Abstract
Macro-algae are a good source of agar oligosaccharides, which can be obtained through bacterial enzymatic hydrolysis. The agarase enzyme secreted by the micro-organisms cleaves the cell wall of the algae and releases agar oligosaccharides as degradation products with various applications. Agarolytic bacteria were isolated from the marine algae Kappaphycus sp., and Sargassum sp., and studied for their agar-degrading properties. Among the 70 isolates, 2 isolates (A13 and Sg8) showed agarase activity in in vitro assays. The maximum agarolytic index was recorded in the isolate Sg8 (3.75 mm and 4.29 µg ml−1 agarase activity), followed by the isolate A13 (2.53 mm and 2.6 µg ml−1 agarase activity). Optimum agarase production of isolate Sg8 was observed at pH7 and at a temperature of 25 °C in 24–48 h, whereas for isolate A13 the optimum production was at pH7 and at a temperature of 37 °C in 48 h. The identities of the agarolytic isolates (Sg8 and A13) were confirmed based on microscopy, morphological, biochemical and molecular analysis as Shewanella algae [National Center for Biotechnology Information (NCBI) GenBank accession number MK121204.1] and Microbulbifer elongatus [NCBI GenBank accession number MK825484.1], respectively.
Keywords: agar-hydrolysing bacteria, Kappaphycus sp., marine macro-algae, Microbulbifer elongatus, Sargassum sp., Shewanella algae
Introduction
The marine ecosystem is ubiquitously distributed with a wide variety of organisms such as seaweeds, zooplankton, bacteria, fungi and crustaceans, containing various insoluble complex polysaccharides. Agar is one of the most significant and productive cell-wall polysaccharides obtained from the red seaweed Rhodophyaceae. The chemical constituents and composition of the agar were determined by Araki and Arai [1], and the agar has been defined as belonging to a family of polymers with a common backbone of neoagarobiose (O–3, 6-α-anhydro-l-galactopyranosyl O-β-d-galactopyranose [1–3]) linked through β 1, 4 bonds [2]. These polysaccharides present in the cell walls of some red macro-algae can be degraded by a number of bacterial strains from marine and other sources. The majority of the agar-hydrolytic bacteria (AHB) are from aquatic, particularly marine, environments. The efficiency of agar hydrolysis depends on the properties and relative concentrations of the agarase enzymes produced by AHB. These bacteria are divided into two groups based on agarase enzyme activity and liquefaction of the agar. Type 1 soften and cause depression of solid agar, while type 2 cause extensive liquefaction of agar [3]. Many agar-hydrolysing bacteria have been identified and reported from seawater, marine sediments, marine algae, marine molluscs, freshwater and soil [4], but the bacteria associated with seaweeds show a major ecological role in polysaccharide carbon recycling; the close association of AHB, nitrogen-fixing bacteria and algae co-existence have been also specified previously [5]. The first agar-degrading marine bacteria, 'Bacillus gelaticus' from the Norwegian coast, was isolated by Gran in 1902 [6]. Many researchers have reported several agarolytic bacteria, such as Vibrio FLB-17, Vibrio sp. JT0107, Bacillus cereus ASK 202, Pseudoalteromonas agarivorans , Agarivorans JA-1, Pseudoalteromonas CY-24, Thalassomonas agarivorans , Pseudomonas aeruginosa AG LSL-11, Vibrio sp. V134 and Simiduia agarivorans strain SA1, that were isolated from different seawater samples [7–14]. Several authors also reported that Pseudoalteromonas sp. strain CKT-1, Microscillia sp., Microbulbifer thermotolerans strain JAMB-A94, Agarivorans sp. JAMB-All, Thalassomonas sp. strain JAMB-A33 and Microbulbifer agarilyticus strain JAMB-A3 were isolated from different marine sediments [15–17]. Agarase is mainly utilized for the production of oligosaccharides that have potential applications in agar and medical industries. The agarase enzymes produced by the AHB have several properties and applications, such as the release of protoplasts for development of commercially valuable products [18], and purification of DNA molecules [7]. Some agar oligosaccharides obtained with agarase enzyme have anti-oxidative, anti-bacterial, anti-mutagenic and immune-modulating properties [19]. In a continuation of our earlier research [20] on the isolation of potential seaweed-associated micro-organisms (SWAM) for Plant growth promoting regulator (PGPR) potential, the present study was carried out to isolate AHB, from red and brown marine macro-algae, and screen for agarolytic activity; thus, considering a wide range of properties.
Methods
Seaweed collection and isolation of AHB
Seaweeds were collected from Munaikadu, Mandapam coast and Rameshwaram, Ramanathapuram district of India, and identified by the Centre of Advanced Study in Marine Biology (CASMB), Annamalai University, Chidambaram, Tamil Nadu, India, as Kappaphycus sp. and Sargassum sp. Both the seaweeds were washed with sterile water and stored at 4 °C.
Ten grams of washed Kappaphycus sp. and Sargassum sp. were homogenized in a sterile mortar and pestle, individually, under aseptic conditions. The samples were enriched in sterilized Bushnell Haas broth (BHB; HiMedia) containing 50 % seawater along with 0.2 % agar (HiMedia) as the sole carbon and energy source. The flasks were incubated in an orbital rotating shaker at 150 r.p.m. for 48 h at 28 °C. Following incubation, the BHB broth was serially diluted and plated on nutrient agar (HiMedia) with 50 % seawater for screening the AHB. A clear zone around a colony indicates the presence of AHB [21].
Optimizing growth conditions for AHB
The potent AHB were identified based on the zone of clearance and sub-cultured into selective medium, Zobell marine agar (ZMA; HiMedia), and stored at 4 °C. The growth and agarase activity of the isolates were studied at different pH values (3, 5, 7 and 9) and temperatures (25, 30, 37 and 42 °C) in Zobell marine broth (ZMB; HiMedia). Overnight culture was inoculated in ZMB medium, adjusted to different pH levels and incubated at the respective temperatures for 24–72 h. After incubation, the optical density value at 540 nm was determined in a spectrophotometer and agarase determination was carried out with a standardized procedure.
Qualitative screening for agarase enzyme
A qualitative test for agarase enzyme was performed with iodine using Lugol’s staining process. An identified isolate was grown in ZMB pH 7.6±0.2, an 8 mm well was cut in the agar, and 100 µl broth culture was added into the well and incubated at 30 °C for 3 days. After incubation, the agarolytic activity was tested by adding 2 ml Lugol’s iodine on the entire surface of the plate and leaving it for 15 min. The other process used for screening was streak plating – a single line streak from the broth culture was inoculated onto the agar medium and incubated at 30 °C for 3 days. After incubation, the plates were observed for zones of clearance on flooding with Lugol’s iodine. The agarolytic index (AI) was calculated as the ratio between the diameter of the clear zone and the colony. It is the index that denotes the ability of the bacteria to produce agarase enzymes [2]. AI (mm) = clear zone diameter (mm) – colony diameter (mm)/colony diameter (mm).
Determination for agarase activity
The quantitative estimation of agarase enzyme activity was carried out in standardized production medium (glycerol – 8.00 ml l–1, yeast extract – 2.00 g l–1, bacteriological peptone – 3.00 g l–1, beef extract – 0.50 g l–1, NaCl – 4.00 g l–1, MgSO4 – 20.00 g l–1, MgCl2 – 1.50 g l–1, KCl – 0.20 g l–1, CaCl2 – 0.01 g l–1, CaCO3 − 1.00 g l–1) by incubating at 30 °C with 150 r.p.m. for 3 days. Subsequently, the culture broth was centrifuged for 15 min at 10 000 r.p.m. at 4 °C. The agarase enzyme activity was estimated by the 3,5-dinitrosalicylic acid method [22] with 2.5 ml agarose (0.1, 0.25 and 0.5%) in 20 mM Tris HCl buffer pH 8.00. The OD540 value was determined using a spectrophotometer. The agarase activity was expressed as units ml–1. One unit of agarase enzyme was expressed as the amount of enzyme required to release 1 µM galactose equivalent min–1 at 30 °C [2].
Biochemical characterization for A13 and Sg8
The identities of the potent AHB (A13 and Sg8) were studied through microscopy, biochemical tests and molecular analysis. Microscopic views of both isolates were analysed by Gram staining and culture morphology was observed on ZMB. Biochemical tests included indole production, methyl red, Voges Proskauer, citrate utilization, starch hydrolysis, cellulose hydrolysis, pectin hydrolysis, lipase hydrolysis, triple sugar iron, catalase, oxidase and lactose fermentation.
16S rRNA gene sequence analysis
The genomic DNA of the isolates (A13 and Sg8) was extracted [23, 24] and the 16S rRNA gene for each was amplified using 27F and 1492R primers (Sigma-Aldrich) in a Bio-Rad T100 thermal cycler. The amplified products were purified and sequenced by Macrogen (South Korea). The resulting 16S rRNA gene sequences were evaluated by comparing them with those sequences previously submitted to public databases using the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) Basic Local Alignment Search Tool (blast) and Ribosomal Database Project (RDP) sequence match tools. The phylogenetic trees were reconstructed with the closely related sequences retrieved from the NCBI nucleotide databases using the maximum-likelihood algorithm with bootstrap values based on 1000 replications in mega version 5.1.
Extracellular crude agarase enzyme production
The extracellular agarase enzyme was prepared in standardized production medium supplemented with agar. After incubation, the medium was centrifuged at 6000 r.p.m. for 30 min. The supernatant was used as crude extracellular agarase enzyme, and examined for its agarase activity both qualitatively and quantitatively.
Results
Isolates Sg8 and A13
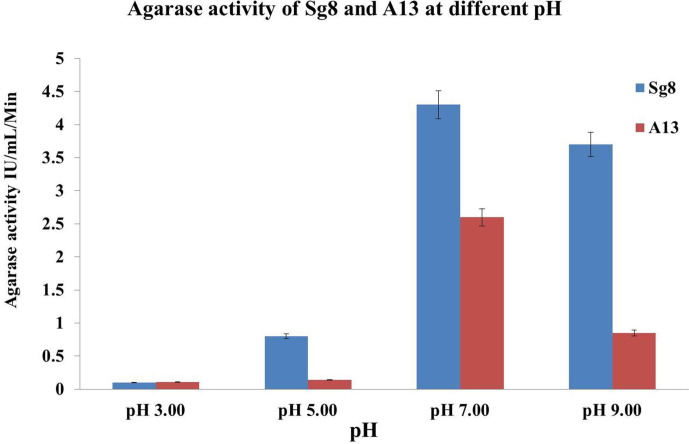
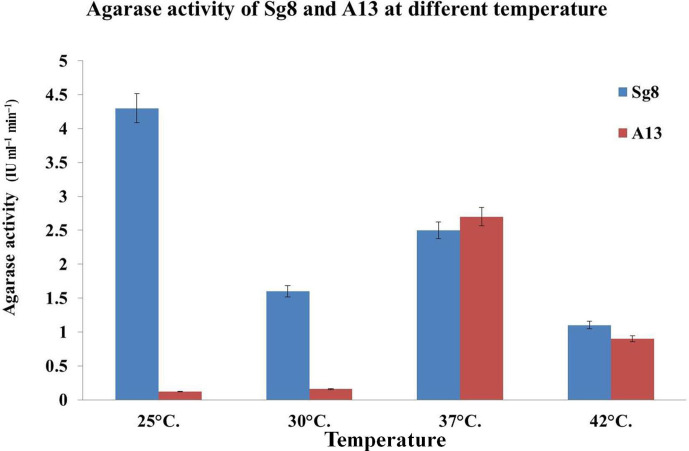
Among the microbes isolated [20] from Kappaphycus sp. and Sargassum sp., two isolates (Sg8 and A13) showed significant agarolytic activity, and it was evident by the formation of a visible depression and softening of the agar medium with the formation of a brown colour after staining with Lugol’s iodine solution. The optimum growth of the bacterial isolates (Sg8 and A13) was recorded at pH 7 after 72 h of incubation. The optimum temperature for the growth and agarase activity of the isolates (Sg8 and A13) was found to be 25 and 37 °C for 48 h, respectively.
Qualitative test for agarase enzyme activity of the selected isolates
The clear zone demonstrated that the isolates were able to hydrolyse agar and is expressed in terms of AI. The AI values of the Sg8 and A13 isolates were found to be 3.75 and 2.53 mm, respectively.
Determination of agarase activity
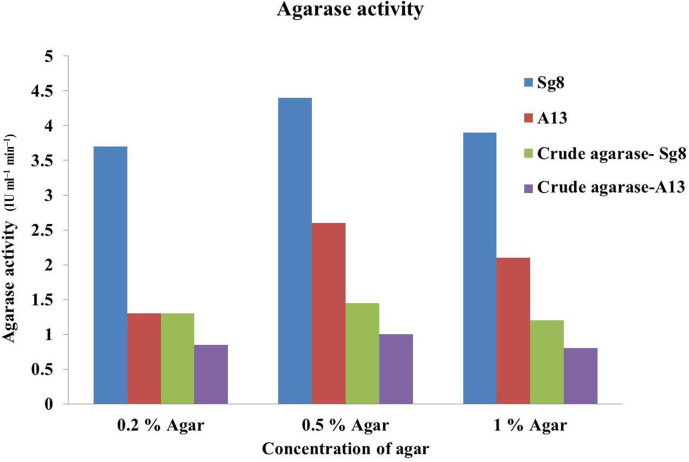
The isolates showed agarase enzyme activity in all concentrations of agar, but production was found to be high at 0.5 % (Sg8 4.29 µg ml−1, A13 2.6 µg ml−1) when compared with 0.2 % (Sg8 3.84 µg ml−1, A13 1 µg ml−1) and 1 % (Sg8 3.9 µg ml−1, A13 2.4 µg ml−1). The AI of the Sg8 crude agarase enzyme was 3.4 mm, and the activity of the agarase was higher in Sg8 compared to A13 (Figs 1 and 2).
Fig. 1.
Effect of pH on the growth and agarase activity of the isolates (Sg8 and A13) after 48 h incubation. The cultures showed a broad range of pH (5 to 9). Good growth and enzyme production were seen at pH 7.00. The enzyme activity was low at pH 3 due to denaturation.
Fig. 2.
Effect of temperature on the growth and agarase activity of the isolates (Sg8 and A13) after 48 h incubation. The highest growth and agarase enzyme activity generated by Sg8 was at 25 °C, whereas A13 showed highest growth and activity at 37 °C.
16S gene sequence analysis and biochemical characterization
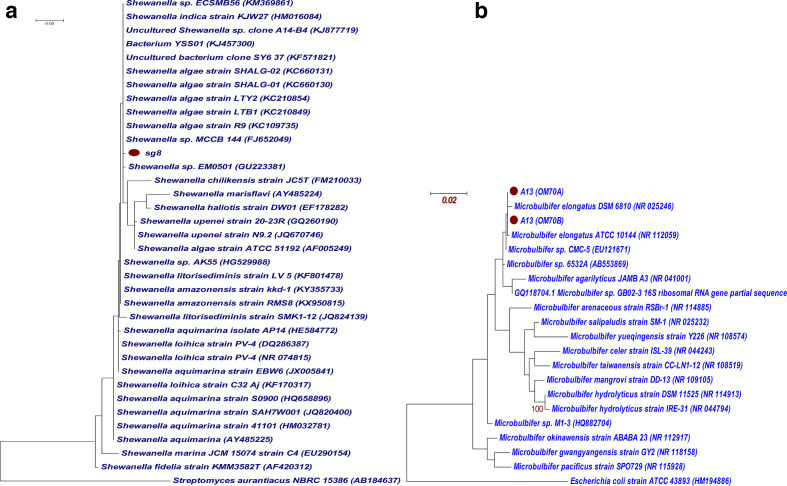
Based on the microscopic view, and morphological, biochemical and molecular analysis, the isolates Sg8 and A13 were identified as Shewanella sp. and Microbulbifer sp., respectively. The Shewanella sp. (Sg8) was observed as Gram-negative, rod-shaped bacteria showing negative results for IMViC tests and with alkaline slants being positive for H2S production in triple sugar iron (TSI) agar. The 16S rRNA gene sequences of Sg8 showed 99 % similarity with the genus Shewanella and 0.0 E values. Phylogenetically, Sg8 was clustered with different species of Shewanella with the distance of 0.002, showing the closest similarity with Shewanella algae (Fig. 3a), and was checked for its biochemical characteristics. Both the strains shared the same morphology and characteristics – growth temperature, pH, catalase and oxidase reactions with H2S production.
Fig. 3.
Maximum-likelihood phylogenetic trees of (a) Sg8 and (b) A13, and their NCBI blastn relatives based on 16S rRNA gene sequences. ●, Sg8 and A13 sequences. Bootstrap values (1000 resamples) are given as per cent values at the nodes of the tree (>80 and >90). Bars, 0.02 nucleotide substitutions per nucleotide position.
The isolate A13 was observed as Gram-negative, rod-shaped bacteria, positive for amylase and catalase enzyme production, and able to utilize citrate, ferment lactose and produce acid slant and butt in TSI medium; and belonged to the Microbulbifer clade, which was further confirmed through molecular analysis. A homology search indicated that the sequence of the isolate (A13) was homologous to the members of the gamma subclass of Proteobacteria , and in particular to the genus Microbulbifer . Phylogenetically, A13 clustered within the clade of type strains of Microbulbifer elongatus with 99.9 % similarity, with the distance values of 0.003 and 0.001 (Fig. 3b). The 16S rRNA sequences of the isolates Sg8 and A13 have been submitted to the NCBI under the GenBank accession numbers MK121204.1 and MK825484.1 respectively.
Extracellular crude enzyme for Sg8 and A13
A clear zone was observed on agar plates with crude enzyme of either isolate, indicating the agar had been hydrolysed and which is expressed in terms of AI. The AI values of the crude enzymes for Sg8 and A13 were found to be 3.4 and 3 mm, respectively (Fig. 4). The agarase enzyme activity was determined for crude enzyme in 0.2, 0.5 and 1.0 % agar (Sg8 1.1, 1.45 and 1.2 µg ml−1 and A13 0.85, 1.00 and 0.75 µg ml−1, respectively).
Fig. 4.
Determination of the agarase activity for the crude enzymes for Sg8 and A13.
Discussion
Marine environments with miscellaneous niches are unrefined sources of bacterial enzymes that can be subjugated for novel applications. Carrageenan and agar are algal polysaccharides extensively present in the cell wall of red algae that are utilized by various seaweed-associated micro-organisms (SWAM) for carbon recycling [25]. In the present study, bacterial communities associated with seaweed for degrading agar were isolated using the enrichment method. Fresh seaweeds were aseptically rinsed and subjected to subsequent enrichment in mineral medium supplemented with seawater and agar. For selective isolation and primary screening of AHB, ZMA was used. As a result, the growth of 70 bacterial isolates was observed that had no clearance or zone around the colonies, whereas 2 isolates (Sg8 and A13) showed significant depression and zone clearance. Most AHB are consistently screened by flooding Lugol’s iodine on the surface of agar plates to visibly confirm the zone of clearance or depression of the medium. The isolates Sg8 and A13 on consequent subculture and screening showed zone clearance and depression by softening of the agar at all given concentrations (0.1, 0.25 and 0.5 % agar). This indicates the utilization of the agar by both isolates through the production of agarase enzyme [3]. The biochemical and molecular investigation helped in the classification of the isolates from vast microbial communities as Shewanella algae (Sg8) and M. elongatus (A13). The isolates were able to grow in an extensive range of pH from 6.00 to 9.00 and showed optimum growth at pH 7.00. The optimum temperature for Sg8 was recorded as 25 °C and for A13 as 37 °C; the enzyme activity was found to increase with temperature, but later decrease at higher temperatures due to denaturation [26]. The agarase enzyme produced by the isolates was induced by the agar present in the culture medium, signifying the increase in agarase activity through increase in agar concentration. The optimum concentration of agar for higher enzyme production was recorded as 0.5 %; however, on additional increase of the agar concentration, the gel proportion of the medium increases and complexity in hydrolysis was found [27].
In conclusion, the agarolytic bacteria isolated from red and brown marine seaweeds were identified as S. algae (Sg8) and M. elongatus (A13), respectively. The agricultural use of these isolates needs further investigation, and the evaluation of the agar oligosaccharides generated as carrageenan and galactans for induction of systemic-acquired resistance in plants are planned in forthcoming work.
Funding information
The authors are thankful to the management of T. Stanes & Company Limited, Coimbatore and BIRAC (BT/SBIRI/1394/31/16) for funding the research.
Acknowledgements
The authors are thankful to Dr V. Karuppiah (former senior scientist at T. Stanes and Company Limited) – for isolation and molecular characterization of the A13 strain; and Dr Anantharaman and team, Centre of Advanced Study in Marine Biology (CASMB), Annamalai University, Chidambaram, Tamil Nadu, India – for assistance with seaweed collection identification. We acknowledge Dr Brindha (microbiologist, T. Stanes and Company Limited) for proof-reading the manuscript.
Author contributions
Conceptualization, ideas; formulation or evolution of overarching research goals and aims: K. P. K., R. K. S., R. S., S. R., K. L. Methodology, development or design of methodology; creation of models: K. P. K., R. K. S., R. S., S. R., K. L. Software, in silico analysis, molecular confirmation of the organisms: R. K. S. Validation, verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs: K. P. K., R. K. S., R. S. Formal analysis, application of statistical, mathematical, computational or other formal techniques to analyse or synthesis of study data: K. P. K., R. K. S., R. S., S. R., K. L. Investigation, conducting a research and investigation process, specifically performing the experiments or data/evidence collection: K. P. K., R. K. S., R. S., S. R., G. B., M. G., K. L.
Conflicts of interest
The authors declare that there are no conflicts of interest
Footnotes
Abbreviations: AHB, agar-hydrolytic bacteria; AI, agarolytic index; NCBI, National Center for Biotechnology Information.
References
- 1.Araki C, Arai K. Studies on the chemical constitution of agar-agar. XVIII. Isolation of a new crystalline disaccharide by enzymatic hydrolysis of agar-agar. Bull Chem Soc Jpn. 1956;29:339–345. doi: 10.1246/bcsj.29.339. [DOI] [PubMed] [Google Scholar]
- 2.Kawaroe M, Pratiwi I, Sunudin A. Isolation and characterization of marine bacteria from macroalgae Gracilaria salicornia and Gelidium latifolium on agarolitic activity for bioethanol production. IOP Conf Ser Earth Environ Sci. 2017;65:012025. doi: 10.1088/1755-1315/65/1/012025. [DOI] [Google Scholar]
- 3.Hodgson DA, Chater KF. A chromosomal locus controlling extracellular agarase production by Streptomyces coelicolor A3(2), and its inactivation by chromosomal integration of plasmid SCP1. J Gen Microbiol. 1981;124:339–348. [Google Scholar]
- 4.Fu XT, Kim SM. Agarase: review of major sources, categories, purification method, enzyme characteristics and applications. Mar Drugs. 2010;8:200–218. doi: 10.3390/md8010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waksman SA, Bavendamm W. On the decomposition of agar-agar by an aerobic bacterium. J Bacteriol. 1931;22:91–102. doi: 10.1128/JB.22.2.91-102.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goresline HE. Studies of agar-digesting bacteria. J Bacteriol. 1933;26:435–457. doi: 10.1128/JB.26.5.435-457.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugano Y, Terada I, Arita M, Noma M, Matsumoto T. Purification and characterization of a new agarase from a marine bacterium, Vibrio sp. strain JT0107. Appl Environ Microbiol. 1993;59:1549–1554. doi: 10.1128/AEM.59.5.1549-1554.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukasawa S, Dunlap PV, Baba M, Osumi M. Identification of an agar-digesting, luminous bacterium. Agric Biol Chem. 1987;51:265–268. doi: 10.1080/00021369.1987.10868011. [DOI] [Google Scholar]
- 9.Kim B-C, Poo H, Lee KH, Kim MN, Park D-S, et al. Simiduia areninigrae sp. nov., an agarolytic bacterium isolated from sea sand. Int J Syst Evol Microbiol. 2012;62:906–911. doi: 10.1099/ijs.0.031153-0. [DOI] [PubMed] [Google Scholar]
- 10.Romanenko LA, Zhukova NV, Rohde M, Lysenko AM, Mikhailov VV, et al. Pseudoalteromonas agarivorans sp. nov., a novel marine agarolytic bacterium. Int J Syst Evol Microbiol. 2003;53:125–131. doi: 10.1099/ijs.0.02234-0. [DOI] [PubMed] [Google Scholar]
- 11.Sie Y-F, Yang H-C, Lee Y. The discovery of agarolytic bacterium with agarase gene containing plasmid, and some enzymology characteristics. Int J Appl Sci Eng. 2009;7:25–41. [Google Scholar]
- 12.Lakshmikanth M, Manohar S, Patnakar J, Vaishampayan P, Shouche Y, et al. Optimization of culture conditions for the production of extracellular agarases from newly isolated Pseudomonas aeruginosa AG LSL-11. World J Microbiol Biotechnol. 2006;22:531–537. doi: 10.1007/s11274-005-9068-2. [DOI] [Google Scholar]
- 13.Jean WD, Shieh WY, Liu TY. Thalassomonas agarivorans sp. nov., a marine agarolytic bacterium isolated from shallow coastal water of An-Ping Harbour, Taiwan, and emended description of the genus Thalassomonas . Int J Syst Evol Microbiol. 2006;56:1245–1250. doi: 10.1099/ijs.0.64130-0. [DOI] [PubMed] [Google Scholar]
- 14.Leon O, Quintana L, Peruzzo G, Slebe JC. Purification and properties of an extracellular agarase from Alteromonas sp. strain C-1. Appl Environ Microbiol. 1992;58:4060–4063. doi: 10.1128/AEM.58.12.4060-4063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xavier Chiura H, Kita-Tsukamoto K. Purification and characterisation of a novel agarase secreted by a marine bacterium, Pseudoalteromonas sp. strain CKT1. Microbes Environ. 2000;15:11–22. doi: 10.1264/jsme2.2000.11. [DOI] [Google Scholar]
- 16.Zhong Z, Toukdarian A, Helinski D, Knauf V, Sykes S, et al. Sequence analysis of a 101-kilobase plasmid required for agar degradation by a Microscilla isolate. Appl Environ Microbiol. 2001;67:5771–5779. doi: 10.1128/AEM.67.12.5771-5779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohta Y, Hatada Y, Nogi Y, Li Z, Ito S, et al. Cloning, expression, and characterization of a glycoside hydrolase family 86 β-agarase from a deep-sea Microbulbifer-like isolate. Appl Microbiol Biotechnol. 2004;66:266–275. doi: 10.1007/s00253-004-1757-5. [DOI] [PubMed] [Google Scholar]
- 18.Yeong H-Y, Khalid N, Phang S-M. Protoplast isolation and regeneration from Gracilaria changii (Gracilariales, Rhodophyta) J Appl Phycol. 2008;20:641–651. doi: 10.1007/s10811-007-9249-5. [DOI] [Google Scholar]
- 19.Kong JY. Proceedings of Symposium on Scientific Study and Industrialization of Health Food. Taipai: Health Food Society of Taiwan; 2001. Production and functional properties of agarooligosaccharides; pp. 60–81. [Google Scholar]
- 20.Srinivasan R, Sengali Ragunath K, Karuppiah V, Radhesh Krishnan S, Gracy M, et al. Isolation and screening of seaweed associated microbes for development of marine based agri – inputs. Seaweed Res Utiln. 2017;39:39–46. [Google Scholar]
- 21.Jannasch HW. Enrichments of aquatic bacteria in continuous culture. Archiv Mikrobiol. 1967;59:165–173. doi: 10.1007/BF00406328. [DOI] [PubMed] [Google Scholar]
- 22.Miller GL, Blum R, Glennon WE, Burton AL. Measurement of carboxymethylcellulase activity. Anal Biochem. 1960;1:127–132. doi: 10.1016/0003-2697(60)90004-X. [DOI] [Google Scholar]
- 23.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, et al. Current Protocols in Molecular Biology. New York: Wiley; 1988. pp. 241–242. [Google Scholar]
- 24.Sun FJ, Harish A, Caetano-Anollés G. Phylogenetic utility of RNA structure: evolution’s arrow and emergence of early biochemistry and diversified life. In: Caetano-Anollés G, editor. Evolutionary Bioinformatics and Systems Biology. Hoboken, NJ: Wiley–Blackwell; 2010. pp. 329–360. [Google Scholar]
- 25.Kloareg B, Quatrano RS. Structure of the cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanogr Mar Biol Ann Rev. 1988.;26:259–315. [Google Scholar]
- 26.Rajeswari S, Jaiganesh R, Muthukumar R, Jaganathan MK. Isolation and characterization of an agarase producing bacteria from marine sediment. Int J Chemtech Res. 2016;9:437–446. [Google Scholar]
- 27.Hu Z, Lin B-K, Xu Y, Zhong MQ, Liu G-M. Production and purification of agarase from a marine agarolytic bacterium agarivorans sp. HZ105. J Appl Microbiol. 2009;106:181–190. doi: 10.1111/j.1365-2672.2008.03990.x. [DOI] [PubMed] [Google Scholar]