Abstract
Background
The efficacy of coronavirus disease 2019 (COVID-19) convalescent plasma (CCP) is primarily ascribed as a source of neutralizing anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies. However, the composition of other immune components in CCP and their potential roles remain largely unexplored. This study aimed to describe the composition and concentrations of plasma cytokines and chemokines in eligible CCP donors.
Methods
A cross-sectional study was conducted among 20 prepandemic healthy blood donors without SARS-CoV-2 infection and 140 eligible CCP donors with confirmed SARS-CoV-2 infection. Electrochemiluminescence detection-based multiplexed sandwich immunoassays were used to quantify plasma cytokine and chemokine concentrations (n = 35 analytes). A SARS-CoV-2 microneutralization assay was also performed. Differences in the percentage of detection and distribution of cytokine and chemokine concentrations were examined by categorical groups using Fisher’s exact and Wilcoxon rank-sum tests, respectively.
Results
Among CCP donors (n = 140), the median time since molecular diagnosis of SARS-CoV-2 was 44 days (interquartile range = 38–50) and 9% (n = 12) were hospitalized due to COVID-19. Compared with healthy blood donor controls, CCP donors had significantly higher plasma levels of interferon (IFN)-γ, interleukin (IL)-10, IL-15, IL-21, and macrophage-inflammatory protein-1, but lower levels of IL-1RA, IL-8, IL-16, and vascular endothelial growth factor-A (P < .0014). The distributions of plasma levels of IL-8, IL-15, and IFN-inducible protein-10 were significantly higher among CCP donors with high (≥160) versus low (<40) anti-SARS-CoV-2 neutralizing antibody titers (P < .0014). The median levels of IL-6 were significantly higher among CCP donors who were hospitalized versus nonhospitalized (P < .0014).
Conclusions
Heterogeneity in cytokine and chemokine composition of CCP suggests there is a different inflammatory state among the CCP donors compared with SARS-CoV-2 naive, healthy blood donors.
Keywords: cytokine, convalescent plasma, COVID-19, neutralizing antibodies, SARS-CoV-2
Compared to controls, COVID-19 convalescent plasma (CCP) had higher distributions of IFN-γ, IL-10, IL-15, IL-21, and MCP-1 but lower IL-1RA, IL-8, IL-16, and VEGF-A. Between CCP with low versus high neutralizing antibody titers, IL-8, IL-15 and IP-10 distributions varied significantly.
The ongoing coronavirus disease 2019 (COVID-19) pandemic is a challenging global health crisis. Although the incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which causes COVID-19, continues to surge, the scientific community is exploring effective prophylactic and therapeutic options. Coronavirus disease 2019 convalescent plasma (CCP) is currently one of the leading therapies and was recently granted hospital Emergency Use Authorization by the US Food and Drug Administration (FDA) [1–4]. Although the efficacy of CCP is primarily thought to be ascribed to neutralizing anti-SARS-CoV-2 antibodies, the composition and potential roles of other immune components in the plasma remain largely unexplored. In this study, the composition and concentrations of plasma cytokines and chemokines were evaluated in eligible CCP donors with confirmed SARS-CoV-2 infection and compared with that in healthy blood donor controls.
METHODS
Study Participants
A cross-sectional study of eligible CCP donors (hereinafter referred to as CCP donors) was conducted as previously described [5–7]. From April to May 2020, eligible CCP donors (n = 140) from the Baltimore/Washington DC area who had a confirmed molecular diagnosis of SARS-CoV-2 ribonucleic acid (polymerase chain reaction [PCR]+), were at least 18 years old, and met the eligibility criteria for community blood donation (eg, no pregnancy within last 6 weeks, never been diagnosed with human immunodeficiency virus, hepatitis B virus, or hepatitis C virus) were included. During this time period, the FDA did not require a specific titer for CCP donors. Plasma was separated from whole blood within 12 hours of collection and stored at −80°C until further processing. In addition, a convenience sample of plasma from 20 platelet blood donors (New York Blood Center, New York, NY) collected before December 2019 served as healthy controls (pre-COVID-19 pandemic blood donors hereinafter referred to as controls). Self-reported demographic and clinical information was collected and available for the CCP donors. However, except for ABO blood group, no other information was available for the controls.
Patient Consent Statement
The design of the work was approved by the Johns Hopkins University School of Medicine Institutional Review Board. All study participants (both CCP donors and controls) provided written informed consent.
Microneutralization Assay
Severe acute respiratory syndrome coronavirus 2 neutralizing antibody (nAb) titers against 100 50% tissue culture infectious doses (TCID50) per 100 μL were determined using a microneutralization (NT) assay, as previously described [7]. The nAb titer was the highest plasma dilution that prevented cytopathic effect in 50% of the wells tested. The nAb area under the curve (AUC) values were estimated using the exact number of wells protected from infection at every plasma dilution.
Multiplexed Sandwich Immunoassays
Highly sensitive, multiplexed sandwich immunoassays using MULTI-ARRAY electrochemiluminescence detection technology (MesoScale Discovery, Gaithersburg, MD) were used for the quantitative evaluation of 35 different human cytokine and chemokine analytes in plasma samples from CCP donors and controls following the manufacturer’s instructions. Based on previously published reports [8] and preliminary discussions with COVID-19 researchers at Johns Hopkins University, the analytes evaluated included the following: colony-stimulating factors (granulocyte-macrophage colony-stimulating factor [CSF], granulocyte CSF [G-CSF] [CSF3]); eosinophil chemotactic proteins (Eotaxin, Eotaxin-3); interleukins (IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-12/IL23p40, IL-13, IL-15, IL-16, IL-17A, IL-18, IL-21, IL-33, and receptor antagonist IL-1RA); interferons (IFN-γ, IFN-α2a); IFN-γ-inducible protein-10 (IP-10); macrophage-inflammatory protein (MIP-1α, MIP-1β); macrophage-derived chemokine (MDC); monocyte chemoattractant protein (MCP-1, MCP-4); tumor necrosis factors (TNF-α, TNF-β); thymus- and activation-regulated chemokine; and vascular endothelial growth factor (VEGF-A). Analyte concentrations were calculated per the manufacturer’s protocol (MSD DISCOVERY WORKBENCH analysis software) and were considered “detectable” if both runs of each sample had a signal greater than the analyte- and plate-specific lower limit of detection (LLOD) (ie, 2.5 standard deviations of the plate-specific blank). Analyte concentrations (pg/mL) from both runs of each analyte were averaged for analysis.
Statistical Analysis
Cytokine and chemokine analytes with <80% detectability in the overall sample were analyzed as binary variables (detectable vs not detectable) and analytes with ≥80% detectability in the overall sample were analyzed continuously, as previously published [9]. Values for analytes with results below the LLOD were imputed using a single stochastic imputation from truncated log-normal distributions fitted to the detectable values of each analyte (ie, random values were drawn from the extrapolated tail of the distribution below the LLOD). The CCP donors were categorized into groups based on the level of nAb (AUC cutoffs of <40, 40–159, and ≥160 as low, middle, and high titer, respectively). Differences in analyte detectability were examined between categorical groups using Fisher’s exact tests. Differences in the distribution of continuous analytes were examined between categorical groups using Wilcoxon rank-sum tests.
To assess whether the distribution of log2-transformed cytokine and chemokine analytes differed by nAb groups (<40, 40–159, or ≥160 AUC) among CCP donors, least-squares linear regression was performed separately for each analyte (dependent variable). For each analyte, adjusted β coefficients were estimated from a multivariable model that included adjustment for age (continuous), sex, and days from PCR+ diagnosis (continuous). As a sensitivity analysis, a second model was also constructed that additionally included adjustment for history of COVID-19 hospitalization status as a measure of severity of illness. Similar analyses were performed using modified Poisson regression for binary analytes. Adjustment for multiple comparisons across analytes was performed by using a family-wise Bonferroni correction to control the family-wise error rate at an α of 0.05 [10]; a 2-sided P < .0014 (0.05/35 analytes) was considered statistically significant. Analyses were performed in R statistical software (version 4.0.2).
RESULTS
Study Participants Characteristics
The sample of CCP donors (n = 140) was 54% male with a median age of 42 years (Supplementary Table 1). The COVID-19 convalescent plasma donors were predominantly white (n = 108), followed by Asian (n = 14), Hispanic (n = 5), African American (n = 4), and mixed or unknown race (n = 9). Although most CCP donors had minor symptoms and did not require any hospitalization, 12 (9%) donors had previously been hospitalized due to COVID-19. At the time of blood collection, a median of 44 days (interquartile range = 38–50) had elapsed since participants had been diagnosed with SARS-CoV-2 infection by PCR. There were 60 CCP donors in the low nAb group (43%), 35 in the middle nAb group (25%), and 45 in the high nAb group (32%), for whom epidemiologic characteristics are given in Supplementary Table 1.
Plasma Cytokine and Chemokine Levels in Coronavirus Disease 2019 Convalescent Plasma Donors
For each of the cytokine and chemokine analytes tested, LLOD range (pg/mL) and total number of detectable samples in CCP donors and controls were calculated. For all analytes, the overall mean coefficient of variation between runs was <15% (Supplementary Tables 2 and 3). The correlation between cytokines and chemokines varied substantially and is shown in (Figure 1).
Figure 1.
Correlation heatmap between cytokines and chemokines among coronavirus disease 2019 convalescent plasma donors. NOTE: This analysis is restricted to analytes that had ≥80% overall detectability.
Among the analytes with ≥80% overall detectability, the median levels of IL-6, IL-8, TNF-α, IL-12/IL23p40, and MDC were higher among CCP donors who were hospitalized versus nonhospitalized (Wilcoxon rank-sum P < .05) (Supplementary Table 4); the median levels of Eotaxin were lower among those hospitalized versus nonhospitalized (Wilcoxon rank-sum P < .05) (Supplementary Table 4). However, only median IL-6 levels differed significantly by hospitalization status after adjusting for multiple comparisons (Wilcoxon rank-sum P < .0014). For analytes with <80% detectability, no significant difference in percentage of detection was observed among CCP donors by hospitalization status (Supplementary Table 5).
Among the analytes with ≥80% detectability, the distribution of plasma IFN-γ, IL-10, IL-15, IL-21, and MCP-1 was significantly higher in CCP donors compared with the controls, whereas the distribution of IL-1RA, IL-8, IL-16, and VEGF-A levels was significantly lower in CCP donors than the controls after adjusting for multiple comparison using Bonferroni correction (Wilcoxon rank-sum P < .0014) (Figure 2). Among CCP donors and the analytes with ≥80% detectability, the distribution of IL-8, IL-15, and IP-10 were significantly higher in the high nAb titer group (≥160 AUC) compared with the low nAb titer group (<40 AUC) (Wilcoxon rank-sum P < .0014) (Figure 3). In multivariable regression analysis, only the distribution of IL-15 was significantly higher in the high nAb titer group than those in the low nAb titer group (AUC < 40) after adjusting for age, sex, days since PCR+ diagnosis, as well as for multiple comparisons (P < .0014) (Supplementary Table 6). Similar results were obtained in the model that adjusted for prior hospitalization status.
Figure 2.
Distribution of log2 cytokine and chemokine levels among pre-coronavirus disease 2019 (COVID-19) pandemic blood donors (controls) to COVID-19 convalescent plasma (CCP) donors. NOTE: There were 20 controls and 140 CCP donors. P values were determined from Wilcoxon rank-sum test. Only P < .05 are presented. Unit of the analytes: log2-transformed pg/mL. *, Significant P values after adjusting for multiple comparison using Bonferroni correction (P < .0014).
Figure 3.
Distribution of log2 cytokine and chemokine levels by neutralizing antibody (nAb) titer group among coronavirus disease 2019 convalescent plasma donors. NOTE: There were 60 samples in the <40 group, 35 in the 40–159 group, and 45 in the ≥160 group. P values were determined by Wilcoxon rank-sum test. Only P < .05 are presented. Unit of the analytes: log2-transformed pg/mL. *, Significant P values after adjusting for multiple comparison using Bonferroni correction (P < .0014).
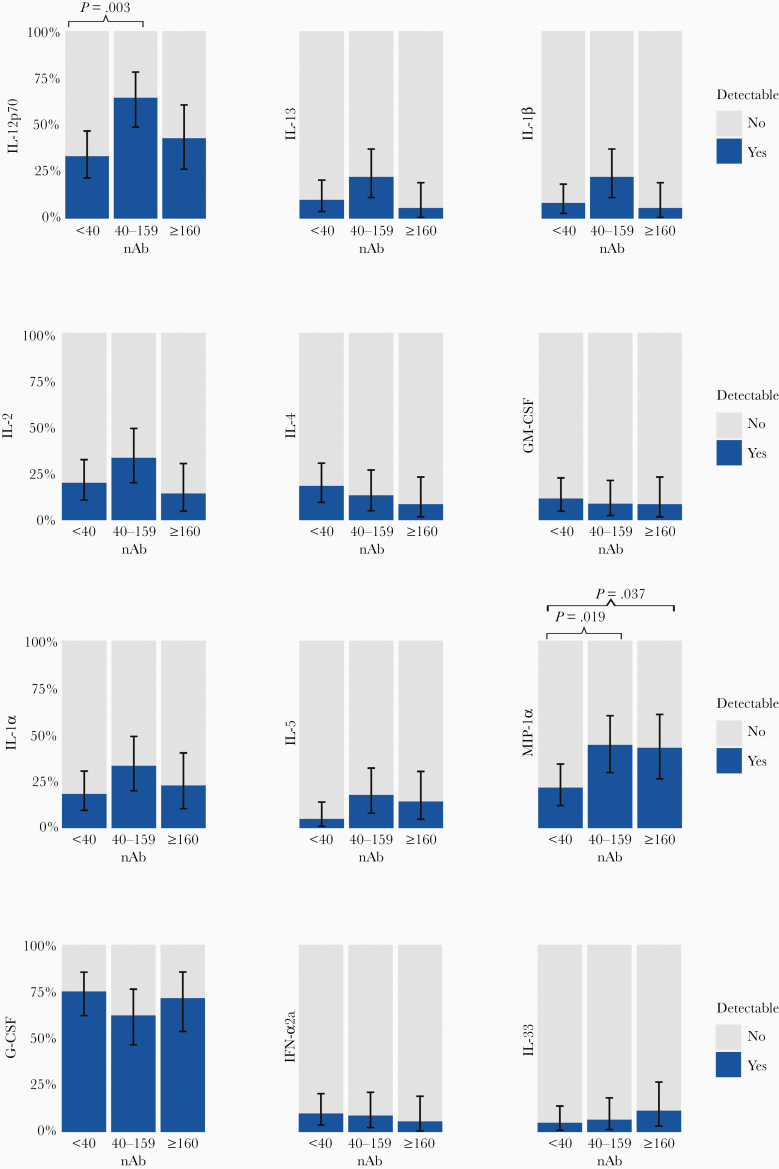
Among analytes with <80% detectability, the percent detection was significantly lower for IL-13, IL-1β, IL-4 but higher for G-CSF among CCP donors than controls after adjusting for multiple comparisons (Fisher’s exact P < 0.0014) (Figure 4). However, there were no statistically significant differences by nAb titer group among CCP donors after adjusting for multiple comparisons (Figure 5; Supplementary Table 7).
Figure 4.
Comparisons of percentage of detection of cytokines and chemokines among pre-coronavirus disease 2019 (COVID-19) blood donors (controls) to COVID-19 convalescent plasma (CCP) donors. NOTE: This analysis is restricted to analytes that had <80% detectability. There were 20 controls and 140 CCP donors. P values were determined by Fisher’s exact test. Only P < .05 are presented. *, Significant P values after adjusting for multiple comparison using Bonferroni correction (P < .0014).
Figure 5.
Comparisons of percentage of detection of cytokines and chemokines by neutralizing antibody (nAb) titer group among coronavirus disease 2019 convalescent plasma donors. NOTE: This analysis is restricted to analytes that had <80% detectability. P values were determined by Fisher’s exact tests. Only P < .05 are presented. No P value was significant after adjusting for multiple comparison using Bonferroni correction (P < .0014).
DISCUSSION
In addition to nAbs, convalescent plasma may contain cytokines (pro- and/or anti-inflammatory), clotting factors, natural antibodies, defensins, pentraxins, and other undefined proteins [11]. Antibodies in convalescent plasma may also mediate their therapeutic effects through a variety of mechanisms (eg, direct virus neutralization, antibody-mediated complement activation, antibody-dependent cellular cytotoxicity, and/or phagocytosis) [11, 12]. Although studies have evaluated the acute phase plasma of COVID-19 patients [13–15], sparse data exist on recovered COVID-19 patients and the components in convalescent plasma. In addition, little is known regarding whether a particular cytokines or chemokines profile is associated with higher nAb. This study demonstrates that CCP has a different cytokine and chemokine profile than that of the plasma of SARS-CoV-2-naive controls, and that IL-8, IL-15, and IP-10 were associated with higher nAb among CCP donors.
Distributions of IFN-γ, IL-10, IL-15, IL-21, and MCP-1 were significantly higher in CCP compared with control plasma. Although no significant difference in median levels of these cytokines and chemokines has been observed in the hospitalized CCP donors (n = 12), this may still reflect residual effects of the heightened immune response that were present during acute phase of the disease in severe as well as moderately ill COVID-19 patients as previously reported [16, 17]. From a functional standpoint, some of these cytokines (IL-10, IL-15, and IL-21) may be involved in B cell survival, differentiation into plasma cells, and class switching, whereas others may impart an inhibitory effect on B cells in certain situations (eg, IL-8, IFN-γ) [18–20]. In addition, it has recently been reported that individuals with inborn errors of type I IFN immunity is associated with life-threatening COVID-19 pneumonia [21]. Of note, some of the analytes tested (eg, IL-1RA, IL-8, IL-16, and VEGF-A) were significantly lower in CCP donors than those from the control cohort, the biological relevance of which requires further investigation. This is in contrast to a previous report by Chi et al [17] where levels of all 48 cytokines and chemokines tested were very similar between a small sample of convalescent (n = 4) and control subjects (n = 4).
In a well controlled immune response, cytokine and chemokine expression is tightly regulated, and loss of this control may have unintended consequences. For instance, IL-10, a key regulator of immune system homeostasis and an anti-inflammatory cytokine, was also associated with sustained chronic infection when expressed aberrantly [22]. Virus-induced aberrant inflammatory responses have been associated with the pathogenesis of many viral diseases including the coronaviruses [8]. Excessive cytokine production, also known as hypercytokinemia, has been linked to pulmonary inflammation and acute lung injury in SARS, Middle East respiratory syndrome-CoV, and SARS-CoV-2 infected patients [8, 23–26]. Longitudinal immune profiling of COVID-19 has revealed an inflammatory cluster of IL-1α, IL-1β, IL-6, IL-10, IL-12 p70, IL-17A, IFNα, thrombopoietin, IL-33, IL-16, IL-21, IL-23, IFNλ, Eotaxin, and Eotaxin-3 in patients with severe disease [16]. Transfusion of plasma with elevated levels of some of these cytokines could contribute to some of the biological effects associated with CCP administration in COVID-19 patients. In this regard, the administration of these cytokine and chemokines may have an immunomodulatory effect in less critical patients via amelioration of the severe inflammatory response (eg, anti-inflammatory IL-10) and/or antibody production (eg, IL-21 in plasma cell generation).
There are limitations to this study. The study is cross-sectional and not designed to infer causal associations. The number of available controls was relatively low, and limited demographic and clinical data were available for the control group, which may have resulted in unmeasured confounding. Nevertheless, findings of this study suggest heterogeneity in cytokine and chemokine composition and levels in CCP. Indeed, the biological significance of the variations in several cytokine and chemokine levels observed in this study requires investigation.
CONCLUSIONS
The markedly different inflammatory states in the CCP donors observed in this study may have particular significance to long-term recovery from COVID-19 and also the therapeutic potential of CCP. A recent study of 55 people recovering from COVID-19 in China showed abnormal lung scans and lung function 3 months after discharge from the hospital, and elevated serum levels of D-dimer was associated to people with more lasting lung problems [27]. Even in outpatients with milder COVID-19 illness, delay in complete recovery with prolonged symptoms such as cough, fatigue, or shortness of breath has been reported in the United States [28]. These studies demonstrate that individuals have different inflammatory states long after symptoms have resolved, and these may contribute to the lingering symptoms (eg, shortness of breath, cough, gastrointestinal problems, headache, or fatigue) reported in the COVID-19 long-haulers. Thus, the levels of CCP cytokines and chemokines provide important insights into overall recovery status of the convalescing patients, as well as its suitability as a therapy for COVID-19 patients with different disease severity. Larger and longer term CCP studies are also necessary to evaluate their immunomodulatory effects in COVID-19 patients.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Table 1. Characteristics of the COVID-19 convalescent plasma donors.
Supplementary Table 2. Cytokine and chemokine detection among COVID-19 convalescent plasma (CCP) donors and pre-COVID-19 blood donors (controls).
Supplementary Table 3. Median (IQR) of cytokine and chemokine levels among COVID-19 convalescent plasma (CCP) donors and pre-COVID-19 blood donors (controls).
Supplementary Table 4. Median (IQR) of cytokines and chemokines by status of hospitalization among COVID-19 convalescent plasma (CCP) donor.
Supplementary Table 5. Detection of cytokines and chemokines by status of hospitalization among COVID-19 convalescent plasma (CCP) donors.
Supplementary Table 6. Association between nAb group and cytokine/chemokine levels among COVID-19 convalescent plasma donors.
Supplementary Table 7. Association of NT group with cytokine/chemokine detection in convalescent plasma donors.
Acknowledgments
We are grateful to all participants who enrolled in this study and donated plasma. We thank the National Institute of Infectious Diseases, Japan, for providing VeroE6TMPRSS2 cells and acknowledge the Centers for Disease Control and Prevention, BEI Resources, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) for severe acute respiratory syndrome-related coronavirus 2, isolate USA-WA1/2020, NR-5228.
Disclaimer. Any views or opinions that are expressed in this manuscript are that of the author’s, based on his own scientific expertise and professional judgment; they do not necessarily represent the views of either the Blood Products Advisory Committee or the formal position of US Food and Drug Administration (FDA), and also do not bind or otherwise obligate or commit either Advisory Committee or the Agency to the views expressed.
Financial support. This work was funded in part by the following: National Institute of Allergy and Infectious Diseases (NIAID) (R01AI120938, R01AI120938S1, and R01AI128779; to A. A. R. T.); NIAID (AI052733, AI15207, and N272201400007C; to A. C.); NIAID (T32AI102623; to E. U. P.); the Division of Intramural Research, NIAID, National Institutes of Health (to O. L., A. R., and T. Q.); National Heart Lung and Blood Institute (1K23HL151826-01 [to E. M. B.] and R01HL059842 [to A. C.]); Bloomberg Philanthropies (to A. D. C.); and Department of Defense (W911QY2090012; to D. S.).
Potential conflicts of interest. E. M. B. reports personal fees and nonfinancial support from Terumo BCT, personal fees and nonfinancial support from Grifols Diagnostics Solutions, outside of the submitted work; E. M. B. is a member of the FDA Blood Products Advisory Committee. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest 2020; 130:2757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US Food and Drug Administration. Recommendations for Investigational COVID-19 Convalescent Plasma. Available at: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma. Accessed 16 September 2020.
- 3. Tobian AAR, Shaz BH. Earlier the better: convalescent plasma. Blood 2020; 136:652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bloch EM, Goel R, Montemayor C, et al. Promoting access to COVID-19 convalescent plasma in low- and middle-income countries. Transfus Apher Sci 2020; 102957. doi: 10.1016/j.transci.2020.102957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benner SE, Patel EU, Laeyendecker O, et al. SARS-CoV-2 antibody avidity responses in COVID-19 patients and convalescent plasma donors. J Infect Dis 2020; 222:1974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel EU, Bloch EM, Clarke W, et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol 2020. doi: 10.1128/JCM.02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klein SL, Pekosz A, Park HS, et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest 2020; 130:6141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noroozi R, Branicki W, Pyrc K, et al. Altered cytokine levels and immune responses in patients with SARS-CoV-2 infection and related conditions. Cytokine 2020; 133:155143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKay HS, Bream JH, Margolick JB, et al. Host factors associated with serologic inflammatory markers assessed using multiplex assays. Cytokine 2016; 85: 71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonferroni CE Teoria statistica delle classi e calcolo delle probabilitá. Pubblicazioni del R Istituto Superiore di Scienze Economiche e Commerciali di Firenze. 1936; 8:3–62. [Google Scholar]
- 11. Garraud O, Heshmati F, Pozzetto B, et al. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus Clin Biol 2016; 23:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Natarajan H, Crowley AR, Butler SE, et al. SARS-CoV-2 antibody signatures robustly predict diverse antiviral functions relevant for convalescent plasma therapy. medRxiv 2020; doi: 10.1101/2020.09.16.20196154. [DOI] [Google Scholar]
- 13. Wang K, Long QX, Deng HJ, et al. Longitudinal dynamics of the neutralizing antibody response to SARS-CoV-2 infection. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laing AG, Lorenc A, Del Barrio ID, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 2020; 26:1623–35. [DOI] [PubMed] [Google Scholar]
- 15. Kox M, Waalders NJ, Kooistra EJ, et al. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA 2020; 324:1565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lucas C, Wong P, Klein J, et al. ; Yale IMPACT Team Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020; 584:463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chi Y, Ge Y, Wu B, et al. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 in China. J Infect Dis 2020; 222: 746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moens L, Tangye SG. Cytokine-mediated regulation of plasma cell generation: IL-21 takes center stage. Front Immunol 2014; 5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kimata H, Yoshida A, Ishioka C, et al. Interleukin 8 (IL-8) selectively inhibits immunoglobulin E production induced by IL-4 in human B cells. J Exp Med 1992; 176:1227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armitage RJ, Macduff BM, Spriggs MK, Fanslow WC. Human B cell proliferation and Ig secretion induced by recombinant CD40 ligand are modulated by soluble cytokines. J Immunol 1993; 150:3671–80. [PubMed] [Google Scholar]
- 21. Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020. doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol 2009; 9:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Li J, Zhan Y, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect Immun 2004; 72:4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep 2016; 6:25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020; 55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020; 25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tenforde MW, Kim SS, Lindsell CJ, et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. Morb Mortal Wkly Rep 2020; 69:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.