Abstract
We compared symptoms and characteristics of 4961 ambulatory patients with and without laboratory-confirmed severe acute respiratory syndrome coronavirus 2 infection. Findings indicate that clinical symptoms alone would be insufficient to distinguish between coronavirus disease 2019 and other respiratory infections (eg, influenza) and/or to evaluate the effects of preventive interventions (eg, vaccinations).
Keywords: influenza, case definitions, COVID-19, observational research studies, vaccine effectiveness
Since early 2020, pre-established influenza surveillance systems have contributed substantially to monitoring the coronavirus disease 2019 (COVID-19) pandemic in the United States. For syndromic surveillance, COVID-like illness is defined as acute illness with fever and either cough or shortness of breath, or a COVID-19 diagnostic code [1]. To evaluate the effectiveness of interventions to prevent mild illness associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, standardized clinical criteria in addition to laboratory confirmation of infection will be needed. In March 2020, participating sites in the US Influenza Vaccine Effectiveness (Flu VE) research network revised study inclusion criteria to prospectively enroll persons with acute febrile or respiratory illness, characterized by reported fever or cough or shortness of breath, who had been tested for SARS-CoV-2 infection. Here we compare symptoms and characteristics of persons with and without laboratory-confirmed SARS-CoV-2 infection.
METHODS
At the study sites of the US Flu VE Network in Michigan, Pennsylvania, Texas, Washington, and Wisconsin, research staff screened for study eligibility among persons of all ages who had sought medical care (eg, telehealth, primary care, urgent care, and emergency departments) and/or COVID-19 testing for an acute respiratory illness. Eligible individuals reported acute illness with fever/feverishness, cough, or shortness of breath/difficulty breathing and had a respiratory specimen collected for SARS-CoV-2 testing within 10 days of illness onset. At 4 sites, research staff contacted and screened patients for eligibility by telephone; at 1 site (Pennsylvania), a sample of potentially eligible patients was screened for eligibility by phone or online survey [2]. Standardized questionnaires collected demographic information, general health status, health-related behaviors such as cigarette smoking or vaping, health care–related employment, and contact with a person with confirmed COVID-19. Questionnaires also solicited information about 12 specific symptoms experienced since illness onset (Figure 1).
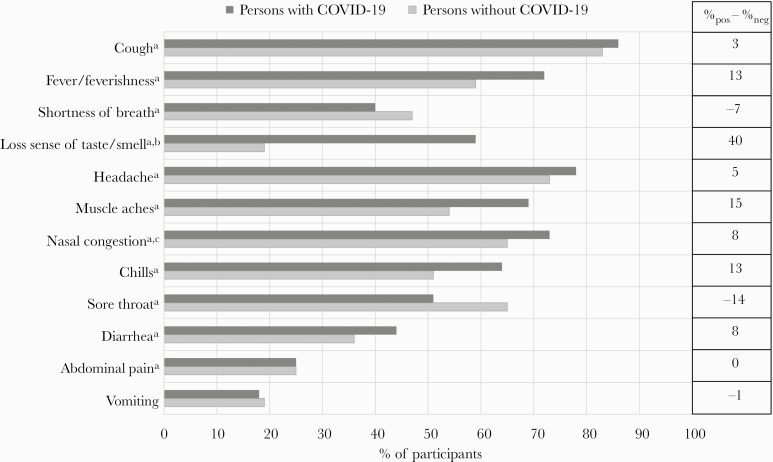
Figure 1.
Self-reported presence of clinical symptoms among 4961 ambulatory persons with acute respiratory illness, by clinical SARS-CoV-2 RT-PCR test result, US Influenza Vaccine Effectiveness Network, March 23–August 15, 2020. aIndicates a statistically significant difference between persons with and without COVID-19 (ie, P < .05). bA subset of participants were asked about loss or decreased sense of taste/smell including 850 persons with COVID-19 and 3252 persons without COVID-19. cA subset of participants were asked about nasal congestion including 613 persons with COVID-19 and 2687 persons without COVID-19. Abbreviations: COVID-19, coronavirus disease 2019; RT-PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Respiratory specimens (from nasal or nasopharyngeal swabs) were tested for SARS-CoV-2 RNA using reverse transcription polymerase chain reaction (RT-PCR) assays at study sites. Days from illness onset to testing were calculated using the clinical specimen collection date. We classified persons with and without COVID-19 based on SARS-CoV-2 RT-PCR test results; persons tested by antigen detection assays alone were excluded. Underlying medical conditions associated with increased risk of severe illness from COVID-19 (eg, cancer, diabetes, chronic obstructive pulmonary disease, or hypertension) [3] were obtained for participants at 4 study sites from electronic medical records based on International Classification of Diseases (ICD)–10 diagnostic codes from medical encounters during the past year, as previously described [4]. Comparisons between persons with and without COVID-19 were made using chi-square tests (categorical variables) or Wilcoxon rank-sum tests (continuous variables). The P value for significance was set at .05. This study was approved by institutional review boards at the Centers for Disease Control and Prevention and all participating sites.
RESULTS
Between March 26 and August 15, 2020, 5760 patients were interviewed and 4961 were included in analyses. Of 799 patients excluded, 402 (50%) were swabbed, tested, or interviewed more than 10 days after symptom onset, 31 (4%) had inconclusive RT-PCR results or had a SARS-CoV-2 antigen test (n = 11), 82 (10%) were asymptomatic before enrollment, and 284 (36%) did not meet clinical criteria during the enrollment interview (ie, no reported cough, fever/feverishness, or shortness of breath/difficulty breathing). Of 284 not meeting clinical criteria, 50 (18%) reported decreased taste or smell.
Of 4961 included, 916 persons with laboratory-confirmed COVID-19 and 4045 with symptoms but without COVID-19 were included. Patient age and race/ethnicity differed by case status (P < .01 for both) (Supplementary Table 1). Compared with persons without COVID-19, persons with COVID-19 were more likely to be adults aged 18–49 years (65% vs 56%) and less likely to be younger than 18 years (6% vs 14%). Persons with COVID-19 more frequently self-identified as Hispanic/Latino (16% vs 7%) or non-Hispanic/Latino Black or African American (8% vs 6%) and less frequently as non-Hispanic/Latino White or Caucasian (70% vs 81%). Among patients with information on underlying chronic medical conditions, persons with COVID-19 less frequently had documented underlying medical conditions than persons without COVID-19 (42% vs 47%; P = .02). Among adults aged ≥18 years, similar percentages of persons with and without COVID-19 identified as health care workers (25% vs 26%; P = .62); persons with COVID-19 less frequently reported cigarette smoking or vaping (13% vs 21%; P < .01). During the 14 days before symptom onset, contact with a person with confirmed COVID-19 was reported by 59% of persons with COVID-19 vs 18% of persons without COVID-19 (P < .01).
Overall, patients reported a median (interquartile range [IQR]) of 3 (2–5) days of symptoms before specimen collection for diagnostic SARS-CoV-2 testing and a median (IQR) of 6 (4–8) days of symptoms at the time of interview. Time between symptom onset and specimen collection did not differ between persons with and without COVID-19 (P = .17). Among qualifying symptoms (fever/feverishness, cough, or shortness of breath/difficulty breathing), cough was most commonly reported (86% of persons with and 83% of persons without COVID-19; P < .01) (Figure 1). Shortness of breath/difficulty breathing was reported less frequently by persons with COVID-19 than without (40% vs 47%; P < .01). Overall, 4898 (99%) patients meeting clinical screening criteria for inclusion in this research study reported fever/feverishness and/or cough. Persons with COVID-19 reported a median (IQR) of 7 (5–8) of the assessed symptoms vs 6 (4–7) for persons without COVID-19 (P < .01). Among 4102 participants asked, 59% of persons with COVID-19 vs 19% of persons without COVID-19 reported diminished taste or smell (P < .01). Generalized symptoms (muscle aches or headache) and gastrointestinal symptoms (vomiting, diarrhea, or abdominal pain) were more common among persons with COVID-19 (91% and 57%, respectively) than among those without COVID-19 (83% and 50%, respectively; P < .01 for both).
DISCUSSION
For control of the COVID-19 pandemic, the Centers for Disease Control and Prevention (CDC) recommends testing symptomatic persons when there is a concern of potential COVID-19 [5]. Clinical signs and symptoms consistent with COVID-19 are common among patients seeking health care for mild respiratory illness and may not inform decisions on who should be tested for SARS-CoV-2 infection. However, future evaluation of effectiveness of interventions, including treatment and vaccination, for prevention of illness or progression to severe disease will require systematic testing of medically attended illnesses meeting standard clinical criteria [6]. In this study conducted in 5 US locations, persons with mild illness seeking clinical care or COVID-19 testing who met simple, standardized screening criteria (reported fever, cough, or shortness of breath of ≤10 days’ duration) commonly reported other respiratory, gastrointestinal, or systemic symptoms. Although persons with COVID-19 were more likely than those without COVID-19 to report gastrointestinal symptoms (vomiting, diarrhea, or abdominal pain) or other symptoms such as muscle aches or headache, the largest difference was observed in diminished or complete loss of taste or smell, reported by more than half of persons with laboratory-confirmed COVID-19 vs 1 in 5 persons without COVID-19. Because of the wide overlap in COVID-19 symptoms with those of other respiratory illnesses, laboratory confirmation of SARS-CoV-2 infection will be critical, not only for limiting disease spread, contact tracing, and monitoring clinical course, but also for assessing the effectiveness of interventions during periods of co-circulation of SARS-CoV-2 and other respiratory viruses, including influenza.
Rapid identification of SARS-CoV-2 infection among symptomatic patients seeking health care is critical to limit disease spread. Participants in this study, including symptomatic health care workers and patients reporting contact with a person with confirmed COVID-19, reported a median of 3 days of illness before collection of respiratory specimens for viral testing; 25% reported ≥5 days. Delays in seeking health care may result in disease transmission without strict adherence to recommended preventive measures, especially among persons with a low index of suspicion for COVID-19. While contact with a known COVID-19 case was strongly associated with SARS-CoV-2 infection, almost half of people with COVID-19 did not report exposure from another person with known COVID-19.
One limitation of this analysis is that only patients meeting screening criteria were included; thus the sensitivity and specificity of other research case definitions were not assessed. In addition, symptom lists at some study sites did not assess symptoms commonly reported by outpatients with confirmed COVID-19 (such as fatigue). Because enrollment in this research study occurred after a clinical swab was obtained for testing, some patients might have been aware of their test results at the time of enrollment and symptom assessment, which could have influenced responses to some questions. Finally, persons without COVID-19 (ie, negative for SARS CoV-2 by RT-PCR) may have had false-negative test results; however, to reduce misclassification, cases were classified based on results of molecular assays, and participants were tested within 10 days of illness onset.
In addition to establishing clinical criteria for mild illness to evaluate interventions to prevent COVID-19, systematic testing for SARS-CoV-2 and influenza will be needed when both viruses circulate for assessing the effectiveness of 2020–2021 seasonal influenza vaccines [2, 7]. Symptom criteria that can be rapidly assessed before enrollment in research studies of influenza and COVID-19 would help ensure systematic testing for both viruses. The sensitivity and specificity of screening criteria, including subjective fever, cough, and loss of taste or smell, should be assessed among ambulatory persons with COVID-19 for use in epidemiologic studies [7].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Elizabeth Armagost, Deanna Cole, Terry Foss, Hannah Gourdoux, Erica Graves, Kayla Hanson, Linda Heeren, Lynn Ivacic, Jacob Johnston, Julie Karl, Diane Kohnhorst, Erik Kronholm, Karen McGreevey, Huong McLean, Jennifer Meece, Vicki Moon, Rebecca Pilsner, DeeAnn Polacek, Martha Presson, Carla Rottscheit, Jackie Salzwedel, Julian Savu, Sandy Strey, Melissa Wendt, and Gail Weinand from Marshfield Clinic Research Institute; Theresa M. Sax, Klancie Dauer, G. K. Balasubramani, Leah McKown, Emily Welsh, Michael Susick, Andrew Ho, and Meredith Axe from University of Pittsburgh; Joshua G. Petrie, Lois E. Lamerato, Ryan E. Malosh, E. J. McSpadden, Hannah Segaloff, Caroline K. Cheng, Rachel Truscon, Emileigh Johnson, Armanda Kimberly, Anne Kaniclides, Amy Getz, Kim Beney, Sarah Bauer, Michelle Groesbeck, Joelle Baxter, Rebecca Fong, Sarah Davenport, Miranda Viars, Micah Wildes, Regina Lehmann-Wandell, Kayla Morse, Rachel Phillips, Nicole Hermes from University of Michigan, Ann Arbor, and Henry Ford Health System, Detroit, Michigan; C. Hallie Phillips, Erika Kiniry, Stacie Wellwood, Brianna Wickersham, Suzie Park, Matt Nguyen, Rachael Burganowski from Kaiser Permanente Washington Health Research Institute; Kayan Dunnigan, Kempapura Murthy, Chandni Raiyani, Marcus Volz, Kimberley Walker, Martha Zayed, Mary Kylberg, Jeremy Ray, Deborah Price, Natalie Settele, Jennifer Thomas, Jaime Walkowiak, Michael Reis, Madhava Beeram, Arundhati Rao, and Alejandro Arroliga from Baylor Scott & White Health.
Financial support. This work was supported through cooperative agreements funded by the US Centers for Disease Control and Prevention and, at the University of Pittsburgh, by infrastructure funding from the National Institutes of Health (UL1 TR001857).
Disclaimer. The findings and conclusions are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. The authors have no financial relationships relevant to this article to disclose. Dr. Monto reports personal fees from Sanofi Pasteur and Seqirus outside the submitted work. Dr. Martin reports personal fees from Pfizer outside the submitted work. Dr. Lisa Jackson reports grants from Novavax outside the submitted work. Dr. Gaglani reports grants from the Centers for Disease Control and Prevention–Abt Associates outside the submitted work. Dr. Michael Jackson and Dr. Zimmerman report grants from Sanofi Pasteur outside the submitted work. Dr. Nowalk reports grants from Merck & Co. outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. Patients provided written or verbal consent/assent for study enrollment as determined by local institutional review board (IRB) requirements. Institutional review boards approved or waived this study as follows. Approved or waived by the Centers for Disease Control and Prevention IRB with reliance on:
1. University of Michigan IRB (Approved): CDC protocol 6238, University of Michigan protocol HUM00119183;
2. University of Pittsburgh IRB (Approved): CDC protocol 6219, University of Pittsburgh protocol STUDY19070407;
3. Baylor Scott and White Health IRB (Approved): CDC protocols 7125 and 7277, Baylor Scott and White protocols 160145 and 020-153;
4. Kaiser Permanente Washington Research Institute (Waived);
5. Marshfield Clinic Research Institute (Approved): CDC protocol 6197, Marshfield Clinic Research Institute protocol BEL10511.
References
- 1. Centers for Disese Control and Prevention. Symptoms of coronavirus Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 25 September 2020.
- 2. Zimmerman RK, Nowalk MP, Bear T, et al. Proposed clinical indicators for efficient screening and testing for COVID-19 infection from classification and regression trees (CART) analysis. medRxiv 2020.05.11.20097980 [Preprint]. 2020. Available at: 10.1101/2020.05.11.20097980. Accessed 14 September 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. People with certain medical conditions Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed 14 September 2020.
- 4. Jackson ML, Chung JR, Jackson LA, et al. Influenza vaccine effectiveness in the United States during the 2015–2016 season. N Engl J Med 2017; 377:534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Overview of testing for SARS-CoV-2 (COVID-19) Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Accessed 14 September 2020.
- 6. Heaton PM. The Covid-19 vaccine-development multiverse. N Engl J Med 2020; 383:1986–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.