Summary
Immunotherapies are disease management strategies that target or manipulate components of the immune system. Infectious diseases pose a significant threat to human health as evidenced by countries continuing to grapple with several emerging and re-emerging diseases, the most recent global health threat being the SARS-CoV2 pandemic. As such, various immunotherapeutic approaches are increasingly being investigated as alternative therapies for infectious diseases, resulting in significant advances towards the uncovering of pathogen–host immunity interactions. Novel and innovative therapeutic strategies are necessary to overcome the challenges typically faced by existing infectious disease prevention and control methods such as lack of adequate efficacy, drug toxicity, and the emergence of drug resistance. As evidenced by recent developments and success of pharmaceuticals such as monoclonal antibodies (mAbs), immunotherapies already show abundant promise to overcome such limitations while also advancing the frontiers of medicine. In this review, we summarize some of the most notable inroads made to combat infectious disease, over mainly the last 5 years, through the use of immunotherapies such as vaccines, mAb-based therapies, T-cell-based therapies, manipulation of cytokine levels, and checkpoint inhibition. While its most general applications are founded in cancer treatment, advances made towards the curative treatment of human immunodeficiency virus, tuberculosis, malaria, zika virus and, most recently COVID-19, reinforce the role of immunotherapeutic strategies in the broader field of disease control. Ultimately, the comprehensive specificity, safety, and cost of immunotherapeutics will impact its widespread implementation.
Keywords: immunotherapy, infectious disease, vaccine, checkpoint inhibition, T-cells
Introduction
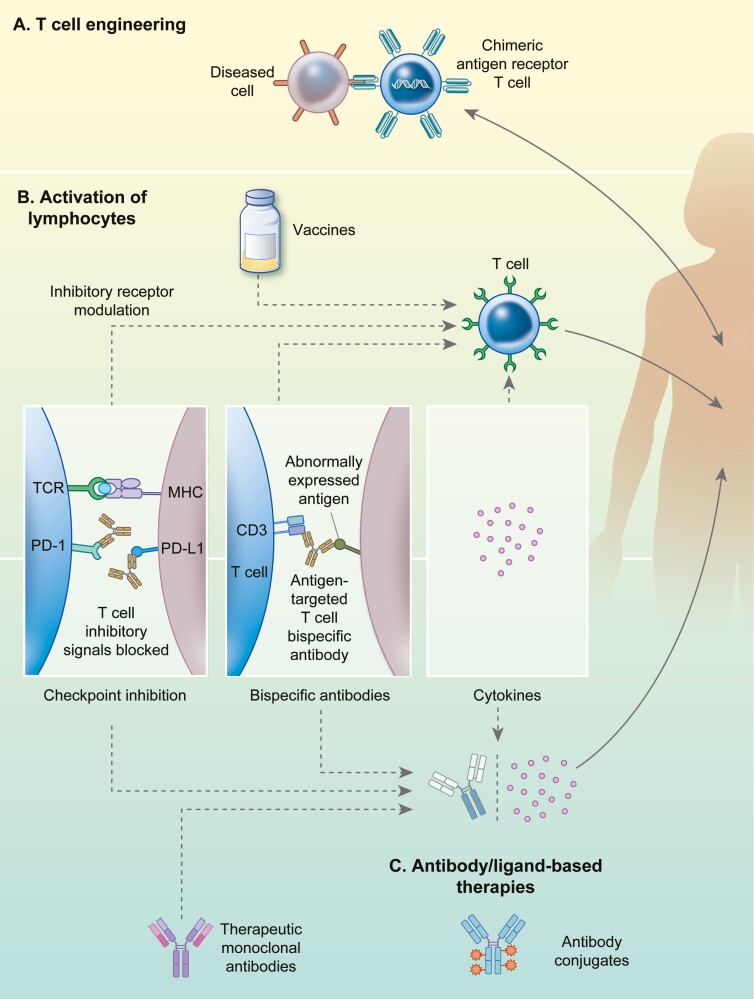
Immunotherapies manipulate components of the immune system to target and eliminate pathogens or diseased host cells to offer protection against disease or alleviate symptoms. Based on their mechanism of action, they are classified as passive immunotherapies which employ constituents produced ex vivo (immune cells or recombinant antibody derivatives) that are administered to patients, and active immunotherapies which trigger components of the host immunological memory using virulence factors that activate effectors (T-cells or humoral response) [1]. Over the decades, several approaches have been described and successfully employed, the most prominent among which have been summarized in Fig. 1 [2].
Figure 1.
Immunotherapeutic advances employed against infectious disease. Most prominent types of immunotherapies described fall under three major categories, namely: (A) T-cell engineering strategies that use genetically modified patient-derived T-cells which are transiently cultured in vitro to express CARs. Such CAR T-cells provides a non-major histocompatibility complex driven recognition of abnormal cells and thus aid in enhanced targeting and elimination of diseased cells. The activity of native lymphocytes such as T-cells and natural killer (NK) cells can be enhanced through multiple ways as illustrated in panel B. (B) Activation of lymphocytes is accomplished through approaches such as vaccinations, that trigger immune memory response to combat invading pathogens. Checkpoint inhibition therapy aims at overcoming inhibitory signals (such as PD-1 or PD-L1) and enhances recognition of abnormal or diseased cells. Checkpoint inhibition also counteracts regulatory T-cells (Treg) that may dampen host cytotoxic T cell responses to infections. Bispecific monoclonal antibodies (BsmAbs) can bind to 2 targets: an antigen on a diseased cell and an antigen on an immune effector like a cytotoxic T-cell (e.g. the CD3 antigen), thus bringing a cytotoxic T-cell in proximity to the cell that requires elimination. Administering proinflammatory cytokines serves to increase the immune activation of patients’ T-cells. (C) Antibody/ligand-based therapies make use of monoclonal antibodies (mAbs) or ligands that function through controlled modulation of other immune system components such as lymphocytes. Such approaches include checkpoint inhibition, BsmAbs, and cytokines. Additionally, therapeutic mAbs are used to neutralize antigen that contribute to pathogenesis such as host or pathogen surface antigens, toxins etc. Appropriately modified mAbs may also be conjugated with agents such a small molecule toxins for their targeted delivery.
Vaccines represent the oldest and most successful form of immunotherapy [3]. They may confer protection through two broad means: (i) immunological memory through the administration of immunogens to induce clonal proliferation of antigen-specific lymphocytes, allowing the host immune system to respond more rapidly and effectively against pathogens that it has previously encountered [4], and (ii) conferring passive protection, post-infection, through the delivering neutralizing agents such as antibodies binding to e.g. bacterial antigens/toxins [2]. The success of immunization programs over the years has contributed to the near or complete eradication of communicable diseases such as smallpox and polio. In the last three decades, scientific advances have impacted the establishment of new vaccine platforms using recombinant antibodies, nucleic acid-based vaccines and improving adjuvants [3].
Monoclonal antibodies (mAbs) have been approved for therapeutic use since 1986 and currently have the most widespread applications among immunotherapies [2, 5]. mAbs rely on the specificity and selectivity of antibodies to their antigen and exert their function through the following mechanisms: (i) by binding to cell surface receptors and inducing a signaling cascade, leading to cell death, (ii) the interference of ligand–receptor interactions necessary for continued cell growth or viability, (iii) antibody-dependent cellular cytotoxicity, which involves the Fc region of the antibody helping to recruit constituents of cell-mediated immunity [such as natural killer (NK) cells, monocytes, and macrophages], and (iv) by complement-dependent cytotoxicity arising from the activation of the complement cascade after binding to the target structure. Antibody conjugates use the targeting domain of a mAb fused to a toxic payload, such as small molecules or apoptosis-inducing toxins to target disease-associated antigens. Once bound to its target, they are internalized and release their payload, triggering cell death while aiming at minimal damage to healthy tissues [2]. Bi-specific antibodies containing two binding domains, in general, one specific for an antigen the other for an effector cell, have also been developed. By interfering with multiple surface receptors/ligands, bispecific antibodies can affect molecules involved in cell proliferation or inflammatory processes, bring targets into close proximity to support protein complexation in the clotting cascade or recruit immune cells to the diseased site circumventing major histocompatibility complex (MHC) engagement [2, 6].
One of the most notable immunotherapies of recent times has been checkpoint blockade therapy which involves the use of mAbs to disrupt the interaction between immune inhibitory receptor-ligand pairs. Immune checkpoints are cellular processes that prevent the host immunity from attacking otherwise healthy cells indiscriminately. Blocking disease-associated abnormal immune checkpoint activation restores normal immune system function, thus permitting enhanced immune responses against upregulated ligands. Prominent checkpoint blockade mAbs target cytotoxic T-lymphocyte-associated protein 4 (CTLA4), programmed cell death 1 ligand 1 (PD-L1), programmed cell death protein 1 (PD1), T-cell immunoglobulin, and mucin domain-containing protein 3 (TIM3) and OX40, which prevent T-cell inhibition and promote effector T-cell activation. Numerous drugs using these checkpoint inhibitors have been approved by the U.S Food and Drug Administration for intervention against various cancers since their first regulatory approval in 2011. Their use in combination with other treatments has reported promising outcomes against human immunodeficiency virus (HIV), tuberculosis (TB), and malaria and are further described in the sections ‘Checkpoint inhibition’ and ‘Checkpoint blockade’ [7, 8].
Cytokines are soluble proteins that mediate intercellular communication for a variety of biological processes including cell proliferation, inflammation, immunity, angiogenesis, wound healing, and repair. Cytokines mediate signaling fundamental to both disease spread and control and have been approved for therapeutic use since 1986 [7]. Their use is infectious disease immunotherapy have been exemplified in the section ‘Cytokine therapy’ and in Fig. 3.
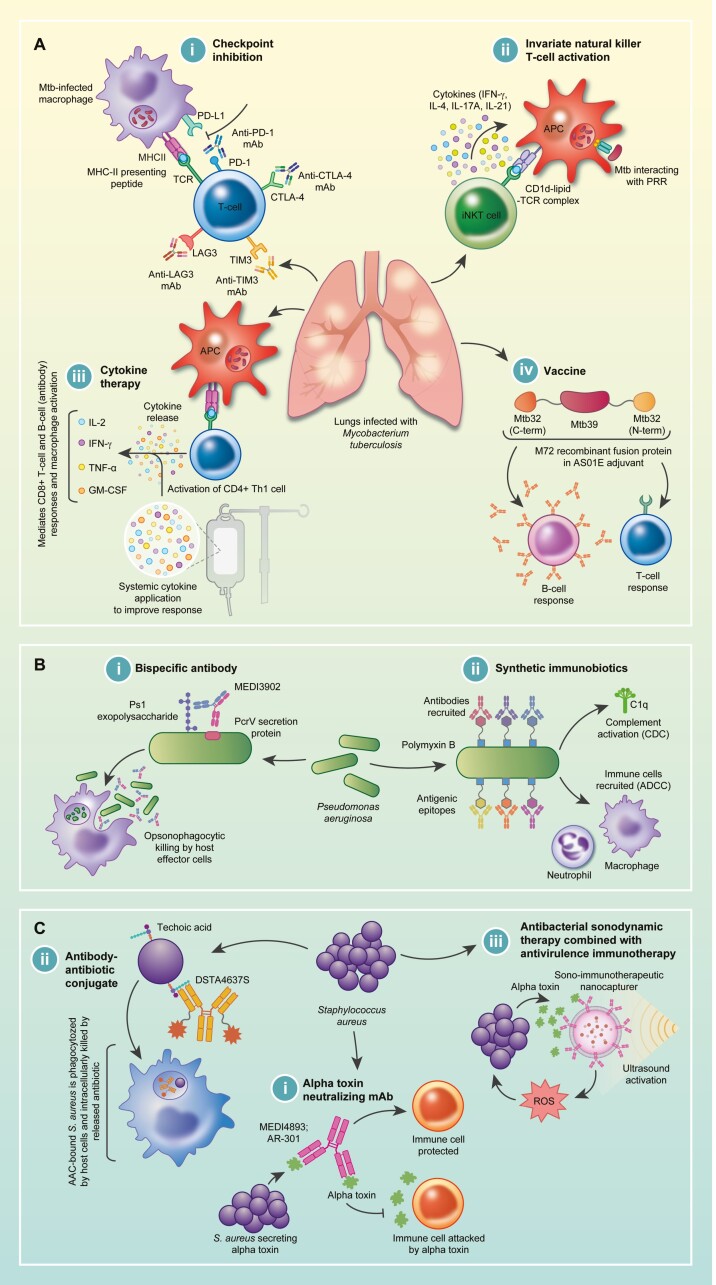
Figure 3.
A: (i) Mycobacterium tuberculosis can induce the expression of ligands for PD-1, CTLA-4, TIM3, and LAG3 on the surfaces of infected macrophages, thereby inhibiting T-cell activation. Using mAb targeting either receptors on the T-cell or ligands on antigen-presenting cells (APC) can disrupt the interaction between receptors and ligands, resulting in T-cell activation. (ii) Invariant natural killer T-cells recognize mycobacterial lipids presented by CD1d on APCs and subsequently secrete cytokines to mediate an immune response. (iii) Various cytokines (IL-2; IFN-γ; TNF-α; GM-CSF) are involved in the Th1 response to Mtb infection, and this effect can be supplemented by systemically administered cytokines. (iv) The novel M72/AS01E vaccine consists of an immunogenic fusion protein (M72) derived from two Mtb antigens (Mtb32 and Mtb39) in the AS01E adjuvant. Upon application, the vaccine mounts humoral (B-cell) and cell-mediated (T-cell) responses, conferring protection against active TB infection. B: (i) The bispecific mAb, MEDI3902, targets two P. aeruginosa virulence factors, part of the type-3 secretion system (PcrV) and the Psl exopolysaccharide. Binding to PcrV prevents cytotoxicity while binding to Psl favors complement-dependent opsonophagocytic killing by host effector cells. (ii) Synthetic immunobiotiocs involve the application of polymyxin B (antibiotic) conjugated to antigenic epitopes. Polymyxin B attaches to the cell surface of Gram-negative bacteria while the antigenic epitopes recruit antibodies in human serum, thereby re-engaging components of the immune system (complement system and antibody-dependent cellular cytotoxicity) against the pathogen. C: (i) Various mAbs can be used to target the S. aureus alpha-toxin, resulting in a protective strategy against the alpha-toxin-mediated killing of host immune cells. (ii) DSTA4637S, an antibody-antibiotic conjugate, specifically binds to the cell surface of S. aureus, followed by opsonophagocytosis of the conjugate, resulting in the intracellular delivery of the antibiotic to S. aureus within the host cell, ensuring more effective antibiotic bactericidal effects. (iii) The combination of antimicrobial sonodynamic therapy with anti-virulence immunotherapy involves the use of toxin-neutralizing antibodies on the surface of a nanovesicle, which is simultaneously loaded with sonosensitizers [meso-tetrakis (4-sulfonatophenyl) porphyrin (TPPS)] that produce reactive oxygen species capable of inducing bacterial cell death upon ultrasound activation.
A recently approved immunotherapeutic approach involves enhancing T-cell function via a chimeric antigen receptor (CAR) [9]. CAR-T-cells are engineered to express a recombinant receptor, usually incorporating a T-cell specificity determining antibody derivative binding to a specific receptor expressed on targeted cells fused to a transmembrane signaling domain, thus allowing MHC-independent T-cell activation. CAR T-cell therapy is a form of adoptive cell therapy, which involves isolating a patient’s peripheral blood T-cells, modifying it to express a CAR ex vivo, then administering the CAR-T-cells into the patient. As many first-generation CARs were anergic, subsequent modifications allowed engineering of not only targeting and transmembrane signaling domains such as a CD3 chain but also by incorporation of a co-stimulatory receptor-like CD28. The third- and fourth-generation CARs were developed with the addition of a second co-stimulatory molecule and an inducible gene to express pro-inflammatory or pro-proliferative cytokines, respectively [2].
In the present review, using examples of diseases that have had the most significant burden and impact on human health in the last decade, we aim to illustrate the advances made and the relevance of the most important immunotherapeutic strategies employed, from literature on research in the field of infectious disease and immunotherapy, published over mainly the last 5 years. Among the vast repertoire of information available, this has allowed us to narrow the viral pathogens discussed to Ebola, HIV, Zika virus, and SARS-CoV-2. Similarly, we have limited the antibacterial therapeutics presented, mostly to TB, with Pseudomonas aeruginosa and Staphylococcus aureus being specifically mentioned to highlight the importance of immunotherapy in circumventing drug resistance in such pathogens. Finally, the antiparasitic therapies have been limited to immunotherapies against malaria, leishmaniasis, and trypanosomiasis which are public health threats in Sub-Saharan Africa and Latin America. Fungal pathogen affects a small population of immunocompromised individuals such as patients with HIV and was thus considered beyond the scope of this review [2].
Immunotherapies of viral disease
Vaccines
Nucleic acid vaccines include mRNA or plasmid DNA (pDNA) vaccines [10]. Alphavirus genomes are commonly modified into mRNA vaccines where the genes encoding for structural proteins are replaced with genes encoding target antigens while conserving the RNA replication machinery. mRNA vaccines utilize the host’s cellular machinery to amplify the antigen-encoded RNA and post-translationally modify the resulting antigens, thereby mimicking natural infection [10]. The innate immune response is therefore stimulated, and the adaptive immune system is activated. There are currently no licensed RNA vaccines for human application, however, clinical trials are currently underway to develop mRNA vaccines expressing the pre-membrane (prM) and envelope (E) proteins of the Zika virus (ZIKV) (NCT04064905; NCT03014089) [11, 12]. Shortly after the discovery of the SARS-CoV-2 genome, an mRNA vaccine encoding the stabilized perfusion SARS-CoV-2 spike protein entered clinical trial (NCT04283461) [13]. During phase I and II, all participants developed anti-SARS-CoV-2 immune responses without developing trial-limiting side effects [14]. The vaccine has subsequently progressed to phase III (NCT04470427) and is currently under investigation for its efficacy against COVID-19. DNA vaccines are primarily based on pDNA backbones encoding for viral antigen with an inserted eukaryotic expression cassette [15]. Upon in vivo cellular uptake, the vaccine-induced high-capacity target gene expression, initiating an antigen-specific immune response [10]. Although there are currently no licensed human DNA vaccines, there are several ongoing clinical trials (Supplementary Table S1).
Viral vaccines use recombinant viral-based vectors, either in live attenuated or in non-replicative forms to express a target antigen [10]. There are numerous viral vector vaccines in clinical trials, including an adenovirus-based vaccine for ZIKV (NCT03356561) and two viral vaccines in phase III for SARS-CoV-2 [15]. The former is based on a type 5 adenovirus expressing SARS-CoV-2 spike (S) protein (NCT04341389) and the latter, expressing the same protein, is based on a chimpanzee adenoviral vector (ISRCTN89951424).
Recombinant protein-based vaccines (rPV) consist of immunogenic proteins from target pathogens. The lack of pathogenic genetic material and the absence of live pathogens make rPVs safer than other vaccine platforms [10]. It may also be the platform of choice for rapid production such as in the case of an emerging epidemic. Many rPV comprise recombinant-virus subunits derived from viral capsids that can self-assemble into viral-like particles [10]. Viral-like particles maintain their original conformation by displaying high numbers of antigen epitopes, thereby preserving viral immunogenicity, allowing crosslinking of B-cell receptors on B-cell surfaces while also permitting uptake into antigen-presenting cells. When self-assembly is not possible, target antigens are expressed as chimeric proteins [10]. There are multiple rPV undergoing pre-clinical and clinical evaluation for the prevention of SARS-CoV-2 (Supplementary Table S1).
Antibody-based therapies
To address the lack of clinically approved targeted therapy for ZIKV, antibody-based therapies have been developed. ZIKV-195, a potent human mAb that binds to the E protein protected mice against a lethal strain of the ZIKV [16]. Other notable E-protein-specific mAbs (Fig. 2D) include ZIKV-117, ZKA190, and the bispecific antibody FIT-1, which is using paratopes of two E-protein-specific mAbs, ZKA190, and ZKA185, was shown to retain the potency of the parental antibodies while simultaneously preventing the generation of resistant mutant strains [16–20].
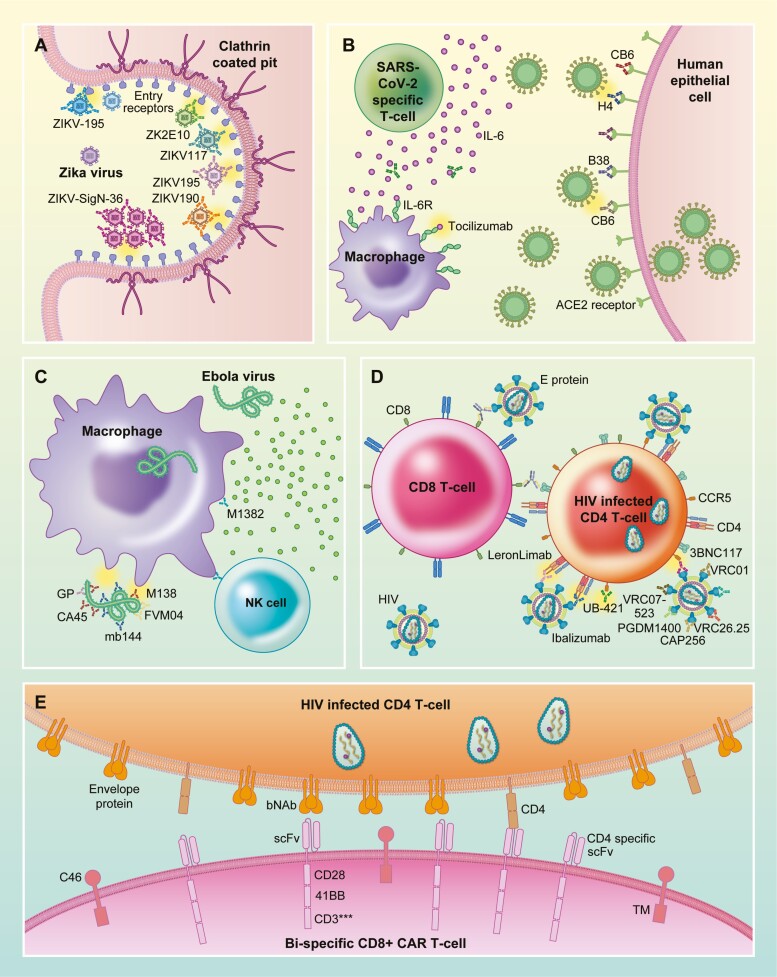
Figure 2.
Immunotherapy for viral diseases. (A) Zika virus E trimers bind to entry receptors found in clathrin coated pits of target cells during the initial stages of viral infection. Monoclonal antibodies ZK2E10, ZIKV117, ZIKV195, and ZIKV190 bind to the entry receptors preventing viral binding and infection. ZIKV-195 crosslinks E protein preventing the formation of E trimers needed for viral entry. ZIKV-SigN-36 binds to the E protein resulting in the formation of aggregates which prevents viral entry. (B) IL-6 secreted by SARS-CoV-2 activated T-cells contributes to the pathogenesis related to cytokine storms during infection. Tocilizumab binds to IL-6 preventing activation of IL-6 receptor, reducing inflammation resulting from the cytokine storm. SARS-CoV-2 binding to ACE2 receptor induces viral entry into target cells. Monoclonal antibodies CB6, H4, and B38 bind to ACE2 prevent viral binding and entry. (C) Attachment of Ebola virus to the surface of macrophages is the first step of viral infection. Monoclonal antibodies M138, CA45, mb144, and FVM04 specific for Ebola glycoproteins (GP) bind to the GP and prevent their interaction with macrophages thus preventing infection. Monoclonal antibody M1382-mediated antibody-dependent cellular cytotoxicity through the recruitment of NK-cells which degranulate and activate death signaling by binding to death receptors expressed on the cell membranes of Ebola-infected macrophages. (D) The first step of HIV infection is the binding of GP120 to CD4 receptor on target cells. CD4-specific monoclonal antibodies Leronlimab, Ibalizumab, and UB-421 bind to CD4 receptor on T-cells prevent viral GP120 from binding to the receptor, thus prevent viral entry and infection. GP120-specific antibodies VRC07, VRC01, 3BNC117, VRC26.25, CAP256, and PGDM1400 bind to GP120 on the viral envelop and prevent GP120 from binding to CD4 thus prevent viral entry into target cells. CD8 and GP120 bi-specific antibodies bind to CD8 with one arm and GP120 with the second arm bringing HIV into close proximity of cytotoxic T-cells, enhancing their capacity to target and kill the virus. (E) Dual CD4 and HIV E protein-specific CD8 CAR-T-cell binds to both CD4 and E proteins on CD4 infected T-cells, inducing cell death of the infected T-cell. The expression of C46 on the surface of the CAR-T-cell prevent the CAR T-cell itself from being infected by the HIV virus.
Passive immunization has proven to be a feasible treatment approach for the Ebola virus (EBOV) (Fig. 2B). A mAb cocktail containing the glycoprotein trimer-specific Abs FVM04 and CA45 was able to neutralize various EBOV strains and conferred complete protection in non-human primates [21]. Furthermore, FVM04 improved the potency and breadth of the ZMappTM antibody cocktail composed the glycoprotein-specific paratopes c2G4, c4G7, and c13C6, which originally failed to meet the targets in humans when [21, 22]. MBP134, which is composed of two broadly neutralizing antibodies (bNAbs) that binds to the base subdomain of the glycoprotein was shown to be effective against EBOV, was further modified to improve efficacy and engage NK cells [23]. Promising antibody monotherapy includes anti-EBOV mAb (M318) which neutralized EBOV and induced antibody-dependent cellular cytotoxicity, and EBOV-520, which neutralized EBOV in animal models [24, 25].
Individuals with SARS-CoV2 possess activated macrophages and T-cells which produce IL-6, eventually resulting in a cytokine storm [26]. The anti-IL-6 mAb, Tocilizumab, has shown promise in clinical studies, demonstrating efficacy in patients presenting with cytokine storm (Fig. 2A). Although it may reduce the necessity mechanical ventilation for infected people or prevent their death, conclusive results from ongoing trials are awaited [27–29]. CB6, a human mAb that binds to the SARS-CoV-2 receptor binding domain, inhibited the infection in rhesus monkeys, showing promise for human application [30]. Furthermore, another study described two antibodies, B38 and H4, which both bind to the receptor-binding domain, and thus neutralize SARS-CoV-2 in mice by preventing the interaction between the virus and the cell receptor thus blocking uptake [31].
Despite the efforts made to curb the overwhelming effects of the HIV/AIDS epidemic, the disease burden remains substantial [32]. The use of combination antiretroviral therapy (cART) has reduced transmission of the virus, the progression toward AIDS, and reduced viremia to below the limit of detection by standard test methods [31]. cART is, however, not curative and requires lifelong sustenance. Cessation of cART results in a rapid relapse in viremia, but lifetime use of cART is costly and may lead to drug-related toxicity emphasizing the need for safer, long-lasting treatments [10]. Antibody-based therapies targeting epitopes exclusive to diseased cells or foreign pathogens have shown promise (Fig. 2C). Ibalizumab, a CD4-directed mouse-derived recombinant humanized mAb received Food and Drug Administration approval for the treatment of multi-drug-resistant HIV-1 in adults [33]. Another CD4-directed humanized mAb, UB-421, was able to maintain viral suppression in the absence of ART in phase II clinical trial [34]. Rather than neutralizing multi-drug-resistant HIV-1 strains, bNAbs binding to the CD4 binding region or the V3 loop, have demonstrated a broader depth of antiviral activity by neutralizing myriads of HIV-1 strains. Promising anti-HIV-1 bNAbs include VRC01, 3BNC117, and 10–1074 [35]. VRC01 was well tolerated as a monotherapy in adults and new-born infants; however, viral suppression was not sustained, and relapse occurred following rapid clearance of the antibody from serum [36, 37]. Modifications were then made to VRC01, generating VRC01-LS, as well as other mAbs to extend the serum half-life [38]. The efficacy of the bNAbs was further improved by use in combination therapy. 3BNC117 used in combination with 10–1074 was well tolerated in healthy adults and achieved prolonged viral suppression in some patients [39]. The combination therapy further potentiated increased HIV group-specific antigen CD8+ T-cell response and increased the CD4+ T-cell response [38]. These strides to combat HIV have shown promise but would require further development before realizing clinical application.
CAR T-cell immunotherapy
Some of the very first CAR T-cells developed for HIV envelope protein (Env)-targeted treatment were generated by replacing the extracellular T cell receptor domain by CD4 (CD4-CAR). While the CAR treatment was safe and feasible in clinical trials, it failed to reduce viral burden permanently [40].
Second-generation CARs containing an intracellular CD28 domain exhibited higher cytokine production and better control over HIV replication in vitro but were susceptible to HIV infection. To overcome this, CD4-CARs were equipped with a viral fusion inhibitor or small hairpin RNAs which degrade viral RNA and knock down CCR5, an HIV-1 co-receptor [39]. Both strategies rendered the CD4-CARs resistant to infection and provided persistent control of infection in animal models. Several other editing techniques have been used to knock out CCR5 including zinc finger nucleases (NCT00842634; NCT01044654; NCT01252641), transcription activators and CRISPR-Cas9. Novel second-generation CARs targeting the HIV CD4 binding site or glycoprotein 120 (gp120) antigens were designed based on single-chain variable fragments derived from Env-specific bNAbs [39]. While these CARs demonstrated specific killing of HIV infected cells, their antiviral efficacy was highly variable and strain-dependent. This was improved by combining second-generation glycan CARs, targeting variable glycans regions on the surface of HIV with CCR5 ablation, enabling superior control of viral replication over the CAR alone. First-generation anti-gp120 CARs, efficiently stimulated activation, and cytokine secretion mediating lysis of Env-expressing HIV-1 infected CD4 T-cells in vitro [39]. Third-generation gp120-specific CARs had superior lysis over CD4 CARs and remained uninfected upon interaction with the cell-free virus. Furthermore, the CAR-induced cytolysis of reactivated HIV reservoirs isolated from infected patients [39]. The main drawback of this approach is viral escape mutants which render the therapy inefficient. To improve treatment efficacy, bi- and tri-specific CARs targeting up to three HIV antigens were designed. Two bi-specific CARs comprising a CD4 domain fused to gp120 or a carbohydrate recognition domain C-type human lectin that binds to conserved glycans on Env, showed superior suppressive activity compared to CD4-CAR [39]. Recently, CAR T-cells with three functionally distinct HIV Env-binding domains were engineered to express two distinct CARs on the same T-cell or one CAR with two targeting elements. Targets included gp120 CD4 binding site, a CD4-induced gp120 epitope and C46 peptide or C34-CXCR4 [41]. C46 peptide and C34-CXCR4 inhibit viral fusion preventing infection of the CAR T-cell [42]. Bi- and tri-specific CARs were able to prevent HIV infection of the CARs while efficiently killing other HIV-positive cells in humanized mouse models as noted in Fig. 2 [43]. There are currently two human clinical trials trying to eradicate the latent HIV reservoir; one is a bNAb-based CAR T-cell therapy (NCT03240328); the other a CD4-CAR T-cell therapy in conjunction with CCR5 ablation (NCT03617198).
In addition to HIV, two cytomegalovirus (CMV)-specific CARs were recently described. One CAR, based on a 21E9 glycoprotein subunit H targeting antibody, had superior activity in all functional tests. Surprisingly, it had 10-fold less binding affinity compared to other CARs targeting the same protein suggesting affinity not to be the main determinant of effectiveness. The 21E9-CAR however binds to a unique epitope suggested to be more accessible [41]. While the CAR showed only modest CD8+ T-cell killing of CMV-infected cells it provided support as a potential candidate for immunotherapy of CMV since they also stimulated cytokine release, the proliferation of effectors and the suppression of viral replication [41].
Aside from engineering CARs, T-cells can also be manipulated in different ways and used for adoptive cell transfer. In Zika virus models, a study generated CD4+ and CD8+ ZIKV-specific T-cell in clinically relevant numbers ex vivo. These T-cells expressed Th1 type cytokines and successfully killed human leukocyte antigen (HLA)-matched ZIKV-infected monocytes. Epitope mapping revealed that these T-cells bound to multiple novel HLA class I and class II epitopes on the NS1 antigen [42]. NS1 is essential to viral replication and immune evasion. This study provided a proof-of-concept for T-cell therapy as a potential treatment strategy against ZIKV infections.
Immunotherapies of bacterial infections
Vaccines
Mycobacterium tuberculosis (Mtb) is the etiological agent of TB and the leading cause of infectious disease-related deaths [44]. While Bacillus Calmette-Guérin (BCG), the only approved TB vaccine, provides consistent protection against severe extrapulmonary forms of pediatric TB, it confers limited protection against pulmonary TB in adults [45]. Furthermore, despite its widespread administration as a TB vaccine, its failure to prevent active TB infection emphasizes the need for novel strategies. An effective TB vaccine should ideally provide greater protective efficacy than BCG and prevent disease thereby disrupting Mtb transmission [44]. Unfortunately, many of the TB vaccines developed in the past have failed to achieve this. Notably, the MVA85A vaccine failed to improve the protective efficacy of BCG in infants and HIV-1-infected adults [46, 47]. Several novel vaccine candidates are currently advancing through or have recently completed clinical trials, with variable success (Supplementary Table S2). In a recent three year analysis of a prevention-of-disease study, the M72:AS01E subunit vaccine (Fig. 3A), composed of the immunogenic fusion protein (M72) derived from two Mtb antigens and the GlaxoSmithKline adjuvant AS01E, displayed a 49.7% efficacy in inducing protection against TB disease in HIV-negative individuals with latent TB infection, showing evident promise for this vaccine (NCT01755598) [48]. The limited success achieved in clinical trials investigating vaccines against infections caused by antibiotic-resistant P. aeruginosa and S. aureus (Supplementary Table S2) is partly attributed to suboptimal study designs that disregard the heterogeneity of patients, hospital epidemiology, specific bacterial strains, and disease progression, highlighting the need for detailed characterization of these parameters to ensure meaningful results [49].
Monoclonal antibody therapy
mAbs are being reconsidered for the treatment of bacterial infections [50]. Antibodies play an important role in immunomodulation during TB, evidenced by antibody profiles during latent TB infection which show enhanced Fc-mediated immune effector function and drive macrophage destruction of intracellular pathogens, highlighting the protective role of these antibodies [51]. However, to date, the development of protective mAbs against Mtb has failed. In contrast, several engineered mAbs for P. aeruginosa and S. aureus have progressed to clinical trials (Supplementary Table S2). MEDI3902 (AstraZeneca PLC), a bispecific IgG1 antibody targeting the PcrV protein (host cell cytotoxicity) and Psl exopolysaccharide (colonization and tissue adherence) of P. aeruginosa (Fig. 3B), is under development for the prevention of pneumonia in high-risk patients (NCT02696902) [52]. Furthermore, the targets are conserved across global isolates of P. aeruginosa and may mediate broad coverage [53]. AR-301 (Aridis Pharmaceuticals), a mAb with alpha-toxin (virulence factor) neutralizing capability (Fig. 3C), conferred protection against alpha toxin-mediated host cell damage when administered as adjunctive treatment to patients with methicillin-resistant S. aureus (MRSA) pneumonia (NCT03816956) [54]. Furthermore, MEDI4893 (AstraZeneca PLC), a novel, long-acting mAb targeting alpha-toxin (Fig. 3C) provided effective immunoprophylaxis against S. aureus disease in addition to sustaining serum levels after intravenous administration to healthy individuals and is currently in phase II clinical trial (NCT02296320) [55].
Checkpoint inhibition
While immune checkpoint inhibitors have revolutionized cancer therapeutics, varied outcomes exist regarding their efficacy in the management of TB. Despite the protective roles of CD4+ and CD8+ T-cells in the containment of Mtb, increasing evidence suggests their progressive dysfunction in patients with active TB infection, often resulting from the expression of inhibitory receptors (PD-1, CTLA-4, LAG3, and TIM3) causing T-cell exhaustion [56, 57]. While mAbs targeting PD-1 and its ligand (PD-L1) have been shown to restore tumor-specific T-cell function, it remains unclear whether this would be advantageous in the treatment of human TB [58, 59]. For instance, Mtb-infected PD-1 knockout mice are dramatically susceptible to new TB infections, which are characterized by higher mycobacterial loads and fatalities [59, 60]. Similarly, Tezera et al. (2020) demonstrate that the inhibition of PD-1 (in a 3D cell culture model of human TB) accelerates Mtb growth via excessive tumor necrosis factor-alpha (TNF-α) secretion [61]. Additionally, in vitro blockade of the PD-1/PD-L1 pathway, may result in enhanced production of IFN-γ, however, this may be insufficient to restore the proliferative potential of Mtb-specific CD4+ T-cells [57]. The development of checkpoint blockade-associated TB and atypical Mtb infections in patients receiving anti-PD-1/PD-L1 mAbs as cancer therapy support these findings [59, 62]. The role of TIM3 has also been evaluated in chronic Mtb infection; wherein functionally impaired TIM3+ T-cells co-expressed other inhibitory receptors while accumulating during infection [63]. Notably, the treatment of chronically infected mice with anti-TIM3 mAb improved T-cell function and achieved better control of the bacterial load [62]. Furthermore, LAG3 is promulgated as a more superior target than PD-1, since inhibiting its action activates T-cells and abrogates the suppressive activity induced by regulatory T-cells [64]. In summary, immune checkpoint expression in TB may be regarded as a physiological response to persistent Mtb pathogen, and its inhibition could potentially enhance infection and pathology, as observed in PD-1 inhibition in knockout mice, cellular, and epidemiological studies. The decision to implement immune checkpoint inhibition for TB treatment will therefore, most likely depend on various aspects such as the host (immunocompetence and HIV status), as well as specific mycobacterial factors (Mtb strain and drug resistance) [65].
T-cell-based immunotherapies
With the need to develop more effective therapeutic interventions for TB (with or without HIV coinfection), the relevance and applicability of T-cell-based immunotherapies are being actively investigated. Unconventional T-cells [natural killer T-cells (NKT), mucosal-associated invariant T-cells (MAIT), γδ T-cells, and HLA-E-restricted T-cells] – a heterogeneous group of T lymphocytes that are not limited to antigen recognition via the classical MHC – could be instrumental candidates in the development of TB-directed T-cell-based therapies [66]. Invariant NKT (iNKT) cells can recognize several lipids associated with the mycobacterial cell and produce different cytokines (IFN-γ, IL-4, IL-17A, and IL-21) that can mount an immune response against Mtb (Fig. 3A) [65]. The potential of iNKT cells is being investigated in phase I and II clinical trials for TB patients concurrently presenting with malignant solid tumors (NCT03551795).
Cytokine therapy
With a better understanding of their contributing roles in important biological processes, various cytokines are being manipulated to alter diseased states (Fig. 3A) [2]. A preclinical in vivo study demonstrated how a novel albumin-fused granulocyte-macrophage colony-stimulating factor (GM-CSF) enhanced its biostability and increased the dendritic cell populations responsible for inducing a potent immune response against Mtb [67]. Furthermore, adjunctive immunotherapy using recombinant human interleukin-2 (rhIL-2) is under clinical assessment in multi-drug-resistant TB patients, aiming to improve treatment efficiency and shortening treatment course (NCT03069534).
Emerging technologies against bacterial pathogens
Several new technologies are emerging against bacterial infections. Antibody-antibiotic conjugates promote the targeted delivery of the antibiotics while ensuring the maintenance of its bactericidal properties (Fig. 3C). DSTA4637S (Genentech), an anti-S. aureus antibody-antibiotic conjugates consisting of a mAb directed against S. aureus-specific wall-teichoic acids linked to an antibiotic showed favorable safety and pharmacokinetic profiles in phase I clinical trial (NCT03162250) [68]. A recent proof-of-concept study combined antimicrobial sonodynamic therapy with anti-virulence immunotherapy, in the form of a nanocapturer (Fig. 3C). These are composed of a neutralizing antibody on the surface nanovesicles loaded with sonosensitizers that produce reactive oxygen species upon ultrasound activation, thereby killing the bacteria and accelerating virulence clearance to eradicate MRSA in mice [69]. Furthermore, synthetic immunobiotics (Fig. 3B), consisting of polymyxin B (an antibiotic that attaches to the surface of Gram-negative bacteria) conjugated to antibody-recruiting antigenic epitopes to induce a targeted immune response, are under investigation [70]. These studies are promising for future treatment strategies for bacterial infections.
Immunotherapies of parasitic disease
Vaccines
The first and only human vaccine in use against a parasitic disease is RTS,S/AS01 (Mosquirix, GlaxoSmithKline) that showed limited efficacy (<50%) against Plasmodium falciparum among children. The vaccine was piloted in Ghana, Kenya, and Malawi in 2019 [71, 72]. Several subunit and irradiated sporozoite vaccines against malaria are currently under development and a comprehensive pipeline of notable anti-malarial vaccines in development are listed in a review by Philips et al. [73]. A recent review further elaborates on progress made in subunit-based vaccines for malaria [70]. Vaccines can be a useful tool against zoonotic transmission as several licensed vaccines for dogs have shown high efficacy against canine leishmaniasis [74]. Combinations vaccination therapy strategies are proving to be effective to treat parasitic disease by improving drug efficacy with a reduced dose. Recombinant Tc24 C4 antigen formulated with a toll-like receptor 4 (TLR4) agonist EC6020 exhibited synergism with benznidazole (one of the two chemotherapeutic agents used to treat Chagas disease), to abrogate parasite load in mice through the induction of a balanced Th1/Th2 response and the antigen-specific release of IFN-γ and IL-4 [75]. The development of nucleic acid-based vaccines can complement peptide vaccines especially to circumvent the limitations the latter possess. Such vaccines can elicit both innate and adaptive responses and would be beneficial to add to the vaccine compendium. Several mRNA vaccines against malaria, leishmaniasis, and toxoplasmosis are presently under investigation [76]. Treatment of Trypanosoma cruzi through DNA-based immunization using cruzipain (Cz)-encoding plasmid with GM-CSF was shown to induce a Th1 response through IgG2a production in mice and promote survival in a mouse infection study [77].
Monoclonal antibodies
Antibodies characterized by the humoral immune response of volunteers enrolled in clinical trials have led to the identification of potent mAbs such as mAbs 2530, 2544, 2586, and 2587 against P. falciparum transmission-blocking vaccine candidate Pfs25 [78]. Transmission-blocking vaccines aim to break the loop of vector to host transmission by targeting the sexual stage of P. falciparum, limiting infected host to vector parasite spread or by blocking the transmission of pre-erythrocytic P. falciparum from Anopheles mosquito to humans [77]. Similarly, Fab binding of the mAbs 311 and 317, derived from human donors enrolled in an RTS,S/AS01B clinical trial, to P. falciparum circumsporozoite protein inhibits in vivo parasite development in C57BL/6 mice [8]. mAbs such as humABAMA1 and humAB10.1–10.3, derived from semi-immune donors against the merozoite antigens Apical Membrane Antigen 1 (AMA1) and Merozoite Surface Protein 10 (MSP10), respectively, have shown promising in vitro inhibition of P. falciparum [79, 80]. The role of opsonizing antibodies is another area of research as recently demonstrated in vitro by the use of an MSP1 subunit antigen to induce opsonization-driven neutrophilic respiratory burst response to merozoites [81].
Checkpoint blockade
Blocking of the PD1/PD-L1 interaction using mAbs was shown to have therapeutic potential in mice to treat malaria and leishmaniasis. The mAbs used against PD1 receptor-targeted CD4+ lymphocytes and PD-L1 in dendritic cells in both diseases and the studies highlight the therapeutic potential of such antibodies [8, 82]. PD-L2 on the other hand plays a protective role in malarial infections through inhibition of PD1/PD-L1 interaction and mediating Th1 immunity. Multimeric PDL2 fused with the Fc region of immunoglobulin (PDL2–Fc) has been shown to reduce infections in mice infected with lethal or cerebral malaria [8].
Antibody conjugates
One of the most direct evidence of infectious disease benefiting from cancer immunotherapy can be illustrated using the example of MacGregor and colleagues who demonstrated receptor-dependent internalization and killing of Trypanosoma brucei using human haptoglobin-hemoglobin receptor (HpHbR) mAbs conjugated to a pyrrolobenzodiazepine toxin in vitro [83].
Future directions and conclusions
Despite the diversity of strategies in existence, besides vaccines, approved immunotherapies for human use against infectious disease trail behind those available to treat different cancers. Nevertheless, immunotherapies of infectious diseases have benefited from advances in cancer immunotherapy. For example, insights into immune evasion mechanisms exhibited by disease cells have led to the exploration of the role of checkpoint inhibition and T-cell exhaustion, which are in turn used as predictive markers of immune suppression to evolve treatment strategies and improve patient responses [8]. Drug and vaccine development against infectious disease are ongoing efforts that are perplexed in large part by multivariate factors such as diversity of pathogenic species causing disease, the clinical manifestation of the disease in different forms, the array of surface antigens that they express to circumvent host immunity and the various survival mechanisms (such as latency) they have evolved. Such facets challenge both the development and the efficacy of broad range vaccines and immunotherapeutic strategies employed against them [10, 70].
Immune-based approaches are particularly promising and aim to overcome limitations posed by conventional chemotherapeutics including efficacy, toxicity, and the looming challenge of drug resistance [73, 84]. As with most therapies for any disease, it has become increasingly evident that a multi-faceted approach for the treatment of various infectious diseases is required. Future pre-clinical and clinical studies would have to incorporate the best combinatorial strategies that will result in optimal patient outcomes. This may include a combination of immunotherapeutic approaches together with traditional treatment options to ensure sterilization of a disease like TB, which is defined by mycobacteria at varying states of replication, or malaria, which requires control measures on multiple levels to prevent infection and spread [73, 85]. The advantage of such an approach that prioritizes optimal clinical outcomes encourages the development of therapeutics that have a high degree of specificity and selectivity and advances medicine to an era of precision medicine [84, 86].
While it might be intuitive that such advances might come at a high cost, technological breakthroughs such as the use of phage display libraries for large-scale antibody fragment screening, improvements in molecular methods to fine-tune and improve antibody longevity and potency (thereby reducing the required dosage), identification of suitable hosts of expression, and optimization of cell culture conditions have all lead to decreasing costs of mAb-based therapies which have now have become comparable to the cost of essential chemotherapeutics over the last three decades, with concurrent improvements in their safety and efficacy. These have in turn spurred the regulatory approval and increased use of therapeutic antibodies thus overcoming multiple factors that previously discouraged their wider use [74, 84]. Novel vaccine platforms such as the use of DNA, mRNA, and viral vector vaccines provide alternative approaches that may lead to rapid and cheaper vaccine development pipelines, overcoming the limitations previously posed by peptide-based vaccines [33, 76, 84]. The parallel development of targeted delivery and improvements in vehicle technologies show the potential to overcome the drug safety issues that stem from systemic immunotherapy administration, expanding options that would be available for experimental interventions. Immunotherapeutic advances are hence increasingly becoming appealing options for infectious disease treatment.
State-of-the-art therapies such as CAR T-cell therapies are still very expensive treatment options costing several hundred thousand dollars per patient and year, in large part due to the approach having a complex process of development before delivery and administration to the patient. This limits the broad applicability of this approach to be used in resource-limited or poor settings, while also limiting their access due to economic disparities [87]. An ideal immunotherapeutic strategy would employ a means that can be easily manufactured, transported and administered easily (including e.g. subcutaneous or oral routes) with minimal numbers of doses to individuals, aiming to confer long-lasting protection that ensures maximum benefit with low costs and off-target effects. Achieving prolonged biological activity to confer protection while also having to ensure reduced costs for equitable access will be a recurring theme that will continue to push the limits and applicability of immunotherapies. Recombinant peptides such as vaccines, mAbs, and antibody conjugates seem like ideal candidates that may accomplish all such goals in the future. Making such a generalization however would mean skimming over the complexities of the underlying diverse biological mechanisms that contribute to disease progression such as factors contributing to host and pathogen heterogeneity, limitations in technology platforms and study models available; all of which are active areas of exploration and yet to be thoroughly understood.
The importance of vaccines and the need for novel therapeutic approaches to combat communicable disease is especially evidenced in recent times by the emergence of the SARS-CoV-2 pandemic and the subsequent, unprecedented clinical development of approximately a hundred vaccine candidates, and the repurposing of existing approved therapeutics such as the IL-6 inhibiting mAbs, Siltuximab, and Tocilizumab [88]. Access to prophylactic and therapeutic medication is still unfortunately very much affected by socio-economic differences. This is quite evident from how middle- and low-income countries bear the heaviest burden of infectious disease. Furthermore, low-income and indigenous populations in wealthier countries are disproportionately affected by infectious diseases [76]. A recent meta-analysis performed by Norris et al. showed that socio-economic status also affects the patient use of predictive biomarkers and precision medicine for therapy [89]. Though this was applicable to cancer immunotherapies, it would not be a stretch to infer that the broad applicability immunotherapies for infectious disease would have suffer from similar shortcomings. Collaborative efforts at local levels between hospitals, academia, and industry coupled with funding that encourages innovation, grants equitable access to technological and production platforms and coordinated global efforts would pave the way for the advancement of the use of immunotherapeutic approaches and hopefully contribute to the eradication of several communicable diseases in the coming decades.
Supplementary Material
Acknowledgements
The Editor-in-Chief and editorial team would like to thank the handling editor, Adriana Bonomo, and the following reviewers, Paul Elkington and Herbert Guedes, for their contribution to the publication of this article. All figures were designed by the authors and illustrated by Alison Schroeer.
Glossary
Abbreviations
- AMA 1
Apical membrane antigen 1
- BCG
Bacillus Calmette-Guérin
- bNAbs
Broadly neutralizing antibodies
- CAR
Chimeric antigen receptor
- cART
Combination antiretroviral therapy
- CMV
Cytomegalovirus
- CTLA4
Cytotoxic T-lymphocyte-associated protein 4
- Cz
Cruzipai
- E or Env
Envelope protei
- EBOV
Ebola virus
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- gp120
Glycoprotein 120
- HIV
Human immunodeficiency virus
- HLA
Human leukocyte antigen
- HpHb
Haptoglobin-hemoglobin receptor
- iNKT
Invariant natural killer T-cells
- mAbs
Monoclonal antibodies
- MAIT
Mucosal-associated invariant T-cells
- MHC
Major histocompatibility complex
- MRSA
Methicillin-resistant S. aureus
- MSP
Merozoite surface protein
- Mtb
Mycobacterium tuberculosis
- NK
Natural killer
- NKT
Natural killer T-cells
- PD1
Programmed cell death protein 1
- PD-L1
Programmed cell death 1 ligand 1
- pDNA
Plasmid DNA
- prM
Pre-membrane protein
- rhIL-2
Recombinant human interleukin-2
- rPV
Recombinant protein-based vaccines
- S
Spike protein
- TB
Tuberculosis
- TIM3
T-cell immunoglobulin and mucin domain-containing protein 3
- TLR4
Toll-like receptor 4
- TNF-α
Tumor necrosis factor-α
- ZIKV
Zika virus
Contributor Information
Dharanidharan Ramamurthy, Medical Biotechnology and Immunotherapy Research Unit, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Trishana Nundalall, Medical Biotechnology and Immunotherapy Research Unit, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Sanele Cingo, Medical Biotechnology and Immunotherapy Research Unit, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Neelakshi Mungra, Medical Biotechnology and Immunotherapy Research Unit, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Maryam Karaan, Medical Biotechnology and Immunotherapy Research Unit, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Krupa Naran, Medical Biotechnology and Immunotherapy Research Unit, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Stefan Barth, Medical Biotechnology and Immunotherapy Research Unit, Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa; Cancer Biotechnology, Department of Integrative Biomedical Sciences, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Funding
The authors would like to thank for in part support and funding of S.B. by the National Research Foundation of South Africa (Grant Number 47904).
Author contributions
D.R. contributed to the development of the article and authored the section on antiparasitic immunotherapies; T.N. and S.C. authored the section on antiviral immunotherapies. N.M. and M.K. contributed to the writing of immunotherapies for bacterial infections. K.N. and S.B. directly and substantially contributed through their critical intellectual input.
Conflict of interest
The authors of this review article declare that they have no conflicts of interest to disclose.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Papaioannou NE, Beniata OV, Vitsos P et al. Harnessing the immune system to improve cancer therapy. Ann Transl Med 2016;4:261. 10.21037/atm.2016.04.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naran K, Nundalall T, Chetty S et al. Principles of immunotherapy: implications for treatment strategies in cancer and infectious diseases. Front Microbiol 2018;9:3158. 10.3389/fmicb.2018.03158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rappuoli R, Pizza M, Giudice GD et al. Vaccines, new opportunities for a new society. Proc Natl Acad Sci USA 2014;111:12288–93. 10.1073/pnas.1402981111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janeway CA Jr, Travers P, Walport M et al. Immunobiology: The Immune System in Health and Disease. Immunological Memory. 5th edn. New York: Garland Science; 2001. [Google Scholar]
- 5. Hooks MA, Wade CS, Millikan WJ Jr. Muromonab CD-3: a review of its pharmacology, pharmacokinetics, and clinical use in transplantation. Pharmacotherapy 1991;11:26–37. [PubMed] [Google Scholar]
- 6. Suurs FV, Lub-de Hooge MN, de Vries EGE et al. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol Ther 2019;201:103–19. 10.1016/j.pharmthera.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 7. Riley RS, June CH, Langer R et al. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov 2019;18:175–96. 10.1038/s41573-018-0006-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 2018;18:91–104. 10.1038/nri.2017.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Update on CAR T Cell Therapies for the Treatment of Cancer. https://news.cancerconnect.com/treatment-care/update-on-car-t-cell-therapies-for-the-treatment-of-cancer-WxNkRaXV_Eeiq9Yy8hhm3A (10 October 2020, date last accessed).
- 10. Pitman MC, Lau JSY, McMahon JH et al. Barriers and strategies to achieve a cure for HIV. Lancet HIV 2018;5:e317–28. 10.1016/S2352-3018(18)30039-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson NAC, Kester KE, Casimiro D et al. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines 2020;5:11. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Makhluf H, Shresta S. Development of Zika Virus vaccines. Vaccines 2018;6:7. 10.3390/vaccines6010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim YC, Dema B, Reyes-Sandoval A. COVID-19 vaccines: breaking record times to first-in-human trials. NPJ Vaccines 2020;5:34. 10.1038/s41541-020-0188-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thanh Le T, Andreadakis Z, Kumar A et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov 2020;19:305–6. 10.1038/d41573-020-00073-5 [DOI] [PubMed] [Google Scholar]
- 15. Trovato M, Sartorius R, D’Apice L et al. Viral emerging diseases: challenges in developing vaccination strategies. Front Immunol 2020;11:2130. 10.3389/fimmu.2020.02130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esquivel RN, Patel A, Kudchodkar SB et al. In vivo delivery of a DNA-encoded monoclonal antibody protects non-human primates against Zika Virus. Mol Ther 2019;27:974–85. 10.1016/j.ymthe.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Long F, Doyle M, Fernandez E et al. Structural basis of a potent human monoclonal antibody against Zika virus targeting a quaternary epitope. Proc Natl Acad Sci USA 2019;116:1591–6. 10.1073/pnas.1815432116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erasmus JH, Archer J, Fuerte-Stone J et al. Intramuscular delivery of replicon RNA encoding ZIKV-117 human monoclonal antibody protects against Zika Virus infection. Mol Ther Methods Clin Dev 2020;18:402–14. 10.1016/j.omtm.2020.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang S, Loy T, Ng TS et al. A human antibody neutralizes different flaviviruses by using different mechanisms. Cell Rep 2020;31:107584. 10.1016/j.celrep.2020.107584 [DOI] [PubMed] [Google Scholar]
- 20. Wang J, Bardelli M, Espinosa DA et al. A human bi-specific antibody against Zika Virus with high therapeutic potential. Cell 2017;171:229–241.e15. 10.1016/j.cell.2017.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brannan JM, He S, Howell KA et al. Post-exposure immunotherapy for two ebolaviruses and Marburg virus in nonhuman primates. Nat Commun 2019;10:105. 10.1038/s41467-018-08040-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howell KA, Qiu X, Brannan JM et al. Antibody treatment of Ebola and Sudan Virus infection via a uniquely exposed epitope within the glycoprotein receptor-binding site. Cell Rep 2016;15:1514–26. 10.1016/j.celrep.2016.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wec AZ, Bornholdt ZA, He S et al. Development of a human antibody cocktail that deploys multiple functions to confer Pan-Ebolavirus protection. Cell Host Microbe 2019;25:39–48.e5. 10.1016/j.chom.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Q, Fan C, Li Q et al. Antibody-dependent-cellular-cytotoxicity-inducing antibodies significantly affect the post-exposure treatment of Ebola virus infection. Sci Rep 2017;7:45552. 10.1038/srep45552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gilchuk P, Kuzmina N, Ilinykh PA et al. Multifunctional Pan-ebolavirus antibody recognizes a site of broad vulnerability on the Ebolavirus glycoprotein. Immunity 2018;49:363–374.e10. 10.1016/j.immuni.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xu X, Han M, Li T et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 2020;117:10970–5. 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guaraldi G, Meschiari M, Cozzi-Lepri A et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2020;2:e474–84. 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Luo P, Liu Y, Qiu L et al. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol 2020;92:814–8. 10.1002/jmv.25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Biran N, Ip A, Ahn J et al. Tocilizumab among patients with COVID-19 in the intensive care unit: a multicentre observational study. Lancet Rheumatol 2020;2:e603–12. 10.1016/S2665-9913(20)30277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shi R, Shan C, Duan X et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020;584:120–4. 10.1038/s41586-020-2381-y [DOI] [PubMed] [Google Scholar]
- 31. Wu Y, Wang F, Shen C et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020;368:1274–8. 10.1126/science.abc2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. GBD 2017 HIV collaborators . Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV 2019;6:e831–59. 10.1016/S2352-3018(19)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu RM, Hwang YC, Liu IJ et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 2020;27:1. 10.1186/s12929-019-0592-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang CY, Wong WW, Tsai HC et al. Effect of anti-CD4 antibody UB-421 on HIV-1 rebound after treatment interruption. N Engl J Med 2019;380:1535–45. 10.1056/NEJMoa1802264 [DOI] [PubMed] [Google Scholar]
- 35. Promsote W, DeMouth ME, Almasri CG et al. Anti-HIV-1 antibodies: an update. Biodrugs 2020;34:121–32. 10.1007/s40259-020-00413-2 [DOI] [PubMed] [Google Scholar]
- 36. Cunningham CK, McFarland EJ, Morrison RL et al. ; IMPAACT P1112 team. Safety, tolerability, and pharmacokinetics of the broadly neutralizing human immunodeficiency virus (HIV)-1 monoclonal antibody VRC01 in HIV-exposed newborn infants. J Infect Dis 2020;222:628–36. 10.1093/infdis/jiz532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cale EM, Bai H, Bose M et al. ; RV397 Study Group. Neutralizing antibody VRC01 failed to select for HIV-1 mutations upon viral rebound. J Clin Invest 2020;130:3299–304. 10.1172/JCI134395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu Y, Cao W, Sun M et al. Broadly neutralizing antibodies for HIV-1: efficacies, challenges and opportunities. Emerg Microbes Infect 2020;9:194–206. 10.1080/22221751.2020.1713707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Niessl J, Baxter AE, Mendoza P et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat Med 2020;26:222–7. 10.1038/s41591-019-0747-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seif M, Einsele H, Löffler J. CAR T cells beyond cancer: hope for immunomodulatory therapy of infectious diseases. Front Immunol 2019;10:2711. 10.3389/fimmu.2019.02711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maldini CR, Claiborne DT, Okawa K et al. Dual CD4-based CAR T cells with distinct costimulatory domains mitigate HIV pathogenesis in vivo. Nat Med 2020;26:1776–87. 10.1038/s41591-020-1039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hanajiri R, Sani GM, Hanley PJ et al. Generation of Zika virus-specific T cells from seropositive and virus-naïve donors for potential use as an autologous or “off-the-shelf” immunotherapeutic. Cytotherapy 2019;21:840–55. 10.1016/j.jcyt.2019.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ali A, Chiuppesi F, Nguyen M et al. Chimeric antigen receptors targeting human cytomegalovirus. J Infect Dis 2020;222:853–62. 10.1093/infdis/jiaa171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019; 1. License: CCBY-NC-SA3.0IGO. [Google Scholar]
- 45. Sable SB, Posey JE, Scriba TJ. Tuberculosis vaccine development: progress in clinical evaluation. Clin Microbiol Rev 2019;33. 10.1128/CMR.00100-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tameris MD, Hatherill M, Landry BS et al. ; MVA85A 020 Trial Study Team. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013;381:1021–8. 10.1016/S0140-6736(13)60177-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ndiaye BP, Thienemann F, Ota M et al. ; MVA85A 030 trial investigators. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2015;3:190–200. 10.1016/S2213-2600(15)00037-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tait DR, Hatherill M, Van Der Meeren O et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med 2019;381:2429–39. 10.1056/NEJMoa1909953 [DOI] [PubMed] [Google Scholar]
- 49. Bekeredjian-Ding I. Challenges for clinical development of vaccines for prevention of hospital-acquired bacterial infections. Front Immunol 2020;11:1755. 10.3389/fimmu.2020.01755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Motley MP, Banerjee K, Fries BC. Monoclonal antibody-based therapies for bacterial infections. Curr Opin Infect Dis 2019;32:210–6. 10.1097/QCO.0000000000000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lu LL, Chung AW, Rosebrock TR et al. A functional role for antibodies in tuberculosis. Cell 2016;167:433–443.e14. 10.1016/j.cell.2016.08.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ali SO, Yu XQ, Robbie GJ et al. Phase 1 study of MEDI3902, an investigational anti-Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults. Clin Microbiol Infect 2019;25:629.e1–6. 10.1016/j.cmi.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 53. Tabor DE, Oganesyan V, Keller AE et al. Pseudomonas aeruginosa PcrV and Psl, the molecular targets of bispecific antibody MEDI3902, are conserved among diverse global clinical isolates. J Infect Dis 2018;218:1983–94. 10.1093/infdis/jiy438 [DOI] [PubMed] [Google Scholar]
- 54. François B, Mercier E, Gonzalez C et al. ; MASTER 1 study group. Safety and tolerability of a single administration of AR-301, a human monoclonal antibody, in ICU patients with severe pneumonia caused by Staphylococcus aureus: first-in-human trial. Intensive Care Med 2018:1787–96. 10.1007/s00134-018-5229-2 [DOI] [PubMed] [Google Scholar]
- 55. Ruzin A, Wu Y, Yu L et al. Characterisation of anti‐alpha toxin antibody levels and colonisation status after administration of an investigational human monoclonal antibody, MEDI4893, against Staphylococcus aureus alpha toxin. Clin Transl Immunol 7:e1009. 10.1002/cti2.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wherry EJ. T cell exhaustion. Nat Immunol 2011;12:492–9. 10.1038/ni.2035 [DOI] [PubMed] [Google Scholar]
- 57. Day CL, Abrahams DA, Bunjun R et al. PD-1 expression on Mycobacterium tuberculosis-specific CD4 T cells is associated with bacterial load in human tuberculosis. Front Immunol 2018;9:1995. 10.3389/fimmu.2018.01995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Godfrey MS, Friedman LN. Tuberculosis and biologic therapies: anti-tumor necrosis factor-α and beyond. Clin Chest Med 2019;40: 721–39. 10.1016/j.ccm.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 59. Barber DL, Sakai S, Kudchadkar RR et al. Tuberculosis following PD-1 blockade for cancer immunotherapy. Sci Transl Med 2019;11(475):eaat2702. 10.1126/scitranslmed.aat2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lázár-Molnár E, Chen B, Sweeney KA et al. Programmed death-1 (PD-1)-deficient mice are extraordinarily sensitive to tuberculosis. Proc Natl Acad Sci USA 2010;107:13402–7. 10.1073/pnas.1007394107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tezera LB, Bielecka MK, Ogongo P et al. Anti-PD-1 immunotherapy leads to tuberculosis reactivation via dysregulation of TNF-. Elife 2020;9:e52668-70. 10.7554/eLife.52668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anand K, Sahu G, Burns E et al. Mycobacterial infections due to PD-1 and PD-L1 checkpoint inhibitors. ESMO Open 2020;5:e000866. 10.1136/esmoopen-2020-00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jayaraman P, Jacques MK, Zhu C et al. TIM3 Mediates T cell exhaustion during Mycobacterium tuberculosis infection. PLoS Pathog 2016;12:e1005490. 10.1371/journal.ppat.1005490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Phillips BL, Gautam US, Bucsan AN et al. LAG-3 potentiates the survival of Mycobacterium tuberculosis in host phagocytes by modulating mitochondrial signaling in an in-vitro granuloma model. PLoS One 2017;12:e0180413. 10.1371/journal.pone.0180413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Langan EA, Graetz V, Allerheiligen J et al. Immune checkpoint inhibitors and tuberculosis: an old disease in a new context. Lancet Oncol 2020;21:e55–65. 10.1016/S1470-2045(19)30674-6 [DOI] [PubMed] [Google Scholar]
- 66. La Manna MP, Orlando V, Tamburini B et al. Harnessing unconventional T cells for immunotherapy of tuberculosis. Front Immunol 2020;11:2107. 10.3389/fimmu.2020.02107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chuang YM, He L, Pinn ML et al. Albumin fusion with granulocyte-macrophage colony-stimulating factor acts as an immunotherapy against chronic tuberculosis. Cell Mol Immunol 2020. 10.1038/s41423-020-0439-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Peck M, Rothenberg ME, Deng R et al. A phase 1, randomized, single-ascending-dose study to investigate the safety, tolerability, and pharmacokinetics of DSTA4637S, an anti-Staphylococcus aureus thiomab antibody-antibiotic conjugate, in healthy volunteers. Antimicrob Agents Chemother 2019;63:1–12. 10.1128/AAC.02588-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pang Z, Raudonis R, Glick BR et al. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv 2019;37:177–92. 10.1016/j.biotechadv.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 70. Feigman MS, Kim S, Pidgeon SE et al. Synthetic immunotherapeutics against Gram-negative pathogens. Cell Chem Biol 2018;25:1185–1194.e5. 10.1016/j.chembiol.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Skwarczynski M, Chandrudu S, Rigau-Planella B et al. Progress in the development of subunit vaccines against malaria. Vaccines (Basel) 2020;8:373. 10.3390/vaccines8030373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. WHO | Q&A on the malaria vaccine implementation programme (MVIP). WHO. http://www.who.int/malaria/media/malaria-vaccine-implementation-qa/en/ (10 October 2020, date last accessed).
- 73. Phillips MA, Burrows JN, Manyando C et al. Malaria. Nat Rev Dis Primers 2017;3:17050. 10.1038/nrdp.2017.50 [DOI] [PubMed] [Google Scholar]
- 74. Ikeogu NM, Akaluka GN, Edechi CA et al. Leishmania immunity: advancing immunotherapy and vaccine development. Microorganisms 2020;8:1201. 10.3390/microorganisms8081201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jones K, Versteeg L, Damania A et al. Vaccine-linked chemotherapy improves benznidazole efficacy for acute Chagas disease. Infect and Immun. 2018;86:15. 10.1128/IAI.00876-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Versteeg L, Almutairi MM, Hotez PJ et al. Enlisting the mRNA vaccine platform to combat parasitic infections. Vaccines 2019;7:122. 10.3390/vaccines7040122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cerny N, Sánchez Alberti A, Bivona AE et al. Coadministration of cruzipain and GM-CSF DNAs, a new immunotherapeutic vaccine against Trypanosoma cruzi infection. Hum Vaccin Immunother 2016;12:438–50. 10.1080/21645515.2015.1078044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scally SW, McLeod B, Bosch A et al. Molecular definition of multiple sites of antibody inhibition of malaria transmission-blocking vaccine antigen Pfs25. Nat Commun 2017;8:1568. 10.1038/s41467-017-01924-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Maskus DJ, Królik M, Bethke S et al. Characterization of a novel inhibitory human monoclonal antibody directed against Plasmodium falciparum Apical Membrane Antigen 1. Sci Rep 2016;6:39462. 10.1038/srep39462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maskus DJ, Bethke S, Seidel M et al. Isolation, production and characterization of fully human monoclonal antibodies directed to Plasmodium falciparum MSP10. Malar J 2015;14:276. 10.1186/s12936-015-0797-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jäschke A, Coulibaly B, Remarque EJ et al. Merozoite surface protein 1 from Plasmodium falciparum is a major target of opsonizing antibodies in individuals with acquired immunity against malaria. Wilkins PP (ed.). Clin Vaccine Immunol 2017;24:e00155–17. 10.1128/CVI.00155-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. da Fonseca-Martins AM, Ramos TD, Pratti JES et al. Immunotherapy using anti-PD-1 and anti-PD-L1 in Leishmania amazonensis-infected BALB/c mice reduce parasite load. Sci Rep 2019;9:20275. 10.1038/s41598-019-56336-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. MacGregor P, Gonzalez-Munoz AL, Jobe F et al. A single dose of antibody-drug conjugate cures a stage 1 model of African trypanosomiasis. PLoS Negl Trop Dis 2019;13:e0007373. 10.1371/journal.pntd.0007373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hooft van Huijsduijnen R, Kojima S, Carter D et al. Reassessing therapeutic antibodies for neglected and tropical diseases. PLoS Negl Trop Dis 2020;14:e0007860. 10.1371/journal.pntd.0007860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ramos-Espinosa O, Islas-Weinstein L, Peralta-Álvarez MP et al. The use of immunotherapy for the treatment of tuberculosis. Expert Rev Respir Med 2018;12:427–40. 10.1080/17476348.2018.1457439 [DOI] [PubMed] [Google Scholar]
- 86. Mahon RN, Hafner R. Applying precision medicine and immunotherapy advances from oncology to host-directed therapies for infectious diseases. Front Immunol 2017;8:688. 10.3389/fimmu.2017.00688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yadav RK, Ali A, Kumar S et al. CAR T cell therapy: newer approaches to counter resistance and cost. Heliyon 2020;6:e03779. 10.1016/j.heliyon.2020.e03779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Margolin E, Burgers WA, Sturrock ED et al. Prospects for SARS-CoV-2 diagnostics, therapeutics and vaccines in Africa. Nat Rev Microbiol 2020;18:690–704. 10.1038/s41579-020-00441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Norris RP, Dew R, Sharp L et al. Are there socio-economic inequalities in utilization of predictive biomarker tests and biological and precision therapies for cancer? A systematic review and meta-analysis. BMC Med 2020;18:282. 10.1186/s12916-020-01753-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analysed in support of this research.