Abstract
Background
This study was conducted to evaluate the impact of low-molecular-weight heparin (LMWH) on the outcome of patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia.
Methods
This is a prospective observational study including consecutive patients with laboratory-confirmed SARS-CoV-2 pneumonia admitted to the University Hospital of Pisa (March 4–April 30, 2020). Demographic, clinical, and outcome data were collected. The primary endpoint was 30-day mortality. The secondary endpoint was a composite of death or severe acute respiratory distress syndrome (ARDS). Low-molecular-weight heparin, hydroxychloroquine, doxycycline, macrolides, antiretrovirals, remdesivir, baricitinib, tocilizumab, and steroids were evaluated as treatment exposures of interest. First, a Cox regression analysis, in which treatments were introduced as time-dependent variables, was performed to evaluate the association of exposures and outcomes. Then, a time-dependent propensity score (PS) was calculated and a PS matching was performed for each treatment variable.
Results
Among 315 patients with SARS-CoV-2 pneumonia, 70 (22.2%) died during hospital stay. The composite endpoint was achieved by 114 (36.2%) patients. Overall, 244 (77.5%) patients received LMWH, 238 (75.5%) received hydroxychloroquine, 201 (63.8%) received proteases inhibitors, 150 (47.6%) received doxycycline, 141 (44.8%) received steroids, 42 (13.3%) received macrolides, 40 (12.7%) received baricitinib, 13 (4.1%) received tocilizumab, and 13 (4.1%) received remdesivir. At multivariate analysis, LMWH was associated with a reduced risk of 30-day mortality (hazard ratio [HR], 0.36; 95% confidence interval [CI], 0.21–0.6; P < .001) and composite endpoint (HR, 0.61; 95% CI, 0.39–0.95; P = .029). The PS-matched cohort of 55 couples confirmed the same results for both primary and secondary endpoint.
Conclusions
This study suggests that LMWH might reduce the risk of in-hospital mortality and severe ARDS in coronavirus disease 2019. Randomized controlled trials are warranted to confirm these preliminary findings.
Keywords: ARDS, COVID-19, low-molecular weight, mortality, SARS-CoV-2
We show that low-molecular-weight heparin (LMWH) is associated with reduced risk of mortality. LMWH may reduce microthrombosis of pulmonary small vessels in patients with COVID-19. Randomized clinical trials are warranted to confirm the role of LMWH in COVID-19.
Since its initial detection, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) rapidly spread worldwide, causing more than 66 million cases of coronavirus disease 2019 (COVID-19) and 1 530 000 deaths [1]. The mortality rate of hospitalized patients with COVID-19 ranges from 11.7% to 28.3% in published studies [2–4], with the highest mortality reported in patients with acute respiratory distress syndrome (ARDS), septic shock, and disseminated intravascular coagulopathy [5, 6]. The dramatic clinical impact of SARS-CoV-2 pneumonia has prompted the medical community to identify effective treatments before a vaccine is developed and made widely available. In the absence of reliable evidence from large-scale randomized clinical trials, there is great uncertainty about the effectiveness of several treatment options in patients with COVID-19. The scientific community addressed concerns about consistency and reliability of clinical data that have also lead to retraction of published studies [7]. Dexamethasone is the only therapeutic agent showing a benefit on the outcome of patients with COVID-19 in the RECOVERY randomized clinical trial [8]. However, the reduction of 28-day mortality is higher in patients who received invasive mechanical ventilation and not confirmed in patients not receiving oxygen therapy.
Recruitment in randomized clinical trials has slowed down in some countries due to a reduction in the pandemic. While awaiting results from randomized clinical trials, we aimed to explore the impact of different treatments, in particular low-molecular-weight heparin (LMWH), on the outcome of hospitalized patients with SARS-CoV-2 pneumonia.
METHODS
Study Design and Data Collection
This prospective observational study included patients consecutively admitted to the tertiary-care, University Hospital of Pisa, Italy from March 4 to April 30, 2020. Patients with pneumonia and laboratory-confirmed COVID-19, diagnosed by a positive SARS-CoV-2 real-time polymerase chain reaction test on a nasopharyngeal swab, were included in the study. Patients’ identification was performed in real time: a dedicated staff of research fellows identified patients with SARS-CoV-2 pneumonia as soon as they arrived at the emergency department, followed the patients during the hospital stay, and collected all data prospectively without interfering with the therapeutic decisions. Epidemiological and demographic information, medical history, comorbidities, information on clinical symptoms at admission, treatments, and interventions, including need for oxygen or invasive mechanical ventilation support, received during the hospital course were prospectively collected. Venous and arterial blood samples for standard biochemistry and arterial blood gas analysis were collected at the time of hospitalization and repeated according to clinical practice and depending on the patient clinical conditions. To assess comorbidity burden, the age-adjusted Charlson comorbidity index was calculated [9]. The severity of disease at hospital admission was estimated by the Sequential Organ Failure Assessment (SOFA) score [10]. Development of moderate to severe ARDS was defined as the acute onset of hypoxemia, manifestations of pneumonia on chest computed tomography imaging of a noncardiac origin, and a PaO2/FiO2 ratio of less than 200 mmHg according to the Berlin Definition [11]. Major bleeding was defined as follows: fatal bleeding and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, or pericardial or intramuscular with compartment syndrome and/or bleeding causing a fall in hemoglobin level of 2 g/dL or more or leading to transfusion of 2 or more units of whole blood or red cells [12]. During the study period, there was limited evidence for any anti-COVID-19 treatment. A panel of experts of our hospital elaborated a guide for the management of COVID-19 patients, which was regularly updated according to any new release from scientific literature (Supplementary Material, Supplementary Figure S1). The majority of COVID-19 patients were treated following indications of the above-mentioned guide. Drugs such as LMWH, dexamethasone, tocilizumab, baricitinib, remdesivir, and antivirals were prescribed only in patients who needed hospitalization of at least 1 day. The LMWH dosage was classified as prophylactic if subcutaneous enoxaparin 40–60 mg daily was administered, or therapeutic if a subcutaneous enoxaparin dosage of 40–60 mg twice daily was used [13]. The decision to adopt standard prophylactic or therapeutic dosages was decided by the attending physician.
Remdesivir was allowed at our center as compassionate use in patients undergoing invasive mechanical ventilation (ClinicalTrials.gov Identifier NCT04323761). Patients were followed-up until death or 30 days after hospital admission.
Outcome
The primary objective of the study was to evaluate the impact of different treatments on the outcome of patients with SARS-CoV2 pneumonia. In particular, the research question was to evaluate whether LMWH is effective in reducing the risk of 30-day all-cause mortality (main outcome measure). The research question was framed before the data collection and the database creation.
The secondary objective was to explore the impact of LMWH and other treatments on a composite endpoint of death or severe ARDS. Low-molecular-weight heparin, hydroxychloroquine, antibiotics including doxycycline and macrolides, antivirals (lopinavir/ritonavir [LPV/r] or darunavir/ritonavir [DRV/r], remdesivir), baricitinib, tocilizumab, and steroids were evaluated as treatment exposures of interest.
Other Study Variables
Other variables evaluated as potential confounders were defined a priori. Covariates that could be associated with outcome were chosen based on clinical judgment and on previous published studies [2, 4]: age, male sex, comorbidities assessed by Charlson comorbidity index, severity of disease evaluated by the SOFA score at admission, lymphocytes, platelets count, troponin values during the first 48 hours, D-dimer, and PiO2/FiO2 ratio on admission were considered as potential confounders and included in the propensity score (PS) analysis (see below).
Statistical Analysis
Sample size estimation was performed on the basis of previous studies showing a decreased mortality of severe COVID-19 patients with coagulopathy who received anticoagulant treatment [14]. Assuming that 75% of patients would be treated with LMWH, with an overall mortality of 30% and a hazard ratio (HR) of 0.5, a sample size of 290 patients would guarantee a power of at least 80% to a Cox regression model when type I error rate is fixed at 5%.
Continuous variables are reported as mean ± standard deviation and medians and interquartile ranges (IQRs) according to their distribution. The normality of distributions was assessed by the Kolmogorov-Smirnov test. Continuous variables were compared by the Student t test or the Mann-Whitney U test, as appropriate. Categorical data were expressed as frequency distributions, and the χ 2 test or Fisher exact test was used to determine whether differences existed between groups.
According with the study objectives, we used 2 different methods to evaluate the impact of different treatments on both the primary and secondary endpoints. It shall be noted that in our data, treatments are not necessarily started at baseline time for all patients; therefore, there might be immortal time bias if this is not taken into account. First, we presented results from multivariate Cox regression analyses, where all treatments and interventions that were potentially associated to the outcome on univariate analysis (P < .1) were added as covariates in the model. To avoid issues with immortal time bias, treatment status was always introduced as a time-dependent covariate. To confirm the study results, a logistic regression analysis was also performed, including all treatments and interventions that were potentially associated to the outcome. More specifically, hydroxychloroquine, LMWH, doxycycline, macrolides, proteases inhibitors, remdesivir, baricitinib, tocilizumab, steroids, and noninvasive ventilation were included as covariates of the regression analysis.
Second, we performed a PS-matched analysis. The PS method attempts to balance treated and nontreated groups to reduce confounding by indication in observational designs, thereby creating a quasi-randomized experiment, according to reporting guidelines on PS analysis [15]. To resolve possible immortal time bias, we performed a time-dependent PS analysis according to Lu [16]. The PS analyses were performed using a multivariable Cox regression model for time-to-treatment. Covariates that could be associated with outcome or allocation to each treatment group (clinically relevant based on previous published studies [4–6] or chosen through univariate analyses) were used to generate the PS: age, male sex, Charlson comorbidity index, lymphocytes, platelets count, troponin value during the first 48 hours, PiO2/FiO2 ratio on admission, all treatments including antiretroviral, remdesivir, steroids, hydroxychloroquine, doxycycline, macrolides, LMWH, baricitinib, tocilizumab, excluding the current treatment of interest.
Longitudinally PS-matched cohorts (1:1 matching ratio) were then built: each patient receiving the treatment of interest was matched with a patient among those who, at the time of treatment, were at risk, eligible, and not treated previously. Matching was based on the nearest-neighbor algorithm, without replacement, with a caliper of 1%. A caliper of 1% was fixed a priori because it was deemed as a compound difference that would not be clinically relevant. To assess whether adequate matching was achieved, we evaluated standardized differences. We also tested (using 2-sample t tests or χ 2 tests, as appropriate) differences in confounders between the 2 groups after matching. Finally, we performed a Cox regression analysis with the matched cohort to test the association between each treatment and primary and secondary outcome and reported the results as HR and 95% confidence interval (CI). As described before, treatment was a time-dependent covariate to take into account the possibility of immortal time bias.
The variance inflation factor (VIF) value was calculated to control the influence of collinearity. We assumed lack of multicollinearity if all variables had a VIF value < 2. Missing data were handled via listwise exclusion. Because the proportion of excluded patients was moderate (21%), we also performed a multiple imputation analysis alongside the complete case analyses. We used Fully Conditional Specification for Multivariate Imputations by Chained Equations, generating m = 10 completed data sets.
Statistical significance was established at P ≤ .05. All reported P values are 2 tailed. The results obtained were analyzed using statistical software packages (SPSS 22.0, IBM, Armonk, NY; and R 3.5.1, Vienna, Austria).
Patient Consent Statement
This observational study was conducted according to the principles stated in the Declaration of Helsinki, and it conforms to standards currently applied in our country. The study was approved by the Comitato Etico Area Vasta Nord Ovest (Internal Review Board, IRB number 230320). The patient’s informed consent was obtained.
RESULTS
Study Population
Overall, 315 consecutive patients with SARS-CoV-2 pneumonia were included in the study. The median age was 70 (IQR, 57–80) years old and the majority of patients (76.2%) were males. Eighty-five (26.9%) patients were admitted to the intensive care unit (ICU). The overall median length of hospital stay was 15 (IQR, 7–25) days. Sixty-eight (21.6%) patients underwent noninvasive ventilation and 55 (17.5%) patients underwent invasive mechanical ventilation. The 30-day hospital mortality rate was 22.2%. The mortality rate was similar in patients admitted or not to the ICU (25.9% vs 20.9%, P = .342).
Compared to patients who survived, nonsurvivors were older, with higher median Charlson comorbidity index and SOFA score on admission (Table 1). Among laboratory findings, lymphopenia, thrombocytopenia, and an increase in aspartate transaminase >3 upper limit of normality were more frequently detected in nonsurvivors. Higher levels of D-dimer and troponin were also significantly higher in nonsurvivors (Table 1).
Table 1.
Demographics and Clinical Characteristics of Patients With SARS-CoV-2 Pneumonia in Surviving Versus Nonsurviving Patients
| Variable | Survivors N = 245 | Nonsurvivors N = 70 | P Value |
|---|---|---|---|
| Age, years, median (IQR) | 66 (54.5–76) | 81 (76–82.25) | <.001 |
| Male sex, n (%) | 155 (63.3%) | 55 (78.6%) | .017 |
| Ethnicity | |||
| Caucasian | 242 (98.8%) | 69 (98.6%) | .594 |
| Black | 2 (0.8%) | 1 (1.4%) | .642 |
| Hispanic | 1 (0.4%) | 0 | .592 |
| Coexisting Comorbidities, n (%) | |||
| COPD | 19 (7.8%) | 19 (27.1%) | <.001 |
| Arterial hypertension | 101 (41.2%) | 44 (62.9%) | .001 |
| Cardiovascular disease | 69 (28.2%) | 37 (52.9%) | <.001 |
| Diabetes mellitus | 45 (18.4%) | 17 (24.3%) | .272 |
| Cerebrovascular disease | 17 (6.9%) | 15 (21.4%) | <.001 |
| Hemodialysis | 4 (1.6%) | 2 (2.9%) | .509 |
| Solid cancer | 32 (13.1%) | 12 (17.1%) | .385 |
| Chronic kidney disease | 14 (5.7%) | 13 (18.6%) | .001 |
| Days from symptoms to admission, median (IQR) | 6 (2–10) | 3 (0–7) | .001 |
| Charlson comorbidity index, median (IQR) | 2 (1–4) | 5 (4–6.25) | <.001 |
| SOFA score on admission, median (IQR) | 2 (1–3) | 4 (3–5.25) | <.001 |
| Vital signs on admission, n (%) | |||
| Systolic blood pressure <90 mmHg | 15 (6.1%) | 11 (15.7%) | .010 |
| Heart rate >110 bpm | 22 (9%) | 19 (27.1%) | <.001 |
| Respiratory rate >24 bpm | 78 (31.8%) | 41 (58.6%) | <.001 |
| PaO2/FiO2, ratio <300 | 127 (51.8%) | 61 (87.1%) | <.001 |
| Laboratory findings on admissiona, n (%) | |||
| Glucose >140 mg/dL | 60 (24.5%) | 24 (34.3%) | .102 |
| Lymphopenia (L < 800/mcL) | 101 (41.2%) | 42 (60%) | .005 |
| Thrombocitopenia (<150<103/mcL) | 74 (30.6%) | 35 (51.5%) | .001 |
| Aspartate transaminase ×3 ULN | 7 (2.9%) | 9 (12.9%) | .001 |
| Alanine transaminase, ×3 ULN | 10 (4.1%) | 6 (8.6%) | .131 |
| Creatinine >1.5 mg/dL | 22 (9%) | 26 (37.1%) | <.001 |
| D-dimer, mg/L | 0.35 (0.2–0.8) | 0.77 (0.47–3.5) | .006 |
| High sensitivity troponin T, ng/L | 12 (7–25) | 60 (32–126) | .029 |
| Procalcitonin, ng/mL | 0.1 (0.06–0.26) | 0.27 (0.13–0.85) | .053 |
| C-reactive protein, mg/dL | 5.8 (2.2–12.9) | 8.7 (5.3–17.1) | .112 |
| Medications | |||
| Doxycycline | 126 (51.4%) | 24 (34.3%) | .011 |
| Macrolides | 39 (15.9%) | 3 (4.3%) | .012 |
| Proteases inhibitors (LPV/r or DRV/r) | 167 (68.2%) | 34 (48.6%) | .003 |
| Remdesivir | 12 (4.9%) | 1 (1.4%) | .198 |
| Hydroxychloroquine | 198 (80.8%) | 40 (57.1%) | <.001 |
| Steroids | 114 (46.5%) | 27 (38.6%) | .238 |
| Low-molecular-weight heparin | 206 (84.1%) | 38 (54.3%) | <.001 |
| Baricitinib | 35 (14.3%) | 5 (7.1%) | .113 |
| Tocilizumab | 11 (4.5%) | 2 (2.9%) | .545 |
| Interventions | |||
| Noninvasive MV, n (%)b | 52 (21.2%) | 16 (22.9%) | .770 |
| Invasive MV, n (%) | 37 (15.1%) | 18 (25.7%) | .039 |
| Time from admission to invasive MV, median (IQR) | 0 (0–2) | 1 (0–4) | .324 |
Abbreviations: bpm, beats per minute; COPD, chronic obstructive pulmonary disease; DRV/r, darunavir/ritonavir; IQR, interquartile range; LPV/r, lopinavir/ritonavir; MV, mechanical ventilation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOFA, Sequential Organ Failure Assessment; ULN, upper limit of normal.
aWorst value during the first 48 hours after admission.
bNoninvasive mechanical ventilation does not include patients who received NIV after weaning from invasive mechanical ventilation.
Treatments
We analyzed the following treatment variables: (1) LMWH (244 patients, 77.5%), (2) hydroxychloroquine (238 patients, 75.5%), (3) LPV/r or DRV/r (201 patients, 63.8%), (4) doxycycline (150 patients, 47.6%), (5) steroids (141 patients, 44.8%, 46 receiving dexamethasone, 54 prednisone, 34 methylprednisolone, 7 hydrocortisone), (6) macrolides (42 patients, 13.3%), (7) baricitinib (40 patients, 12.7%), (8) tocilizumab (13 patients, 4.1%), and (9) remdesivir (13 ICU patients, 4.1%). Of the 71 patients not receiving LMWH, 5 (7%) received new oral anticoagulants. Table 2 shows the time (expressed in days) from the start of symptoms to the start of each treatment in survivors versus nonsurvivors.
Table 2.
Time From Symptoms and Start of Each Treatment in Survivors and in Nonsurvivorsa
| Treatment or Intervention | Time From Symptoms to Treatment in Survivors | Time From Symptoms to Treatment in Nonsurvivors | P Value |
|---|---|---|---|
| Doxycycline | 7 (2.75–10) | 6 (3–8) | .579 |
| Macrolides | 9 (3–15) | 4 (2–6) | .345 |
| Proteases inhibitors (LPV/r or DRV/r) | 1 (1–5.75) | 3 (2–3) | .333 |
| Remdesivir | 16.5 (11–25) | 19b | 1.0 |
| Hydroxychloroquine | 6 (2–10) | 6.5 (3–9.75) | .857 |
| Steroids | 13 (8–21) | 9 (5–16) | .036 |
| Low-molecular-weight heparin | 5 (1–10) | 5 (2–9) | .977 |
| Tocilizumab | 11 (5–15) | 9 (5–9) | .769 |
| Baricitinib | 9 (7–13) | 8 (3.5–13.5) | .473 |
| Noninvasive ventilation | 8.5 (6–12.75) | 7 (3–9) | .076 |
Abbreviations: DRV/r, darunavir/ritonavir; LPV/r, lopinavir/ritonavir.
aData are expressed as days, median (interquartile range).
bOnly 1 patient who died received remdesivir.
Study Endpoints
Tables 3 and 4 show univariate and multivariate Cox models (with treatments as time-dependent covariates) about the association between interventions and primary or secondary endpoint, respectively. As shown, at multivariate analysis, proteases inhibitors (HR, 0.54; 95% CI, 0.3–0.97; P = .039) and LMWH (HR, 0.36; 95% CI, 0.21–0.6; P < .001) were factors associated with reduced risk of death. With regards to the composite endpoint, LMWH remained a protective factor (HR, 0.61; 95% CI, 0.39–0.95; P = .029), whereas steroids were associated with an increased risk of death or occurrence of severe ARDS (HR, 2.55; 95% CI, 1.54–4.21; P < .001).
Table 3.
Univariate and Multivariate Cox Regression Analysis Evaluating the Association Between Interventions and 30-Day Mortality (All Predictors Are Included as Time-Dependent Covariates)
| Intervention | HR (95% CI) | P Value | aHR (95% CI) | P Value |
|---|---|---|---|---|
| Medications During Hospital Course | ||||
| Doxycycline | 0.57 (0.34–0.94) | .028 | 0.92 (0.49–1.69) | .78 |
| Macrolides | 0.33 (0.10–1.04) | .058 | 0.32 (0.09–1.03) | .05 |
| Proteases inhibitors (LPV/r or DRV/r) | 0.41 (0.25–0.66) | <.001 | 0.54 (0.3–0.97) | .039 |
| Remdesivir | 0.28 (0.04–2.08) | .218 | - | - |
| Hydroxychloroquine | 0.43 (0.26–0.70) | <.001 | 0.75 (0.4–1.39) | .36 |
| Steroids | 0.25 (0.78–2.10) | .315 | - | - |
| Low-molecular-weight heparin | 0.24 (0.15–0.40) | <.001 | 0.36 (0.21–0.6) | <.001 |
| Immunosuppressants | ||||
| Tocilizumab | 0.51 (0.13–2.11) | .36 | - | - |
| Baricitinib | 0.14 (0.03–0.56) | .006 | 0.69 (0.27–1.78) | .45 |
| Other Interventions | ||||
| Noninvasive ventilation | 0.84 (0.47–1.53) | .57 | - | - |
| ECMO | 1.90 (0.26–13.73) | .52 | - | - |
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; DRV/r darunavir/ritonavir; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; LPV/r, lopinavir/ritonavir.
aNoninvasive ventilation (NIV) does not include patients who received NIV after weaning from invasive mechanical ventilation.
Table 4.
Univariate and Multivariate Cox Regression Analysis Evaluating the Association Between Interventions and a Composite Endpoint of Death or Severe ARDS (All Predictors Are Included as Time-Dependent Covariates)
| Intervention | HR (95% CI) | P Value | aHR (95% CI) | P Value |
|---|---|---|---|---|
| Medications During Hospital Course | ||||
| Doxycycline | 0.89 (0.61–1.30) | .56 | - | - |
| Macrolides | 0.42 (0.19–0.97) | .043 | 0.78 (0.33–1.81) | .56 |
| Proteases inhibitors (LPV/r or DRV/r) | 0.81 (0.55–1.18) | .28 | - | - |
| Remdesivir | 0.25 (0.03–1.80) | .17 | - | - |
| Hydroxychloroquine | 0.96 (0.64–1.45) | .85 | - | - |
| Steroids | 2.00 (1.26–3.17) | .003 | 2.55 (1.54–4.21) | <.001 |
| Low-molecular-weight heparin | 0.53 (0.35–0.79) | .002 | 0.61 (0.39–0.95) | .029 |
| Immunosuppressants | ||||
| Tocilizumab | 1.07 (0.39–2.91) | .89 | - | - |
| Baricitinib | 0.53 (0.25–1.18) | .12 | - | - |
| Other Interventions | ||||
| Noninvasive ventilationa | 1.74 (1.11–2.75) | .016 | 1.15 (0.69–1.94) | .059 |
| ECMO | NE | - | - | - |
Abbreviations: aHR, adjusted hazard ratio; ARDS, acute respiratory distress syndrome; CI, confidence interval; DRV/r, darunavir/ritonavir; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; LPV/r, lopinavir/ritonavir; NE, not evaluable.
aNoninvasive ventilation (NIV) does not include patients who received NIV after weaning from invasive mechanical ventilation.
The logistic regression analysis confirmed that LMWH was associated with reduced risk of death (odds ratio [OR], 0.33; 95% CI, 0.17–0.66; P = .002) and death or occurrence of severe ARDS (OR, 0.37; 95% CI, 0.19–0.72; P = .003) (Supplementary Table 1 and Supplementary Table 2, respectively). None of the variables included in the multivariate models showed multicollinearity.
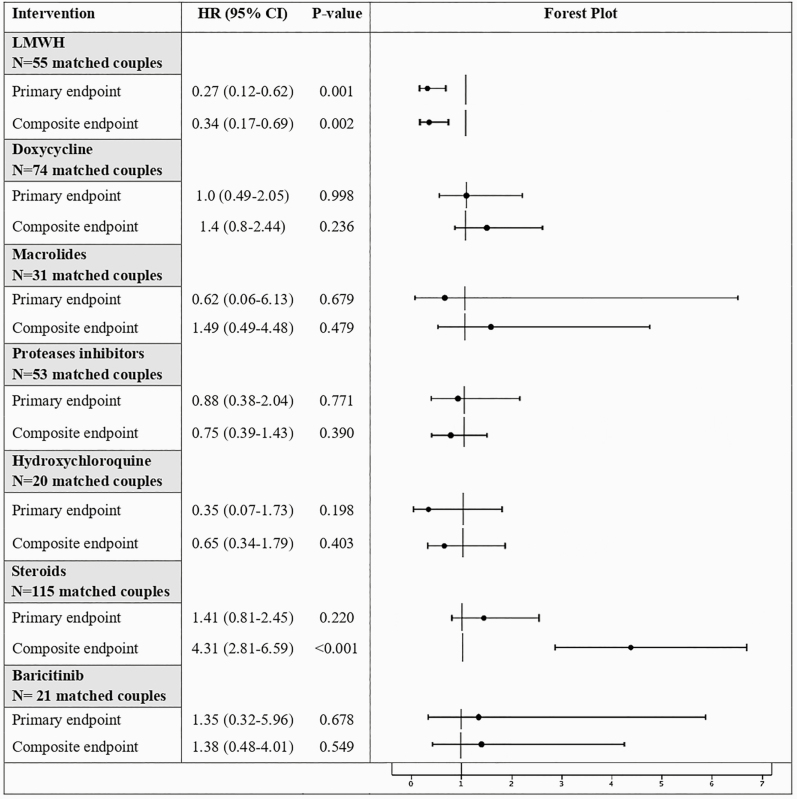
As described in Figure 1, the PS-matching analysis confirmed the findings of multivariate analysis. In the LMWH PS-matched cohort, the use of LMWH was associated with lower risk of 30-day mortality (HR, 0.27; 95% CI, 0.12–0.62; P = .001) and composite endpoint (HR, 0.34; 95% CI, 0.17–0.69; P = .002). The groups matched by PS were well balanced on all included variables as demonstrated by the standardized differences (uniformly below 10%) and comparison tables (Supplementary Materials). Multiple imputation confirmed the results of the complete case analysis (primary endpoint: HR = 0.425, 95% CI = 0.246–0.735, P = .002; secondary endpoint: HR = 0.491, 95% CI = 0.309–0.782, P = .003).
Figure 1.
Impact of Different Treatments on the Risk of All-Cause Mortality (Primary Endpoint) and a Composite of Mortality or Severe ARDS (Composite Endpoint) in PS-Matched Patients With SARS-CoV-2 Pneumonia. Abbreviations: ARDS, acute respiratory distress syndrome; CI, confidence interval; HR, hazard ratio; LMWH, low-molecular-weight heparin; PS, propensity score; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; hsTnT, high-sensitivity troponin T (ng/L). NOTE: PS matched includes the following: age, male sex, Charlson comorbidity index, severity of illness at hospital admission assessed by Sequential Organ Failure Assessment (SOFA) score, lymphocytes, platelets count, and hsTnT values during the first 48 hours after admission, PiO2/FiO2 on admission, all medications (including LMWH, doxycycline, macrolides, proteases inhibitors, hydroxychloroquine, steroids, baricitinib, tocilizumab, and remdesivir), excluding the current treatment of interest.
Risk of Bleeding in Patients Receiving Low-Molecular-Weight Heparin
Among 244 patients treated with LMWH, 187 (76.6%) received a prophylactic dosage and 57 (23.4%) received a therapeutic dosage. Eleven patients (4.5%) developed major bleeding episodes: 3 had hematuria, 3 had bleeding from the respiratory tract, 2 had bleeding from the gastrointestinal tract, 2 had hematoma that needed embolization, and 1 had bleeding of the subclavian vein. All patients who developed a major bleeding received therapeutic dosages of LMWH. Three deaths were attributed to a major bleeding episode.
DISCUSSION
Our study shows that after adjusting for age, sex, baseline comorbidities, degree of respiratory dysfunction, and the receipt of other treatments, LMWH was the only factor associated with reduced risk of developing severe ARDS and mortality.
Considering the observational nature of the study, this finding should be considered as an hypothesis that serves as the basis for randomized controlled trials. Our study has some limitations. First, because it was monocentric, the results might be affected by local practice in the management of the COVID-19 infection; moreover, almost all patients (except for 4) were of Caucasian ethnicity, and our findings cannot be generalized to other healthcare settings and/or populations. Thus, external validation of our results is needed. However, it should be considered that all patients underwent similar treatment and interventions, according to the approved internal guide for COVID-19 patients admitted to our hospital. Second, our study was not powered to analyze differences in subgroups of patients receiving treatments other than LMWH, and the limited number of patients assigned to some treatments (eg, remdesivir, steroids, tocilizumab, baricitinib) might underestimate the role of these therapies in the management of COVID-19. Third, although criteria for ICU admission in our hospital were based on the degree of respiratory impairment expressed by FiO2/PO2 ratio, elderly sick patients with ultimately fatal diseases were excluded from ICU admission, and this may impact the interpretation of some interventions. Finally, the analysis on the beneficial effects of treatments should be interpreted cautiously, because it was not conducted on randomized groups and might therefore be affected by several measured and unmeasured confounding factors. Randomized controlled trials are needed to confirm our preliminary findings.
The potential role of LMWH in patients with COVID-19 seems to be plausible and supported by recent studies. Occlusion and microthrombosis formation in pulmonary small vessels have been reported in dead patients with COVID-19, and typical microvascular platelet-rich thrombotic depositions has been also described in other tissues, such as the myocardium [17]. High rates of thromboembolic events have been reported in patients with severe SARS-CoV-2 pneumonia admitted to ICUs [18]. This is in line with the demonstration of platelet activation and artery dysfunction in patients with community-acquired pneumonia [19–21], but it may also represent a specific pathogenetic finding in patients with SARS-CoV-2 pneumonia. As a matter of fact, the radiological demonstration of contiguity of filling defects to the parenchymal opacities suggests a link between the SARS-CoV-2-induced lung inflammation and vascular occlusion [18]. Previous observations suggested the potential role of LMWH in COVID-19. In a retrospective study including 449 patients with severe COVID-19 in China (99 of whom received heparin), it was found that 28-day mortality of heparin users was lower than that of nonusers in patients with D-dimer levels more than 6-fold the upper limit of normal and sepsis-induced coagulopathy score ≥4 [14]; the major limitations of this report are the small sample size, the retrospective design, and the fact that some patients were still hospitalized at the time of manuscript submission (thus outcome cannot be definitively assessed) [14]. More recently, an observational study investigated a large cohort of 2773 COVID-19 patients hospitalized within the Mount Sinai Health System in New York City: in patients who required mechanical ventilation, the in-hospital mortality was 29.1% with a median survival of 21 days for those treated with anticoagulant therapy compared with 62.7% with a median survival of 9 days in patients who did not receive anticoagulant treatment. In the multivariate model, duration of anticoagulant treatment was associated with a reduced risk of mortality (HR of 0.86 per day; 95% CI, 0.82–0.89; P < .001) [22]. The International Society of Thrombosis and Haemostasis suggests to use LMWH at prophylactic dosages in all patients with 3- to 4-fold increase in D-dimer value, prolonged prothrombin time, fibrinogen levels <2.0 g/L, and platelet count <100 × 109/L [23]. Of interest, a recent paper found that among COVID-19 patients, the development of clinically significant thrombosis was associated with abnormal thromboelastographic (TEG) parameters [24]. The TEG results outside reference ranges were detected in 62% of thrombosis events, suggesting that TEG may be useful in accurately identifying patients at increased thrombosis risk and thereby necessitating anticoagulation [24].
CONCLUSIONS
Nevertheless, for other diseases with high prevalence in older population, the prescription of an anticoagulation treatment should be weighed against the risk of bleeding. This is particularly important because we observed several cases of major bleeding and 3 subsequent deaths were directly attributed to the consequences of anticoagulant therapy. Therefore, it is crucial to identify clinical or laboratory parameters able to select those patients who could receive prophylactic versus therapeutic dosages of anticoagulant therapy, thus minimizing the risk of bleeding. Ongoing multicenter, randomized, controlled trials will be able to address this question (ClinicalTrials.gov Identifier NCT04372589, NCT04367831, NCT04345848, and NCT04366960).
In conclusion, for modulating the activation of the coagulopathy pathways, LMWH may be beneficial in patients with COVID-19. Randomized clinical trials are warranted to confirm these preliminary results and identify the most adequate LMWH dosage.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Author contributions.M. F. and F. M. designed the study; G. T. created the case report form, developed the database, and analyzed and interpreted data; G. T. and G. B. coordinated the data collection; G. B. and V. G. recruited patients and collected clinical data; M. F. and F. M. wrote the study; A. F. performed the statistical analysis and propensity score matching; A. R., A. V., F. F., F. C., F. G., L. C., A. C., M. S., F. M., S. D. M., and M. P., L. G. revised and contributed to the critical revision of the final manuscript.
Potential conflicts of interest. M. F. received speakers honoraria from Angelini, MSD, Pfizer, and Nordic Pharma. F. M. has participated in advisory boards and/or received speaker honoraria from Angelini, Correvio, MSD, Pfizer, Astellas, Gilead, BMS, Jansenn, ViiV, BioMerieux, Biotest, Becton-Dickinson, Nordic Pharma, Pfizer, and Shionogi. L. G. reports other from Quipu, grants from Pfizer, other from Boehringer Ingelheim, other from Corman, other from Sanofi-Aventis, and other from Sevier. All conflicts of interest are outside the submitted study. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
PISA COVID-19 Study Group
Agostini o Degl’Innocenti Sabrina1, Antognoli Rachele2, Baldassarri Rubia3, Bertini Pietro3, Biancalana Martina1, Borselli Matteo1, Brizzi Giulia3, Calsolario Valeria2, Carpene Nicoletta4, Cinotti Francesco5, Cipriano Alessandro5, Della Rocca Alessandra3, Desideri Massimiliano4, Forotti Giovanna6, Gherardi Marco4, Maggi Fabrizio7, Mengozzi Alessandro6, Malacarne Paolo3, Masi Stefano6, Monfroni Marco3, Morea Alessandra8, Nencini Elia1, Park Naria5, Paterni Simone2, Piagnani Chiara3, Ruberti Francesca6, Sciuto Maria6, Serradori Massimiliano4, Spinelli Stefano1
1Emergency Medicine Unit, Azienda Ospedaliera Universitaria Pisana; 2Geriatrics Unit, Azienda Ospedaliera Universitaria Pisana; 3Department of Anaesthesia and Intensive Care, Azienda Ospedaliera Universitaria Pisana; 4Respiratory Unit, Azienda Ospedaliera Universitaria Pisana; 5Emergency Department, Azienda Ospedaliera Universitaria Pisana; 6Internal Medicine Department, Azienda Ospedaliera Universitaria Pisana; 7Virology Unit, Department of Laboratory Medicine Pisa University Hospital
Contributor Information
Pisa COVID-19 Study Group:
Agostini o Degl’Innocenti Sabrina, Antognoli Rachele, Baldassarri Rubia, Bertini Pietro, Biancalana Martina, Borselli Matteo, Brizzi Giulia, Calsolario Valeria, Carpene Nicoletta, Cinotti Francesco, Cipriano Alessandro, Della Rocca Alessandra, Desideri Massimiliano, Forotti Giovanna, Gherardi Marco, Maggi Fabrizio, Mengozzi Alessandro, Malacarne Paolo, Masi Stefano, Monfroni Marco, Morea Alessandra, Nencini Elia, Park Naria, Paterni Simone, Piagnani Chiara, Ruberti Francesca, Sciuto Maria, Serradori Massimiliano, and Spinelli Stefano
References
- 1. World Health Organization. Novel coronavirus. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/. Accessed 7 December 2020.
- 2. Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J 2020; 55:2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ji D, Zhang D, Xu J, et al. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis 2020; 71:1393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46:846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 2020; 323:1824–36. [DOI] [PubMed] [Google Scholar]
- 8. Horby P, Lim WS, Emberson JR, et al. ; RECOVERY Collaborative Group Dexamethasone in hospitalized patients with covid-19–preliminary report. N Engl J Med 2020: NEJMoa2021436. [Google Scholar]
- 9. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 10. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–10. [DOI] [PubMed] [Google Scholar]
- 11. Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–33. [DOI] [PubMed] [Google Scholar]
- 12. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005; 3:692–4. [DOI] [PubMed] [Google Scholar]
- 13. Smythe MA, Priziola J, Dobesh PP, et al. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis 2016; 41:165–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020; 18:1094–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins GS, Le Manach Y. Comparing treatment effects between propensity scores and randomized controlled trials: improving conduct and reporting. Eur Heart J 2012; 33:1867–9. [DOI] [PubMed] [Google Scholar]
- 16. Lu B. Propensity score matching with time-dependent covariates. Biometrics 2005; 61:721–8. [DOI] [PubMed] [Google Scholar]
- 17. Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 2020; 8:681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Minuz P, Mansueto G, Mazzaferri F, et al. High rate of pulmonary thromboembolism in patients with SARS-CoV-2 pneumonia. Clin Microbiol Infect 2020; 26:1572–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cangemi R, Casciaro M, Rossi E, et al. ; SIXTUS Study Group; SIXTUS Study Group Platelet activation is associated with myocardial infarction in patients with pneumonia. J Am Coll Cardiol 2014; 64:1917–25. [DOI] [PubMed] [Google Scholar]
- 20. Violi F, Cangemi R, Falcone M, et al. ; SIXTUS (Thrombosis-Related Extrapulmonary Outcomes in Pneumonia) Study Group Cardiovascular complications and short-term mortality risk in community-acquired pneumonia. Clin Infect Dis 2017; 64:1486–93. [DOI] [PubMed] [Google Scholar]
- 21. Falcone M, Russo A, Cangemi R, et al. Lower mortality rate in elderly patients with community-onset pneumonia on treatment with aspirin. J Am Heart Assoc 2015; 4:e001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 2020; 76:122–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thachil J, Juffermans NP, Ranucci M, et al. ISTH DIC subcommittee communication on anticoagulation in COVID-19. J Thromb Haemost 2020; 18:2138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mortus JR, Manek SE, Brubaker LS, et al. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open 2020; 3:e2011192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.