Abstract
Hydrogen sulfide (H2S), an endogenously generated and regulated signaling gas, plays a vital role in a variety of (patho)physiological processes. In the past few years, different kinds of H2S-releasing compounds (often referred to as H2S donors) have been developed for H2S delivery, but it is still challenging to make H2S donors with tunable payloads in a simple and efficient manner. Herein, a series of peptide-H2S donor conjugates (PHDCs) with tunable donor loadings are designed for controlled H2S release. The PHDCs self-assemble into nanoribbons with different geometries in aqueous solution. Upon addition of cysteine, these nanostructures release H2S, delivering their payload into H9C2 cells, as visualized using an H2S-selective fluorescent probe. Beyond imaging, in vitro studies show that the ability of PHDCs to mitigate doxorubicin-induced cardiotoxicity in H9C2 cardiomyocytes depends on their nanostructures and H2S release profiles. This strategy may enable the development of sophisticated H2S-releasing biomaterials for drug delivery and regenerative medicine.
Keywords: Hydrogen Sulfide, Self-assembly, Drug delivery, Controlled release, Peptide
Graphical Abstract
INTRODUCTION
As a biological signaling molecule, hydrogen sulfide (H2S) is involved in many physiological and/or pathological processes such as vasodilation and angiogenesis.1–4 Although the majority of foundational reports in this area used inorganic salts such as sodium sulfide (Na2S) or sodium hydrosulfide (NaSH) as H2S sources,5–6 these molecules are not ideal compounds for studying H2S biology due to their instantaneous release profiles and the transient nature of this signaling gas. In order to provide tools for studying H2S biology with slow and sustained rates of H2S delivery, several groups have recently developed many H2S-releasing compounds (so-called H2S donors).7–12 These donors respond to specific triggers, releasing H2S after application of a stimulus such as light, biologically-relevant thiols, changes in pH, enzymatic activity, and many others. However, despite this variety of synthetic H2S donors, several issues still need to be addressed. Low water solubility, few methods to tune release kinetics, and in most cases no capacity for delivery to a specific biological target limit the use of many H2S donors both as chemical tools for investigating the biological roles of H2S and as potential therapeutics.
One promising strategy to address these limitations is to incorporate H2S donors into materials, either through physical encapsulation13–14 or by covalent conjugation to hydrophilic polymers.15–18 While these methods can be effective, the short- and long-term biological activities, combined with potential toxicity arising from the synthetic polymers, may be cause for concern.19 Additionally, polydispersity must be considered, referring here both to polymer length and the amount of conjugated H2S donor, making polymeric drug delivery vehicles of all kinds consistently vulnerable to batch-to-batch variability. To circumvent these problems, we began designing and evaluating H2S-releasing materials based on self-assembling peptides.20–24 Peptides are useful materials from which to build self-assembled nanostructures for biomedical applications due to their inherent biodegradability and biocompatibility under many circumstances.25 Moreover, they can be quickly synthesized and purified with direct sequence control.26–27 Previously, our group showed that H2S-donating S-aroylthiooximes (SATOs) appended to the hexapeptide Ile-Ala-Val-Glu-Glu-Glu (IAVEEE) assembled into nanofibers in water.20 The nanostructures protected the SATO groups from hydrolytic decomposition and prolonged the release time in water as compared to small molecule SATOs. Very recently, we found that conjugation of SATOs to short tetrapeptides not only extended their H2S release profiles, but also modulated their release behaviors as a result of their different self-assembled morphologies.21
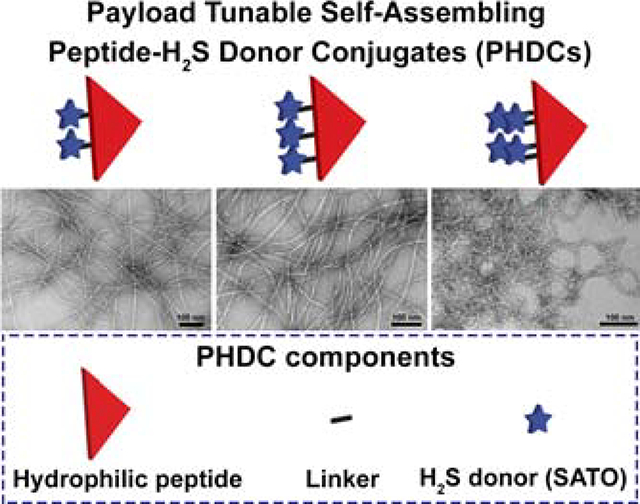
Despite progress in developing peptide-based H2S donors, a remaining downside of existing constructs is their limited tunability in terms of donor loading. A series of H2S-donating peptides with variable numbers of SATO groups per peptide has not been explored. In this context, we report here a group of peptide-H2S donor conjugates (PHDCs) that self-assemble in water to form stable supramolecular nanostructures with an ability to tune the SATO loading by molecular design. We aimed to evaluate how donor loading affects the self-assembled morphology, H2S release profiles, and biological activity of these PHDCs.
To achieve this goal, we appended SATOs, a thiol-triggered H2S donor developed in our lab,28 onto peptide sequences of 2–7 amino acids. Specifically, S-benzoylthiohydroxylamine (SBTHA) was added to three peptides, each with the same pentapeptide, Phe-Glu-Glu-Glu-Glu (FE4), but with different numbers of 4-formylbenzoic acid (FBA) units attached to Lys residues on the side chain ε-amine or the N-terminus. This molecular design allowed us to precisely attach two, three, or four SATOs onto the peptides, corresponding to respective SATO loadings of 24%, 28% and 31% by weight (Eq. S1–3). The structures of these three PHDCs are shown in Figure 1 and are labeled dSATO-FE4, tSATO-FE4, and qSATO-FE4 for short. Synthetic procedures and characterization details are included in the Supporting Information (Figure S1).
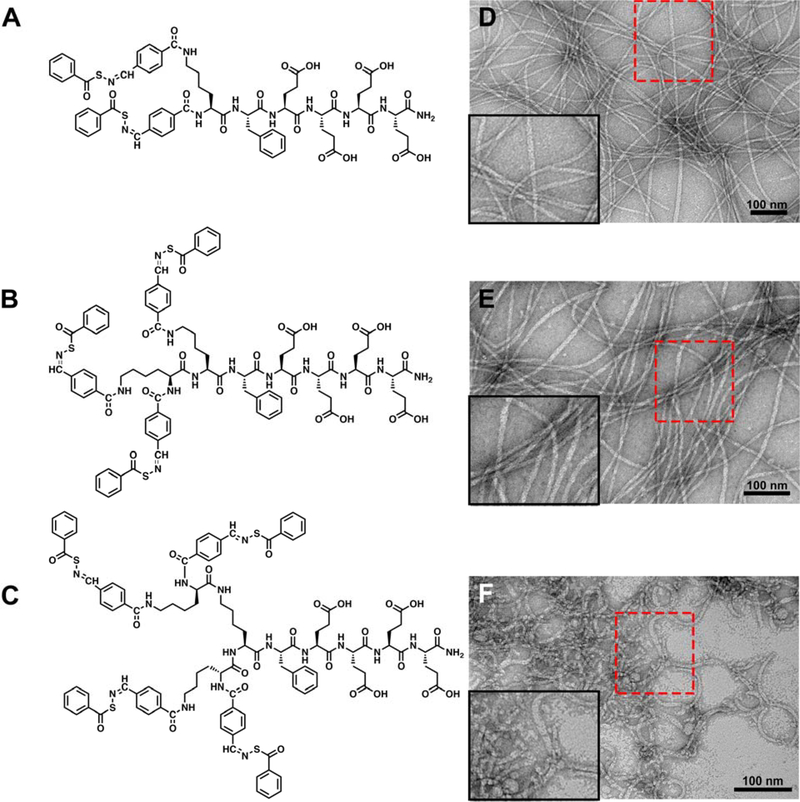
Figure 1.
(A-C) Molecular structures of PHDCs studied in the present work. (A) dSATO-FE4; (B) tSATO-FE4; (C) qSATO-FE4. (D-F) Conventional TEM images illustrate the effect of SATO numbers on the self-assembled morphologies of the PHDCs in phosphate buffer (10 mM, pH 7.4). (D) Twisted ribbons formed by dSATO-KFE4; (E) Twisted ribbons formed by tSATO-FE4; (F) Curved ribbons formed by qSATO-FE4. Inserts in the bottom left corners of panels D-F show zoomed-in images of the areas outlined by the red rectangles. Solution concentration: 100 μM peptides in phosphate buffer (pH 7.4). Uranyl acetate (UA) was used to stain all grids prior to imaging.
RESULTS AND DISCUSSION
We first probed each PHDC after dissolution in aqueous 10 mM phosphate buffer (pH 7.4) by conventional transmission electron microscopy (TEM). Images showed that all three PHDCs assembled into long, one-dimensional nanostructures (Figure 1D–F). The dominant morphology observed for PHDC dSATO-FE4 was twisted ribbons (Figure 1D). The widths were 7±1 nm, and average lengths were a few micrometers. PHDC tSATO-FE4 also formed twisted ribbons 7±1 nm in width and several micrometers in length (Figure 1E). In contrast, a different morphology was observed for PHDC qSATO-FE4. It associated into curved ribbons with average widths of 6±1 nm (Figure 1F). Although not as long as the twisted ribbons formed by dSATO-FE4 and tSATO-FE4, the curved ribbons tended to form bundles, which we expected could be beneficial for prolonging H2S release. These results show how incorporating different numbers of SATOs into the PHDC structure allows for tuning of both the SATO loading content and the self-assembled morphologies.
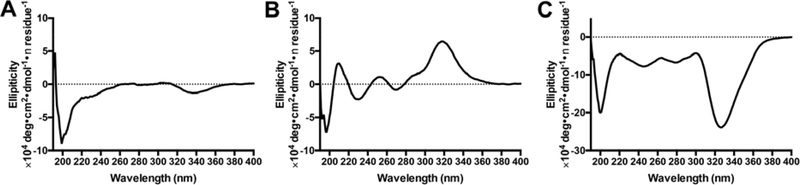
To probe the differences among these PHDCs in their self-assembled states, we began by conducting Nile Red assays to measure their critical aggregation concentrations (CACs), followed by circular dichroism (CD) spectroscopy to study the secondary structures of the self-assembled PHDCs. The Nile Red assay showed that the CAC value for each of the three PHDCs was between 20 and 30 μM (Figure S2 and Table S1). This is consistent with previous CAC measurements on PHDCs.21 Despite their similar self-assembled morphologies, CD spectroscopy revealed that the secondary structures of these PHDC nanostructures were quite different. All three exhibited signals in the peptide region (190−240 nm), where amide bonds in peptides absorb, and in the SATO region (300−360 nm). Absorptions in the SATO region are consistent with UV-vis absorption peaks (Figure S3). In the peptide region, the spectra for both dSATO-FE4 (Figure 2A) and qSATO-FE4 (Figure 2C) showed primarily random coil structure with some amount of α-helix, although the intensity in the peptide region for qSATO-FE4 was substantially greater than that for dSATO-FE4. In contrast, the CD spectrum for tSATO-FE4 was consistent with both a β-sheet structure, as indicated by the positive peak at 215 nm, and some random coil contribution (Figure 2B). These data indicate that secondary structures of these nanoassemblies are quite sensitive to the nature of the hydrophobic component, even though the peptide sequences are similar. The absorption of each PHDC varied in the SATO region as well. PHDCs dSATO-FE4 and qSATO-FE4 showed negative peaks near 330 nm, with qSATO-FE4 exhibiting a much more intense peak than dSATO-FE4. Unlike the negative absorption peaks of dSATO-FE4 and qSATO-FE4 in the SATO region, tSATO-FE4 exhibited a positive peak in the same range, possibly due to different handedness within these nanoribbons.29 The intensity of the SATO peak for tSATO-FE4 was between that of dSATO-FE4 and qSATO-FE4. We speculate that the observed intensity differences in this region among the three PHDCs is a result of increased hydrophobic effects and therefore stronger molecular packing among SATO groups as more SATO groups are introduced into the molecular design.
Figure 2.
CD spectra of (A) dSATO-FE4, (B) tSATO-FE4, and (C) qSATO-FE4 in phosphate buffer (75 μM, pH 7.4).
We next examined how the H2S release profiles of the three PHDCs was affected by their supramolecular structure. Because these PHDCs self-assemble in water, we expected that the nanostructures would shield SATO components from the aqueous environment, providing a method to control release of H2S triggered by cysteine (Cys). We typically assess release profiles from H2S donors by an H2S-selective microelectrode probe. This method provides a real-time measurement of H2S solution concentration; however, it is not capable of providing cumulative release curves because H2S volatilizes and oxidizes as it is produced. Therefore, H2S release profiles obtained with this electrochemical probe-based method are often compared using peaking times, which provide an approximate quantification of relative release rates among similar samples.
H2S release from these PHDCs was triggered by Cys, which we and others use commonly to trigger release of H2S from SATO-based materials.16, 20–21, 30 In order to eliminate the false response from the electrochemical probe generated by Cys, we used a specially made vial, reported previously,21 to evaluate PHDC release profiles. In these experiments, the releasing solution was loaded into the inner well of the vial; next the well was sealed with a gas-permeable membrane (Figure S4). We kept PHDC concentration in the inner well at 1 mM while varying the amounts of Cys (4 mM total Cys for dSATO-FE4, 6 mM for tSATO-FE4, and 8 mM for qSATO-FE4) to keep the molar ratio of SATO to Cys at 1:2. The concentration of H2S as it passed from the inner well into a large volume of PBS was then monitored over time.
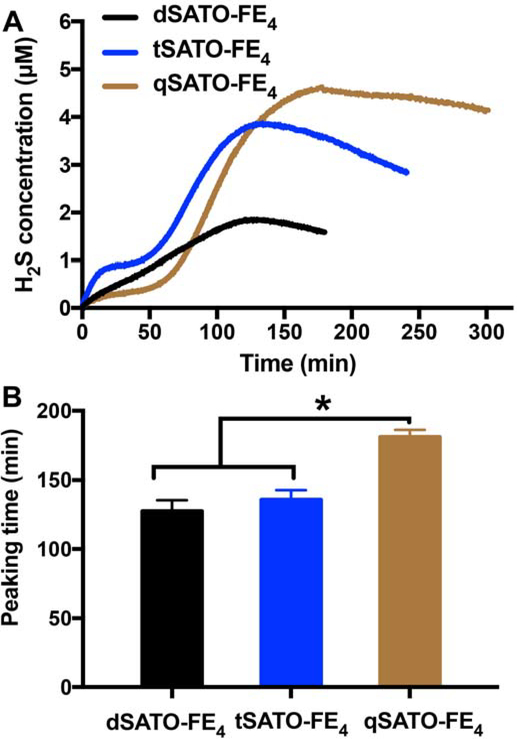
All three PHDCs showed steady and consistent H2S release lasting for several hours (Figure 3A). Although all three PHDCs formed nanoribbons, the H2S release profiles were quite different. Both dSATO-FE4 and tSATO-FE4 released H2S immediately after addition of Cys. Compared with the steady release profile of dSATO-FE4, there was a plateau for tSATO-FE4 after 20 min, and then the release rate accelerated. This interesting release profile may result from a morphology change during the H2S release process. With three SATOs toward the N-terminal end of the peptide, Cys penetration into the core of the assemblies would not lead to reaction of all three SATOs at the same time, leading to a decrease in the magnitude of the hydrophobic and π−π interactions, changing the morphology. These newly formed nanostructures would therefore be expected to release H2S faster as the packing among SATOs becomes weaker and the nanostructures loosen. Therefore, the observed release profile would be a combination from different nanostructures. Despite the different profiles, peaking times for dSATO-FE4 and tSATO-FE4 were quite similar, at 127±8 and 136±7 min, while the peaking time for qSATO-FE4 was significantly longer at 181±4 min (Figure 3B). Interestingly, unlike the profiles for dSATO-FE4 and tSATO-FE4, we observed an induction period of 50 min for qSATO-FE4 where H2S was slowly released, after which the rate of release increased sharply. This induction time phenomenon was also observed in a different PHDC system reported by our group recently.21 We speculate that this initial period of slow release from qSATO-FE4 results from Cys diffusing more slowly into the short, curved nanoribbon bundles formed by qSATO-FE4 (Figure 1F) compared with the separated nanoribbons in the other two PHDCs. In order to investigate the concentration effect on H2S release, we performed H2S release experiments with PHDC concentrations of 100 μM in the inner well were carried out. As shown in Figure S5 and Table S2, shorter peaking times were observed at 100 μM than at 1 mM despite the 10-fold dilution. Additionally, no significant difference in peaking times was observed, and no induction time was noted for any of the three PHDCs. These results resemble those from other drug-releasing self-assembling peptides31–32 and our recently reported PHDC tetrapeptides,21 where the release rate increases upon dilution of the samples. These data collectively indicate that Cys penetrated into these nanoribbons in a similar manner regardless of the varying number of SATO groups when the concentration of PHDC was low, but that rates of Cys diffusion varied at higher PHDC concentrations.
Figure 3.
(A) H2S release profiles and (B) corresponding peaking times of dSATO-FE4, tSATO-FE4, and qSATO-FE4 triggered by Cys at rt in PBS (pH = 7.4). Data were obtained on an H2S-sensitive electrochemical probe from a solution of PHDC (1 mM, 110 μL total) and Cys (4 mM for dSATO-FE4, 6 mM for tSATO-FE4, 8 mM for qSATO-FE4) sealed in an inner well with a gas-permeable membrane inside a vial containing PBS (5 mL). Error bars indicate standard deviations of three separate experiments. * indicates p<0.05 for a comparison of the groups indicated as determined by a one-way analysis of variance (ANOVA) with a Student-Newman-Keuls comparisons post-hoc test (n=3).
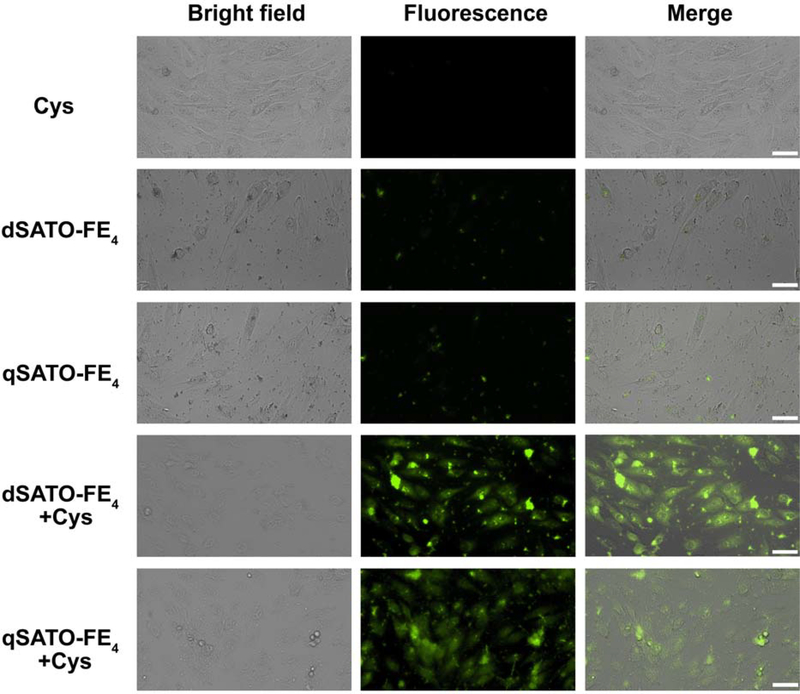
We then turned our attention to biological studies on PHDCs dSATO-FE4 and qSATO-FE4, which had the shortest and longest peaking times. Fluorescence microscopy was used to investigate whether dSATO-FE4 and qSATO-FE4 could be deliver H2S into cells. We used WSP-5,33 an H2S-selective fluorescent probe, to monitor H2S accumulation from these PHDCs in H9C2 cells. As expected, Cys alone provided no fluorescent signal, because WSP-5 responds to H2S but not to Cys (Figure 4, first row).33 Treating cells with PHDCs without Cys provided a weak fluorescent signal, which likely resulted from a small amount of H2S generated from hydrolysis of PHDCs (Figure 4, second and third rows). In sharp contrast, co-addition of dSATO-FE4 or qSATO-FE4 with Cys resulted in a significant increase in WSP-5 fluorescence (Figure 4, fourth and fifth rows), demonstrating that both dSATO-FE4 and qSATO-FE4 can be successfully activated to release H2S in vitro and that the released H2S can be imaged using an H2S-responsive fluorescent probe.
Figure 4.
Bright field, fluorescence, and merged images showing fluorescence in H9C2 cells pre-incubated with H2S probe WSP-5 (50 μM) for 30 min and then treated with the following groups: Cys (800 μM), PHDC (200 μM for dSATO-FE4; 100 μM for qSATO-FE4) or PHDC and Cys for 2 h. Cells were then washed, and fluorescence images were taken in PBS. Scale bars are 50 μm.
Given that both PHDCs were capable of delivering H2S into cells, we next explored their ability to protect against cardiotoxicity induced by doxorubicin (Dox), a common chemotherapeutic. Dox, an anthracycline with widespread antitumor activity, has potent therapeutic effects on a variety of cancers, including lymphomas, leukemias, soft-tissue sarcomas, and various types of solid tumors.34 However, its clinical use is limited by its dose-limiting and at times life-threatening cardiotoxicity.35 Previous studies showed that H2S, delivered as instantaneously releasing Na2S, rescues cardiomyocytes treated with Dox by mitigating stress in the endoplasmic reticulum,36–37 or depressing the p38 MAPK pathway,38–39 but this cardioprotective capacity has not been tested widely on sustaained H2S donors.21 More importantly, slow-releasing H2S donors frequently lead to enhanced biological effects compared to instantaneously releasing Na2S.16, 40 Thus, we envisioned that the PHDCs developed here might rescue cardiomyocytes in the presence of Cys.
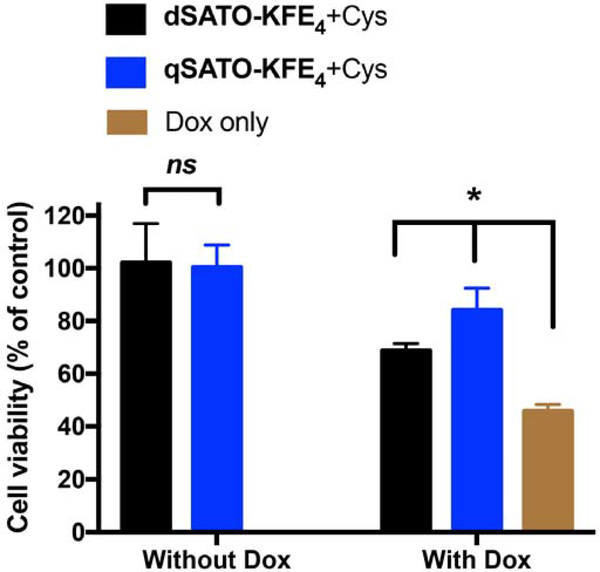
First, we confirmed that both dSATO-FE4 at 200 μM and qSATO-FE4 at 100 μM (both 400 μM SATO) were nontoxic to H9C2 cells in the presence of Cys (left two columns in Figure 5). In treatment studies, H9C2 cells were pretreated with dSATO-FE4 and qSATO-FE4 in the presence of Cys for 30 min.21, 36, 41 Without removing the PHDC/Cys solution, Dox was added and cells were incubated for another 24 h. Compared to the Dox only treatment group (46% viability, brown column in Figure 5), cell viability increased significantly when cells were treated with dSATO-FE4 (69% viability) or qSATO-FE4 (84% viability) (black and blue columns on the right in Figure 5). More importantly, qSATO-FE4 was significantly more effective in rescuing cells than dSATO-FE4. Because PHDCs were added in amounts to keep SATO loadings equal, this difference in bioactivity is likely due to the following two phenomena: 1) PHDC stock solutions (2 mM dSATO-FE4 and 4 mM qSATO-FE4) were added to cell media immediately before adding media to the cells, preserving their original morphologies; 2) The length of qSATO-FE4 curved ribbons are much shorter than that the dSATO-FE4 nanoribbons (Figure 1), which may facilitate cell uptake. Finally, we also confirmed that sustained H2S release was key for rescuing cardiomyocytes treated with Dox through comparisons to other H2S donors and control compounds (Figure S6), including Cys alone (49% viability), GYY4137 (38% viability), and Na2S (56% viability). Taken together, these results highlight the importance of release rate on H2S bioactivity.
Figure 5.
Cell viability of H9C2 cardiomyoctyes pretreated with dSATO-FE4 (200 μM) or qSATO-FE4 (100 μM) in the presence of Cys (800 μM) for 30 min before Dox addition (5 μM) or not (control). * indicates p<0.01. Error bars indicate standard deviations of three separate experiments with five replicates per experiment. Group comparisons are indicated as determined by a one-way ANOVA with a Student-Newman-Keuls comparisons post-hoc test.
CONCLUSIONS
In summary, we discuss here the synthesis and self-assembly of discrete PHDC nanostructures with tunable H2S donor content by controlling the number of H2S-releasing SATO units near the peptide N-terminus. The release behaviors depended strongly on the self-assembled morphology of the PHDCs, which relied heavily on molecular design, with the peaking time prolonged as the SATO loading percentage increased. In vitro fluorescence studies showed that in the presence of Cys, H2S released from PHDCs could be delivered into cells and visualized using an H2S-responsive fluorescent probe. In addition, the released H2S mitigated Dox-induced toxicity in H9C2 cardiomyoctyes, in which the PHDC with more SATO loading was more effective than its counterparts. These results highlight how variation in payload loading in amphiphilic peptides not only regulates the resulting self-assembled morphology, but also can modulate the bioactivity of these molecules. As the number of bioactive or drug molecules on a peptide can be easily changed by tuning the molecular design, we believe this strategy offers a general method to fabricate multiple types of nanostructures carrying a particular payload, enabling nanostructure-activity studies, such as this one, to be conducted on a wide variety of systems.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Science Foundation (DMR‐1454754) and the National Institutes of Health (R01GM123508). We also acknowledge the Dreyfus foundation for supporting these studies through a Camille Dreyfus Teacher-Scholar Award to J.B.M. We acknowledge Chadwick R. Powell for synthesizing GYY4137, Kearsley M. Dillon for synthesis of WSP-5, Prof Tijana Grove and her students for experimental assistance, and Samantha J. Scannelli and Kearsley M. Dillon for careful readings of the manuscript. The authors also acknowledge use of facilities within the Nanoscale Characterization and Fabrication Laboratory at Virginia Tech.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website. Detailed experimental section, further cell studies, and additional characterization (circular dichroism, CAC measurements, ESI-MS, and UV−vis).
REFERENCES
- (1).Wang R, Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [DOI] [PubMed] [Google Scholar]
- (2).Szabo C, Gasotransmitters in cancer: from pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016, 15, 185–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).King AL; Polhemus DJ; Bhushan S; Otsuka H; Kondo K; Nicholson CK; Bradley JM; Islam KN; Calvert JW; Tao YX; Dugas TR; Kelley EE; Elrod JW; Huang PL; Wang R; Lefer DJ, Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 3182–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Kimura H, Production and physiological effects of hydrogen sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Zhao WM; Zhang J; Lu YJ; Wang R, The vasorelaxant effect of H2S as a novel endogenous gaseous K-ATP channel opener. Embo J. 2001, 20, 6008–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Jha S; Calvert JW; Duranski MR; Ramachandran A; Lefer DJ, Hydrogen sulfide attenuates hepatic ischemia-reperfusion injury: role of antioxidant and antiapoptotic signaling. Am. J. Physiol.-Heart Circul. Physiol. 2008, 295, H801–H806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hartle MD; Pluth MD, A practical guide to working with H2S at the interface of chemistry and biology. Chem. Soc. Rev. 2016, 45, 6108–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Powell CR; Dillon KM; Matson JB, A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Zhao Y; Biggs TD; Xian M, Hydrogen sulfide (H2S) releasing agents: chemistry and biological applications. Chem. Comm. 2014, 50, 11788–11805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Zhao Y; Henthorn HA; Pluth MD, Kinetic insights into hydrogen sulfide delivery from caged-carbonyl sulfide isomeric donor platforms. J. Am. Chem. Soc. 2017, 139, 16365–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Chauhan P; Bora P; Ravikumar G; Jos S; Chakrapani H, Esterase activated carbonyl sulfide/hydrogen sulfide (H2S) donors. Org. Lett. 2017, 19, 62–65. [DOI] [PubMed] [Google Scholar]
- (12).Zhao Y; Wang H; Xian M, Cysteine-activated hydrogen sulfide (H2S) donors. J. Am. Chem. Soc. 2011, 133, 15–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Feng S; Zhao Y; Xian M; Wang Q, Biological thiols-triggered hydrogen sulfide releasing microfibers for tissue engineering applications. Acta Biomater. 2015, 27, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Connal LA, The benefits of macromolecular hydrogen sulfide prodrugs. J. Mater. Chem. B 2018, 6, 7122–7128. [DOI] [PubMed] [Google Scholar]
- (15).Urquhart MC; Ercole F; Whittaker MR; Boyd BJ; Davis TP; Quinn JF, Recent advances in the delivery of hydrogen sulfide via a macromolecular approach. Polym. Chem. 2018, 9, 4431–4439. [Google Scholar]
- (16).Foster JC; Radzinski SC; Zou XL; Finkielstein CV; Matson JB, H2S-releasing polymer micelles for studying selective cell toxicity. Mol. Pharm. 2017, 14, 1300–1306. [DOI] [PubMed] [Google Scholar]
- (17).Hasegawa U; van der Vlies AJ, Design and synthesis of polymeric hydrogen sulfide donors. Bioconjugate Chem. 2014, 25, 1290–1300. [DOI] [PubMed] [Google Scholar]
- (18).Takatani-Nakase T; Katayama M; Matsui C; Hanaoka K; van der Vlies AJ; Takahashi K; Nakase I; Hasegawa U, Hydrogen sulfide donor micelles protect cardiomyocytes from ischemic cell death. Mol. Biosyst. 2017, 13, 1705–1708. [DOI] [PubMed] [Google Scholar]
- (19).Dobrovolskaia MA; Mcneil SE, Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007, 2, 469–478. [DOI] [PubMed] [Google Scholar]
- (20).Carter JM; Qian Y; Foster JC; Matson JB, Peptide-based hydrogen sulphide-releasing gels. Chem. Commun. 2015, 51, 13131–13134. [DOI] [PubMed] [Google Scholar]
- (21).Wang Y; Kaur K; Scannelli SJ; Bitton R; Matson JB, Self-assembled nanostructures regulate H2S release from constitutionally isomeric peptides. J. Am. Chem. Soc. 2018, 140, 14945–14951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kaur K; Qian Y; Gandour RD; Matson JB, Hydrolytic decomposition of S-aroylthiooximes: effect of pH and N-arylidene substitution on reaction rate. J. Org. Chem. 2018, 83, 13363–13369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Qian Y; Kaur K; Foster JC; Matson JB, Supramolecular tuning of H2S release from aromatic peptide amphiphile gels: effect of core unit substituents. Biomacromolecules 2019, 20, 1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Longchamp A; Kaur K; Macabrey D; Dubuis C; Corpataux J-M; Déglise S; Matson JB; Allagnat F, Hydrogen sulfide-releasing peptide hydrogel limits the development of intimal hyperplasia in human vein segments. Acta Biomater. 2019, DOI: 10.1016/j.actbio.2019.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Matson JB; Stupp SI, Self-assembling peptide scaffolds for regenerative medicine. Chem. Commun. 2012, 48, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wang Y; Cheetham AG; Angacian G; Su H; Xie LS; Cui HG, Peptide-drug conjugates as effective prodrug strategies for targeted delivery. Adv. Drug Deliv. Rev. 2017, 110, 112–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Cui HG; Webber MJ; Stupp SI, Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers 2010, 94, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Foster JC; Powell CR; Radzinski SC; Matson JB, S-aroylthiooximes: a facile route to hydrogen sulfide releasing compounds with structure-dependent release kinetics. Org. Lett. 2014, 16, 1558–1561. [DOI] [PubMed] [Google Scholar]
- (29).Xing Q; Zhang J; Xie Y; Wang Y; Qi W; Rao H; Su R; He Z, Aromatic motifs dictate nanohelix handedness of tripeptides. ACS Nano 2018, 12, 12305–12314. [DOI] [PubMed] [Google Scholar]
- (30).Lin LH; Qin HR; Huang JB; Liang H; Quan DP; Lu J, Design and synthesis of an AIE-active polymeric H2S-donor with capacity for self-tracking. Polym. Chem 2018, 9, 2942–2950. [Google Scholar]
- (31).Cheetham AG; Zhang PC; Lin YA; Lock LL; Cui HG, Supramolecular nanostructures formed by anticancer drug assembly. J. Am. Chem. Soc. 2013, 135, 2907–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lin R; Cheetham AG; Zhang PC; Lin YA; Cui HG, Supramolecular filaments containing a fixed 41% paclitaxel loading. Chem. Commun. 2013, 49, 4968–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Peng B; Chen W; Liu CR; Rosser EW; Pacheco A; Zhao Y; Aguilar HC; Xian M, Fluorescent probes based on nucleophilic substitution-cyclization for hydrogen sulfide detection and bioimaging. Chem.-Eur. J 2014, 20, 1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Muggia FM; Green MD, New anthracycline antitumor antibiotics. Crit. Rev. Oncol. Hematol. 1991, 11, 43–64. [DOI] [PubMed] [Google Scholar]
- (35).Scully RE; Lipshultz SE, Anthracycline cardiotoxicity in long-term survivors of childhood cancer. Cardiovas. Toxicol. 2007, 7, 122–128. [DOI] [PubMed] [Google Scholar]
- (36).Wang XY; Yang CT; Zheng DD; Mo LQ; Lan AP; Yang ZL; Hu F; Chen PX; Liao XX; Feng JQ, Hydrogen sulfide protects H9c2 cells against doxorubicin-induced cardiotoxicity through inhibition of endoplasmic reticulum stress. Mol. Cell. Biochem. 2012, 363, 419–426. [DOI] [PubMed] [Google Scholar]
- (37).Chegaev K; Rolando B; Cortese D; Gazzano E; Buondonno I; Lazzarato L; Fanelli M; Hattinger CM; Serra M; Riganti C; Fruttero R; Ghigo D; Gasco A, H2S-donating doxorubicins may overcome cardiotoxicity and multidrug resistance. J Med Chem 2016, 59, 4881–4889. [DOI] [PubMed] [Google Scholar]
- (38).Guo RM; Lin JC; Xu WM; Shen N; Mo LQ; Zhang CR; Feng JQ, Hydrogen sulfide attenuates doxorubicin-induced cardiotoxicity by inhibition of the p38 MAPK pathway in H9c2 cells. Int. J. Mol. Med. 2013, 31, 644–650. [DOI] [PubMed] [Google Scholar]
- (39).Guo R; Wu K; Chen J; Mo L; Hua X; Zheng D; Chen P; Chen G; Xu W; Feng J, Exogenous hydrogen sulfide protects against doxorubicin-induced inflammation and cytotoxicity by inhibiting p38MAPK/NFkB pathway in H9c2 cardiac cells. Cell. Physiol. Biochem. 2013, 32, 1668–1680. [DOI] [PubMed] [Google Scholar]
- (40).Kang J; Li Z; Organ CL; Park C-M; Yang C.-t.; Pacheco A; Wang D; Lefer DJ; Xian M, pH-controlled hydrogen sulfide release for myocardial ischemia-reperfusion injury. J. Am. Chem. Soc. 2016, 138, 6336–6339. [DOI] [PubMed] [Google Scholar]
- (41).Chen Y; Sun L; Wang Y; Zhao X, A dual-fluorescent whole-well imaging approach for screening active compounds against doxorubicin-induced cardiotoxicity from natural products. RSC Adv 2015, 5, 106431–106438. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.