Abstract
Background
Concerns about false-negative (FN) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid amplification tests (NAATs) have prompted recommendations for repeat testing if suspicion for coronavirus disease 2019 (COVID-19) infection is moderate to high. However, the frequency of FNs and patient characteristics associated with FNs are poorly understood.
Methods
We retrospectively reviewed test results from 15 011 adults who underwent ≥1 SARS-CoV-2 NAATs; 2699 had an initial negative NAAT and repeat testing. We defined FNs as ≥1 negative NAATs followed by a positive NAAT within 14 days during the same episode of illness. We stratified subjects with FNs by duration of symptoms before the initial FN test (≤5 days versus >5 days) and examined their clinical, radiologic, and laboratory characteristics.
Results
Sixty of 2699 subjects (2.2%) had a FN result during the study period. The weekly frequency of FNs among subjects with repeat testing peaked at 4.4%, coinciding with peak NAAT positivity (38%). Most subjects with FNs had symptoms (52 of 60; 87%) and chest radiography (19 of 32; 59%) consistent with COVID-19. Of the FN NAATs, 18 of 60 (30%) were performed early (ie, ≤1 day of symptom onset), and 18 of 60 (30%) were performed late (ie, >7 days after symptom onset) in disease. Among 17 subjects with 2 consecutive FNs on NP NAATs, 9 (53%) provided lower respiratory tract (LRT) specimens for testing, all of which were positive.
Conclusions
Our findings support repeated NAATs among symptomatic patients, particularly during periods of higher COVID-19 incidence. The LRT testing should be prioritized to increase yield among patients with high clinical suspicion for COVID-19.
Keywords: coronavirus, COVID-19 testing, false-negative
Among 2699 patients with an initial negative SARS-CoV-2 NAAT who were retested while symptomatic, 60 (2.2%) were positive on repeat testing. We report clinical, laboratory, and radiologic characteristics of patients with false-negative SARS-CoV-2 NAATs in a high-prevalence setting.
Prompt and accurate diagnosis of coronavirus disease 2019 (COVID-19) is critical to guide isolation and management of persons under investigation, so as to reduce transmission, conserve personal protective equipment, and determine treatment eligibility [1]. The mainstay of COVID-19 diagnosis is detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) ribonucleic acid from nasopharyngeal (NP) or lower respiratory tract (LRT) specimens using nucleic acid amplification tests (NAATs) [2]. However, sensitivity of a single SARS-CoV-2 NAAT for COVID-19 diagnosis ranges widely from 70% to 95%, due to a combination of viral, host, and laboratory factors [2–8]. The NAAT sensitivity from an NP specimen is highest within 5 days after symptom onset at 80%–95% and declines below 80% after 5 days of symptoms [3, 4, 7, 9–13]. Poor NP specimen quality due to suboptimal collection technique further compromises NAAT sensitivity [8]. The LRT specimens have better overall sensitivity for SARS-CoV-2 detection [2, 13, 14, 15], but many clinical laboratories do not have validated testing platforms for SARS-CoV-2 NAAT from LRT specimens [1].
Concern for potential false-negative SARS-CoV-2 NAATs (FNs) has led the Infectious Diseases Society of America (IDSA) to recommend repeated NAATs for diagnosis when clinical suspicion for COVID-19 is moderate to high [2]. However, overall frequency of FNs and characteristics of patients with FNs remain poorly understood [5, 7, 16, 17]. We created a definition of FNs, determined the frequency of FNs among all patients with repeat NAATs for diagnosis, and characterized the clinical, radiologic, and laboratory features of patients with FNs at a large academic medical center during the Spring 2020 COVID-19 surge in Boston, Massachusetts.
METHODS
Study Design and Participants
We conducted a retrospective cohort study of adults ≥18 years old with SARS-CoV-2 NAATs performed between March 3–May 18, 2020 at Massachusetts General Hospital (MGH) and affiliated outpatient settings. Until April 26, 2020, testing was prioritized for patients with COVID-19 symptoms and asymptomatic patients admitted with epidemiologic risk factors (eg, undomiciled, exposure to a confirmed COVID-19 case, or presenting from a skilled nursing facility). Beginning April 27, 2020, all patients underwent SARS-CoV-2 testing at admission; outpatients continued to have symptom-driven testing, although testing of asymptomatic individuals expanded to include confirmed exposures. Throughout the study period, symptomatic hospitalized individuals with moderate to high suspicion for COVID-19 infection and outpatients who re-presented to care with worsening symptoms underwent a repeat NP NAAT after an initial negative test. Subsequent NP, expectorated sputum, or tracheal aspirate NAATs were performed in high suspicion cases. Subjects with at least 1 negative NP NAAT followed by a first positive NAAT from any specimen within 14 days (reflecting the SARS-CoV-2 incubation period) during the same illness episode were defined as having a FN. Subjects with documentation of a prior positive NAAT before the study window were excluded.
Patient Consent Statement
This study was approved by the Mass General Brigham Institutional Review Board (2020P000829) with a waiver of written informed consent.
Data Collection
We extracted demographic, clinical, laboratory, and radiologic data by chart review from the electronic medical record into a standardized data collection form. Two reviewers (among C.M.D., J.J.C., J.E.L., S.M.M., or E.P.H.) independently verified the date of symptom onset and symptoms present at the time of specimen collection for any FN. We adapted the classification of “typical” symptoms from IDSA guidelines and included either cough or dyspnea, or any 2 of the following: fevers/chills, myalgias, headache, sore throat, or new anosmia/ageusia [2]. We reported radiologic findings on chest computed tomography (CT) using standardized Radiological Society of North America (RSNA) criteria [18].
Severe Acute Respiratory Syndrome Coronavirus 2 Nucleic Acid Amplification Tests
Severe acute respiratory syndrome coronavirus 2 nucleic acid amplification tests were performed using US Food and Drug Administration emergency use-authorized assays with comparable sensitivity [19, 20]: the Roche COBAS SARS-CoV-2 assay (targets the E- and ORF1 a/b-regions), the Cepheid Xpert Xpress SARS-CoV-2 assay (targets regions of the E-and N2-genes), or a laboratory-developed quantitative polymerase chain reaction test targeting the N1- and N2-genes [21]. Commercial tests were performed and reported according to the manufacturer’s instructions. For most NAATs performed, we collected cycle threshold (Ct) values for each SARS-CoV-2 target, as a proxy for SARS-CoV-2 viral load, to describe patterns of viral burden trajectory, and to identify possible causes of FNs. We separated positive NP NAATs into 4 categories based on the lower Ct value for each assay: strong (Ct < 25), moderate (Ct 25–30), weak (Ct > 30), and unknown [21].
Identifying False-Negative Results
To separate subjects with FNs from those with true initial negative results (with the subsequent positive result reflecting an additional exposure and infection), we reviewed clinical and laboratory data to determine whether the initial negative and subsequent positive test(s) were performed during the same illness episode. During clinical review, we judged that tests were performed during the same illness episode if the subject had persistence or evolution of symptoms between the initial negative test and the positive test without an asymptomatic interval. We excluded subjects whose tests were performed during separate illness episodes or for whom a reliable history of symptoms could not be obtained. Subjects with an interval of ≥7 days between the initial negative and first positive NAAT also underwent laboratory review with evaluation of first positive NAAT Ct values. If the Ct values fell into the “strong” category, consistent with early COVID-19 infection, and the initial negative test was performed more than 7 days prior, the initial test was judged unlikely to have been performed during the same illness episode and was excluded [6, 7, 22, 23]. Before their exclusion, all cases were discussed between 2 clinical microbiologists (M.N.A. and S.E.T.) and 2 infectious disease (ID) physicians (C.M.D. and E.P.H.). Reviewers discussed any conflicting assessments until consensus was reached; if consensus could not be reached, an additional ID physician adjudicated the case (S.M.M. and J.E.L.).
Frequency of Positive and False-Negative Nucleic Acid Amplification Tests
We examined the weekly proportion of initial (first) NAATs performed at MGH and affiliated outpatient settings that were positive. We then measured the proportion of subjects with an initial FN among all subjects with repeat SARS-CoV-2 testing within 14 days; FN frequency was not calculated for the weeks of May 5 and May 16, 2020 because a 14-day testing window was not available within the study period.
Statistical Analysis
Because SARS-CoV-2 NP NAATs have better sensitivity within the first 5 days of illness, we compared subjects whose initial negative NAAT was obtained within 5 days of symptom onset to subjects whose initial test was obtained after 5 days of illness [3, 5]. We compared categorical variables using the Fisher’s exact test and continuous variables using the Wilcoxon rank-sum (Mann-Whitney U) test. We considered a 2-sided P < .05 to be statistically significant. All statistical analyses were conducted with Stata version 15.1 (StataCorp, College Station, TX).
RESULTS
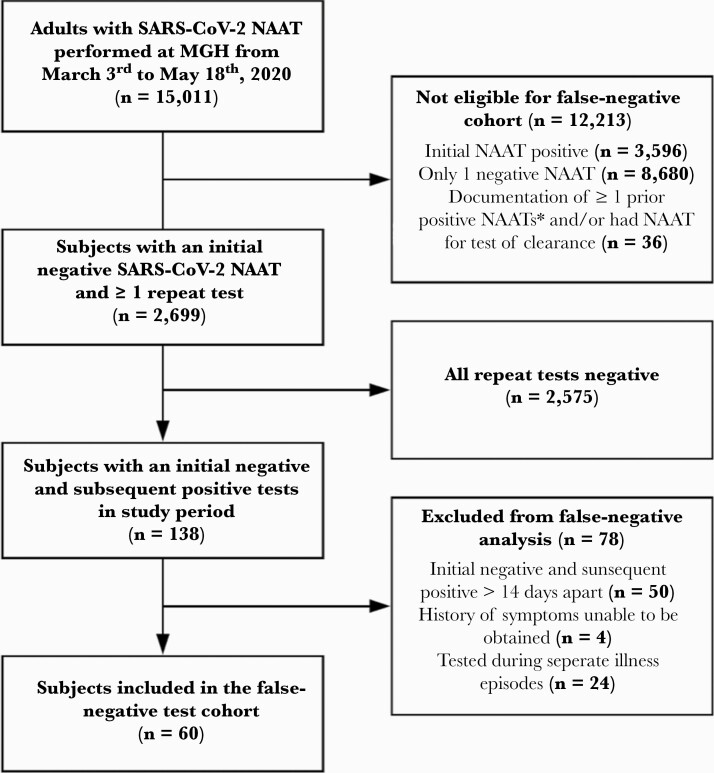
Between March 3 and May 18, 2020, 15 011 adult subjects had ≥1 SARS-CoV-2 NAATs performed, 3596 (24%) of whom had an initial positive test and 36 were excluded due to having a prior positive test or undergoing testing for disease clearance (Figure 1). Of 11 379 subjects with an initial negative test, 2699 (24%) underwent repeat testing due to ongoing symptoms and moderate or high suspicion for COVID-19 infection. Of subjects who underwent repeat testing, 138 of 2699 (5.1%) had a subsequent positive test during the study period (138 of 11 379 or 1.2% of all subjects with an initial negative NAAT). We excluded 50 of 138 (36%) subjects because the testing interval was >14 days, 4 of 138 (2.9%) subjects had given inadequate symptom history, and 24 of 138 (17%) subjects because testing occurred during separate episodes of illness. Thus, 60 subjects were classified as having an FN (60 of 2699 or 2.2% of those who had additional testing after an initial negative NAAT). Considering all subjects with positive tests during the study period, 60 of 3734 (1.6%) had initial false-negative tests.
Figure 1.
Cohort flow chart demonstrating inclusion and exclusion criteria of subjects in the study. We evaluated subjects who underwent a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid amplification test (NAAT) at Massachusetts General Hospital between March 3 and May 18, 2020 for inclusion in the false-negative test cohort. Subjects with only 1 negative NAAT, an initial positive NAAT at Massachusetts General Hospital (MGH), or documentation of ≥1 prior positive NAATs were not eligible. We excluded subjects with ≥1 repeat SARS-CoV-2 NAATs if all of their NAATs were negative, the initial negative and subsequent positive tests were >14 days apart, a history of symptoms could not be obtained, or 2 reviewers reached consensus that the subject’s discordant tests were performed across separate illness episodes. A total of 60 subjects were included in the false-negative NAAT cohort. *, Initial positive NAAT performed at an outside facility or performed before the study window.
Relationship of False-Negative Nucleic Acid Amplification Tests and Coronavirus Disease Incidence
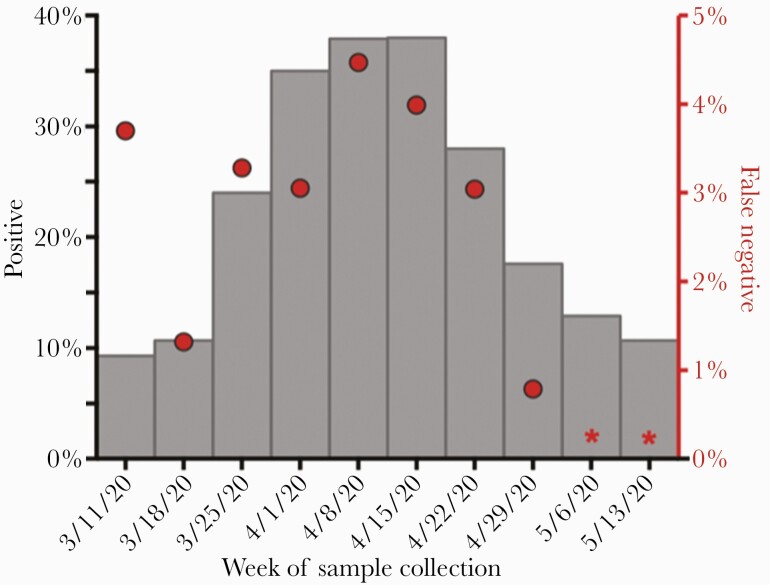
The proportion of initial NAATs that were positive rose during the first few weeks of the study period, reaching its peak at 38% the week of April 15, 2020 and declining to 18% the week of April 29, 2020 (Figure 2). The frequency of FNs among all subjects with repeat testing for diagnosis followed a similar pattern, with its peak at 4.4% the week of April 8, 2020, which declined to 0.8% during the week of April 29, 2020. The week of March 11, 2020 was an outlier, with an FN frequency of 3.6%, despite initial test positivity of only 9%, and may be attributable to repeat testing only offered to the highest risk patients in the setting of test scarcity before the availability of in-house testing. Throughout the study period, repeat SARS-CoV-2 testing also became more common as test availability improved (Supplemental Table 1) [2].
Figure 2.
Weekly proportions of initial positive and false-negative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid amplification tests (NAATs) during the coronavirus disease 2019 (COVID-19) surge. We examined the percentage of subjects with positive SARS-CoV-2 NAATs (gray bars, left y-axis) among all subjects with SARS-CoV-2 NAATs in relation to the percentage of false-negative (ie, initially negative, repeat-positive) NAATs (red circles, right y-axis) each week throughout the initial COVID-19 surge in Boston, Massachusetts. We calculated the percentage of false-negative tests as the proportion of subjects with an initial negative NAAT who had a subsequent positive NAAT during the same episode of illness among all subjects who had repeat SARS-CoV-2 testing within a 14-day window. Subjects with multiple negative tests before a positive test were counted only once, on the date of their first negative test in the series. (*) indicates weeks with omitted proportion calculations because a 14-day testing window was not available within the study period.
Features of Subjects With False-Negative Nucleic Acid Amplification Tests
Among the subjects with FNs, 38 of 60 (63%) underwent initial NAAT within 5 days of symptom onset, and 22 of 60 (37%) underwent initial NAAT > 5 days after illness onset (Table 1).
Table 1.
Clinical Characteristics and Presenting Symptoms of Subjects With Discordant COVID-19 NAATs Within 14 Days
| Symptom Onset to First NAAT | ||||
|---|---|---|---|---|
| Characteristic, n (%) or Median (IQR) | Total (n = 60) | 0–5 Days (n = 38) | >5 Days (n = 22) | P Value |
| Clinical Characteristics | ||||
| Age, years | 54 (38–65) | 55 (38–65) | 52 (38–59) | .50 |
| Male | 36 (60) | 24 (63) | 12 (55) | .59 |
| Race/Ethnicity | ||||
| White, non-Hispanic | 18 (30) | 15 (40) | 3 (14) | .07 |
| Black, non-Hispanic | 6 (10) | 4 (11) | 2 (9) | |
| Latinx | 22 (37) | 13 (34) | 9 (41) | |
| Other, non-Hispanica | 10 (17) | 3 (8) | 7 (32) | |
| Not reported | 4 (7) | 3 (8) | 1 (5) | |
| Healthcare worker | 6 (10) | 5 (13) | 1 (5) | .40 |
| Lives in congregate settingb | 16 (27) | 16 (42) | 0 (0) | <.01 |
| Known COVID-19+ contactc | 28 (47) | 22 (58) | 6 (27) | .01 |
| Location of First Test | ||||
| ED/inpatient setting | 39 (65) | 23 (61) | 16 (73) | .41 |
| Outpatient | 21 (35) | 15 (39) | 6 (27) | |
| Admitted to the hospital | 45 (75) | 26 (68) | 19 (86) | .22 |
| Required ICU care | 9 (20) | 3 (12) | 6 (32) | .14 |
| Required intubation | 7 (16) | 2 (8) | 5 (26) | .21 |
| Died | 3 (5) | 3 (8) | 0 (0) | .29 |
| Symptoms Present at the Time of Specimen Collection of the First Negative NAATd | ||||
| Typical symptoms present at time of first teste | 52 (87) | 32 (84) | 20 (91) | .70 |
| Respiratory symptoms (any) | 54 (90) | 35 (92) | 19 (86) | .66 |
| Cough | 38 (63) | 22 (58) | 16 (73) | .28 |
| Dyspnea | 30 (50) | 15 (39) | 15 (68) | .06 |
| Rhinorrhea/congestion | 19 (32) | 12 (32) | 7 (32) | 1.00 |
| Sore throat | 19 (32) | 12 (32) | 7 (32) | 1.00 |
| Chest pain/tightness | 13 (22) | 8 (21) | 5 (23) | 1.00 |
| GI symptoms (any) | 18 (30) | 5 (13) | 13 (59) | <.01 |
| Nausea/vomiting | 11 (18) | 2 (5) | 9 (41) | <.01 |
| Diarrhea | 12 (20) | 3 (8) | 9 (41) | <.01 |
| Anorexia | 11 (18) | 1 (3) | 10 (45) | <.01 |
| Abdominal pain | 3 (5) | 1 (3) | 2 (9) | .58 |
| Neurologic symptoms (any) | 23 (38) | 9 (24) | 14 (64) | <.01 |
| Headache | 14 (23) | 4 (11) | 10 (45) | <.01 |
| Anosmia/ageusia | 8 (13) | 2 (5) | 6 (27) | .04 |
| Altered mental status | 6 (10) | 3 (8) | 3 (14) | .66 |
| Dizziness | 3 (5) | 2 (5) | 1 (5) | 1.00 |
| Other symptoms (any) | 38 (63) | 19 (50) | 19 (86) | <.01 |
| Fever/chills | 35 (58) | 19 (50) | 16 (73) | .11 |
| Myalgias | 31 (52) | 18 (47) | 13 (59) | .43 |
| Fatigue | 24 (40) | 14 (37) | 10 (45) | .59 |
| Weakness | 9 (15) | 5 (13) | 4 (18) | .71 |
Bold indicates statistically significant values.
Abbreviations: COVID-19, coronavirus disease 2019; ED, emergency department; GI, gastrointestinal; ICU, intensive care unit; IQR, interquartile range; NAAT, nucleic acid amplification test.
aIncludes patients who self-reported their race as “Asian” or “other.”
bCongregate settings included the following: skilled nursing facilities; psychiatric, substance abuse, or rehabilitation hospital; hospice; or correctional facilities.
cContact with someone with confirmed COVID-19 infection, irrespective of symptoms.
dThese categories reflect any symptoms of that type reported at the time of first NAAT; patients could present with symptoms in multiple categories and be counted in each.
eTypical symptoms include either cough or dyspnea, or 2 of the following: fever/chills, myalgias, headache, sore throat, or new anosmia/ageusia.
Clinical Features
The median age was 54 years (interquartile range [IQR], 38–65), and 36 (60%) were male. Approximately half of the subjects (47%) reported recent contact with someone with known COVID-19; SARS-CoV-2 exposure was more often reported among subjects whose initial negative test was within 5 days of symptom onset (P = .01). The majority of subjects (45 of 60, 70%) were admitted to the hospital within 30 days of their initial NAAT; 9 of 60 (20%) required intensive care, and 3 of 60 (5%) died. Presentation with typical COVID-19 symptoms at the time of the initial negative NAAT was common (52 of 60, 87%) and did not differ by time from symptom onset (P = .38). However, subjects who reported symptoms for more than 5 days at the time of the initial negative NAAT were more likely to endorse gastrointestinal symptoms (59% vs 13%, P < .01) and neurologic symptoms (64% vs 24%, P < .01) than those tested within 5 days of symptom onset.
Radiologic Features
Many subjects with FN NAATs had a chest x-ray (CXR) performed within 24 hours of the initial negative NAAT (32 of 60, 53%) (Table 2). It is notable that 9 of 32 (28%) CXRs showed no abnormalities around the time of the initial negative NAAT, whereas 19 of 32 (59%) demonstrated bilateral opacities and 4 of 32 (13%) revealed other abnormalities. The CXR findings did not differ by time from symptom onset to initial NAAT (P = .18). The CT chest scan was obtained in 14 of 60 (23%) subjects within 24 hours of the initial FN (Table 2) and in 22 of 60 (37%) subjects during the testing window (Supplemental Table 2). No patients had CT chest imaging that was negative for pneumonia.
Table 2.
Radiologic and Laboratory Findings of Subjects With False-Negative NAATs
| Symptom Onset to First NAAT | ||||
|---|---|---|---|---|
| Finding, n (%) or median (IQR) | Total (n = 60) | 0–5 Days (n = 38) | >5 Days (n = 22) | P Value |
| Radiologic Characteristics | ||||
| CXR performed within 24 hours of the initial negative test | 32 (53) | 22 (58) | 10 (45) | .43 |
| No abnormalities | 9 (28) | 5 (28) | 4 (29) | .18 |
| Bilateral opacity | 19 (59) | 9 (50) | 10 (71) | |
| Other abnormality | 4 (13) | 4 (22) | 0 (0) | |
| CT chest performed within 24 hours of initial negative testa | 14 (23) | 10 (26) | 4 (18) | .54 |
| Negative for pneumonia | 0 (0) | 0 (0) | 0 (0) | .13 |
| Atypical | 5 (36) | 5 (50) | 0 (0) | |
| Indeterminate | 4 (29) | 3 (30) | 1 (25) | |
| Typical | 5 (36) | 2 (20) | 3 (75) | |
| Laboratory Characteristics | ||||
| Number of Tests Required for Diagnosis | ||||
| 2 tests | 43 (72) | 28 (74) | 15 (68) | .61 |
| 3 tests | 12 (20) | 8 (21) | 4 (18) | |
| 4 tests | 5 (8) | 2 (5) | 3 (14) | |
| Time between onset of symptoms and final diagnosis, days | 10 (5–14) | 6 (3–10) | 14 (10–16) | <.01 |
| Time between first test and final diagnosis, days | 2 (1–7) | 4 (1–7) | 1 (1–8) | .18 |
| Ct Value at Diagnosis | ||||
| Strong on NP | 10 (17) | 8 (21) | 2 (9) | .21 |
| Moderate on NP | 6 (10) | 4 (11) | 2 (9) | |
| Weak on NPb | 30 (50) | 17 (45) | 13 (59) | |
| Unknown on NP | 5 (8) | 5 (13) | 0 (0) | |
| Diagnosed on NAAT from LRT specimen | 9 (15) | 4 (11) | 5 (23) | |
Bold indicates statistically significant values.
Abbreviations: CT chest, computed tomography of the chest; Ct, cycle threshold; CXR, chest x-ray; LRT, lower respiratory tract; IQR, interquartile range; NAAT, nucleic acid amplification test; NP, nasopharyngeal.
aCT chest findings as reported using the Radiological Society of North America (RSNA) criteria [18].
bIncludes patients for whom only the E target was detected at the time of the first positive test.
Laboratory Features
Subjects required 2–4 NAATs for diagnosis (Table 2), and the first positive NAAT was performed a median of 2 days (IQR, 1–7) after the initial negative NAAT. At diagnosis, among the 60 subjects with FNs, 30 (50%), 6 (10%), 10 (17%), and 5 (8%) had weak, moderate, strong, and unknown Ct values on NP NAAT, whereas 9 (15%) were diagnosed by LRT specimen testing.
Of subjects with initial NP FNs, 9 of 9 (100%) who provided LRT specimens were positive on LRT NAAT. Subjects whose initial test was >5 days after symptom onset were not more likely to be diagnosed on LRT specimen than subjects tested within 5 days of symptoms (P = .27). Among 17 subjects with 2 consecutive negative NP NAATs and repeat positive testing, 9 (53%) were diagnosed via LRT testing.
Viral Burden Trajectories and Suspected Cause of False-Negative Tests
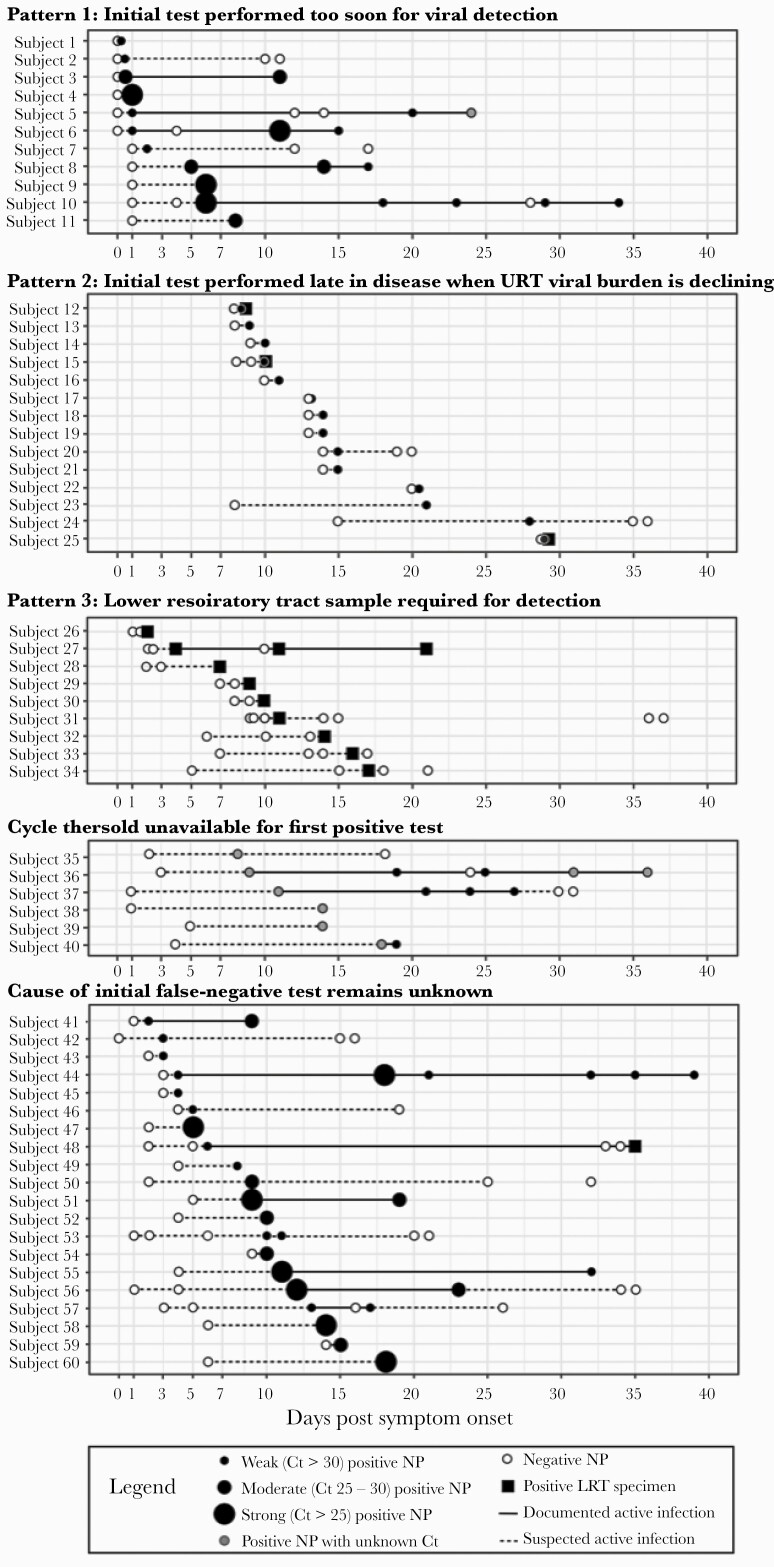
We observed several patterns of viral burden trajectories that varied by time from symptom onset (Supplemental Appendix, Supplemental Table 3). In Pattern 1 (Figure 3, Subjects 1-11 or 18%), the initial negative NAAT was performed within 1 day of symptom onset and was followed by either a weak positive test within 1 day of symptom onset or a subsequent moderate-strong positive test. Pattern 1 suggests that the initial FN NAAT was performed too early in the disease course for viral detection, although viral load usually peaks around symptom onset [5, 7, 12]. In Pattern 2 (14 of 60 [23%] subjects), the initial NAAT was performed more than 7 days after symptom onset with weak Ct values at diagnosis (Figure 3, Subjects 12–25). Pattern 2 suggests that the FN was obtained when the upper respiratory tract viral burden was declining, late in the course of infection [3, 5, 7]. In Pattern 3, 9 of 60 subjects (Figure 3, Subjects 26–34, [15%]) required LRT specimen NAAT for diagnosis. Pattern 3 suggests LRT-predominant disease; the FN results using an NP specimen may be attributable to sampling a less-affected body site. Several subjects with Pattern 3 also had initial NP NAATs performed late in illness when viral burden in the upper respiratory tract may be lower [13, 14, 15, 23].
Figure 3.
Cycle threshold (Ct) trajectories categorized by most likely cause of initial false-negative nucleic acid amplification test (NAAT). Among the 60 subjects with false-negative NAATs, we plotted all available severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) NAATs obtained after their initial false-negative test, by number of days postsymptom onset. Tests obtained from a nasopharyngeal (NP) specimen are shown as dots of increasing size based on increasing SARS-CoV-2 viral load. Positive tests are depicted in black (if the Ct value was known) or gray (if the Ct value was unknown). Negative tests are depicted in white. The intervals from the initial negative test to the first positive test, and from the last positive test to any subsequent negative tests, are shown as dotted lines; these intervals signify times of suspected active infection. Intervals between positive tests are shown as solid lines to depict documented active infection. Test patterns were depicted in 5 categories corresponding to the observed viral burden pattern. LRT, lower respiratory tract; URT, upper respiratory tract.
The Ct values were unavailable for the first positive test in 6 of 60 (10%) subjects (Subjects 35–40). The remaining 20 of 60 (32%) subjects had a viral burden trajectory that did not fall into 1 of the 3 patterns (Subjects 41–60). We suspected poor specimen quality or incorrect date of symptom onset in these cases but could not confirm.
DISCUSSION
Improved understanding of the frequency of and features associated with false-negative SARS-CoV-2 NAATs is critical to crafting COVID-19 diagnostic approaches that balance test sensitivity, test availability, and risks of potential exposure from undiagnosed infection. We found a low frequency (2.2%) of newly positive SARS-CoV-2 NAATs on repeat testing during the same episode of illness despite 25% COVID-19 prevalence during the study period. The frequency of FNs in any given week correlated with the weekly positive rate, supporting the hypothesis that the yield of repeat testing is proportional to pretest probability [24]. Most patients with FNs had typical symptoms and radiologic findings of COVID-19 at the time of their initial negative test, and when performed, all chest CTs showed evidence of pneumonia. Although the cause of FNs remained unknown for some subjects, our findings suggest that 18% were tested so early postsymptom onset (0–1 days) that NP viral burden was undetectable, and 38% had late-stage or LRT-predominant disease, situations in which SARS-CoV-2 viral burden in the upper respiratory tract is lower [13, 14, 15, 23].
The frequency of initial false-negative tests was lower in our study compared with other reports [4, 7, 15, 16, 25, 26]. Jamal et al [27] observed that 27 of 91 (30%) hospitalized patients with laboratory-confirmed COVID-19 had a false-negative NP NAAT; however, time from symptom onset to testing was prolonged in the study (median, 12 days; IQR, 9–15). In a cohort from Wuhan, China, 9 of 76 (12%) patients with confirmed COVID-19 had initially negative NP NAATs, but time from symptom onset to testing was not assessed [28]. However, our overall frequency of initial FNs is similar to that observed by investigators in Washington, California, and Illinois during periods of high COVID-19 prevalence (3.0%–3.5%) [17, 29].
There are several potential reasons why we observed a lower frequency of FNs. First, our study population had a higher frequency of repeat SARS-CoV-2 NAAT (24%) than some others (3%–6%) [17, 29] and was therefore more likely to include patients with only moderate suspicion for COVID-19. Second, we limited the testing window to 14 days to avoid misclassifying results as false-negative, when they more likely reflect true-negative results followed by a subsequent infection preceding the first positive test. Even when limiting the testing window to 14 days, we found that 27% of patients with discordant tests had testing performed across separate illness episodes. Studies that do not account for potential incident infections during testing intervals of 7 days or more may substantially overestimate the frequency of false-negative NAATs.
Despite retesting 24% of patients with an initial negative NAAT, we may have underestimated COVID-19 incidence among patients with mild or rapidly resolving symptoms, or among asymptomatic patients without repeat testing within the MGH system. Other studies have reported high SARS-CoV-2 positivity among asymptomatic or presymptomatic individuals when community prevalence is high [30, 31], but data regarding the frequency of FNs among asymptomatic patients are lacking. We also observed a high proportion of patients with FNs who had typical symptoms and radiologic findings of COVID-19; however, it is possible that patients with atypical symptoms or imaging findings underwent repeat testing less frequently and, therefore, are more likely to have evaded diagnosis.
Our results demonstrate that 2 negative NP NAATs may not be sufficient for diagnosis when clinical suspicion for COVID-19 is high. Among patients with initial FNs, 17 of 60 (28%) required more than 2 NP NAATs to detect SARS-CoV-2. However, after 2 negative NP NAATs, the yield of additional NP testing decreases, and LRT testing may be preferred among patients with persistent or worsening symptoms, although not all patients can provide adequate expectorated sputum samples for analysis. In our cohort, 15% of patients with any FN, and the majority (53%) of patients with 2 NP FNs, were ultimately diagnosed through LRT testing. The viral load in the LRT is reported to be higher than in the nasopharynx in patients with pulmonary involvement, which may contribute to the better sensitivity of LRT specimens throughout the disease [14, 15, 23]. It is critically important that manufacturers of commercially available SARS-CoV-2 NAAT platforms validate LRT specimens to increase access to this modality and overcome limitations of NP NAAT sensitivity.
In reviewing all available NAAT results and associated Ct values, several viral trajectory patterns emerged that informed the likely etiology of the false-negative tests in our study. Many initial negative NAATs were performed either very early or late in disease when viral load in the upper respiratory tract may be lower, resulting in lower NP NAAT sensitivity. In most individuals, viral load is highest around the time of symptom onset [5, 7, 12, 23]. However, published reports document considerable heterogeneity in viral trajectories both between and within individuals [7, 12, 22]; some individuals start with a low viral load that rises later in the first few days after symptom onset [7, 12, 22]. Our findings suggest that when testing patients for COVID-19 within the first 24 hours of symptoms, a single repeat NP NAAT within 7 days is likely to offer reliable SARS-CoV-2 detection. On the other hand, if the initial negative NAAT was obtained after 7 days of symptoms, clinicians should pursue further diagnostic testing after a second negative NAAT if clinical suspicion for COVID-19 infection remains high, including an LRT NAAT when feasible and/or SARS-CoV-2 serologies. Uncertainty regarding timing of symptom onset or adequacy of NP NAAT specimen collection should also prompt consideration of repeat SARS-CoV-2 testing.
It remains unclear whether patients who have false-negative NP NAATs can transmit COVID-19, particularly if the NAATs are obtained more than 10 days after symptom onset [10, 32]. The Ct values show strong inverse correlation with cell culture positivity, which would suggest that patients with negative NAATs due to very low viral load are minimally or not infectious [32, 33]. Therefore, retesting patients who present late in disease may not substantially reduce the likelihood of onward transmission. However, a case report from Wuhan, China described a healthcare worker who had 2 negative NP NAATs and yet transmitted SARS-CoV-2 to 3 other people [34]. In our study, half of patients with initial FNs had subsequent positive NP NAATs with strong Ct values, reflecting high viral burden, and potentially high risk of transmission. Therefore, symptomatic patients with negative NAATs, but high clinical suspicion, should continue isolation precautions until active COVID-19 infection is no longer suspected [1].
To overcome limitations of NAATs, chest CT can be helpful in patients for whom there is high suspicion for COVID-19. All of the 14 subjects in our study who had CT chest scan performed concurrent with their initial FN had radiologic evidence of pneumonia [18]. This finding is consistent with other reports of patients with initial FN NAATs that similarly describe imaging findings concerning for COVID-19 before diagnosis [35–38]. However, although chest CT is highly sensitive for COVID-19 (97%; 95% CI, 95%–98%), it is poorly specific (25%; 95% CI, 22%–30%), suggesting that CT results must be interpreted in the clinical context, because these findings can also be seen with many other disease processes, including other viral infections [38, 39]. When clinical suspicion is high, chest CT findings typical for COVID-19 pneumonia should prompt consideration of additional NAAT(s), including an LRT NAAT when possible, for diagnosis.
This study has several limitations. First, we conducted a single-center retrospective review of patients’ medical records, which did not allow for direct questioning about symptoms or exposures. Deriving the date of symptom onset from the medical record may be unreliable due to inconsistency of reporting, variable patient recall, and the nonspecific and sometimes mild nature of initial COVID-19 symptoms [40]. Second, COVID-19 serology was not widely available during the study period (Supplemental Table 3); therefore, the prevalence of false-negative test results may be underestimated [41]. We only described the yield of repeat SARS-CoV-2 NAAT for COVID-19 diagnosis among patients under investigation; our analysis does not address the sensitivity, potential yield, or role of repeat testing for the purpose of population surveillance among asymptomatic individuals. Finally, we were unable to identify a clear cause of FNs in some subjects given an inability to formally assess specimen quality, missing Ct values (including for most LRT NAATs), and the challenges of determining an accurate date of symptom onset.
CONCLUSIONS
In conclusion, our study supports IDSA recommendations to repeat NAATs among patients with symptoms or imaging findings typical of COVID-19, particularly if initial testing is performed very early (within 1 day) or late (>1 week) after symptom onset, or in settings where community COVID-19 prevalence is high. Because a substantial proportion of patients with false-negative tests were diagnosed only through LRT testing, expansion of LRT testing should be prioritized to improve testing yield among patients for whom there is high clinical suspicion for COVID-19.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank the many Massachusetts General Hospital (MGH) Infectious Diseases physicians who volunteered their time to review patient records during the initial coronavirus disease 2019 (COVID-19) surge, and the infection preventionists of the MGH Infection Control Unit. We also appreciate Giulia Park’s help with manuscript formatting and proofreading.
Disclaimer. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This project was funded by Grant Numbers T32 AI007433 and R37AI058736-16S1 from the National Institute of Allergy and Infectious Diseases, Grant Number K08 HD101342 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Harvard Catalyst Medial Research Investigator Training fellowship.
Potential conflicts of interest. J. A. B. has received grant support from Zeus Scientific, bioMerieux, Immunetics, the Bay Area Lyme Foundation, and the Lyme Disease Biobank Foundation, for work unrelated to this study, and has been a consultant for T2 Biosystems, DiaSorin, and Roche Diagnostics. S. E. T. receives funding from the Centers for Disease Control for COVID-19-related work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Coronavirus (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/index.html. Accessed 3 July 2020.
- 2. Infectious Diseases Society of America. Guidelines on the diagnosis of COVID-19. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/. Accessed 5 July 2020. [DOI] [PMC free article] [PubMed]
- 3. Miller TE, Garcia Beltran WF, Bard AZ, et al. Clinical sensitivity and interpretation of PCR and serological COVID-19 diagnostics for patients presenting to the hospital [published online ahead of print August 28, 2020]. Fed Am Soc Exp Biol J 2020; doi: 10.1096/fj.202001700RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu L, Liu W, Zheng Y, et al. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. Microbes Infect 2020; 22:206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Green DA, Zucker J, Westblade LF, et al. Clinical performance of SARS-CoV-2 molecular testing. J Clin Microbiol 2020; 58:e00995-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan W, Lu Y, Zhang J, et al. Viral kinetics and antibody responses in patients with COVID -19. medRxiv 2020; doi: 2020.03.24.20042382. [Google Scholar]
- 7. Kucirka LM, Lauer SA, Laeyendecker O, et al. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med 2020; 173:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kinloch NN, Ritchie G, Brumme CJ, et al. Suboptimal biological sampling as a probable cause of false-negative COVID-19 diagnostic test results. J Infect Dis 2020; 222:899–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis 2020; 71:778–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 581:465–9. [DOI] [PubMed] [Google Scholar]
- 11. Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect 2020; 81:357–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020; 26:672–5. [DOI] [PubMed] [Google Scholar]
- 13. Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis 2020; 71:793–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020; 323:1843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang Y, Yang M, Shen C, et al. Laboratory diagnosis and monitoring the viral shedding of SARS CoV-2 infection. Innovation 2020; 1:100061 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rong Y, Wang F, Liu J, et al. Clinical characteristics and risk factors of mild-to-moderate COVID-19 patients with false-negative SARS-CoV-2 nucleic acid. J Med Virol 2020. doi: 10.1002/jmv.26242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Long DR, Gombar S, Hogan CA, et al. Occurrence and timing of subsequent SARS-CoV-2 RT-PCR positivity among initially negative patients. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. endorsed by the society of thoracic radiology, the American College of Radiology, and RSNA–Secondary Publication. J Thorac Imaging 2020; 35:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anahtar MN, Shaw B, Slater D, et al. Development of a qualitative real-time RT-PCR assay for the detection of SARS-CoV-2: A guide and case study in setting up an emergency-use, laboratory-developed molecular assay. medRxiv 2020. doi: 10.1101/2020.08.26.20157297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. U.S. Food and Drug Administration. SARS-CoV-2 reference panel comparative data. Available at: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-reference-panel-comparative-data. Accessed 12 October 2020.
- 21. Mitchell SL, St. George K, Rhoads DD, et al. Understanding, verifying and implementing Emergency Use Authorization molecular diagnostics for the detection of SARS-CoV-2 RNA. J Clin Microbiol 2020; 58:e00796-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020; 382:1177–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang Y, Chen S, Yang Z, et al. SARS-CoV-2 viral load in clinical samples from critically ill patients. Am J Respir Crit Care Med 2020; 201:1435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection–challenges and implications. N Engl J Med 2020; 383:e38. [DOI] [PubMed] [Google Scholar]
- 25. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology 2020; 296:E115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Y, Yao L, Li J, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol 2020; 92:903–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jamal AJ, Mozafarihashjin M, Coomes E, et al. Sensitivity of nasopharyngeal swabs and saliva for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020. doi: 10.1093/cid/ciaa848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X, Tan L, Wang X, et al. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis 2020; 94:107–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ridgway JP, Pisano J, Landon E, Beavis KG, Robicsek A. Clinical sensitivity of severe acute respiratory syndrome coronavirus 2 nucleic acid amplification tests for diagnosing coronavirus disease 2019. Open Forum Infect Dis 2020; 7:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patel MC, Chaisson LH, Borgetti S, et al. Asymptomatic SARS-CoV-2 infection and COVID-19 mortality during an outbreak investigation in a skilled nursing facility. Clin Infect Dis 2020; 69:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prabhu M, Cagino K, Matthews KC, et al. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG Int J Obstet Gynaecol 2020. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. medRxiv 2020. doi: 2020.06.08.20125310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis 2020; 39:1059–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cao G, Tang S, Yang D, et al. The potential transmission of SARS-CoV-2 from patients with negative RT-PCR swab tests to others: two related clusters of COVID-19 outbreak. Jpn J Infect Dis 2020. doi: 10.7883/yoken.JJID.2020.165. [DOI] [PubMed] [Google Scholar]
- 35. Xie X, Zhong Z, Zhao W, et al. Chest CT for typical coronavirus disease 2019 (COVID-19) pneumonia: relationship to negative RT-PCR testing. Radiology 2020; 296:E41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu J, Wu R, Huang H, et al. Computed tomographic imaging of 3 patients with coronavirus disease 2019 pneumonia with negative virus real-time reverse-transcription polymerase chain reaction test. Clin Infect Dis 2020; 71:850–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang P, Liu T, Huang L, et al. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology 2020; 295:22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020; 296:e32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bai HX, Hsieh B, Xiong Z, et al. Performance of radiologists in differentiating COVID-19 from non-COVID-19 viral pneumonia at chest CT. Radiology 2020; 296:E46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Davis LL. Determining time of symptom onset in patients with acute coronary syndromes: agreement between medical record and interview data. Dimens Crit Care Nurs 2015; 34:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with COVID-19. Clin Infect Dis Off Publ Infect Dis Soc Am 2020. doi: 10.1093/cid/ciaa461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.