Abstract
OBJECTIVES
Our goal was to evaluate the prevalence of and risk factors for pneumothorax in patients with invasive mechanical ventilation in the intensive care unit (ICU) diagnosed with coronavirus disease 2019 pneumonia.
METHODS
The prevalence of pneumothorax was retrospectively reviewed in 107 patients diagnosed with coronavirus disease 2019 pneumonia and treated in an ICU in Turkey between 11 March 2020 and 30 April 2020.
RESULTS
The patients were aged 19–92 years; 37 (34.6%) were women. Pneumothorax developed in 8 (7.5%) of the intubated patients. Four (50%) of the patients with pneumothorax and 68 (68.7%) of those without it died. In the univariable logistic regression analysis of the presence of comorbid diseases (P = 0.91), positive end-expiratory pressure (P = 0.18), compliance (P = 0.93), peak pressure (P = 0.41) and the Horowitz index (P = 0.13) did not show statistically significant effects in increasing the risk of pneumothorax.
CONCLUSIONS
There was no significant increase or decrease in the risk of pneumothorax in patients treated with invasive mechanical ventilation after the diagnosis of coronavirus disease 2019-related pneumonia/acute respiratory distress syndrome. However, consideration of the risk of pneumothorax in these individuals may have the potential to improve the prognoses in such settings.
Keywords: Pneumothorax, Mechanical ventilation, Coronavirus disease 2019, Positive end-expiratory pressure
Severe acute respiratory syndrome coronavirus 2 is associated with high rates of transmission and death [1].
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 is associated with high rates of transmission and death [1]. In patients diagnosed with coronavirus disease 2019 (COVID-19), chest lesions may progress to confluent bilateral consolidation with the development of lung lesions in the lower lobes of the lungs, resulting in complications such as mediastinal emphysema, subcutaneous emphysema and pneumothorax [2, 3].
Invasive mechanical ventilation is frequently used in patients who receive treatment in the intensive care unit (ICU) following the diagnosis of COVID-19 pneumonia, and the mortality risk is high among patients with severe disease in such settings [4]. However, data on the complications associated with the use of invasive mechanical ventilation in the treatment of COVID-19 are limited. Pneumothorax is among the complications that may present during COVID-19 treatment [2, 3]. In a previous study, 5.9% of 202 COVID-19 patients undergoing emergency tracheal intubation developed pneumothorax, and this rate increased to 10.4% within 24 h [5].
COVID-19 causes serious respiratory problems and is therefore associated with a strong requirement for intensive care and invasive mechanical ventilation [4, 5]. During the COVID-19 pandemic, one of the reasons to consult with thoracic surgeons concerning patients with invasive mechanical ventilation during a stay in the ICU is the development of pneumothorax. In this setting, pneumothorax may develop for various reasons. However, it is not known whether invasive mechanical ventilation is associated with the risk of development of pneumothorax in these patients. Accordingly, our goal was to investigate the frequency of occurrence of pneumothorax in patients with COVID-19-related pneumonia/acute respiratory distress syndrome who were treated with invasive mechanical ventilation.
MATERIALS AND METHODS
Data on patients who received invasive mechanical ventilation in the ICU due to COVID-19 between 11 March 2020 and 30 April 2020 were retrospectively screened. Approval for the use of data from patients with COVID-19 treated in the ICU of the Bakırköy Dr. Sadi Konuk Training and Research Hospital, Turkey was obtained from the Ministry of Health and the ethics committee of the hospital. In addition, approval was obtained from the patients and their relatives to use their data in the study. We included data on patients who received invasive mechanical ventilation in the ICU due to COVID-19 pneumonia and were discharged or who died. Data on patients who continued to receive treatment during the study period were excluded. The inclusion criteria were COVID-19 diagnosis, receiving treatment in the ICU and requiring invasive mechanical ventilation. Exclusion criteria were a negative real-time reverse transcriptase-polymerase chain reaction test result for COVID-19, absence of mechanical ventilator use, diagnosis of pneumothorax before intensive care treatment and diagnosis of pneumothorax before mechanical ventilation.
In terms of the mechanical ventilation strategy applied to the patients in the study, a low tidal volume (6–8 ml/kg) and a sufficient positive end-expiratory pressure (PEEP), which is the lowest PEEP required for optimal oxygenation, were used to reduce the degree of alveolar distention. Low tidal volume is the tidal volume required to prevent atelectasis. The researchers of this study provided all the patients with intensive care treatment. We applied a low tidal volume to prevent negative consequences, such as the poor oxygenation that results from barotrauma and alveolar capillary permeability. In addition, the treatment team avoided the use of an excessive fluid load to protect the lungs.
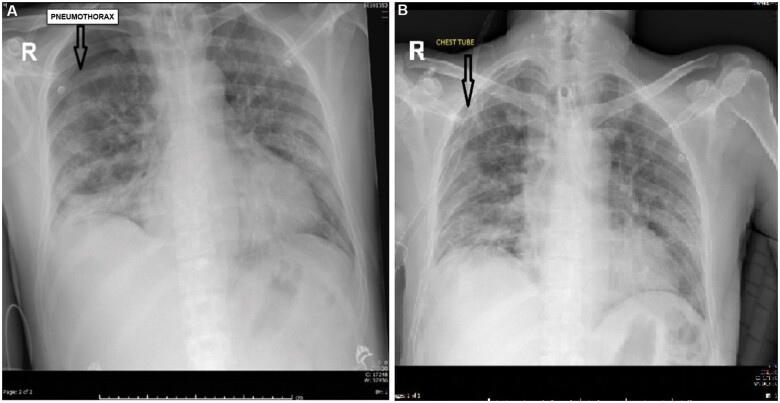
Data from 107 patients who met the inclusion criteria were evaluated. Data on age, sex, comorbid disease, mortality, pneumothorax and mechanical ventilation were collected and registered in a form. In patients who were receiving treatment in the COVID-19 ICU, pneumothorax was diagnosed by a thoracic surgeon who evaluated the clinical and radiological findings. Because the clinical findings associated with pneumothorax tend to vary across patients, the medical records of those diagnosed with pneumothorax were re-examined in detail, and all diagnoses were reviewed by a thoracic surgeon who was not involved in the treatment of the patients to allow for accurate diagnostic confirmation. In addition, data on patients’ blood gas levels, saturation results, chest radiographic findings, ultrasound results and computed tomographic (thorax) findings were examined to check the diagnoses. Bilateral pneumothorax was observed in 1 patient, unilateral pneumothorax was observed in 7 patients (in the right lung in 5 patients and in the left lung in 2 patients). All patients had a chest tube inserted using a conventional method. The necessary care and sensitivity were shown to protect the lung(s) during the surgical procedure (Fig. 1).
Figure 1:
A case of pneumothorax before (A) and after treatment (B).
Mechanical ventilation
PEEP is defined as the positive pressure that remains in the airway at the end of the respiratory cycle, whereas peak pressure is the highest pressure value in the airway with the breath given to the patient by the ventilator in the inspiration. Compliance, in this context, refers to the volume change that occurs against the unit pressure change, reflecting the elastic properties of the respiratory system. In this study, the value that was recorded just before the development of the pneumothorax was considered the PEEP. In other patients, the highest PEEP value that was applied during the treatment process was recorded as the PEEP.
Collins method
The Collins method was used to determine the size of the pneumothorax (%), using the formula Y = 4.2 + [4.7 × (A + B + C)], in which A is the distance between the apex of the partially collapsed lung and the apex of the thoracic cavity, B refers to the midpoint of the upper half of the collapsed lung and C indicates the midpoint of the lower half of the collapsed lung.
Statistical analysis
Descriptive analyses, such as percentage median (minimum–maximum), were used to evaluate the characteristics of the patients diagnosed with COVID-19. A univariable logistic regression analysis was used to evaluate whether the parameters related to mechanical ventilation (compliance, peak pressure, the Horowitz index and PEEP) and demographic features (comorbidities and age) had a significant effect on increasing the risk of pneumothorax. In the univariable logistic regression analysis, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each variable. The significance level was determined at P-value <0.05 for all analyses. The IBM SPSS 22.0 program (IBM Corp., Armonk, NY, USA) was used for the analyses.
RESULTS
The patients were aged between 19 and 92 years; 37 (34.6%) were women. Pneumothorax developed in 8 (7.5%) of the patients who were invasively intubated. The characteristics of those with and without pneumothorax are shown in Table 1.
Table 1:
Comparison of the demographic characteristics of participants with and without pneumothorax
| Pneumothorax present (n = 8) | Pneumothorax absent (n = 99) | |
|---|---|---|
| Age (years) | 61 (53–63.5) | 60 (51–70) |
| Treatment duration (days) | 27.5 (16.5–39.0) | 10 (4–19) |
| Compliance | 33 (28.5–37.5) | 33 (27–37) |
| Peak pressure | 26.5 (24.5–30.0) | 27 (24–29) |
| Horowitz index | 140.5 (92.5–173) | 167 (121–209) |
| PEEP | 10 (9.5–10) | 9 (8–10) |
| Above-PEEP | 14 (14–20) | 17 (15–20) |
| Sex | ||
| Male | 8 (100.0) | 62 (62.6) |
| Female | 0 (0.0) | 37 (37.4) |
| Ventilation modes | ||
| CPAP | 1 (12.5) | 1 (1.0) |
| PCV | 4 (50.0) | 89 (89.9) |
| PRVC | 3 (37.5) | 2 (2.0) |
| PSV | 0 (0.0) | 2 (2.0) |
| SIMV | 0 (0.0) | 5 (5.1) |
| ECMO | ||
| Yes | 1 (12.5) | 2 (2.0) |
| No | 2 (87.5) | 97 (98.0) |
| Deaths | ||
| Yes | 4 (50.0) | 68 (68.7) |
| No | 4 (50.0) | 31 (31.3) |
| Tracheostomy | ||
| Yes | 3 (37.5) | 11 (11.1) |
| No | 5 (62.5) | 88 (88.9) |
| Comorbid disease | ||
| Yes | 6 (75.0) | 76 (76.8) |
| No | 2 (25.0) | 23 (23.2) |
Data are presented as median and interquartile range (25th–75th) or n (%).
CPAP: continuous positive airway pressure; ECMO: extracorporeal membrane oxygenation; PCV: pressure control ventilation; PEEP: positive end-expiratory pressure; PRVC: pressure regulated volume control: PSV: pressure support ventilation: SIMV: synchronized intermittent mandatory ventilation.
A total of 76 (76.8%) of the 99 patients who did not develop pneumothorax had comorbid diseases. Among those without pneumothorax, cancer (nasopharyngeal, testicular, laryngeal, malignant melanoma, colon and chronic lymphocytic leukaemia) was observed in 9 (9.1%) patients, cerebrovascular disease in 7 (7.1%), chronic obstructive pulmonary disease in 8 (8.1%), chronic kidney failure in 17 (17.2%), asthma in 4 (4.0%), coronary artery disease in 21 (21.2%), diabetes mellitus in 46 (46.5%), hypertension in 50 (50.5%), psychiatric disorders (schizophrenia and depression) in 3 (3.0%), neurological diseases (epilepsy, mental retardation, Alzheimer disease, cerebrovascular disorders) in 9 (9.1%) and other diseases (cirrhosis, hepatitis B, hypothyroidism, rheumatoid arthritis, pemphigus vulgaris, familial Mediterranean fever, ankylosing spondylitis, gout, glioblastoma multiforme and gastrointestinal perforation) in 13 (13.1%) patients.
Pneumothorax presented after implementation of mechanical ventilation in 2 (25%) of the 8 patients in whom it was observed in the left lung, 5 (62.5%) of those in whom it was observed in the right lung and 1 (12.5%) who had bilateral pneumothorax. The air leak continued till the end of the first day in 2 of the patients (25.0%) with pneumothorax. Additionally, coronary artery disease was noted in 2 (25.0%) patients, hypertension in 1 (12.5%), chronic hepatitis B infection in 1 (12.5%), both epilepsy and cerebrovascular disease in 1 (12.5%) and both gout and benign prostatic hyperplasia in 1 (12.5%) patient. Patients tended to develop pneumothorax between 3 and 25 days following their admission to the ICU and between 1 and 25 days after intubation. When the pneumothorax states were measured, the Collins formula yielded values ranging from 30.85% to 139.0%. The other clinical features of the pneumothorax cases are shown in Table 2.
Table 2:
Clinical features of patients with pneumothorax
| Cases | Day of pneumothorax development after intensive care unit admission | Day of pneumothorax development after intubation | Location of pneumothorax | Drainage | Air leak | Chest tube follow-up duration (days) | Right (%) | Left (%) | Comorbid diseases |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 3 | L | 0 | 1 | 2 | 15.3 | CAD | |
| 2 | 3 | 1 | L | 200 | 0 | 15 | 50.8 | HT | |
| 3 | 4 | 4 | R | 200 | 0 | 5 | 42.4 | Chronic HB | |
| 4 | 8 | 8 | R | 0 | 0 | 6 | 31.7 | None | |
| 5 | 4 | 4 | R | 1500 | 0 | 2 | 139.0 | None | |
| 6 | 15 | 15 | R | 300 | 0 | 6 | 41.8 | CAD | |
| 7 | 4 | 1 | R | 100 | 0 | 10 | 61.5 | Epilepsy, CD | |
| 8 | 25 | 25 | RL | 200 | 1 |
R (5) L (12) |
30.9 | 31.5 | Gout, BPH |
BPH: benign prostatic hyperplasia; CAD: coronary artery disease; CD: cerebrovascular disease; HB: hepatitis B; HT: hypertension.
The duration of chest intubation among those with pneumothorax varied between 2 and 15 days (median 7). The chest tube was removed on the day of death in 2 patients, and on the day of extubation in 3 patients. In 1 patient, the chest tube was removed 2 days after extubation.
In the univariable logistic regression analysis, pneumothorax and comorbidities were coded as negative conditions. In the analysis, age (OR 0.99, CI 0.93–1.04; P = 0.67), presence of comorbid diseases (OR 0.91, CI 0.17–4.81; P = 0.91), PEEP (OR 1.42, CI 0.86–2.36; P = 0.18), compliance (OR 1.00, CI 0.92–1.08; P = 0.93), peak pressure (OR 0.90, CI 0.70–1.16; P = 0.41) and the Horowitz index (OR 0.99, CI 0.98–1.00; P = 0.13) did not show statistically significant effects in increasing the risk of pneumothorax (Table 3).
Table 3:
Univariable logistic regression analysis of pneumothorax risk
| OR | 95% CI | P-value | |
|---|---|---|---|
| Age | 0.99 | 0.93–1.04 | 0.67 |
| Comorbid diseases (present) | 0.91 | 0.17–4.81 | 0.91 |
| PEEP | 1.42 | 0.86–2.36 | 0.18 |
| Compliance | 1.00 | 0.92–1.08 | 0.93 |
| Peak pressure | 0.90 | 0.70–1.16 | 0.41 |
| Horowitz index | 0.99 | 0.98–1.00 | 0.13 |
CI: confidence interval; OR: odds ratio; PEEP: positive end-expiratory pressure.
DISCUSSION
In this study, the rate of pneumothorax development was 7.5% among patients in the ICU who underwent invasive mechanical ventilation following the diagnosis of COVID-19 pneumonia. Additionally, all the patients who developed pneumothorax were men, and 50% of the patients died. The features associated with mechanical ventilation did not show statistically significant effects in increasing the risk of pneumothorax in patients with COVID-19.
The rate of pneumothorax due to barotrauma has been found to vary between 4% and 15% in patients with intubation, and the frequency of pneumothorax may increase, depending on the duration of mechanical ventilation and the presence of comorbid diseases [6, 7]. In a previous study, the rate of pneumothorax development was 5.9% in the first 24 h following initiation of ventilation, and this rate increased to 10.4% after 1 day [5]. COVID-19 causes severe respiratory problems, often requiring invasive mechanical ventilation. However, the effects of COVID-19 on the human body are still being explored. Based on the existing literature and the findings of our study, it can be concluded that the rate of pneumothorax development in patients with COVID-19 is similar to that among patients without COVID-19 who undergo invasive mechanical ventilation.
In this study, compliance, peak pressure, the Horowitz index and PEEP were not significant in increasing the risk of pneumothorax in patients with COVID-19. Manoeuvres performed to correct the quality of oxygenation with high PEEP values in positive pressure ventilation may cause alveolar rupture due to overvoltage in the alveoli, and this situation may lead to pneumothorax. The risk factors associated with pneumothorax vary across patients treated in the ICU, and various applications, especially thoracentesis, central venous catheter placement, bronchoscopy, pericardiocentesis and tracheotomy, are related to a high risk [7]. In addition, invasive mechanical ventilation leads to different side effects, such as decreased right ventricular preload, respiratory system complications, auto-PEEP, pneumonia and gastrointestinal complications [8]. Therefore, the pneumothorax that develops in COVID-19 patients with mechanical ventilation in the ICU may be caused by different factors.
The length of hospital stay differed across those with and without pneumothorax. Because pneumothorax is among the complications that develop during the follow-up of patients, both the duration of treatment and length of stay in the ICU are likely to be prolonged. Pneumothorax increases the risk of mortality when undiagnosed or untreated [9]. Therefore, patients with pneumothorax tend to require longer periods of treatment. We found that 50% of those with pneumothorax died, and 75% of them had comorbid diseases; the corresponding mortality in the patients without pneumothorax was 68.7%, and comorbid diseases were found in 76.8% of these patients. However, our findings indicate that, although pneumothorax prolongs the treatment period, it does not pose a statistically significant risk in terms of mortality.
Bilateral pneumothorax developed in 1 patient and unilateral pneumothorax in 7 (5 with right lung pneumothorax, 2 with left lung pneumothorax), consistent with other reports in the literature. All the patients were treated with chest drainage tube insertion immediately following detection of the pneumothorax, which provided adequate treatment. In 6 of the patients who had a chest tube inserted, the air leakage ceased after the first day. In the follow-up period, 2 patients showed the absence of drainage after chest tube insertion, whereas 6 exhibited serous fluid drainage. In such patients, it may be useful to monitor the rate of fluid drainage and air leakage after chest tube insertion. In 1 patient, both mediastinal and subcutaneous emphysema was observed with pneumothorax, whereas in another, pneumothorax was accompanied by subcutaneous emphysema; both patients died. Therefore, in the follow-up of such patients, it is important to monitor them for the development of subcutaneous emphysema and mediastinal emphysema.
All the patients who developed pneumothorax were men. In previous studies, the frequency of pneumothorax development was 3 times higher in men [10], and 67% of those who were intubated due to COVID-19 were also men [5]. Therefore, pneumothorax may have a tendency to develop more frequently among male COVID-19 patients who require invasive mechanical ventilation.
Limitations
Our study has some limitations that must be considered when interpreting its results. First, its retrospective design made it difficult to control for the effect of confounding variables, which may affect pneumothorax development and mortality. Additionally, the limited number of patients who developed pneumothorax may have also affected the statistical results.
CONCLUSION
We did not observe any significant increase or decrease in the risk of developing pneumothorax in patients treated with invasive mechanical ventilation after diagnosis of COVID-19-related pneumonia/acute respiratory distress syndrome. The patients in our study showed characteristics similar to those observed in patients treated for diseases other than COVID-19 pneumonia. Therefore, the development of pneumothorax during invasive mechanical ventilation in patients with COVID-19 pneumonia does not pose a risk different from that associated with other diseases. Accordingly, invasive mechanical ventilation can be used safely at appropriate ventilation modes and pressures in patients with COVID-19 pneumonia as in other patients. Consideration of the risk of pneumothorax in the follow-up of patients in the ICU who require invasive mechanical ventilation due to COVID-19 might yield improved treatment outcomes.
Abbreviations
- CI
Confidence interval
- COVID-19
Coronavirus disease 2019
- ICU
Intensive care unit
- OR
Odds ratio
- PEEP
Positive end-expiratory pressure
Conflict of interest: none declared.
Author contributions
Servet Özdemir: Conceptualization; Data curation; Methodology; Writing—original draft. Deniz Özel Bilgi: Data curation; Methodology; Writing—original draft. Selçuk Köse: Data curation; Methodology; Writing—original draft. Gülsüm Oya: Data curation; Writing—original draft.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks the anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
- 1. Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR.. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 2020;55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L.. Radiology perspective of coronavirus disease 2019 (COVID-19): lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. AJR Am J Roentgenol 2020;214:1078–82. [DOI] [PubMed] [Google Scholar]
- 3. Sun R, Liu H, Wang X.. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol 2020;21:541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hua J, Qian C, Luo Z, Li Q, Wang F.. Invasive mechanical ventilation in COVID-19 patient management: the experience with 469 patients in Wuhan. Crit Care 2020;24:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yao W, Wang T, Jiang B, Gao F, Wang L, Zheng H. et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth 2020;125:e28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu CW, Sun SF.. Iatrogenic pneumothorax related to mechanical ventilation. World J Crit Care Med 2014;3:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strange C. Pleural complications in the intensive care unit. Clin Chest Med 1999;20:317–27. [DOI] [PubMed] [Google Scholar]
- 8. Karakoç E. Basic mechanical ventilation modes and adjustments. Yoğun Bakım Dergisi 2007;7:317–22. [Google Scholar]
- 9. Xiang C, Wu G.. SARS-CoV-2 pneumonia with subcutaneous emphysema, mediastinal emphysema, and pneumothorax: a case report. Medicine 2020;99:e20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bobbio A, Dechartres A, Bouam S, Damotte D, Rabbat A, Régnard JF. et al. Epidemiology of spontaneous pneumothorax: gender-related differences. Thorax 2015;70:653–8. [DOI] [PubMed] [Google Scholar]