoid any complications and ensure patients’ accelerated recovery. Lastly, we outline a nurse-led protocol of rapid mobilization and reconditioning as an effective strategy to facilitate safe next-day discharge home. As the pandemic abates, TAVI programmes must facilitate access to care without compromising patient safety, enable hospitals to manage the competing demands created by COVID-19 and establish new processes to support patients living with valvular heart disease.
Keywords: Transcatheter aortic valve implantation, COVID-19, clinical pathway, multidisciplinary team
Background
People living with severe aortic stenosis experience significant and rapid exacerbation of symptoms, increased mortality, morbidity, and hospital readmissions.1 Due to the natural history of aortic stenosis, most patients are elderly, and are burdened with frailty and other multiple comorbidities.2,3 Transcatheter aortic valve implantation (TAVI) is an established minimally invasive treatment option that enables patients to rapidly derive significantly improved quantity and quality of life, regardless of surgical risk profile.4,5
The higher health vulnerabilities associated with aortic stenosis are further compounded in the current era of the COVID-19 pandemic with the challenges of providing timely therapy to primarily older (⩾ 80 years) individuals.6,7 Delays to appropriate therapy for patients with severe aortic stenosis can exacerbate the risks of emergency hospital admission, deteriorating symptoms and the cascade of complications of worsening heart failure related to severe valvular heart disease, including the loss of independent function.8 Developing strategies to provide timely therapy for at risk patients with aortic stenosis is paramount during the pandemic.
To date, much emphasis has been placed on strategies to ‘flatten the curve’ of escalating COVID-19 rates of infection, hospitalization and mortality across regions and populations. As the pandemic begins to slow, balancing competing demands to ‘manage the plateau’ of the on-going prevalence and risks of COVID-19 while addressing the escalating needs of patients with cardiovascular disease awaiting treatment presents the next challenge for health care systems across regions.9,10 There is mounting evidence that treatment-seeking delays and longer wait times for an increasing volume of patients are taking a significant toll on cardiac patients’ survival, morbidity and quality of life, and will create a wave of pressure on health care resources and personnel.11 For TAVI programmes, the interruption of referral pathways, the imperative need to stay connected with patients and the difficulties of resuming or accelerating scheduling pose unchartered challenges. In this context, there is a compelling need to facilitate the rapid adoption of best practices adapted to the unique demands created by COVID-19 and leverage existing evidence to minimize health care resources, facilitate the accelerated treatment of aortic stenosis without compromising patient safety and ensure that patients return home to enjoy the benefits that TAVI affords.12 To this end, we outline recommendations to optimize access to TAVI in rapidly evolving health care systems that will continue to address the devastating effect of the COVID-19 pandemic for the foreseeable future.
Coordinated and streamlined assessment pathway
The pivotal role of the TAVI programme nurse who leads the coordination of patients’ journey of care is made all the more salient in current times. Although well established in some regions, and endorsed in multiple guidelines as an important member of the multidisciplinary team,13,14 this role remains in its infancy or is absent across most of Europe. The competencies of this expert clinician include knowledge of the aortic stenosis patient population, their clinical presentation and disease progression, clinical assessment of symptoms and urgency, patient and family education to facilitate shared decision-making and early discharge planning, and the coordination of complex, and currently altered, processes of care.15 The TAVI programme nurse provides an essential central point of coordination to case manage individual patients while maintaining seamless communication with the multidisciplinary team.16–18 Given the effect of COVID-19 on channels of communication, patients are reporting that access to the TAVI programme nurse is perceived as a ‘help line’ for on-going monitoring, guidance, reassurance and advocacy, and continuity of care. Importantly, the TAVI programme nurse conveys to patients and their family information about how hospitals are instituting protocols and practices to ensure patient safety and comfort during their admission as the pandemic progresses. The new challenges posed by COVID-19 may offer a strong incentive to consider adopting this role in centres that currently rely on a less coordinated approach.
Constrained access to cardiac imaging, cancellation of out-patient valvular heart disease clinic appointments and inability of the multidisciplinary team to meet in person to discuss treatment decision and procedure planning require modifications to conventional TAVI assessment pathways and clinic processes during, and likely after, the COVID-19 crisis. The additional risk posed to elderly patients to undergo pre-procedure consultations and diagnostic testing in the hospital must be carefully considered. Therefore, the historical practice of an in-person assessment pathway must pivot to an expedited work-up to avoid delays in treatment decision (Figure 1). Nimble and easy to implement strategies that guarantee physical distancing without compromising adherence to guidelines include the use of telemedicine consultation for nursing and medical assessments, individualized risk stratification for cardiac imaging requirements and the preferential use of computed tomography as a gate-keeper diagnostic test, and transition to a virtual platform for the multidisciplinary team meeting.19 Screening for the risk of post-TAVI atrio-ventricular conduction delay20 may help anticipate the need for post-procedure monitoring and the consideration of early interventions.
Figure 1.
Vancouver Accelerated TAVI Clinical Pathway adapted for COVID-19.
CT: computed tomography; CCTA: cardiac computed tomography angiography; CAD: coronary artery disease; TF: transfemoral; TAVI: transcatheter aortic valve implantation: MD: Medical Doctor
For patients preparing for admission, the historical practice of a separate pre-procedure appointment in the surgical pre-assessment clinic for a consultation with the anaesthesiologist must now be used more discriminately to mitigate the risks of COVID-19. Separate processes are necessary to complete pre-procedure diagnostics (e.g. chest X-ray, blood tests) and conduct a pre-procedure telephone assessment. COVID-19 initial screening involves reviewing patients’ symptoms, potential community exposure, and travel history, while routine testing prior to TAVI for both the patient and their accompanying support, if required, differs by regions and will likely continue to be influenced by local penetrance of COVID-19, availability of testing and regional health policies. Although systematic screening is widely recommended and established, testing protocols remain more diverse and offer programmes and patients significant challenges related to timing, location and communication of findings. The European Association of Percutaneous Cardiovascular Interventions and the Acute Cardiovascular Care Association recommend that a strategy of testing before an invasive procedure should be prioritized.21
TAVI wait list management
As hospitals prioritize ensuring sufficient capacity for the treatment of COVID-19 patients, the cumulative impact of delayed referrals and the near complete cancellation of elective and most in-patient procedures is rapidly leading to longer wait times and a growing number of people waiting for treatment across regions. Wait time for TAVI is incrementally associated with increased adverse events and worse outcome.22 International experience suggests that a strategy of gradual re-opening of hospital services will be preferred over a full resumption of past schedules as the pandemic abates. To match varying health policy across regions, TAVI programmes must adopt on-going processes to facilitate the active monitoring of patients on the wait list and the appropriate queueing of procedures to reduce the risks of adverse events while on the wait list.23
The TAVI programme nurse is ideally suited to conduct a systematic telephone assessment to ascertain patients’ health and urgency status. In the Vancouver programme, the components of the historical wait time telephone assessment have been revised in light of COVID-19, and include treatment-seeking events (e.g. unscheduled contact with physician, emergency department visit), physical health status (e.g. activity tolerance, mobilization) and symptoms (e.g. fatigue, shortness of breath, angina, syncope/pre-syncope) (Figure 2). In anticipation of procedure scheduling, it is essential to engage patients in discussions about their willingness to come to the hospital given the evolution of the pandemic and the availability of social support for transportation and early recovery.
Figure 2.
Clinical documentation of status on wait list and urgency stratification.
ED: Emergency Department; NYHA: New York Heart Association functional classification
The criteria for triage and prioritization must address the needs of complex patients exhibiting signs and symptoms of acute decompensation at risk for emergency admission and increased mortality while waiting. On-going prioritization based on local or regional urgency criteria may be helpful to coordinate scheduling (Figure 2). Programmes must also consider the potential benefit of scheduling patients with a more predictable hospitalization who can be treated rapidly and supported in their accelerated reconditioning and rapid return home. Combining various urgency categories in the planning of service delivery might offer the most effective strategy to attend to the most urgent patients while optimizing capacity to reduce the overall wait list.
The imperative of a minimalist approach
A minimalist approach refers to a sequence of fully optimized activities aimed achieving an excellent outcome without compromising patient safety.24 The Vancouver Multimodality, Multidisciplinary but Minimalist TAVR study demonstrated the safety and feasibility of a bundle of care grounded in a minimalist peri-procedure approach.25 The goal is to leverage current evidence, to reduce the disruptions to patients’ health status and physiological reserves during their admission and to decrease the intensity of health services requirements to enhance access to TAVI.26 The adoption of a minimalist approach as the preferred default strategy is an imperative to promote access to care in the ‘new normal’ as COVID-19 continues to dictate priorities of care.
A comprehensive peri-procedure minimalist approach begins with the same-day admission of elective patients to delay the deleterious effects of hospitalization on the elderly and convey the important messaging that contemporary TAVI is a same-day procedure with an overnight stay for close monitoring. The use of a cardiac catheterization laboratory and its associated small and versatile structural heart procedure team is particularly appropriate to avoid the competing demands placed on operating rooms and reduce the requirements for human resources and equipment.27
In regions where COVID-19 required the redeployment of cardiac catheterization laboratory and/or operating room nurses to care for patients in the intensive care unit or other in-patient units, strategies to repatriate the human resources to procedure rooms are necessary to reconstitute peri-procedure teams.28 The rapid implementation of protocols and processes to decrease the risk of infection and cross-contamination in laboratories focuses on creating patient and staff pathways that minimize exposure and ensure appropriate cleaning. The availability of personal protective equipment (PPE) is paramount to staff safety and emergency preparedness.29 Similarly to pre-procedure screening for COVID-19, there is significant heterogeneity in practice across programmes and regions related to the PPE protocols. The European Society of Cardiology recommends that health care providers should have adequate supply and training in proper techniques for donning and removing PPE, and that all patients entering the catheterization laboratory should wear a surgical mask.28,30 US professional societies recommend that the treatment team should be limited to essential personnel and that staff who scrub for procedures should use PPE suitable for airborne precautions, including an N95 respirator and a face shield.19
The use of a strategy of local anaesthesia with or without light procedural sedation is associated with improved haemodynamic stability, shorter procedure times, reduced risk of post-procedure delirium, and accelerated mobilization and reconditioning.31 Given the on-going demands placed on anaesthesiology services to attend to the challenging care of unstable COVID-19 patients, it may be judicious to consider a model of physician-directed and nurse-administered local anaesthesia or conscious sedation32 if important safety targets can be met (e.g. ability to convert to general anaesthesia within 5 min in the event of an emergency). An open visual field between the patient and the implanting team promotes seamless communication, early warning of complications, and the inclusion of patients as essential ‘partners’ in minimalist TAVI. The consistent use of ultrasound-guided vascular access further mitigates peri-procedure risks by reducing the incidence of vascular injury and bleeding and is essential to promote early mobilization.33 Together, these practices increase the likelihood of transferring a predictably stable patient who is optimally prepared for an accelerated recovery protocol to the post-procedure team.
Admission and early recovery in the clinical area adjacent to the laboratory further reduces the footprint of TAVI by avoiding the use of critical care units that will continue to face significant duress for the remainder of the pandemic.34
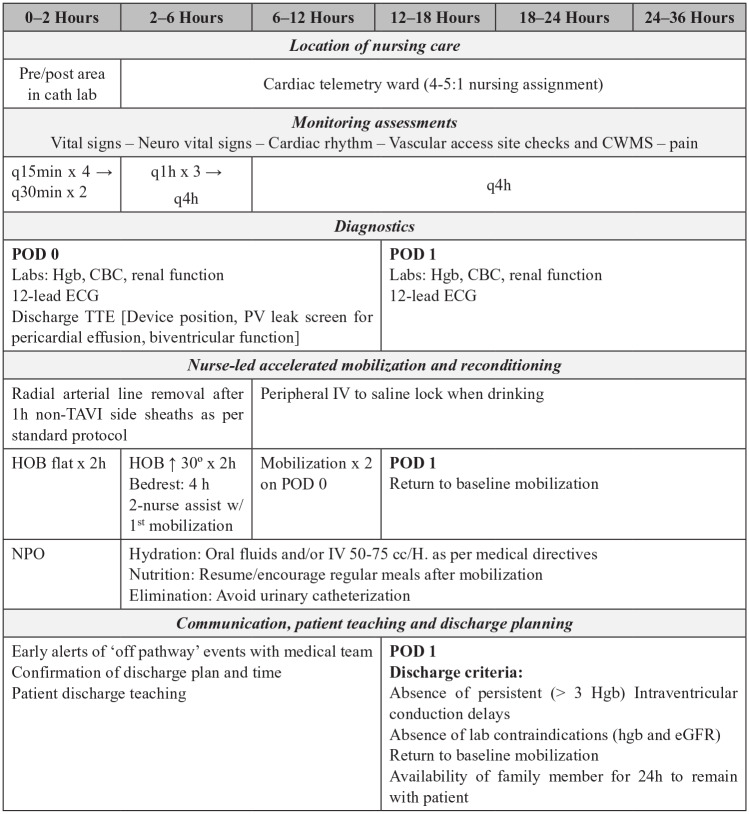
Accelerated reconditioning and next-day discharge home
Priorities of care in the post-procedure phase include close monitoring, early mobilization and accelerated reconditioning, and discharge planning. The post-procedure protocol of 3M TAVR and the Vancouver Accelerated Recovery Clinical Pathway provide useful direction to guide nursing practice.34 Details presented in Figure 3 may serve as a roadmap to nursing education to promote standardization of practice and change management. Critical care nursing competencies are best suited in the very early recovery period to provide close monitoring of haemodynamic and neurological status, cardiac telemetry and vascular access haemostasis. Ideally, the patient is admitted to a unit in close proximity to the TAVI implanting team (e.g. clinical unit that serves the cardiac catheterization laboratory) to promote continuity of medical care in this potentially vulnerable phase of care. Although contingency plans must be in place to secure post-procedure critical care for patients experiencing haemodynamic instability, new conduction delay or vascular access complications, most TAVI patients can be safely transferred to a cardiac telemetry ward under the care of an expert cardiovascular nurse and the on-going medical direction of the implanting team.34 The avoidance of critical care during the COVID-19 pandemic further reduces the competing impact of TAVI on intensive care units that remain under significant strain.28
Figure 3.
Vancouver Accelerated Reconditioning nursing protocol adapted for COVID-19 to facilitate safe-next day discharge home after TAVI.
q: every; h: hour; CWMS: colour, warmth, movement, sensitivity; Hgb: haemoglobin; CBC: complete blood count; TTE: transthoracic echocardiogram; PV: paravalvular; ECG: electrocardiogram; TAVI: transcatheter aortic valve implantation; IV: intravenous; HOB: head of bed; NPO: nothing by mouth; POD: post-operative day; cc: centilitre; eGFR: estimated glomerular filtration rate
Early mobilization is one of the most effective strategies to facilitate patients’ rapid return to baseline status.35 Accelerated time to mobilization is associated with avoidance of functional decline, resumption of activities of daily living and shorter length of stay.36 A standardized protocol of 4-h bedrest followed by nurse-led mobilization at least twice on the procedure day and demonstrated return to baseline mobilization on the morning after the procedure facilitates the goal of readiness for safe next-day discharge.34,27
Timing of discharge hinges on the implanting team’s assessment that patients are safe to transition home. Discharge criteria include the absence of persistent (⩾ 3 h) intraventricular conduction delay, clinically important change in laboratory values (e.g. haemoglobin, renal function), completion of patient teaching and confirmation of availability of a family member to remain with patient for the first 24 h home.25 Given the potential benefits of further minimizing time spent in hospital, the James Cook University Hospital TAVI team (Middlesbrough, UK) has developed a same-day discharge programme that considers additional criteria including pre-existing permanent pacemaker, low contrast use, absence of procedural complications, early full mobilization and availability of social support. Additional safety net measures such as telephone contact with the TAVI nurse on post-discharge day 1 and a check of post-procedural blood tests in the community mitigate the risks of delayed complications. Although not specifically designed for COVID-19, safe same-day discharge is ideally suited to use during the pandemic (Muir D and McCalmont G, personal communication, 2020, manuscript in preparation).
Schedules of standardized in-person 30-day and longer-term follow-up are no longer suited to the on-going need for social distancing and minimizing time spent in hospital. Although telephone follow-up by the TAVI programme nurse can provide essential information about symptoms, recovery and overall health status, the absence of follow-up cardiology consultation and cardiac echocardiography may create gaps in care and monitoring. For example, longitudinal surveillance of transaortic pressure gradients provides important information about valve function. Adaptation of follow-up processes to enable echocardiography close to patients’ homes is required to ensure patient safety and continuity of care.
Managing the implications of COVID-19: setting a new benchmark for TAVI
For patients living with the devastating prognosis of severe aortic stenosis, timely access to valve replacement is the only treatment available. The rapid onset of halting referrals and procedures to create capacity to manage the COVID-19 pandemic will now be followed by the resumption of access to care under drastically different circumstances. TAVI programmes cannot expect a ‘flipping of the switch’ back to historical practices; rather, ‘increasing the dimmer’ requires that programmes leverage the evidence available to implement a series of pre-, peri- and post-procedure best practices to ensure the highest quality of outcomes, the lowest risk of COVID-19 exposure and the most efficient use of health care resources. To this end, we have the collective opportunity to set a new benchmark for contemporary TAVI by promoting the adoption of a clinical pathway that combines the latest evidence and cross-jurisdiction expertise.
Implications for practice
The COVID-19 pandemic is interrupting access to transcatheter aortic valve implantation to treat people with severe aortic stenosis.
There is evidence that adopting a series of best practices can enable transcatheter aortic valve implantation programmes to accelerate access to care and minimize patients’ risk while in hospital.
Cardiovascular nurses play a pivotal role in leading changes in practice and processes of care.
There is an urgent need for transcatheter aortic valve implantation programmes to adopt sustainable strategies to manage the on-going implications of COVID-19.
Declaration of conflicting interests
The authors have no conflicts of interest to declare.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Deharo P, Bisson A, Herbert J, et al. Outcomes in nonagenarians undergoing transcatheter aortic valve implantation: Anationwide analysis. Eurointervention 2020; 15: 1489–1496. [DOI] [PubMed] [Google Scholar]
- 2. Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol 2014; 30: 962–970. [DOI] [PubMed] [Google Scholar]
- 3. Olsson K, Naslund U, Nilsson J, et al. Hope and despair: Patients’ experiences of being ineligible for transcatheter aortic valve implantation. Eur J Cardiovasc Nurs 2019; 18: 593–600. [DOI] [PubMed] [Google Scholar]
- 4. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019; 380: 1695–1705. [DOI] [PubMed] [Google Scholar]
- 5. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019; 380: 1706–1715. [DOI] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020; 368: m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu PP, Blet A, Smyth D, et al. The science underlying COVID-19: Implications for the cardiovascular system. Circulation. Epub ahead of print 16 April 2020. DOI: 10.1161/circulationaha.120.047549. [DOI] [PubMed] [Google Scholar]
- 9. Wood DA, Sathananthan J, Gin K, et al. Precautions and procedures for coronary and structural cardiac interventions during the COVID-19 pandemic: Guidance from Canadian Association of Interventional Cardiology. Can J Cardiol 2020; 36: 780–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jaarsma T, van der Wal M, Hinterbuchner L, et al. Flexibility and safety in times of coronavirus disease 2019 (COVID-19): Implications for nurses and allied professionals in cardiology. Eur J Cardiovasc Nurs 2020; 19: 462–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elbaz-Greener G, Masih S, Fang J, et al. Temporal trends and clinical consequences of wait times for transcatheter aortic valve replacement: A population-based study. Circulation 2018; 138: 483–493. [DOI] [PubMed] [Google Scholar]
- 12. Lauck SB, Wood DA, Baumbusch J, et al. Vancouver transcatheter aortic valve replacement clinical pathway: Minimalist approach, standardized care, and discharge criteria to reduce length of stay. Circ Cardiovasc Qual Outcomes 2016; 9: 312–321. [DOI] [PubMed] [Google Scholar]
- 13. Otto CM, Kumbhani DJ, Alexander KP, et al. 2017 ACC expert consensus decision pathway for transcatheter aortic valve replacement in the management of adults with aortic stenosis: A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2017; 69: 1313–1346. [DOI] [PubMed] [Google Scholar]
- 14. Asgar AW, Ouzounian M, Adams C, et al. 2019 Canadian Cardiovascular Society position statement for transcatheter aortic valve implantation. Can J Cardiol 2019; 35: 1437–1448. [DOI] [PubMed] [Google Scholar]
- 15. Hawkey M, Højberg Kirk B. The valve program clinician. In: Hawkey MC, Lauck SB, Perpetua EM, et al. (eds) Transcatheter aortic valve replacement program development Philadelphia, PA: Wolters Kluwer, 2020. [Google Scholar]
- 16. Hawkey MC, Lauck SB, Perpetua EM, et al. Transcatheter aortic valve replacement program development: Recommendations for best practice. Catheter Cardiovasc Interv 2014; 84: 859–867. [DOI] [PubMed] [Google Scholar]
- 17. Lauck S, Achtem L, Boone RH, et al. Implementation of processes of care to support transcatheter aortic valve replacement programs. Eur J Cardiovasc Nurs 2013; 12: 33–38. [DOI] [PubMed] [Google Scholar]
- 18. Baumbusch J, Lauck SB, Achtem L, et al. Understanding experiences of undergoing transcatheter aortic valve implantation: one-year follow-up. Eur J Cardiovasc Nurs 2018; 17: 280–288. [DOI] [PubMed] [Google Scholar]
- 19. Chung CJ, Nazif TM, Wolbinski M, et al. The restructuring of structural heart disease practice during the Covid-19 pandemic. J Am Coll Cardiol. Epub ahead of print 13 April 2020. DOI: 10.1016/j.jacc.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nazif TM, Dizon JM, Hahn RT, et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: The PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv 2015; 8: 60–69. [DOI] [PubMed] [Google Scholar]
- 21. Chieffo A, Stefanini GG, Price S, et al. EAPCI position statement on invasive management of acute coronary syndromes during the COVID-19 pandemic. Eur Heart J 2020; 41: 1839–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elbaz-Greener G, Yarranton B, Qiu F, et al. Association between wait time for transcatheter aortic valve replacement and early postprocedural outcomes. J Am Heart Assoc 2019; 8: e010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wood DA, Mahmud E, Thourani VH, et al. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: Guidance from North American society leadership. J Am Coll Cardiol. Epub ahead of print 08 May 2020. DOI: 10.1016/j.jacc.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lauck SB, Sathananthan J, Wood DA, et al. Minimalist approaches to TAVR: Options and implications, http://wwwaccorg (2020, Accessed 30 May 2020).
- 25. Wood DA, Lauck SB, Cairns JA, et al. The Vancouver 3M (Multidisciplinary, Multimodality, But Minimalist) clinical pathway facilitates safe next-day discharge home at low-, medium-, and high-volume transfemoral transcatheter aortic valve replacement centers: The 3M TAVR study. JACC Cardiovasc Interv 2019; 12: 459–469. [DOI] [PubMed] [Google Scholar]
- 26. Lauck SB, Baron SJ, Sathananthan J, et al. Exploring the reduction in hospitalization costs associated with next-day discharge following transfemoral transcatheter aortic valve replacement in the United States. Struct Heart 2019; 3: 423–430. [Google Scholar]
- 27. Sathananthan J, Webb JG, Polderman J, et al. Safety of accelerated recovery on a cardiology ward and early discharge following minimalist TAVR in the catheterization laboratory: The Vancouver accelerated recovery clinical pathway. Struct Heart 2019; 3: 229–235. [Google Scholar]
- 28. Tarantini G, Fraccaro C, Chieffo A, et al. Italian Society of Interventional Cardiology (GISE) position paper for cath lab-specific preparedness recommendations for healthcare providers in case of suspected, probable or confirmed cases of COVID-19. Catheter Cardiovasc Interv. Epub ahead of print 31 March 2020. DOI: 10.1002/ccd.28888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Welt FGP, Shah PB, Aronow HD, et al. Catheterization laboratory considerations during the Coronavirus (COVID-19) Pandemic: From ACC’s Interventional Council and SCAI. J Am Coll Cardiol. Epub ahead of print 23 March 2020. DOI: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. European Society of Cardiology. ESC guidance for the diagnosis and management of cv disease during the COVID-19 pandemic, https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance (accessed 21 April 2020).
- 31. Sathananthan J, Webb JG, Lauck SB, et al. Impact of local anesthesia only versus procedural sedation using the vancouver clinical pathway for TAVR: Insights from the 3M TAVR study. JACC Cardiovasc Interv 2019; 12: 1000–1001. [DOI] [PubMed] [Google Scholar]
- 32. Keegan P, Lisko JC, Kamioka N, et al. Nurse led sedation: The clinical and echocardiographic outcomes of the 5-Year Emory experience. Struct Heart 2020; forthcoming. [Google Scholar]
- 33. Kotronias RA, Scarsini R, de Maria GL, et al. Ultrasound guided vascular access site management and left ventricular pacing are associated with improved outcomes in contemporary transcatheter aortic valve replacement: Insights from the OxTAVI registry. Catheter Cardiovasc Interv. Epub ahead of print 20 November 2019. DOI: 10.1002/ccd.28578. [DOI] [PubMed] [Google Scholar]
- 34. Lauck SB, Sathananthan J, Park J, et al. Post-procedure protocol to facilitate next-day discharge: Results of the multidisciplinary, multimodality but minimalist TAVR study. Catheter Cardiovasc Interv. Epub ahead of print 01 December 2019. DOI: 10.1002/ccd.28617. [DOI] [PubMed] [Google Scholar]
- 35. Brown CJ, Redden DT, Flood KL, et al. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc 2009; 57: 1660–1665. [DOI] [PubMed] [Google Scholar]
- 36. Calero-Garcia MJ, Ortega AR, Navarro E, et al. Relationship between hospitalization and functional and cognitive impairment in hospitalized older adults patients. Aging Ment Health 2017; 21: 1164–1170. [DOI] [PubMed] [Google Scholar]