Abstract
A serological survey was carried out in Monteria (500 000 population), a mid-size city in Colombia. An overall prevalence of 55.3% (95% confidence interval, 52.5%–57.8%) was found among a sample of 1.368 people randomly selected from the population. Test positivity was related to economic characteristics with the highest prevalence found in the most impoverished areas, representing 83.8% of the city’s population. We found a prevalence that might be associated with some important level of population immunity.
Keywords: coronavirus, ELISA, infectious disease transmission, poverty areas, socioeconomic status
We present the results of a seroprevalence study carried out in Monteria, Colombia. A 55.3% overall prevalence in a sample of 1.368 people was obtained. We found a prevalence that may be associated with some important level of population immunity.
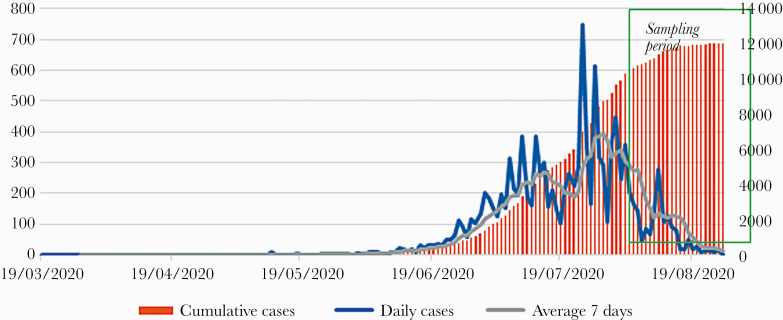
Colombia (503 million inhabitants) identified its first case of coronavirus disease 2019 ([COVID-19] severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection) on March 2, 2020, and by October 14, 2020 it had reported 930 159 cases (incidence, 17 539 per 100 000 people) and 28 306 deaths (mortality, 5339 per 100 000 people), with variations for incidence and mortality by department and region [1]. Monteria is a medium-size city (~500 000 people) in the Caribbean region, with a high proportion of the population living in poverty (~80%). Local SARS-CoV-2 circulation was first detected on March 15, 2020, and by October 14, 2020 it had reported 25 208 cases and 1585 deaths (incidence rate, 13 776 per 100 000 people; mortality rate, 15 258 per 100 000 people) (Figure 1).
Figure 1.
Seroprevalence studies are essential because they allow a more reliable estimation of the population infected after COVID-19 epidemics and estimate the likelihood of a second peak of transmission [2]. Colombia, according to the website https://serotracker.com/data, has no reported seroprevalence studies of SARS-CoV-2.
METHODS
This study aimed to estimate the prevalence of SARS-CoV-2 past infections in Monteria. A population-based serological study was carried out over a random sample (n = 1368) of the population selected from all city areas. The neighborhood population size weighted the sample. The steps to select the sample were as follows: (1) blocks of houses were randomly selected from every neighborhood (10 neighborhoods); (2) a list of houses in every selected block was developed, identifying every house with a particular number; and (3) house-to-house visits were made in the city’s neighborhoods, taking the even numbers. Adjoining houses were not visited. No attempt was made to identify whether participants had antecedents of suspected or confirmed COVID-19 infection or disease, nor whether they had been a contact of the confirmed case(s). The number of samples taken by neighborhoods fluctuated between 55 and 428. The blood samples were taken by health staff trained for epidemiological fieldwork. Once the blood samples were obtained, the tubes were sent to the University of Cordoba laboratory, where they were centrifuged. The enzyme-linked immunosorbent assay (ELISA) test was carried out by microbiologists who are experts in this type of test.
Sera were analyzed using INgezim COVID 19 DR test (Ingenasa; Eurofins, Madrid, Spain), a dual recognition ELISA detecting semiquantitatively total SARS-CoV-2 virus N-protein-specific antibodies (immunoglobulin [Ig]G, IgM, and IgA). The assay was previously validated by both the manufacturer and us [3], with a 90%–93% sensitivity and specificity of 91%–99% (Table 1).
Table 1.
Serology and Cross-Reactivity Using a SARS-CoV-2 Commercial ELISA Test in Patients With Tropical Endemic Diseases From Córdoba, Colombia, and According Assay Manufacturers
| Patient’s Group | N | Diagnosis Period | Positive Samples by ELISA COVID-19 Test (%) | Negative Samples by ELISA COVID-19 Test (%) | |
|---|---|---|---|---|---|
| Patients from Córdoba, Colombia | Confirmed SARS-CoV-2 infectiona | 63 | June–July 2020 | 58 (92) | 5 (8) |
| COVID-19 clinically diagnosed and asymptomatic contactsb | 8 | July 2020 | 6 (75) | 2 (25) | |
| Acute dengue infectionc | 8 | April–December 2019 | 0 (0) | 8 (100) | |
| Recent dengue infectionc | 15 | April–December 2019 | 0 (0) | 15 (100) | |
| Acute Zika infectiond | 19 | November 2015–February 2016 | 5 (26) | 14 (74) | |
| Previous Chikungunya infectione | 9 | November 2015–April 2016 | 0 (0) | 9 (100) | |
| Recent exposure to spotted fever group Rickettsiaef | 5 | February 2013 | 0 (0) | 5 (100) | |
| Data according assay manufacturers (Ingenasa; Eurofins, Madrid, Spain)g | Confirmed RT-qPCR SARS-CoV-2 infection | 162 | Ongoing SARS-CoV-2 pandemic | 150 (92.6) | 12 (7.4) |
| Healthy patients/non-SARS-CoV-2 infection | 249 | Before SARS-CoV-2 pandemic | 2 (0.8) | 247 (99.2) | |
| Seasonal coronavirus (229E, NL63, OC43 HKU1) infection | 121 | No data | 0 (0) | 121 (100) |
Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; RT-qPCR, quantitative reverse-transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aSerum samples were collected 2 weeks before (in 32 patients) and 2 weeks after (in 31 patients) the date of SARS-CoV-2 RT-qPCR-positive collected swab sample.
bSerum samples of symptomatic patients were collected during the first 2 weeks of symptoms in 1 patient and 2 weeks after symptoms in 5 patients.
cSerum samples were collected on the same day as dengue-test (RT-qPCR, NS1 antigen, ELISA immunoglobulin [Ig]M) positive samples.
dSerum samples were collected on the same day as Zika-RT-qPCR-positive samples.
eSerum samples were collected the on same day as Chikungunya-ELISA IgG-positive samples.
fSerum samples were collected the same day as spotted fever group Rickettsiae-immunofluorescence assay-IgG positive samples.
Patient Consent Statement
The research committee of the Institute of Tropical Biological Research of the University of Cordoba approved the ethics protocol, and informed consent was obtained from all patients. Patients were registered using an anonymous numeric code. The study incorporated procedures, management, and conservation of samples, and technical-administrative procedures for health research required by resolution 8430 of the Ministry of Health of Colombia, in 1993 and declaration of Helsinki for ethical and medical research in human subjects. The study was considered as minimal risk.
RESULTS
From 1368 people (male n = 577, n = female 791), we found an overall prevalence of 55.3% (95% confidence interval [CI], 52.5–57.8), with the most massive prevalence among people between 20 to 60 years old (53.9%; 95% CI, 49.4%–58.3%). Prevalence varied by neighborhood, from 11.5% to 75% (Table 2). Three neighborhoods had prevalence above the average (75%, 70.9%, and 57.1%), and all of them were placed in disadvantaged areas of the city. People living in rural areas had a prevalence under the city average (46.8%; 95% CI, 38.6–68.5) (Table 2). There were no differences by sex: males had 57.2% of seroprevalence (95% CI, 53.1%–61.3%), and females had 54.4% of seroprevalence (95% CI, 50.8%–58.0%).
Table 2.
Neighborhoods, Socioeconomic Strata, Size of Population, and Results of Seroprevalence, Monteria, Colombia
| Neighborhoods (Socioeconomic Strata) | Population | Negative Tests | Positives Tests | Total Tests | Prevalence (%) | 95% CI |
|---|---|---|---|---|---|---|
| 1. (Low) | 43.000 | 32 | 37 | 69 | 53.6 | 41.1–66.1 |
| 2. (Low) | 17.000 | 40 | 19 | 59 | 32.2 | 19.4–43.9 |
| 3. (Low) | 39.000 | 53 | 64 | 117 | 54.7 | 45.2–64.1 |
| 4. (Low) | 102.000 | 13 | 39 | 52 | 75.0 | 62.2–87.7 |
| 5. (Middle) | 23.000 | 201 | 72 | 273 | 26.4 | 20.9–31.7 |
| 6. (Low) | 63.000 | 16 | 39 | 55 | 70.9 | 57.9–83.8 |
| 7. (Middle) | 5.000 | 54 | 7 | 61 | 11.5 | 2.6–20.2 |
| 8. (High) | 47.000 | 276 | 152 | 428 | 35.5 | 3.8–40.1 |
| 9. (Low) | 15.000 | 63 | 84 | 147 | 57.1 | 48.8–55.4 |
| 10. Rural (Low) | 110.000 | 55 | 52 | 107 | 48.6 | 38.6–58.5 |
| Total Overall | 464.000 | 803 | 565 | 1368 | a 55.3 | 52.5–57.8 |
Bold text indicates the overall total confidence interval (95% CI).
Abbreviations: CI, confidence interval.
aPrevalence adjusted by population in all 10 neighborhoods.
The present work is the first serological study reported from Colombia, where SARS-CoV-2 has been circulating for more than 7 months. It yields a similar proportion of infection than the study conducted by Del Brutto et al [4] in Atahualpa (Ecuador), a small rural population, where they found a prevalence of 44%. It is remarkable that these Latin American results are higher than those reported from European cities [5, 6] and China [7], where positivity to serological tests ranged from 0.9% to 13%, suggesting a lower attack rate in the population.
Monteria’s high prevalence suggests that there are many people entirely or partially immune to SARS-CoV-2, resulting in some level of population protection against the second wave of infection. During the first wave, Monteria detected 12 226 cases and 709 deaths, and the local hospital nearby could collapse. However, if 55% of the population is already nonsusceptible, the second wave may not occur because the level of population immunity to reach protection against COVID-19 outbreaks is approximately 65% [2].
Low adherence to quarantine recommendation is one of the main reasons behind this unexpected high prevalence. It is not surprising if the socioeconomic conditions of Monteria’s population are reviewed. Employment in the informal sector is as high as 60% in the whole city but may be 83.8% in some areas, which forces people to remain on the streets struggling to gain enough to pay for the everyday food and shelter.
DISCUSSION
This study may help to clarify the true magnitude of COVID-19 mortality in Monteria. Currently, the city has reported 709 deaths, which amount to a case-fatality rate (CFR) of 5.7%, 2 times the national average CFR. However, if our results are valid, the true CFR would be 0.26% because ~275 000 people would have been infected in Monteria during the first peak of transmission.
The study also has some limitations. First, the recombinant antigen N was used for laboratory testing, which has excellent sensitivity; however, recombinant antigens are not usually specific (3). Although we indeed have a large circulation of SARS-CoV-2, other coronaviruses that could have cross-reactions with the N antigen cannot be ruled out (3). Moreover, cross-reactions with arboviruses and SARS-CoV-2 were recently reported [3, 8, 9], and Monteria is an endemic region for dengue, Zika, and Chikungunya. Therefore, false positives could also be possible. In addition, the ELISA used by us detects total gamma globulins (IgM, IgG, IgA), and it is believed that IgA produces false positives [10]. Second, no information about antecedents of COVID-19 nor contact-confirmed cases was collected from participants, which precluded us from analyzing the performance of the test by the presence or absence of clinical symptoms.
The main strength of this study is that it shows, for the first time, the prevalence of SARS-CoV-2 in a small capital city of Colombia using a randomly selected sample. It allows us to extrapolate the sample results to the city’s whole population and contribute epidemiological elements for decision making by municipal public health authorities. Vaccination strategies are already being discussed in Colombia, but a high prevalence of natural infection, such as observed in the present study, may preclude vaccines as a population strategy because it may not be cost effective. However, the possibility of reinfection suggests that people that have been infected once cannot be considered to be immune. Although so far, long-established reinfection cases seem to be very scarce, and additional verification and longer supplement time are essential to apprehend the length of immunity, transmissibility, and the probability and consequences of reinfection [11]. The present assumption is that clinical management, infection prevention/control, and contact tracing concerns are not expected to diverge for a second infection related to people infected for the first time [11].
CONCLUSIONS
We do not know whether immunity provided by the natural infection is long-lasting. However, we will soon find out because on September 1, 2020, the national government opened trade, national and international road and air transportation, and restaurants. If the number of cases in Monteria maintains its current decreasing trend, it could be a sign that the city has reached some vital level of protection by natural infection even if it is below 65%, which is proposed as the cutoff point for herd immunity [2]. If we assume that approximately 50% of the population reached natural immunity in the city of Monteria, we could be moderately optimistic, and we are likely to have less occupancy in intensive care unit beds. Moreover, the possible reinfections could be clinically milder.
Acknowledgments
Author contributions. S. M., N. A.-G., E. G., R. R., A. G., Y. B., F. d. L. H., Á. A. F.-M., H. K., and M. M. design the study. J. M., K. G., C. M., G. A., C. G., H. C., and V. C. carried out the enzyme-linked immunosorbent assay tests and reviewed the article.
Financial support. This work was funded by Ministerio de Ciencia Tecnología e Innovación, Colombia: Research project “Fortalecimiento de capacidades instaladas de ciencia y tecnología de la Universidad de Córdoba para atender problemáticas asociadas con agentes biológicos de alto riesgo para la salud humana en el departamento de Córdoba” (Acta 76, May 5, 2020, to Alcaldia de Monteria for support on collecting the blood samples).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. De la Hoz-Restrepo F, Alvis-Zakzuk NJ, De la Hoz-Gomez JF, et al. Is Colombia an example of successful containment of the 2020 COVID-19 pandemic? A critical analysis of the epidemiological data, March to July 2020. Int J Infect Dis 2020; 99:522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eckerle I, Meyer B. SARS-CoV-2 seroprevalence in COVID-19 hotspots. Lancet 2020; 396:514–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faccini-Martinez A, Rivero R, Garay E, et al. Serological cross-reactivity using a SARS-CoV-2 ELISA test in acute Zika virus infection, Colombia, Int J Infect Dis 2020; 101:191–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Del Brutto OH, Costa AF, Mera RM, et al. SARS-CoV-2 in rural Latin America. A population-based study in coastal Ecuador. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1055/5876901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. ; ENE-COVID Study Group Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med 2020; 382:2302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu X, Sun J, Nie S, et al. Author correction: Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med 2020; 26:1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lustig Y, Keler S, Kolodny R, et al. Potential antigenic cross-reactivity between SARS-CoV-2 and dengue viruses. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spinicci M, Bartoloni A, Mantella A, et al. Low risk of serological cross-reactivity between dengue and COVID-19. Mem Inst Oswaldo Cruz 2020; 115:e200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19. Clin Infect Dis 2020; doi: 10.1093/cid/ciaa760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. European Centre for Disease Prevention and Control. Reinfection with SARS-CoV: considerations for public health response: ECDC; 2020. Available at: https://www.ecdc.europa.eu/sites/default/files/documents/Re-infection-and-viral-shedding-threat-assessment-brief.pdf. Accessed 14 October 2020.