Abstract
Background
To identify the adolescent school girls with risk for polycystic ovarian syndrome (PCOS), assess their risk status, and evaluate the impact of lifestyle modifications on PCOS risk reduction.
Methods
An experimental research was conducted among adolescent girls belonging to two Government run schools in Tiruvallur district of Tamil Nadu state, India, from 6 June to 9 December 2016. A standard risk assessment questionnaire was adopted for risk assessment after making few modifications (Cronbach alpha 0.86). The experimental group received lifestyle modifications (yoga for two months and walking exercise for two months), with no such intervention provided for the control group. The impact of these interventions was assessed in terms of risk minimization and a P value less than .05 was considered statistically significant.
Results
A total of 204 (control—102; experimental—102) girls with statistically insignificant difference in demographic features were studied. During the pretest, 85.2% (n = 87) in the experimental group and 83.3% (n = 85) the controls had “moderate risk” for PCOS. Girls with “high risk” level of PCOS were 14.8% (n = 15) and 15.7% (n = 17) in the experimental group and the control group, respectively. In posttest‐1 (after yoga sessions) risk assessment, 71.6% had “moderate risk,” 5.9% had “high risk” in the experimental group, whereas 87.3% had “moderate risk” and 12.7% had “high risk” in the control group. In posttest‐2 (after exercise sessions) risk assessment, 48% had “moderate risk” and 0% had high risk in the experimental group, whereas 88.2% were “moderate risk” and 11.8% were “high risk” in the control group. Repeated measure ANOVA with Greenhouse‐Geisser correction showed mean risk reduction score statistically significant between pretest and post‐test (33.38 ± 7.28 vs 22.75 ± 12.09, respectively mean difference is 10.63: F = 236.12 P < .001), suggesting a positive correlation with the intervention.
Conclusions
Yoga and exercise were beneficial in minimizing PCOS risk, as reflected in the risk assessment score. More such interventions, covering different schools, could provide larger health benefits.
Keywords: adolescent girls, exercise, lifestyle modifications, polycystic ovarian syndrome, risk assessment, school girls, yoga
1. BACKGROUND
Polycystic ovarian syndrome (PCOS) is a disorder affecting females of reproductive age, characterized by amenorrhea, obesity, and hirsutism, and is associated with enlarged polycystic ovaries. 1 The signs and symptoms include menstrual irregularity, obesity, and excessive hair growth on the face and chest. 2 , 3 Psychological disorder, including depression, anxiety, bipolar disorders, stress, sleep apnea, can worsen the Quality of Life (QoL). 4 The major complications associated with PCOS are infertility, diabetes, cardiovascular diseases, dyslipidemia, hypertension, glucose intolerance, and metabolic syndrome. 5 , 6 , 7 There are several medications available for treating PCOS, with varying success levels and are associated with drug‐related problems. Evidence shows lifestyle modifications as a viable first‐line effective means for preventing PCOS. 8 , 9 , 10 , 11 , 12 Even a small change in lifestyle helps to decrease the risk for PCOS. 13 It is evident that there are no medications that can completely cure PCOS. 14 It has been well documented that lifestyle modifications, such as proper diet, 15 yoga, and exercise, 16 help in decreasing the symptoms and severity of the disease.
In India, the prevalence of PCOS is highly variable, ranging from 2.2% to 26%. 17 The prevalence of PCOS in South Indian states like Andhra Pradesh is 9.13%, 18 within the same state Nellore district has a prevalence of 15.4%. 19 Telangana, a neighboring state in South India, has a prevalence of about 20%. 20 In Bangalore city of Karnataka state, PCOS is gradually increasing and is becoming an epidemic. 21 PCOS prevalence among the age group between 18 and 25 years is 3.7% in Lucknow, a city in North India, 22 46.8% in New Delhi, the capital city of India, and 26.4% in Kerala, a south Indian state. 23 Furthermore, the prevalence rate of PCOS among medical undergraduate girls in Pondicherry was 12.18%, even though they had basic knowledge about the reproductive system and PCOS, 24 and 9.8% in Thiruvananthapuram, 25 both these cities are in South India. These observations show a rising prevalence of PCOS in India.
On a daily observation, one can note a drastic change in the lifestyle of Indian population, which is more often linked to the globalization in India during the past three decades. During ancient days, Indian women were exposed to household physical work such as pulling water from the well, working in the field, washing clothes with hands, making decorative arts in their homes (Rangoli), grinding flour using Indian grinding machine (Ammi Kal), and so on. All these household works are likely to be linked to an improved endocrine function. Due to the advancement in technology, these natural lifestyle exercises are being replaced with modern devices and technologies. Adolescent girls spend their time in watching television, playing video games, smartphone, and other gadgets. Thus, the scope for physical exercises is limited. Lifestyle modifications such as yoga and exercise are associated with less risk, least cost, does not require a visit to a health club or gymnasium, and do not cause any negative effects on the reproductive system of adolescent girls. Thus, it is worth considering lifestyle modifications as a promising method for reducing the risk of PCOS.
Schools can also serve as a platform for providing lifestyle education to students. School children are at the earliest stage of life hood, and hence are open to accept changes, so they can be molded easily by teachers and parents. The school‐going adolescent girls are proven to be attentive and are willing to listen. Schools could also involve the teachers who could be very much valuable in identification, motivation, and training the students. Moreover, children from Government run schools and their parents may not be aware of the risk of PCOS due to their poor socioeconomic background, less exposure to the internet and smartphone devices, and low literacy level. Thus, schools can be considered as an ideal setting to provide education on lifestyle changes and related awareness in an attempt to reduce PCOS risk.
Risk assessment appears to be the most useful method for identifying the condition at the earliest stage and encourage the adolescents to seek for interventions to PCOS development as there is no complete cure for it. 26 There have been many types of research studies done in this area, but no specific research has been conducted to deal with the risk assessment and impact of lifestyle modifications on PCOS among school going adolescent girls. All these scenarios provoked the researcher to undertake this study with the objectives of identifying the adolescent girls with risk for PCOS, and to evaluate the impact of yoga and exercise on PCOS risk among the adolescent girls.
2. METHODS
2.1. Research design
In this research, a “true experimental research” design was adopted, and the test group received interventions with no intervention provided for the experimental group.
2.2. Study population
Adolescent girls studying in Government Girls Higher Secondary School in Keelmanambedu and Government Girls Higher Secondary School, Poonamallee in Thiruvallur district of Tamil Nadu, India. The sample comprised of adolescent girls studying in 10th to 12th grades.
2.3. Ethical consideration
Prior to the study, ethical approval was obtained from the Billroth Hospital Ethical Review Board, Chennai, Tamil Nadu, India. Further school permission was obtained from the Chief Educational Officer of the (CEO) of Thiruvallur district and the Headmistress from Government Girls Higher Secondary School at Keelmanambedu and Government Girls Higher Secondary School Poonamallee. In this study, there were no potential risks that might cause any harm to the adolescent girls. All the information about the adolescent girls was kept private and confidential.
2.3.1. Informed consent from the adolescent girls
All the adolescent girls were given a verbal explanation about the research and were asked to sign the prepared informed consent form made in the local Tamil language. The consent form had information about the study participant, goals of the study, data collection procedure, selection of risk adolescent girls, potential risks, potential benefits, confidentiality of the study participant, voluntary involvement in the study, right to withdraw information, and the contact information, which was explained clearly. This consent form was developed by the researcher and was corrected by the research supervisor. Initially, the consent has been given to three girls and was validated for the ease of understanding the contents.
2.3.2. Informed consent from the parents
A written informed letter was obtained from the parents of the school girls during which they were clarified that the information provided would strictly be anonymous and kept confidential. For any clarification about the study, the principal investigator's contact details were provided.
2.3.3. The right to fair treatment
School girls were selected based on the research requirements and not based on vulnerability, and they had an option to easily approach the research investigator at any point in the study period to clarify their doubts. Written permission was obtained from the girls who were voluntarily ready to provide the pictures. In addition, written permission was also obtained from their parents.
2.4. Sample size determination
The sample size was calculated using a pilot study report. Sample size determination was calculated using power analysis.
To reduce the risk from 25% to 10% with α = 5%, β = 20%

P1 = 25%; P2 = 10%; α = 1.96 (allowable error 5%); β = 0.84 (power of the study 80%); d = 15%.
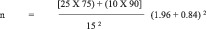
=97 per group = (1875 + 900) (7.84) /225.
The sample size was estimated with the reduction of 15% of risk from baseline (25% of risk), 80% power of the study, 5% allowable error, and 10% of dropout rate. The required sample size 97 + 10 = 107 per group was estimated. Power analysis is illustrated in Figure 1.
FIGURE 1.
Power analysis of the sample population
2.5. Sampling techniques
Nonprobability purposive sampling was used to select the samples. There are a total of 10 Government Girls Higher Secondary Schools functioning in Thiruvallur district. Among these 10 schools, two of them were selected using simple random techniques of lottery method. Out of these two, the first one was chosen for the experimental group (with intervention) and the second one was chosen for the control group. Then, the risk assessment questionnaire was distributed to all the students who fulfilled the inclusion and exclusion criteria of sampling. From these students, “high risk” and “moderate risk” girls were segregated by applying the risk assessment scores developed by Shoba et al. 26
2.6. Sample selection
Both the schools had a total of 1262 students who were studying in the 10th to 12th grade (506 in the experimental group and 756 in the control group). Of these, 442 students fell under the selection criteria in the experimental group (Keelmanambedu School) and 573 in the control group (Poonamallee School). Among these students, sample stratification (segregating only the moderate and high‐risk category group) was done, and the girls with the risk were 105 in the experimental group and 120 in the control group. The remaining adolescent girls fell under the “no risk” and “low‐risk” categories. Of these 102 girls, three girls in the experimental group dropped out from the study as they failed to complete all the follow‐ups and missed the interventions (yoga and exercise).
From the control group, 102 samples were selected randomly from 120 samples for equalizing the sample size in both the groups. Thus, the total number of subjects who were included for data analysis was n = 102 in the experimental group and n = 102 in the control group. The sample selection is depicted in Figure 2.
FIGURE 2.
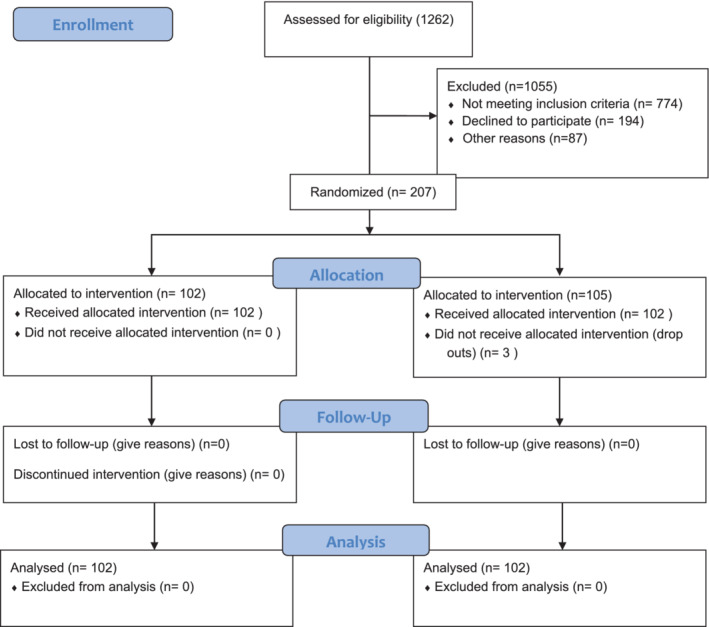
CONSORT flow diagram
2.7. Inclusion criteria
Inclusion criteria were adolescent girls studying in 10th to 12th grades who attained menarche before six months, present at the time of data collection, can read, speak, and understand Tamil or English language, and identified with “moderate” and “high risk” for PCOS by using the risk assessment questionnaire.
2.8. Exclusion criteria
Exclusion criteria were adolescent girls who were not willing to fill the questionnaire, not willing to participate in lifestyle modification interventions, sick at the time of data collection, practicing yoga and exercise regularly, diagnosed with PCOS, and undergoing treatment were excluded.
2.9. Risk assessment questionnaire
The contents of the questionnaire were obtained by the researcher based on the tool originally designed by Shoba et al. 26 This questionnaire had a total of 16 questions. In that, there were 13 “yes” or “no” type questions and three “rating scale” type of statements. In addition, it also had four demographics parameters. Prior to using the questionnaire, written permission was obtained from the author.
2.9.1. Modification of the questionnaire
After getting permission from the author, the researcher modified 13 “yes” or “no type” questions into rating scale (Q7, Q8, Q9, Q10, Q11, Q12, Q13, Q14, Q15, Q16, Q17, and Q 20). The researcher included three rating scale questions in addition to the ones made by the original author (Q6, Q18, and Q19). In addition, the researcher added four statements (Q16, Q18, Q19, and Q20) based on the inputs obtained from the panel of experts that consisted of Nursing professional, Gynecologist, Psychologist, and Biostatistician. The final risk assessment questionnaire had a total of 20 statements.
Apart from the 20 statements related to PCOS, the authors added 16 demography related questions, such as age, class of study, residence, type of family, religion, number of siblings in the family, order of birth among the siblings, nature of food eating, mode of transportation, age at menarche, nature of food eating, father's and mother's education, occupation of father and mother, monthly income, and sources of information on PCOS.
2.9.2. Risk assessment scoring
The interpretation of the score in percentage is given in Table 1.
TABLE 1.
Risk assessment score interpretation
| Level of risk | Score | Percentage population falling under each category (%) |
|---|---|---|
| No risk | 0‐10 | 0‐17 |
| Low risk | 11‐20 | 17.1‐33.3 |
| Moderate risk | 21‐40 | 33.4‐66.6 |
| High risk | 41‐60 | 66.7‐100 |
While performing risk assessment, the Body Mass Index (BMI) was calculated using the following formula:
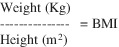
2.10. Validation of the study questionnaire
2.10.1. Content validity
The content validity was obtained from the experts from the field of Nursing, Gynecologist, Medical, Sociologist, Psychologist, and Statistician. Face validity was obtained from five subjects. A checklist has been made and experts were asked to fill their responses. The filled responses were analyzed to finalize the contents of the risk assessment and demographic data. The entire questionnaire was also validated based on adolescent girls' responses on their “ability to read” and “ability to understand”.
2.10.2. Reliability
It is a condition in which the same results will be achieved whenever the same technique is repeated within the study. 27 The reliability was calculated by using Cronbach method. The Cronbach “α” value of risk assessment is 0.86.
2.10.3. Final questionnaire
The final validated questionnaire had 20 questions related to risk assessment on PCOS. The responses used were divided into four categories, where three represented the highest score of “usually”, 2 represented “sometimes”, 1 represented “rarely”, and the lowest score denotes “none” (Appendix S1).
3. DATA COLLECTION PROCEDURE
3.1. Selection of moderate and high‐risk adolescent girls
3.1.1. Experimental group
The school selected for the experimental group was Government Girls Higher Secondary School, Keelmanambedu. In this school, 506 adolescent girls were studying. Out of the 506 girls, 442 were selected for the study based on the selection criteria. The risk assessment questionnaire was given to each class section‐wise. After the risk assessment, the subject that was filtered for the group which came under “no risk, low risk” category was not included in this study. Of the 442 girls, 90 adolescents were grouped under the category “moderate” and 15 girls were grouped under the “high risk” category. Of the 105 (90 + 15 = 105) adolescent girls, three dropped out during the intervention. The remaining 102 girls completed the entire study, and the data of these 102 girls are included in the result analysis.
3.1.2. Control group
The school that was selected for the control group was Girls Higher Secondary School, Poonamallee, Tamil Nadu, which consists of 756 adolescent girls studying at present. Out of the 756 adolescent girls, 573 were selected for the study based on the selection criteria. The risk assessment questionnaire was given to each class section‐wise. After the risk assessment, subjects were filtered and the group that came under “no risk,” “low risk” category was not included in the study. Of the 573 girls, 103 adolescent girls were grouped under the category “moderate” and 17 girls were grouped under “high risk” category. Of the 120 (103 + 17 = 120) girls, 102 girls were selected randomly in order to equalize the experimental group. One hundred and two adolescent girls completed the entire study and the data of these 102 girls are included in the result analysis (Table 2).
TABLE 2.
Selection process of moderate and high‐risk subjects
| Sample selection | Experimental group | Control group |
|---|---|---|
| Total girls from 10th to 12th grade | 506 | 756 |
| Based on selection criteria | 442 | 557 |
| No risk, low risk (excluded) | 224 + 113 = 337 | 265 + 172 = 437 |
| Moderate and high risk (included) | 90 + 15 = 105 | 103 + 17 = 120 (randomization 102) |
| Drop out | 3 | No drop out |
| Final sample for analysis | 102 | 102 |
4. ADMINISTRATION OF QUESTIONNAIRES
The risk assessment and the demographic questionnaire were given to the adolescent girls as a pretest on risk assessment in both the experimental and the control groups. Of the selected girls, the ones categorized under “moderate risk” and “high risk” of PCOS were included, and “low risk” and “no risk” girls were excluded.
The time duration for filling the questionnaires ranged from 30 minutes to 1 hour. After data collection, the researcher held feedback meetings to identify and resolve any problems encountered while filling the questionnaire. The researcher used such meetings as an opportunity for controlling the quality of information by checking the questionnaires for completeness and missing data.
The questionnaire was again administered to both the control and intervention groups' girls after completion of Phase I intervention (yoga) and again after completion of Phase II intervention (Exercise) as described below.
5. INTERVENTION
The intervention had two phases:
5.1. Phase I intervention (yoga sessions for 2 months)
5.1.1. Conceptualization and development of the yoga training module
The concept of yoga intervention was taken from the traditional yoga scriptures of Patanjali yoga sutras. All interventions were related to the holistic health approach. The Yogasanas consisted of Pranayama, Meditations, Bhadrasana (Butterfly pose), and Chakki Chalanasana (moving the grinding wheel). These specific interventions of yoga were developed by a team of experts, which included Gynecologist, yoga therapist, and nursing professionals.
In order to train the girls for the yoga intervention, the researcher also underwent the training program and got certified from “Legends Yoga training Academy,” Porur, Chennai.
For a better understanding of yoga training as an intervention for the adolescent girls, it has been divided into various stages as given below:
Stage I—Preliminary phase to create awareness among stakeholders
In order to initiate yoga training and to get the cooperation and to improve awareness among the girls, a yoga training program was organized by the researcher in cooperation from Sri Patanjali Maharishi Yoga Training Centre, Mangadu, Chennai. The program started at 1.30 pm and ended at 4 pm. All the selected girls and even school teachers participated in this program. All the girls sat on the school ground and the school provided the audio facility for the event.
In this session, the trainer gave a 1‐hour lecture that included an introduction to the importance of yoga and the benefits of yoga in PCOS. Following this, for the next 1.5 hours, the entire adolescent girls were trained on the asanas. Then, the adolescent girls were requested to come at 8.30 am for practicing yoga for two months.
Stage II—Performance of yoga by the adolescent girls
On the first‐day, adolescent girls were made to gather on the ground under a tree on the side of the school and practiced only meditation and pranayama, and on the second day they practiced butterfly pose and chakki chalasana pose. From the third day onward, all the four asanas were practiced by the girls.
Stage III—Continuation phase
From the third day onward, a 1‐minute introduction was given daily by the investigator about yoga, and the girls were motivated to continue yoga. The girls were made to do meditation for 5 minutes then relaxation for 2 minutes, pranayama for 5 minutes then 2 minutes for relaxation, 5 minutes for butterfly asana then 2 minutes for relaxation, chakki chalasana asana for 5 minutes then 2 minutes relaxation finally at the end of the interventions.
Stage IV—Sustenance phase
After two months of yoga intervention, adolescent girls were made to do brisk walking exercise for 30 minutes daily. They were advised to come to school at 8.30 am regularly for 2 months. Log book was maintained by the researcher. Adolescent girls were advised to do yoga even during the holidays.
Note: As an ethical point of view, yoga was taught to the control group at the end of the study.
5.2. Phase II intervention (exercise sessions for 2 months)
5.2.1. Stage 1—Preliminary phase
After reviewing various journals and books, as well as expert opinion, the researcher incorporated to do brisk walking exercises for the girls after the yoga intervention in the experimental group.
5.2.2. Stage II—Performance phase
Girls were requested to come regularly for practicing walking exercise for 2 months in the school ground at 8.30 am daily. The researcher also maintained a log with the help of the school teachers and class representative of each grade. The absentees were requested to do walking exercise on Sunday to compensate the absence, since walking exercise was planned for weekly six days. All of them were also requested to continue the intervention on Government holidays.
Note: As an ethical point of view, brisk walking exercise was taught for the adolescent girls in the control group at the end of the study.
6. DATA PROCESSING AND ANALYSIS
The collected data were checked for consistency and completeness, and it was analyzed using Statistical Package for Social Sciences (SPSS, version 16) and STATA (version 12) software and Epi info (version 3.5.1).
6.1. Statistical analysis
6.1.1. Descriptive statistics
Frequencies, means, and SDs were used to analyze the demographic characteristics. Quantitative risk score was given in mean and SD. Quantitative risk, with respect to different grades of scoring, was presented using frequency and percentages. The similarity of demographic variables, distribution between the experiment and the control was tested using Chi‐square test.
6.1.2. Inferential statistics
The pretest and posttest mean risk score results were compared using the paired t test to evaluate the impact of lifestyle modifications on the risk for PCOS among adolescent girls. Pretest, posttest‐1, and posttest‐2 statistical differences are found.
6.2. Pilot study
Pilot study was conducted in Government Girls Higher Secondary School, Kamaraj Nagar, Avadi, Thiruvallur district. 28 The following modifications were carried out based on the pilot study's findings:
A question (Q9. How do you normally come to school?) was added in the demographic variables.
Initially, six yogasanas were planned and executed for the pilot study. However, considering the time factor and the suggestions by the experts, four yogasanas, namely “pranayama”, “meditations”, “bhadrasana”, and “chakki chalanasana” were executed in the main study, and two yogasanas namely “bharadvajasana” and “sun salutation” were excluded from the main study.
7. RESULTS
7.1. Demographic profile of the study respondents
Altogether 204 (experimental, n = 102; control, n = 102) samples were enrolled in the research as per the inclusion and exclusion criteria of the study. Table 3 shows the demographic features of the study subjects.
TABLE 3.
Demographic profile of the study respondents in both the groups (n = 204)
| Demographic variables | Group | Chi‐square test | ||||
|---|---|---|---|---|---|---|
| Experiment (n = 102) | Control (n = 102) | |||||
| n | % | n | % | |||
|
Age (in years) |
15 16 17 and above |
28 45 29 |
27.5 44.1 28.4 |
21 53 28 |
20.6 52.0 27.4 |
χ2 = 1.67 P = .43 DF = 2 NS |
| Class |
10th grade 11th grade 12th grade |
34 39 29 |
33.3 38.3 28.4 |
29 41 32 |
28.4 40.2 31.4 |
χ2 = 0.59 P = .74 DF = 2 NS |
| Residence |
Rural Semi‐urban Urban |
36 37 29 |
35.3 36.3 28.4 |
28 42 32 |
27.4 41.2 31.4 |
χ2 = 1.46 P = .48 DF = 2 NS |
| Type of family |
Nuclear Joint Extended |
71 26 5 |
69.6 25.5 4.9 |
78 20 4 |
76.5 19.6 3.9 |
χ2 = 1.22 P = .54 DF = 2 NS |
| Religion |
Hindu Christian Muslim |
87 9 6 |
85.3 8.8 5.9 |
80 14 8 |
78.4 13.8 7.8 |
χ2 = 1.66 P = .43 DF = 2 NS |
| Sibling |
None One Two > Two |
3 50 35 14 |
2.9 49.0 34.3 13.8 |
3 44 42 13 |
2.9 43.1 41.3 12.7 |
χ2 = 1.05 P = .79 DF = 3 NS |
| Order of child |
Eldest Second Third Fourth or above |
37 41 21 3 |
36.3 40.2 20.6 2.9 |
36 36 28 2 |
35.3 35.3 27.4 2.0 |
χ2 = 1.59 P = .67 DF = 3 NS |
|
Age at Menarche (in years) |
11 12 13 14 15 and above |
9 33 36 19 5 |
8.8 32.4 35.3 18.6 4.9 |
10 35 23 26 8 |
9.8 34.3 22.5 25.6 7.8 |
χ2 = 4.7 P = .31 DF = 4 NS |
| Type of food | Vegetarian | 24 | 23.5 | 21 | 20.6 |
χ2 = .25 P = .61 DF = 1 NS |
| Nonvegetarian | 78 | 76.5 | 81 | 79.4 | ||
| Mode of transportation | Walking | 24 | 23.5 | 25 | 24.5 |
χ2 = 0.25 P = .61 DF = 3 NS |
| Bus | 71 | 69.7 | 67 | 65.7 | ||
| Cycling | 4 | 3.9 | 5 | 4.9 | ||
| Others | 3 | 2.9 | 5 | 4.9 | ||
| Source of information | No information | 11 | 10.8 | 22 | 21.6 |
χ2 = 0.74 P = .86 DF = 5 NS |
| Television | 41 | 40.2 | 32 | 31.4 | ||
| News paper | 25 | 24.5 | 16 | 15.7 | ||
| Friends | 8 | 7.8 | 13 | 12.7 | ||
| Health personnel | 15 | 14.7 | 17 | 16.6 | ||
| Books | 2 | 2.0 | 2 | 2.0 | ||
Note: NS, No significant; DF, degrees of freedom. No significant P > .05.
7.2. Pretest level of risk assessment
There was no significant difference between the PCOS risk assessment score of participants in the experimental and the control groups (P = .70; χ2 = 0.15). Figure 3 shows the pretest level of risk on PCOS among the adolescent girls in the experimental and control groups.
FIGURE 3.
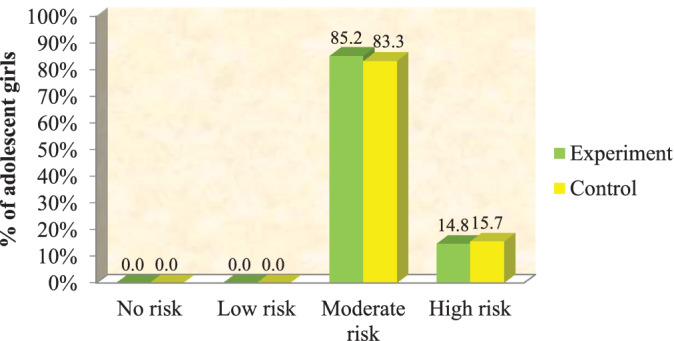
Pretest level of risk assessment in both the groups (n = 204). Note: Chi‐square test = 0.15 DF = 1 P = .70 (no significant P > .05)
7.3. Comparison of the experimental group's and the control group's level of risk assessment score for pretest, posttest‐1, and posttest‐2
There was no significant difference between the PCOS risk assessment score of participants in the experimental and control groups (P = .70; χ2 = 0.15). In both the groups, none of the adolescent girls had either “no risk” or “low risk level.” Table 4 reveals the risk of PCOS among adolescent girls in the experimental and the control groups during pretest, posttest‐1, and posttest‐2.
TABLE 4.
Comparison of the experimental group's and the control group's level of risk assessment score for pretest, posttest‐1, and posttest‐2 (n = 204)
| Group | Risk | Group | Chi‐square test | |||
|---|---|---|---|---|---|---|
| Experiment | Control | |||||
| n | % | n | % | |||
| Pretest | Moderate risk | 87 | 85.2 | 85 | 83.3 |
χ2 = 0.15 P = .70 DF = 1 |
| High risk | 15 | 14.8 | 17 | 15.7 | ||
| Total | 102 | 100.0 | 102 | 100.0 | ||
| Posttest‐1 | No risk | 10 | 9.8 | 0 | 0.0 |
χ2 = 27.15 P = .001*** DF = 3 |
| Low risk | 13 | 12.7 | 0 | 0.0 | ||
| Moderate risk | 73 | 71.6 | 89 | 87.3 | ||
| High risk | 6 | 5.9 | 13 | 12.7 | ||
| Total | 102 | 100.0 | 102 | 100.0 | ||
| Posttest‐2 | No risk | 21 | 20.6 | 0 | 0.0 |
χ2 = 77.09 P = .001*** DF = 2 |
| Low risk | 32 | 31.4 | 0 | 0.0 | ||
| Moderate risk | 48 | 48.0 | 90 | 88.2 | ||
| High risk | 0 | 0.0 | 12 | 11.8 | ||
| Total | 102 | 100.0 | 102 | 100.0 | ||
Note: Not significant P > .05 *** very high significant at P ≤ 0.001.
7.4. Comparison of pretest, posttest‐1, and posttest‐2 risk assessment score in both the groups
Table 5 shows that repeated measure ANOVA with Greenhouse‐Geisser correction showed that mean risk reduction score difference (10.63) was statistically significant (F = 236.12 P < .001) between pretest (33.38 ± 7.28) and posttest (22.75 ± 12.09) in the experimental group's adolescent girls.
TABLE 5.
Six comparison of pretest, posttest‐1, and posttest‐2 risk assessment score in both the groups (n = 204)
| Group | Pretest | Posttest‐1 | Posttest‐2 | Mean difference | Repeated measures ANOVA F‐test | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| Experiment | 33.38 | 7.28 | 27.20 | 10.21 | 22.75 | 12.09 | 10.63 | F = 236.12 P = .001*** |
| Control | 34.84 | 6.37 | 34.52 | 8.06 | 34.22 | 6.93 | 0.62 | F = 1.16 P = .82 |
7.5. Comparison of the experimental group's and control group's level of BMI
Table 6 shows the BMI of adolescent girls in the experimental group and the control group during pretest, posttest‐1, and posttest‐2. Statistically, there was no significant difference found between the BMI of the experimental group and the control group in pretest.
TABLE 6.
Comparison of the experimental group's and the control group's level of BMI (n = 204)
| Test | Weight status | Group | Chi‐square test | |||
|---|---|---|---|---|---|---|
| Experiment | Control | |||||
| n | % | n | % | |||
| Pretest | Underweight | 10 | 9.8 | 13 | 12.7 |
χ2 = 0.71 P = .87 DF = 3 not Significant |
| Normal | 68 | 66.7 | 66 | 64.7 | ||
| Overweight | 22 | 21.6 | 20 | 19.6 | ||
| Obese | 2 | 1.9 | 3 | 3.0 | ||
| Total | 102 | 100.0 | 102 | 100.0 | ||
| Posttest‐1 | Underweight | 8 | 9.8 | 12 | 11.8 |
χ2 = 2.01 P = .57 DF = 3 Significant |
| Normal | 76 | 71.6 | 67 | 65.7 | ||
| Overweight | 16 | 12.7 | 20 | 19.6 | ||
| Obese | 2 | 5.9 | 3 | 2.9 | ||
| Total | 102 | 100.0 | 102 | 100.0 | ||
| Posttest‐2 | Underweight | 6 | 6.0 | 12 | 11.8 |
χ2 = 7.62 P = .05* DF = 3 Significant |
| Normal | 83 | 81.3 | 68 | 66.7 | ||
| Overweight | 13 | 12.7 | 19 | 18.6 | ||
| Obese | 0 | 0.0 | 3 | 2.9 | ||
| Total | 102 | 100.0 | 102 | 100.0 | ||
Note: Not significant P > .05 * significant at P ≤ .05 ** highly significant at P ≤ .01 *** very high significant at P ≤ .001.
7.6. Association between risk assessment score and adolescent girls' demographic variables in experimental group
Table 7 depicts the association between risk assessment score of the experimental group's adolescent girls and their demographic variables. With reference to the above table, only three variables, namely age (F = 3.28 at P = .05), class (F = 3.30 at P = .05), and order of birth (F = 3.13 at P = .03), were significantly associated with their risk assessment score. But there was no statistically significant association observed between the rest of the variables, such as place of residence (F = 1.13 at P = .05), type of family (F = 0.47 at P = .62), religion (F = 0.71 at P = .49), number of siblings (F = 0.71 at P = .54), age at menarche (F = 0.90 at P = .44), with their risk assessment score.
TABLE 7.
Association between risk assessment score and adolescent girls' demographic variables in experimental group (n = 102)
| Demographic variables | n | Risk assessment score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretest | Posttest | Reduction score = pre‐post | One‐way | ||||||
| Mean | SD | Mean | SD | Mean | SD | ANOVA F‐test/t‐test | |||
|
Age (in years) |
15 | 28 | 34.32 | 6.80 | 25.86 | 11.60 | 11.46 | 7.49 |
F = 3.28 P = .05* S |
| 16 | 45 | 33.24 | 7.19 | 22.96 | 12.11 | 13.49 | 7.47 | ||
| 17 and above | 29 | 32.69 | 7.97 | 19.79 | 12.72 | 16.48 | 7.42 | ||
| Class | 10th grade | 34 | 34.38 | 6.30 | 26.18 | 11.36 | 11.21 | 7.76 |
F = 3.30 P = .05* S |
| 11th grade | 39 | 32.90 | 7.70 | 21.85 | 12.62 | 14.51 | 7.69 | ||
| 12th grade | 29 | 32.86 | 7.86 | 20.31 | 12.29 | 15.83 | 6.74 | ||
| Place of residence | Rural | 36 | 32.06 | 6.89 | 20.22 | 12.60 | 15.28 | 7.72 |
F = 1.13 P = .33 (NS) |
| Semi‐urban | 37 | 35.11 | 8.37 | 25.46 | 11.79 | 12.68 | 7.13 | ||
| Urban | 29 | 32.83 | 5.92 | 22.79 | 12.07 | 13.34 | 8.09 | ||
| Type of family | Nuclear | 71 | 33.39 | 7.51 | 22.41 | 11.92 | 14.13 | 7.22 |
F = 0.47 P = .62 NS |
| Joint | 26 | 32.42 | 5.56 | 22.35 | 12.89 | 13.42 | 8.89 | ||
| Extended | 5 | 38.20 | 11.03 | 31.80 | 12.40 | 10.80 | 6.94 | ||
| Religion | Hindu | 87 | 33.52 | 7.44 | 22.95 | 12.34 | 13.86 | 7.60 |
F = 0.71 P = .49 NS |
| Christian | 9 | 32.00 | 7.62 | 19.78 | 11.82 | 15.22 | 7.00 | ||
| Muslim | 6 | 33.50 | 4.42 | 26.00 | 12.52 | 10.50 | 9.35 | ||
| Number of siblings | None | 3 | 30.00 | 4.58 | 21.67 | 14.19 | 11.33 | 9.61 |
F = 0.71 P = .54 NS |
| One | 50 | 34.32 | 6.31 | 24.90 | 11.59 | 12.60 | 7.48 | ||
| Two | 35 | 33.31 | 8.19 | 22.86 | 12.33 | 13.49 | 7.59 | ||
| >Two | 14 | 30.93 | 8.36 | 15.79 | 12.64 | 19.29 | 6.08 | ||
| Order of birth | Eldest | 37 | 35.11 | 7.12 | 22.19 | 11.96 | 12.92 | 7.22 |
F = 3.13 P = .03* (S) |
| Second | 41 | 36.10 | 5.08 | 19.69 | 11.39 | 16.41 | 7.99 | ||
| Third | 21 | 34.86 | 10.11 | 21.67 | 14.02 | 13.19 | 7.38 | ||
| Fourth and above | 3 | 33.00 | 6.24 | 20.33 | 15.89 | 12.67 | 9.87 | ||
|
Age at Menarche (in years) |
11 | 9 | 33.67 | 6.02 | 22.89 | 11.48 | 13.78 | 6.14 |
F = .90 P = .44 (NS) |
| 12 | 33 | 34.70 | 8.13 | 23.85 | 13.23 | 14.36 | 8.20 | ||
| 13 | 36 | 32.42 | 7.84 | 21.64 | 12.41 | 13.78 | 7.22 | ||
| 14 | 19 | 32.63 | 5.34 | 23.16 | 11.34 | 12.47 | 7.37 | ||
| 15 and above | 5 | 34.00 | 6.60 | 23.80 | 13.08 | 15.00 | 11.94 | ||
| Type of food | Vegetarian | 24 | 33.17 | 6.14 | 22.04 | 12.54 | 14.88 | 8.74 |
t = 0.80 P = .43 NS |
| Nonvegetarian | 78 | 33.45 | 7.63 | 23.10 | 12.22 | 13.45 | 7.28 | ||
| Mode of transportation | Walking | 24 | 33.58 | 5.17 | 24.42 | 11.28 | 12.54 | 8.18 |
F = 2.38 P = .07 NS |
| Bus | 71 | 33.61 | 7.97 | 23.32 | 12.32 | 13.52 | 7.35 | ||
| Cycling | 4 | 28.25 | 1.50 | 9.00 | 3.92 | 22.25 | 2.63 | ||
| Others | 3 | 33.33 | 9.24 | 17.67 | 17.62 | 18.67 | 8.39 | ||
| Source of information | No information | 11 | 33.45 | 6.33 | 24.45 | 12.36 | 12.00 | 7.13 |
F = 1.09 P = .37 NS |
| Television | 41 | 34.29 | 7.77 | 23.41 | 12.04 | 13.90 | 7.05 | ||
| News paper | 25 | 33.92 | 7.10 | 25.16 | 12.53 | 12.12 | 8.43 | ||
| Friends | 8 | 33.00 | 9.77 | 19.75 | 14.95 | 17.13 | 8.51 | ||
| Health person | 15 | 30.67 | 5.78 | 18.00 | 11.05 | 16.27 | 7.20 | ||
| Books | 2 | 29.50 | 2.12 | 22.50 | 13.44 | 10.00 | 11.31 | ||
Note: NS, not significant; S, significant not significant P > .05 at * significant at P ≤ .05.
7.7. Association between risk assessment score and demographic variables in the control group
Table 8 shows the association between the risk assessment score and adolescent girls demographic variables in the control group. None of the demographic variables like “age” (F = 0.59 at P = .55), class (F = 1.75 at P = .17), place of residence (F = 3.53 at P = .03), type of family (F = 1.62 at P = .20), religion (F = 0.62 at P = .945), number of siblings (F = 1.73 at P = .16), order of birth (F = 1.14 at P = .34), age at menarche (F = 0.60 at P = .66), type of family (t = 1.99 at P = .08), mode of transportation to school (F = 2.24 at P = .08), and source of information (F = 1.12 at P = .38) had any significant association with the attitude score.
TABLE 8.
Association between risk assessment score and demographic variables in the control group (n = 102)
| Demographic variables | n | Risk assessment score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pretest | Posttest | Gain score = post‐pre | One‐way | ||||||
| Mean | SD | Mean | SD | Mean | SD | ANOVA F‐test/t‐test | |||
| Age | 15 years | 21 | 34.57 | 5.80 | 31.14 | 8.34 | 3.43 | 3.67 |
F = 0.59 P = .55 NS |
| 16 years | 35 | 34.60 | 7.05 | 32.89 | 10.25 | 1.72 | 5.91 | ||
| 17 years and more | 24 | 35.50 | 5.51 | 33.14 | 10.36 | 2.36 | 7.71 | ||
| Class | 10th grade | 26 | 33.69 | 5.70 | 30.55 | 8.67 | 3.14 | 4.17 |
F = 1.75 P = .17 NS |
| 11th grade | 30 | 35.37 | 7.69 | 34.49 | 10.84 | 0.88 | 5.91 | ||
| 12th grade | 24 | 35.22 | 4.98 | 32.03 | 9.38 | 3.19 | 7.46 | ||
| Place of residence | Rural | 28 | 35.39 | 6.58 | 34.86 | 9.91 | 0.54 | 6.41 |
F = 3.53 P = .03 NS |
| Semi urban | 29 | 34.10 | 6.70 | 30.07 | 9.86 | 4.02 | 5.99 | ||
| Urban | 23 | 35.34 | 5.81 | 33.94 | 9.34 | 1.41 | 5.44 | ||
| Type of family | Nuclear | 53 | 34.71 | 6.44 | 32.86 | 10.19 | 1.85 | 6.48 |
F = 1.62 P = .20 NS |
| Joint | 23 | 35.20 | 6.70 | 32.40 | 8.88 | 2.80 | 3.91 | ||
| Extended | 4 | 35.75 | 3.59 | 28.50 | 8.81 | 7.25 | 5.50 | ||
| Religion | Hindu | 69 | 35.23 | 6.65 | 32.88 | 9.87 | 2.35 | 6.18 |
F = 0.06 P = .945 NS |
| Christian | 6 | 33.07 | 5.58 | 31.21 | 11.03 | 1.86 | 6.26 | ||
| Muslim | 5 | 34.13 | 4.36 | 32.25 | 8.46 | 1.88 | 5.49 | ||
| Number of siblings | None | 2 | 35.00 | 10.15 | 37.33 | 13.01 | −2.33 | 3.21 |
F = 1.73 P = .16 NS |
| One | 41 | 35.11 | 6.21 | 32.14 | 9.53 | 2.98 | 5.84 | ||
| Two | 26 | 34.48 | 6.03 | 31.83 | 10.35 | 2.64 | 6.64 | ||
| >Two | 11 | 35.08 | 7.80 | 35.54 | 8.92 | −.46 | 4.59 | ||
| Order of birth | Eldest | 32 | 33.75 | 4.69 | 30.81 | 9.30 | 2.94 | 6.81 |
F = 1.14 P = .34 NS |
| Second | 31 | 35.67 | 5.81 | 32.89 | 9.53 | 2.78 | 6.06 | ||
| Third | 14 | 34.96 | 8.51 | 33.93 | 10.76 | 1.04 | 5.10 | ||
| Fourth or above | 3 | 38.00 | 9.90 | 41.00 | 12.73 | −3.00 | 2.83 | ||
|
Age at Menarche (in years) |
11 | 9 | 35.10 | 4.31 | 30.60 | 7.68 | 4.50 | 5.19 |
F = 0.60 P = .66 NS |
| 12 | 26 | 35.83 | 6.09 | 34.37 | 10.14 | 1.46 | 6.51 | ||
| 13 | 26 | 32.57 | 5.95 | 29.91 | 6.47 | 2.65 | 4.15 | ||
| 14 | 14 | 36.04 | 7.39 | 34.27 | 12.12 | 1.77 | 7.66 | ||
| 15 and above | 5 | 32.88 | 6.53 | 29.63 | 10.14 | 3.25 | 4.10 | ||
|
Type of food |
Vegetarian | 20 | 33.95 | 5.04 | 29.38 | 6.93 | 4.57 | 5.23 |
t = 1.99 P = .05 NS |
| Nonvegetarian | 60 | 35.07 | 6.68 | 33.43 | 10.35 | 1.64 | 6.17 | ||
| Mode of transportation | Walking | 18 | 36.56 | 7.06 | 36.64 | 10.23 | −.08 | 6.01 |
F = 2.24 P = .08 NS |
| Bus | 55 | 34.51 | 6.27 | 31.60 | 9.57 | 2.91 | 6.02 | ||
| Cycling | 4 | 33.20 | 4.15 | 32.00 | 10.68 | 1.20 | 7.29 | ||
| Others. | 3 | 32.40 | 5.18 | 26.40 | 5.55 | 6.00 | 2.65 | ||
| Source of information | No information | 11 | 34.05 | 5.52 | 32.32 | 10.81 | 1.73 | 6.80 |
F = 1.12 P = .38 NS |
| Television | 30 | 35.31 | 7.44 | 32.47 | 9.95 | 2.84 | 5.18 | ||
| News paper | 18 | 35.00 | 7.04 | 31.75 | 7.66 | 3.25 | 4.65 | ||
| Friends | 7 | 35.92 | 6.18 | 36.77 | 8.41 | −.85 | 4.96 | ||
| Health personnel | 12 | 34.76 | 5.04 | 32.12 | 11.32 | 2.65 | 8.34 | ||
| Books | 2 | 28.50 | 3.54 | 21.50 | 3.54 | 7.00 | 0.00 | ||
Note: NS = not significant not significant P > .05.
7.8. Identification of influencing factors for risk assessment score using multivariate logistic regression
Table 9 shows the multivariate logistic regression analysis, which identifies 15‐year‐old girls, 12th grade girls, more educated mothers, and family income of more than Rs. 5000 for adolescent girls have reduced more risk score than others after intervention. Adjusted odds ratio was given with 95% confidence interval.
TABLE 9.
Identification of influencing factors for risk assessment score using multivariate logistic regression
| Demographic variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P value | UnadjustedOR(95%CI) | P value | AdjustedOR(95%CI) | |
| Age (≥15 years vs < 15 years) | .02* | 2.0 (1.0‐7.7) | .05* | 1.8(1.1‐4.1) |
| Class (≥10th grade vs < 10th grade) | .05* | 3.0 (1.1‐7.8) | .05* | 1.7(0.7‐2.3) |
| Birth order (≤Second vs > Second) | .02* | 3.1(1.0‐9.5) | .53 | 1.1(0.6‐2.1) |
| Mother's education (≥ primary vs < primary) | .01** | 2.8 (1.2‐6.9) | .03* | 1.9(1.1‐4.8) |
| Monthly income (≥Rs.5000 vs ≤ Rs.5000) | .02* | 2.7 (1.1‐6.8) | .02* | 1.6(1.0‐5.2) |
Table 10 shows the question‐wise percentage of risk assessment score on PCOS among the experimental group and the control group of adolescent girls during pretest to posttest‐2. Upon a maximum score of 60, the mean risk assessment score was reduced by 17.7% in the experimental group after intervention from a pretest score of 55.6% to a posttest‐2 score of 37.9%. However, the mean risk assessment score was reduced by 1.1% only in the control group from a pretest score of 58.1% to a posttest‐2 score of 57%.
TABLE 10.
Each question‐wise percentage of risk assessment score in both the groups (n = 204)
| Questions | Max score | Experiment | Control | ||||
|---|---|---|---|---|---|---|---|
| Pretest | Posttest‐2 | % gain score | Pretest | Posttest‐2 | % gain score | ||
| Do you have irregular periods? | 3 | 74.3 | 57.7 | 16.6 | 76.0 | 74.7 | 1.3 |
| Do you miss your period in your regular cycle? | 3 | 50.3 | 38.0 | 12.3 | 53.3 | 51.7 | 1.6 |
| Do you get your menstrual cycle after more than 35 days? | 3 | 40.7 | 33.7 | 7.0 | 43.7 | 42.7 | 1.0 |
| Do you have your menstrual flow for more than five days? | 3 | 54.7 | 42.7 | 12.0 | 56.0 | 55.7 | 0.3 |
| Are you changing more than 4 pads per day? | 3 | 62.3 | 49.7 | 12.6 | 64.3 | 63.3 | 1.0 |
| Do you have the habit of eating junk food? | 3 | 75.0 | 48.7 | 26.3 | 77.7 | 76.0 | 1.7 |
| Do you experience nausea/vomiting during menstruation? | 3 | 34.3 | 28.0 | 6.3 | 38.3 | 37.3 | 1.0 |
| Do you experience abdominal pain during menstruation? | 3 | 72.0 | 41.7 | 30.3 | 75.0 | 72.3 | 2.7 |
| Do you experience pain/tenderness in breast during menstruation? | 3 | 41.0 | 27.3 | 13.7 | 42.7 | 43.0 | −0.3 |
| Do you have heavy menstrual flow with clots? | 3 | 59.7 | 39.7 | 20.0 | 62.3 | 60.3 | 2.0 |
| Do you have pimples on your face? | 3 | 65.3 | 37.7 | 27.6 | 67.7 | 66.0 | 1.7 |
| Do you have hair fall? | 3 | 72.3 | 50.3 | 22.0 | 74.0 | 73.3 | 0.7 |
| Do you have problem with hair growth over chest, face or abdomen? | 3 | 16.0 | 15.0 | 1.0 | 18.7 | 18.7 | 0.0 |
| Do you have any darkening and thickening of skin folds around the neck and axillae? | 3 | 45.3 | 38.0 | 7.3 | 46.7 | 46.7 | 0.0 |
| Do you have weight gain? | 3 | 57.0 | 39.3 | 17.7 | 58.7 | 58.7 | 0.0 |
| Do you have difficulty in losing weight? | 3 | 52.7 | 36.0 | 16.7 | 55.3 | 54.7 | 0.6 |
| Do you have frequent thirst and urination? | 3 | 59.0 | 36.3 | 22.7 | 62.0 | 60.0 | 2.0 |
| Do you feel extremely hungry, irritable and sleepy? | 3 | 58.7 | 34.3 | 24.4 | 61.0 | 61.0 | 0.0 |
| Do you have symptoms of giddiness and fatigue? | 3 | 59.7 | 27.7 | 32.0 | 61.7 | 61.0 | 0.7 |
| Do you have stressful and depressive mind? | 3 | 62.3 | 36.7 | 25.6 | 64.7 | 63.0 | 1.7 |
| Total | 60 | 55.6 | 37.9 | 17.7 | 58.1 | 57.0 | 1.1 |
8. DISCUSSION
PCOS affects women's health in multiple ways, ranging from sense of well‐being to impaired reproductive health. Risk assessment for PCOS can largely help early identification and providing better remedies. In this research, a modified version of a standard PCOS risk assessment questionnaire was used to assess the PCOS risk among adolescent school going girls. This assessment mainly focused on their menstruation, eating habits, excessive hair growth, junk foods, and the psychological factors, such as depression and stress. The impact of intervention on the PCOS risk was studied, and the findings showed a reduction in the risks, such as the BMI. In one study, authors found menstrual cycle disorder, bad mood, family history of diabetes, and family history of infertility menstrual irregularity of mother, and lack of physical exercise as risk factors for PCOS. 29 Obesity, central obesity, and insulin resistance are strongly implicated in its etiology, and reduction of these risk factors should be the central focus of the treatment. Short‐term weight loss has been consistently successful in reducing insulin resistance and restoring ovulation and fertility. 30
In the present research, the mean risk assessment score reduced after yoga and continued to reduce after the exercise sessions. It was important to note that the percentage of adolescent girls with high risk of PCOS decreased to 5.9% in posttest 1 and “none” in posttest 2 in experimental groups. One research showed that yoga helped in controlling endocrine function and decreasing the symptoms of PCOS. 31 Other reported studies showed significant reduction in triglycerides levels, decrease in luteinizing/follicle stimulating hormone ratio, and the sex hormone binding globulin levels, and improvement following yoga. 32 , 33 However, in the present research, no laboratory investigators were performed to assess the impact of intervention, and the assessment was based on a risk assessment tool. Few similar studies reported that lifestyle modification helps, to hormonal profile helps, to restore the ovulation for the initial management of the reproductive disorder 34 , 35 , 36 , 37 had also showed that exercise plays an important role in the risk reduction of PCOS.
It was noticed that during the pretest in the experimental group, 85.2% of them had moderate risk and 14.8% had high risk level of PCOS. The percentage of adolescent girls with high risk of PCOS decreased to 5.9% in posttest‐1 and none in posttest‐2 in the experimental group. Yoga is a holistic approach toward better health. In PCOS, it is agreed that yoga can be beneficial. 38 In one research, authors found yoga very effective than even physical exercises in improving glucose, lipid levels, and minimized insulin resistance in adolescent PCOS girls. 8
It is well known that PCOS is linked with menstrual abnormalities. 39 , 40 , 41 In the present research, 50% of girls in the experimental group responded that they have irregular periods “usually” and 48% responded that they “sometimes” miss their periods in their regular cycle. Half of the participants (50%) responded that they “sometimes” get their menstrual cycle after more than 35 days and 40% responded that they “sometimes” get menstrual flow for more than 5 days. Thirty‐six percentage and 34.3% responded that they change more than four pads per day “sometimes” and “usually,” respectively. Similar to the test group, even in the control group, a greater number of the girls, 52%, responded that they have irregular periods “usually” and 48% responded that they “sometimes” miss their periods in their regular cycle. Fifty‐two percentage of the participants responded that they “sometimes” get their menstrual cycle after more than 35 days and 42.2% responded that they “sometimes” get menstrual flow for more than five day. Thirty‐nine percentage and 34.3% responded that they change more than 4 pads per day “sometimes” and “usually,” respectively. In a study from India, 6.3% of the girls had oligomenorrhea with PCOS, 0.22% had clinical hyperandrogenism, and 2.39% had oligomenorrhea in the presence of clinical hyperandrogenism. 18 A similar study found that a greater number of girls had irregular menstrual periods. 42 This indicates that girls with PCOS risk have irregular menstrual periods. If untreated, this condition can affect the reproductive health and can pose a major problem in their family life. So, it is commonly understood that irregular menstrual period is one of the signs and symptoms of PCOS. Thus, the responsibility lies in the hands of health professionals, school teachers, and parents to find out this problem and help them to treat or prevent PCOS. Upon intervention, there was a slight improvement in the menstrual conditions of the students in the experimental group. After the intervention, they knew the importance of regular menstrual period, and they would consider meeting the doctor for better preventive care.
Eating junk foods can be harmful 43 and is known to have a link with PCOS. 44 The present research findings showed a higher number of girls having the habit of eating junk foods. Similarly, higher mean scores of 2.25 and 2.33 were seen for the habit of eating junk food among the experimental group and the control group participants, respectively. Eating junk foods can cause disturbance in carbohydrate metabolism and thus can cause abnormal blood glucose, which is linked with PCOS. A study conducted in India reported a greater number of adolescent girls with PCOS were having the habit of eating junk foods. 26 The schoolgirls in the experimental group received education on the harmful effects of junk food. The students were encouraged to avoid eating junk foods. Many of the students had the habit of eating junk foods sold in front of the school. They agreed with the researcher that they will try to stop eating as much as possible as it was linked to their reproductive health.
Obesity is a well‐known risk factor for PCOS. 45 , 46 , 47 It is well understood that obese girls carry more risk for developing PCOS in future and hence it is important to help them in devising strategies to overcome obesity and associated problems. In the present research, among experiment group, 21.6% (n = 22) were overweight and 1.9% (n = 2) were obese. In the control group, 19.6% (n = 20) of them were overweight and 3.0% (n = 3) were obese. In various studies, significantly higher number of women had a high BMI, suggesting the relationship between obesity and PCOS. 48 , 49 , 50 Upon intervention, in the present research, students agreed to perform exercise and yoga daily and make it as a day to day habit. They may also spread the importance of performing exercise and yoga to their peer groups. There was also a slight improvement in the BMI of intervention group girls. However, this finding is a short‐term observation and it would be interesting to monitor these girls for a long time and see the impact of the awareness program, exercise, and yoga on their BMI.
PCOS is strongly linked to psychological symptoms 51 and these symptoms can further influence the diagnosis and treatment of PCOS. 52 In the present research, at baseline, majority of the girls were “usually” depressive, feeling “irritable and sleepy” giddiness fatigue sometimes in the experimental group and the control group. This could be due to their examinations and other school related stress. However, studies showed that anxiety level and the depression among women with PCOS is more common. 31 , 52 If left unattended it can worsen the quality of life, poor academic performance, and can also later lead to chronic psychiatric problems. The schoolgirls in the experimental group received counseling and education to tackle the psychological stress factors. After the intervention, as mentioned in Table 10, there were improvements in the questions related to psychological factors. Most of the girls showed more energy and were relieved from depression. It is also imperative that exercise and yoga can certainly improve the psychological aspects of any individual, as it involves both body and mind.
9. LIMITATIONS
This study was limited only to “high” risk and “moderate risk” adolescent girls. “Low risk” and “no risk” adolescents' girls were not included in the study and hence can be a limitation. Furthermore, this research was restricted only to adolescent girls who are studying in 10th to 12th grades in two government Girls Higher Secondary Schools and hence may not be representative for the entire school girls. Private schools were exempted and thus possess inclusion bias. This study was limited only to 4 months of lifestyle modifications and only 30 minutes a day. Having more time could have provided better results. The researcher could not supervise the adolescent girls performing yoga and exercise during government holidays. Self‐reported information relies primarily on respondents providing the right information. Misreporting by respondents cannot be ruled out.
10. CONCLUSIONS
This research finding concluded yoga and exercise customized as per school girls schedule involving school teachers can have a positive impact on PCOS risk reduction. As reported in the research, upon interventions, a significant risk reduction occurred among the experimental group, suggesting the usefulness of interventions. This study thus provides a foundation for incorporating lifestyle interventions among adolescent school going girls with PCOS risk. Lifestyle modifications among school going adolescent girls may be a successful strategy in PCOS risk minimization among the Indian population.
ETHICS STATEMENT
The ethical approval was obtained from the Billroth Hospital Ethical Review Board, Chennai, India.
AUTHOR CONTRIBUTIONS
Conceptualization: Valarmathi Selvaraj, Jain Vanitha, Fabiola M. Dhanaraj, Prema Sekar, Anitha Rajendra Babu
Data Curation: Valarmathi Selvaraj
Formal Analysis: Valarmathi Selvaraj, Jain Vanitha
Investigation: Valarmathi Selvaraj, Fabiola M. Dhanaraj
Methodology: Valarmathi Selvaraj, Jain Vanitha, Fabiola M. Dhanaraj, Prema Sekar, Anitha Rajendra Babu
Resources: Valarmathi Selvaraj, Fabiola M. Dhanaraj
Supervision: Valarmathi Selvaraj, Jain Vanitha, Fabiola M. Dhanaraj, Prema Sekar, Anitha Rajendra Babu
Validation: Jain Vanitha, Prema Sekar, Anitha Rajendra Babu
Writing – Review & Editing: Valarmathi Selvaraj, Fabiola M. Dhanaraj, Prema Sekar
All authors have read and approved the final version of the manuscript.
Valarmathi Selvaraj had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
FUNDING
This research was self‐financed by the Principal investigator (VS) as a part of her PhD research with her personal money.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.
CONSENT FOR PUBLICATION
The consent is taken from both the study subjects and their parents.
TRANSPARENCY STATEMENT
Valarmathi Selvaraj affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Appendix S1. Supporting Information.
ACKNOWLEDGEMENTS
The authors express thanks to all the students of Government Girls Higher Secondary School, Keelmanambedu and Government Girls Higher Secondary School, Poonamallee, who agreed to participate in the study. Without their participation, this research would not have been accomplished.
Selvaraj V, Vanitha J, Dhanaraj FM, Sekar P, Babu AR. Impact of yoga and exercises on polycystic ovarian syndrome risk among adolescent schoolgirls in South India. Health Sci Rep. 2020;3:e212 10.1002/hsr2.212
DATA AVAILABILITY STATEMENT
The data of this research can be obtained from the corresponding author with an Email request.
REFERENCES
- 1. Stein IF. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181‐191. [Google Scholar]
- 2. Unfer V, Proietti S, Gullo G, Porcaro G, Carlomagno G, Bizzarri M. Polycystic ovary syndrome: features, diagnostic criteria and treatments. Endocrinol Metab Syndr. 2014;3(3):1000136. [Google Scholar]
- 3. Nicandri KF, Hoeger K. Diagnosis and treatment of polycystic ovarian syndrome in adolescents. Curr Opin Endocrinol Diabetes Obes. 2012;19(6):497‐504. [DOI] [PubMed] [Google Scholar]
- 4. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. 2014;6:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barber TM, Mc Carthy MI, Wass JAH, Franks S. Obesity and polycystic ovarian syndrome. Clinical Endocrinology, Obesity and polycystic ovary syndrome. Clin Endocrinol (Oxf). 2006;65:137‐145. [DOI] [PubMed] [Google Scholar]
- 7. Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;24(3):302‐312. [DOI] [PubMed] [Google Scholar]
- 8. Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Effect of holistic yoga program on anxiety symptoms in adolescent girls with polycystic ovarian syndrome: a randomized control trial. Int J Yoga. 2012;5(2):112‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tang T, Glanville J, Hayden CJ, White D, Barth JH, Balen AH. Combined lifestyle modification and metformin in obese patients with polycystic ovary syndrome. A randomized, placebo‐controlled, double‐blind multicentre study. Hum Reprod. 2005;21(1):80‐89. [DOI] [PubMed] [Google Scholar]
- 10. Domecq JP, Prutsky G, Mullan RJ, et al. Lifestyle modification programs in polycystic ovary syndrome: systematic review and meta‐analysis. J Clin Endocrinol Metab. 2013;98(12):4655‐4663. [DOI] [PubMed] [Google Scholar]
- 11. Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13(6):1502‐1505. [DOI] [PubMed] [Google Scholar]
- 12. Moran LJ, Ko H, Misso M, et al. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence‐based guidelines. J Acad Nutr Diet. 2013;113(4):520‐545. [DOI] [PubMed] [Google Scholar]
- 13. Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13(6):251‐257. [DOI] [PubMed] [Google Scholar]
- 14. Hoeger K, Davidson K, Kochman L, Cherry T, Kopin L, Guzick DS. The impact of metformin, oral contraceptives, and lifestyle modification on polycystic ovary syndrome in obese adolescent women in two randomized, placebo‐controlled clinical trials. J Clin Endocrinol Metab. 2008;93(11):4299‐4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Puurunen J, Piltonen T, Morin‐Papunen L, et al. Unfavorable hormonal, metabolic, and inflammatory alterations persist after menopause in women with PCOS. J Clin Endocrinol Metab. 2011;96(6):1827‐1834. [DOI] [PubMed] [Google Scholar]
- 16. Verma A, Kumar S, Dei L, Dhiman K. Management of PCOS: a psychosomatic disorder by yoga practice. Int J Innov Res Devel. 2015;4(1):216‐219. [Google Scholar]
- 17. Moro C, Pasarica M, Elkind‐Hirsch K, Redman LM. Aerobic exercise training improves atrial natriuretic peptide and catecholamine‐mediated lipolysis in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(7):2579‐2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163‐2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagaraja B, Sandhya G, Medabalmi A, Epari V. Polycystic ovarian syndrome. Prevalence and its correlates among adolescent girls. Trop Med Public Health. 2014;6:632‐636. [Google Scholar]
- 20. Radha P, Devi RS, Madhavi J. Comparative study of prevalence of polycystic ovarian syndrome in rural and urban population. J Adv Med Dent Scie Res. 2016;4(2):90. [Google Scholar]
- 21. Trina R. What is polycystic ovarian syndrome and PCOS treatment. Health. 2013;18 https://www.indiatimes.com/health/healthyliving/what-is-polycystic-ovary-syndrome.26634043 [Google Scholar]
- 22. Gill H, Tiwari P, Dabadghao P. Prevalence of polycystic ovary syndrome in young women from North India: A Community‐based study. Indian J Endocrinol Metab. 2012;16(suppl 2):S389‐S392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vijayan CP, Sonia A. Prevalence of Polycystic Ovary Syndrome among students of a teaching collegiate hospital. Health Sci. 2013;2(1):4. [Google Scholar]
- 24. Vijaya K, Bharatwaj RS. Prevalence and undetected burden of polycystic ovarian syndrome (PCOS) among female medical undergraduate students in South India—a prospective study in Pondicherry. GJRA–Glob J Res Analysis. 2014;3:63–64. [Google Scholar]
- 25. Nair MK, Pappachan P, Balakrishnan S, Leena ML, George B, Russell PS. Menstrual irregularity and poly cystic ovarian syndrome among adolescent girls—a 2‐year follow‐up study. Indian J Pediatr. 2012;79(1):69‐73. [DOI] [PubMed] [Google Scholar]
- 26. Shobha, Devi ES, Prabhu A. An exploratory survey to identify the adolescents with high risk for Polycystic Ovarian Syndrome (PCOS) and to find the effectiveness of an awareness programme among students of selected pre‐university colleges of Udupi District. IOSR Journal of Nursing and Health. Science. 2014;3(3):66‐69. https://www.iosrjournals.org/iosr‐jnhs/papers/vol3‐issue3/Version‐2/H03326669.pdf [Google Scholar]
- 27. Babbie ER. The basics of social research. Cengage Learn. 2013(7th ed). [Google Scholar]
- 28. Valarmathi S, Jain V, Fabiola D, Prema S, Anitha B. Implementation of an awareness program and lifestyle intervention on polycystic ovarian syndrome among adolescent schoolgirls in India. Acta Sci Paediatr. 2020;3:24‐30. [Google Scholar]
- 29. Shan B, Cai JH, Yang SY, Li ZR. Risk factors of polycystic ovarian syndrome among Li People. Asian Pac J Trop Med. 2015;8(7):590‐593. [DOI] [PubMed] [Google Scholar]
- 30. Giallauria F, Palomba S, Maresca L, et al. Francesco Orio. Exercise training improves autonomic function and inflammatory pattern in women with polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2008;69(5):792‐798. [DOI] [PubMed] [Google Scholar]
- 31. Shanthi S. Utilization of Swamiji Vethathri Maharishis Kayakalpa Yoga Practice for women with PCOS. Aayagam Int J Multidiscipl Res. 2013;1:1. [Google Scholar]
- 32. Ulka N, Neela T, Shalia B, Kanchan S, Ghantaili MM. Effect of yogic practices on infertility related to polycystic ovarian syndrome. Int J Yoga. 2010;2(3):5‐7. [Google Scholar]
- 33. Redman LM, Elkind‐Hirsch K, Ravussin E. Aerobic exercise in women with polycystic ovary syndrome improves ovarian morphology independent of changes in body composition. Fertil Steril. 2011;95(8):2696‐2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahmed EM, Salem ME, Sweed MS. Effect of lifestyle modifications on polycystic ovarian syndrome symptoms. J Am Sci. 2012;8(8):535‐544. [Google Scholar]
- 35. Khademi A, Alleyassin A, Aghahosseini M, Tabatabaeefar L, Amini M. The effect of exercise in PCOS women who exercise regularly. Asian J Sports Med. 2010;1(1):35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harrison CL, Stepto NK, Hutchison SK, Teede HJ. The impact of intensified exercise training on insulin resistance and fitness in overweight and obese women with and without polycystic ovary syndrome. Clin Endocrinol (Oxf). 2012;76(3):351‐357. [DOI] [PubMed] [Google Scholar]
- 37. Brown AJ, Setji TL, Sanders LL, et al. Effects of exercise on lipoprotein particles in women with polycystic ovary syndrome. Med Sci Sports Exerc. 2009;41(3):497‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garfinkel M, Schumacher HR Jr. Yoga. Rheum Dis Clin. 2000;26(1):125‐132. [DOI] [PubMed] [Google Scholar]
- 39. Livadas S, Christou M, Economou F, et al. Menstrual irregularities in PCOS. Does it matter when it starts? Exp Clin Endocrinol Diabetes. 2011;119(6):334‐741. [DOI] [PubMed] [Google Scholar]
- 40. Panidis D, Tziomalos K, Chatzis P, et al. Association between menstrual cycle irregularities and endocrine and metabolic characteristics of the polycystic ovary syndrome. Eur J Endocrinol. 2013;168(2):145‐152. [DOI] [PubMed] [Google Scholar]
- 41. Hoyt KL, Schmidt MC. Polycystic ovary (Stein‐Leventhal) syndrome: etiology, complications and treatment. Clin Lab Sci. 2004;17(3):155‐163. [PubMed] [Google Scholar]
- 42. Kala K, Datti SN, Sujatha C, Dayanand R, Kumar Guruprasad GA. A study of clinical manifestations of PCOS among obese and non‐obese rural women. Indian J Basic Appl Med Res. 2013;8:946‐951. [Google Scholar]
- 43. Seth A, Sharma R. Childhood obesity. Indian J Pediatr. 2013;80(4):309‐317. [DOI] [PubMed] [Google Scholar]
- 44. De Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta‐analysis. Hum Reprod Update. 2011;17(4):495‐500. [DOI] [PubMed] [Google Scholar]
- 45. Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes (Lond). 2002;26(7):883‐896. [DOI] [PubMed] [Google Scholar]
- 46. Barry JA, Bouloux P, Hardiman PJ. The impact of eating behavior on psychological symptoms typical of reactive hypoglycemia. A pilot study comparing women with polycystic ovary syndrome to controls. Appetite. 2011;57(1):73‐76. [DOI] [PubMed] [Google Scholar]
- 47. Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006;113(10):1148‐1159. [DOI] [PubMed] [Google Scholar]
- 48. Boyle JA, Cunningham J, OˈDea K, Dunbar T, Norman RJ. Prevalence of polycystic ovary syndrome in a sample of Indigenous women in Darwin, Australia. Med J Aust. 2012;196(1):62‐66. [DOI] [PubMed] [Google Scholar]
- 49. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta‐analysis. Hum Reprod Update. 2010;16(4):347‐363. [DOI] [PubMed] [Google Scholar]
- 50. Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91(2):492‐497. [DOI] [PubMed] [Google Scholar]
- 51. Zangeneh FZ, Jafarabadi M, Naghizadeh MM, Abedinia N, Haghollahi F. Psychological distress in women with polycystic ovary syndrome from Imam Khomeini Hospital. Tehran J Reprod Infertil. 2012;13(2):111‐115. [PMC free article] [PubMed] [Google Scholar]
- 52. Banting LK, Gibson‐Helm M, Polman R, Teede HJ, Stepto NK. Physical activity and mental health in women with polycystic ovary syndrome. BMC Womens Health. 2014;14(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information.
Data Availability Statement
The data of this research can be obtained from the corresponding author with an Email request.


