Abstract
Background:
Race-based differences in efficacy for advanced NSCLC have not been studied due to under-representation of patients of minority background in pivotal trials. We explored real-world differences in outcome in our diverse patient population.
Methods:
We retrospectively analyzed clinical outcomes of patients with advanced NSCLC treated with single-agent immune checkpoint blockade (ICB) between 2013 and July 2018 at Winship Cancer Institute of Emory University. Primary efficacy comparison between Blacks and Whites was performed by bivariate and multivariate analyses for overall survival (OS) and progression free survival (PFS).
Results:
We analyzed data from 257 patients: The median age was 69 years old, 50.6% were female, Whites (W) /Blacks (B)/Other race made up 63.4%/29.5%/7.1% respectively. ICB was 1st line in 51 (19.9%), 2nd line in 161 (62.9%), 3rd line in 33 (12.9%). The most commonly used agents were nivolumab (49.0%), pembrolizumab (25.2%) and atezolizumab (21.3%). No difference in OS (p=0.839) and PFS (p=0.235) between B and W were seen. The sample ORR was 20.6% - 15.2% B and 23.1% for W. No differences in OS (p=0.0812) and PFS (p=0.176) between females and males were seen. The rate of immune-related adverse events (irAE) was similar in B and W (20.0% vs. 29.9%, P-value=0.148). On multivariate analysis, race was not significantly associated with OS or PFS.
Conclusions:
Real-world analysis of our institutional experience showed similar efficacy and tolerability of ICB in Black vs White advanced NSCLC patients. Larger multi-institutional studies to include other US minority populations would make our findings generalizable.
Keywords: Non-small cell lung cancer, immune checkpoint inhibition, racial outcomes, anti-PD1/PDL1, real-world data
PRECIS
We performed a retrospective study of overall survival (OS) and progression free survival (PFS) of non-small cell lung cancer patients treated with single agent immune checkpoint blockade in Atlanta, GA between 2013 to 2018. Our data shows similar outcomes between Blacks and Whites in terms of OS, PFS, and rate of immune-related adverse events. Large studies are needed to make our findings more generalizable.
INTRODUCTION:
Patients from minority racial background in the United States are equally likely as Caucasians to enroll in clinical trials1–3. However, actual participation rate on cancer trials reflect a pervasive racial disparity, with the overrepresentation of Whites and male participants in oncology trials conducted in the recent decades (80% and 59.8%, respectively)4,5,6. Despite regulatory and policy initiatives to address this challenge, only 2% of approximately 10,000 trials sponsored by the US National Cancer Institute included sufficient minority participants to meet the goals of those initiatives7,8. In addition, only 13.4% of published studies reported enrolment data by race or ethnicity, in spite of policy prescription for the FDA to report trial results in specific demographic subgroups9,10. The FDA’s Drug Trials Snapshots for 2018 showed a remarkable imbalance in participants in oncology trials that supported the regulatory approvals and marketing of 17 new drug entities. Out of a total of 5,157 clinical trial enrollees, the majority were males (62%) and White (68%). There was a good representation of Asians (15%) but Black or African American and Hispanic patients were underrepresented at 4% each11. However, more than half the US population will be a race other than non-Hispanic White by 2045 according to the projection by the US Census Bureau12.
Potential differences or similarities between US blacks and Caucasians with respect to the efficacy of immune checkpoint blockade (ICB) for advanced non-small cell lung cancer (NSCLC) have not been previously studied. This is primarily due to the fact that pivotal trials of ICB in NSCLC had an underrepresentation of patients of racial minority background13. In spite of this, ICB adoption in NSCLC was rapid among patients from all racial background despite limited inclusion in clinical trials and limited knowledge of treatment outcomes or toxicity profiles13, 14. Moreover, long and durable responses to ICB are seen only in a subset of treated patients, making the development of reproducible biomarkers of treatment response a critical need along with a better understanding of host differences across racial groups. The neutrophil-to-lymphocyte ratio (NLR) is one biomarker that has shown promise in this setting15.
In order to bridge this knowledge gap, we systematically explored potential differences in ICB efficacy in patients from the different racial backgrounds using clinical outcome data from our diverse lung cancer patient population where Blacks constitutes approximately 35.2%.
METHODS:
Patient Selection
We performed a retrospective review of the clinical outcome of patients with advanced or metastatic NSCLC treated between January 1st, 2013, and July 1st, 2018 with ICB alone for all lines of therapy at the Winship Cancer Institute of Emory University and its affiliated satellite medical oncology clinics. The data cutoff was April 15th, 2019. Patients were identified through the drug administration pharmacy database and associated ICD9 and ICD 10 codes C33.xx and CD34.xx. Unique ICB administration entries without an associated ICD code were also screened to capture all patients with lung cancer. Lung cancer patients should have received at least 1 dose of Anti-PD1/PD-L1 with or without Anti-CTLA-4 inhibitors to be included in our analytic dataset. Exclusion criteria included having received ICB as part of a combination therapy that included other classes of systemic therapy, having received more than 1 line of ICB, initiation of ICB at another institution, chronic use of prednisone>10mg, HIV-positive patients, or having an incomplete medical record. The study was conducted as part of an IRB-approved protocol for chart review and data collection.
Study Design
The patients’ baseline demographics, disease histology, and stage, ICB treatment start and end date, baseline complete blood count at ICB treatment initiation and adverse events were all recorded from the electronic medical record.
The analysis focused on NSCLC with advanced or metastatic disease on ICB alone. Small cell lung cancer patients were not included in the analysis given their distinct biology and small number in our sample. We also excluded those who were treated with durvalumab as consolidation therapy after concurrent chemo-radiation for Stage III NSCLC.
As primary outcomes, the overall survival (OS) and progression free survival (PFS) according to self-reported race [White/Caucasian (Whites) vs. Black/African American (Blacks)] and of OS and PFS according to gender were measured. OS was assessed from the time of ICB initiation to death or censoring at the last clinic visit. PFS was defined as the time between first ICB dose and restaging radiological scan demonstrating progressive disease or death. The response rate followed RECIST criteria and was based on radiological findings along with the documented interpretation of the oncology provider. The outcome was censored if the patient was lost to follow up. As a secondary analysis, the rate of adverse events were retrieved using the Common Terminology of Adverse Events v.4.0. The severity of immune-related AEs (irAEs) were also compared by gender and race.
A proxy to income level was calculated using the median household income at the ZIP code of residence obtained from the US census American Community Survey (ACS) 2013–2017 5-year estimate. Median household income was categorized by tertile (low/medium or high) according to Georgia state distribution of ZIP codes16.
Statistical Analysis
Demographic and clinical characteristics were summarized using descriptive statistics. The Kaplan-Meier method was used to estimate OS and PFS probabilities and were compared by patient characteristics using the log-rank test. We performed bivariate and multivariate analyses to assess factors associated with OS and PFS using the cox proportional hazards model. Multivariate models were selected using backward model selection with an alpha of removal of 0.2. Hazard ratios with 95% confidence intervals (CIs) were generated in the two models. A p-value of <0.05 was considered statistically significant for all analyses. All analyses were conducted using SAS Version 9.4 and SAS macros developed by the Biostatistics and Bioinformatics Shared Resource at Winship Cancer Institute in Atlanta, Georgia17.
RESULTS:
Patient and tumor characteristics
Overall, the study had a total of 257 patients with the following characteristics: mean age of 67.5 years, females 50.6%, Whites 63%, and Blacks 29.2% (Table 1). 40.6% had an ECOG functional status of 0–1. The vast majority were current or former smokers (83.8%). The majority of patients had Medicare insurance (60.9%) and 15 patients (5.8%) were uninsured. Adenocarcinoma was the most common histology, forming 65% of patients. Of those who had PD-L1 status information, close to half (49%) had high PD-L1 expression >=50%. The majority of patients had advanced disease (Stage III 19.8% and Stage IV 67.8%) at presentation and 80.2% received ICB as second-line or more. Brain metastases were present in 35.7% of patients at ICB initiation. Nivolumab was the most commonly used agent (49.2%) followed by pembrolizumab (25.2%) and atezolizumab (21.3%). Supplementary Table 1 outlines the baseline demographic and clinical characteristics according to race. There were no statistically significant differences in PD-L1 expression levels between Blacks and Whites (parametric p=0.491 by Chi-square test).
Table 1:
Baseline demographic and clinical characteristics of patients with advanced or metastatic Non-Small cell Lung Cancer (NSCLC)
| Variable | Level | N (%) = 257 |
|---|---|---|
| Race | Black/African American | 75 (29.5) |
| Other | 18 (7.1) | |
| White/Caucasian | 161 (63.4) | |
| Missing | 3 | |
| Sex | Female | 130 (50.6) |
| Male | 127 (49.4) | |
| ECOG Functional Status | 0 | 18 (9.4) |
| 1 | 84 (43.8) | |
| 2 | 68 (35.4) | |
| 3 | 21 (10.9) | |
| 4 | 1 (0.5) | |
| Missing | 65 | |
| Income | Low/Middle Income | 90 (38.8) |
| High Income | 142 (61.2) | |
| Missing | 25 | |
| Insurance | Medicaid | 9 (3.6) |
| Medicare | 156 (63.2) | |
| Private | 67 (27.1) | |
| Uninsured | 15 (6.1) | |
| Missing | 10 | |
| Histology | Adenocarcinoma NSCLC | 165 (65.2) |
| Adenosquamous NSCLC | 3 (1.2) | |
| Large cell NSCLC | 5 (2.0) | |
| NOS | 17 (6.7) | |
| SCLC | 18 (7.1) | |
| Squamous NSCLC | 45 (17.8) | |
| Missing | 4 | |
| PD-L1 expression level | 1–49% | 20 (20.8) |
| <1% | 29 (30.2) | |
| >=50% | 47 (49.0) | |
| Missing | 161 | |
| Body Mass Index | Underweight/Normal Weight | 132 (52.6) |
| Overweight | 73 (29.1) | |
| Obese | 46 (18.3) | |
| Missing | 6 | |
| Brain metastasis at treatment initiation | No | 154 (64.2) |
| Yes | 86 (35.8) | |
| Missing | 17 | |
| Liver metastasis at treatment initiation | No | 192 (83.1) |
| Yes | 39 (16.9) | |
| Missing | 26 | |
| Neutrophil to lymphocyte ratio at treatment initiation | <5 | 122 (51.7) |
| >=5 | 114 (48.3) | |
| Missing | 21 | |
| EGFR status | Negative | 149 (89.8) |
| Positive | 17 (10.2) | |
| Missing | 91 | |
| ALK status | Negative | 159 (98.1) |
| Positive | 3 (1.9) | |
| Missing | 95 | |
| TP53 status | Negative | 56 (55.4) |
| Positive | 45 (44.6) | |
| Missing | 156 | |
| ICB line | 1 | 51 (19.9) |
| 2 | 161 (62.9) | |
| 3 | 33 (12.9) | |
| 4 | 11 (4.3) | |
| Missing | 1 | |
| ICB agent | Atezolizumab | 55 (21.4) |
| Nivolumab + Ipilimumab | 11 (4.3) | |
| Nivolumab | 126 (49.0) | |
| Pembrolizumab | 65 (25.3) | |
| Median | 69 | |
| Minimum | 34 | |
| Maximum | 90 | |
| Std Dev | 9.52 | |
| Missing | 0 |
Efficacy of ICB by Race
When looking at the overall population, the median OS for the entire NSCLC population was not reached (NR) (95% CI: 30.2, NR), with 12-month survival 70.2% (95% CI: 63.9%, 75.6%) and 24-month survival 57.7% (95% CI: 50.6%, 64.1%) at the time of data cutoff. The median PFS was 5.9 months (95% CI: 4.7, 8.6) and the median follow up time was 15.4 months. The ORR was 20.6% for the entire population, 15.2% for Blacks and 23.1% for Whites. The median duration of response was 3.24 months for the entire population, 3.24 months in blacks and 3.18 months in Whites. Our analysis showed no OS difference between Blacks and Whites (Figure 1A) with non-significant log rank p-value of 0.839 and comparable 12-month survival (66.2% Blacks 70.0% Whites) and 24-month (58.2% Blacks; 54.2% Whites). Similarly, there were no statistically significant differences in PFS between the two races (log rank p=0.235) with a noted numerically shorter PFS in Blacks of 4.4 (95% CI: 2.5, 16.8) compared with 7.7 months (95% CI: 5.4, 10.7) in Whites (Figure 1B). There were comparable 12-month PFS (39.6% Blacks; 40.2% Whites) and 24-month PFS (30.1% Blacks; 32.9% Whites) between the two groups. The censoring rates were similar for OS (60% Blacks; 56% Whites) and PFS (34% Blacks; 37% Whites) regardless of a racial group.
Figure 1A:
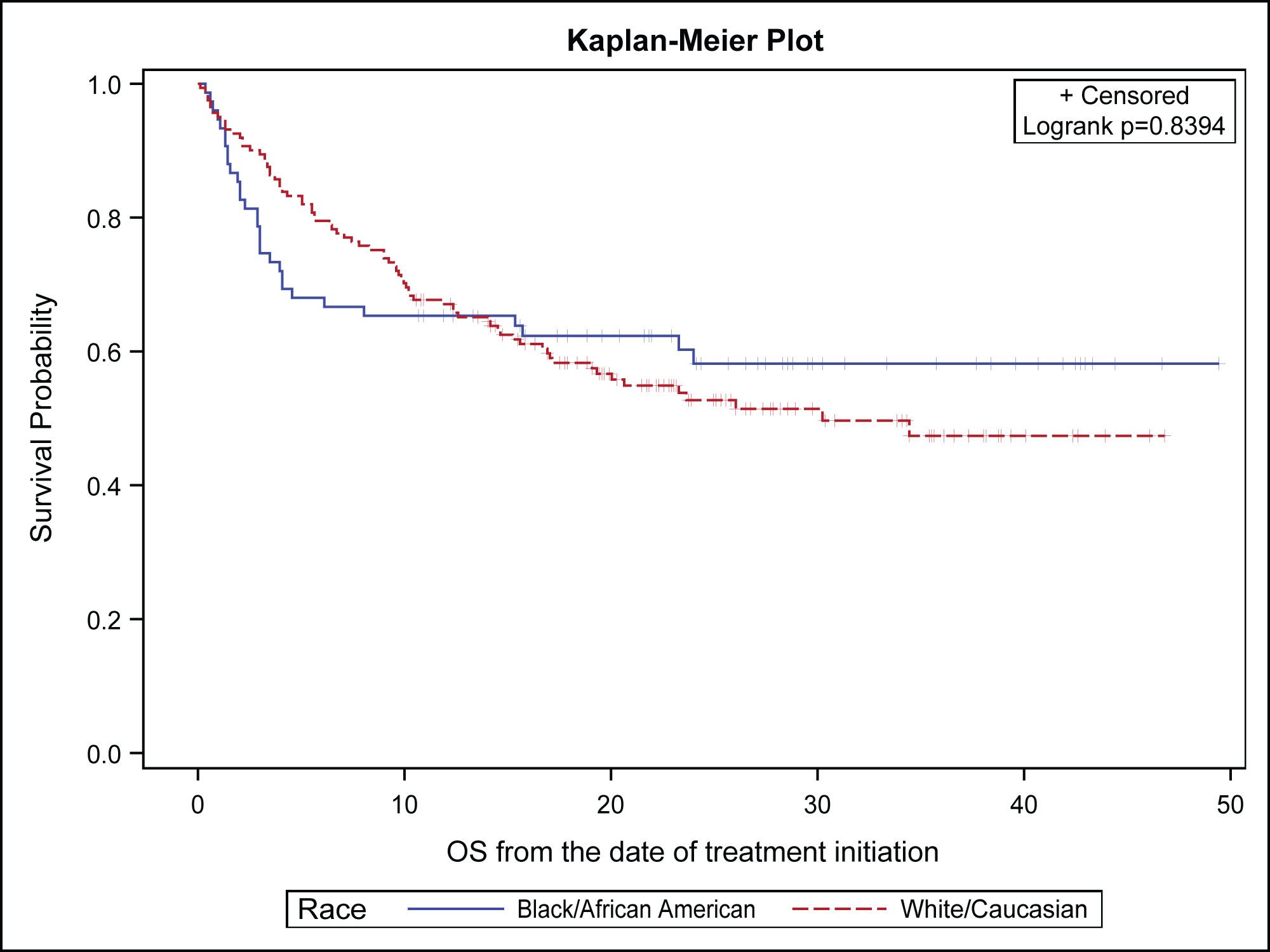
Overall survival of non-small cell lung cancer patients by race
Figure 1B:
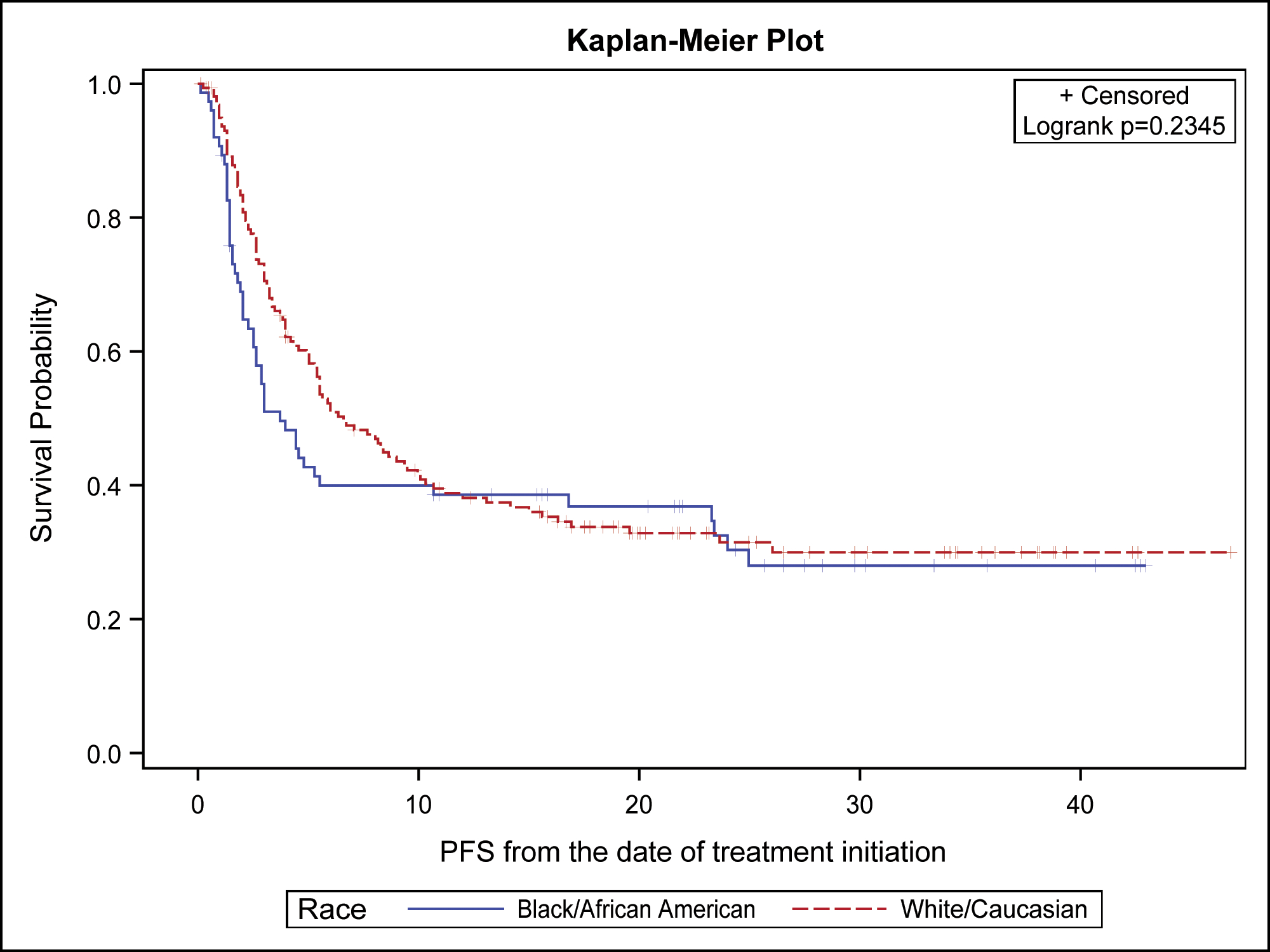
Progression free survival of non-small cell lung cancer patients by race
Efficacy of ICB by Gender
Analysis of the gender difference in clinical outcomes for NSCLC patients treated with ICB showed no statistically significant differences in OS (p=0.0812) and PFS (p=0.176) between females and males. However, there was a noticeable trend in favor of females with respect to OS (Figure 2A) and PFS (Figure 2B). Landmark analysis for OS rates at 12 and 24 months showed a trend in favor of females (73.5% vs. 63.7%) and (60.0% vs. 49.6%) respectively. Similarly, the median PFS was 9 months (95% CI: 4.4, 23.6) for females and 5.9 months (95% CI: 4, 8.3) for males (p=0.176). The 12-month PFS rate was 45.8% for females versus 33.3 % for males while the 24-month PFS rate was 36.9% for females vs. 25.4% for males. Importantly, there were more females censored for both PFS and OS events at the time of the primary data analysis, 63% vs. 49% for OS; and 41% vs. 31% for PFS. When limiting the analysis to females, we found no statistically significant bivariate association with race of OS [HR 0.98 (95% CI: 0.54–1.78), log-rank p-value=0.936] and PFS [HR 1.32 (95% CI: 0.82–2.10), log-rank p-value=0.246] between Black females vs. White females.
Figure 2A:
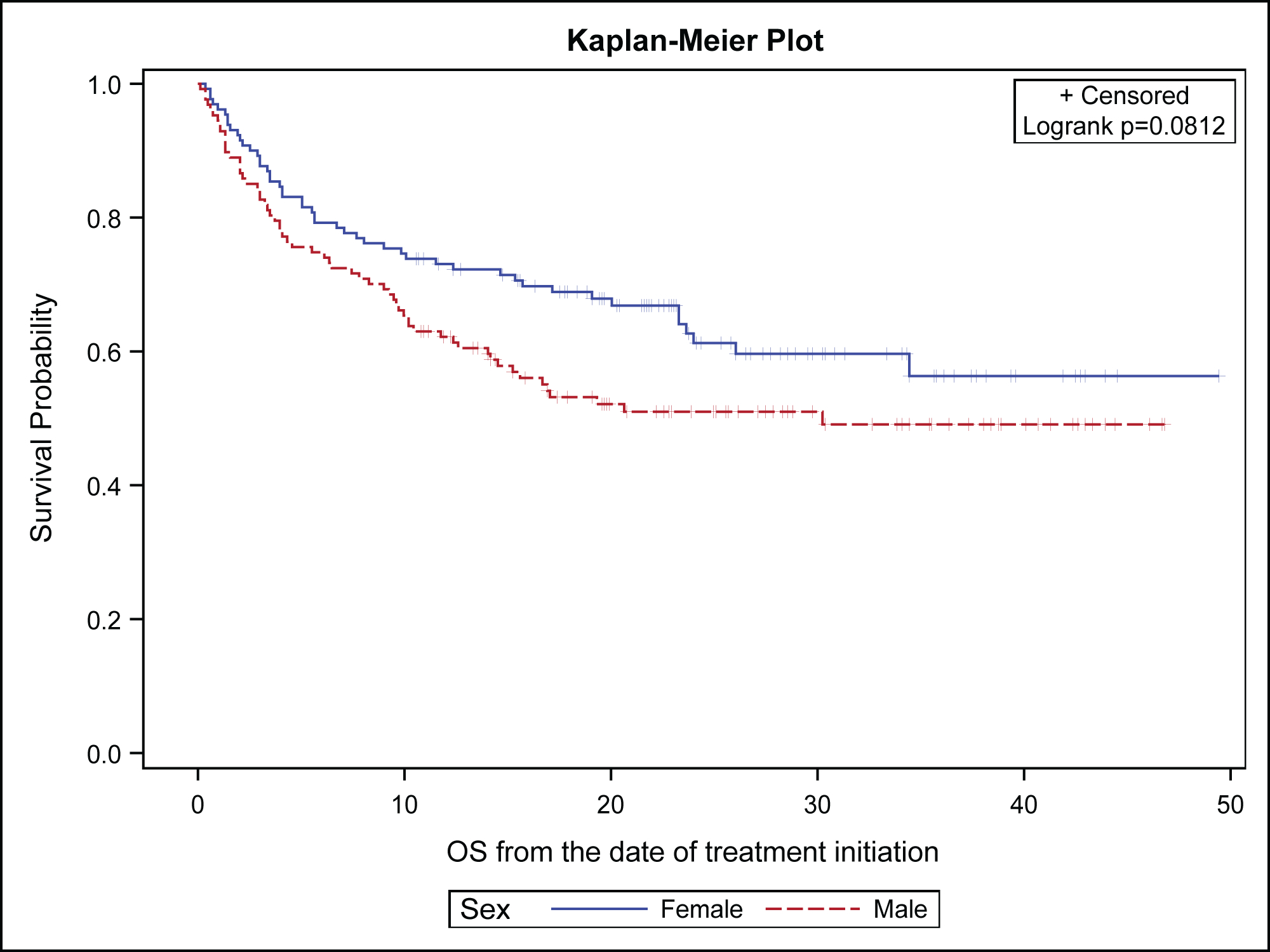
Overall survival of non-small cell lung cancer patients by gender
Figure 2B:
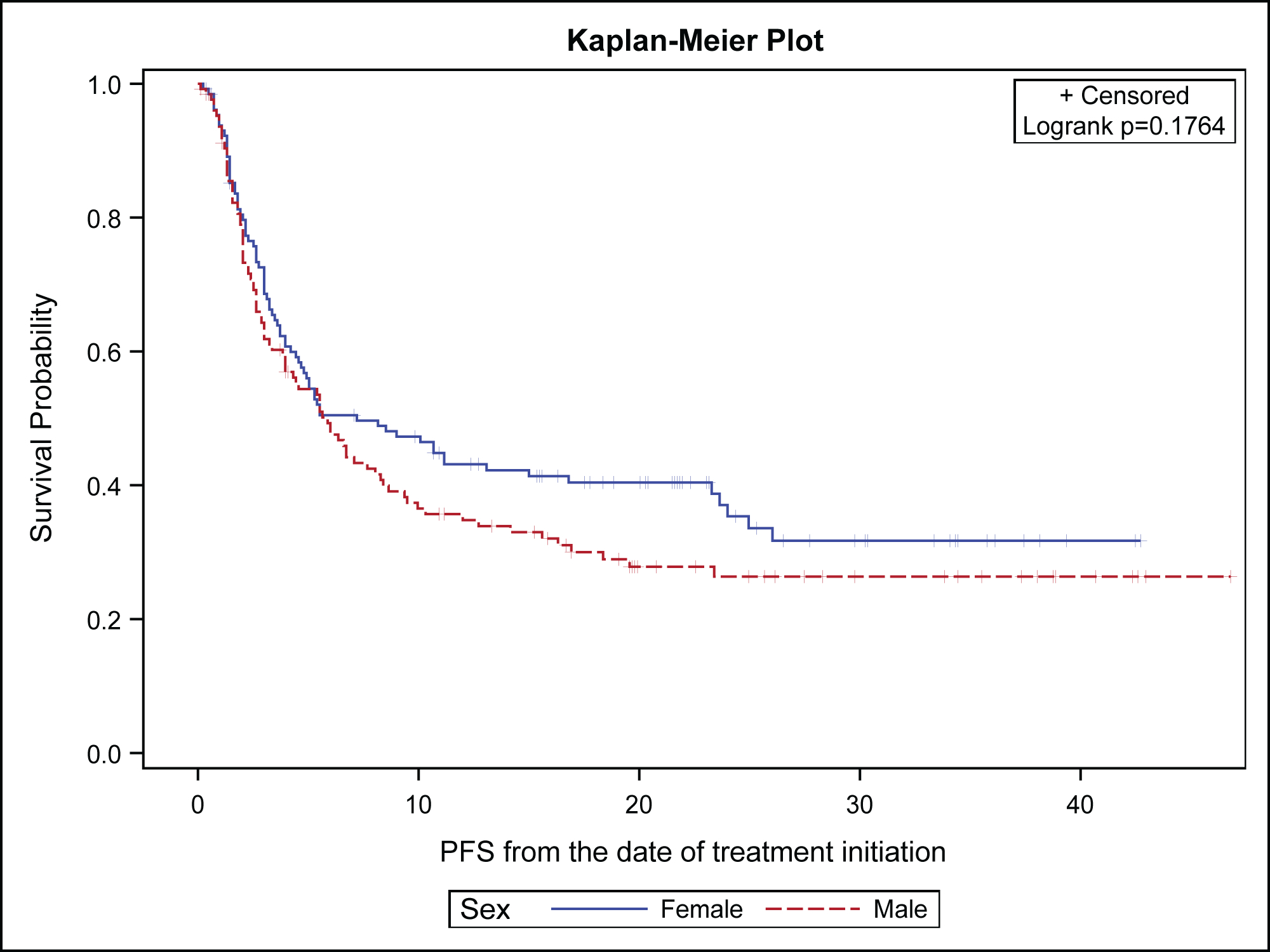
Progression Free Survival of non-small cell lung cancer patients by gender
Bivariate and multivariate regression of overall survival and progression-free survival
Table 2 displays the unadjusted and adjusted association of OS with demographic and clinical characteristics. At the bivariate level, the risk of death was 38% lower among patients with non-squamous histology compared to patients with squamous histology [HR 0.62 (95% CI: 2.5, 16.8), log-rank p-value=0.042] and 33% lower among patients with NLR<5 compared to those with NLR ≥ 5 [HR 0.67 (95% CI 0.46,1.00), log-rank p-value=0.048]. Females had a trend to better OS than males [HR 0.70 (95% CI 0.48, 1.03)], but the association was not statistically significant (log-rank p-value=0.066). All other demographic and clinical characteristics including race were not significantly associated with OS at the bivariate level. In the multivariate model (including sex, ECOG functional status, income level, insurance status, histology, PD-L1 status, and NLR), OS was statistically significantly associated with sex [HR 0.12 (95% CI: 0.04,0.40) for female vs. male], ECOG [lower risk of death for ECOG 0, 1 and 2 compared to 3, p-value=0.002], insurance type [HR 0.08 (95% CI: 0.01–0.56) for government versus private insurance], and NLR [HR 0.05 (95% CI: 0.00–0.91) for NLR <5 versus NLR ≥5].
Table 2:
Bivariate and multivariate association of overall survival with demographic and clinical characteristics in advanced or metastatic Non-Small Cell Lung Cancer (N=257)
| Bivariate model | Multivariate model | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Level | N | Hazard Ratio (95% CI) | HR P-value |
Log-rank P-value |
Hazard Ratio (95% CI) | HR P-value |
Log-rank P-value |
| Race | Black/African American | 75 | 0.90 (0.59–1.37) | 0.62 | 0.133 | - | - | - |
| Other | 18 | 0.33 (0.10–1.04) | 0.059 | - | - | |||
| White/Caucasian | 161 | Ref | ref | - | - | |||
| Sex | Female | 130 | 0.70 (0.48–1.03) | 0.068 | 0.066 | 0.12 (0.04–0.40) | <.001 | <.001 |
| Male | 127 | Ref | ref | ref | ref | |||
| ECOG | 0 | 18 | 0.13 (0.01–1.16) | 0.067 | 0.077 | 0.13 (0.02–0.70) | 0.018 | 0.002 |
| 1 | 84 | 0.23 (0.03–1.70) | 0.15 | 0.05 (0.01–0.22) | <.001 | |||
| 2 | 68 | 0.35 (0.05–2.55) | 0.298 | 0.16 (0.04–0.62) | 0.008 | |||
| 3 | 21 | 0.39 (0.05–3.01) | 0.363 | ref | ref | |||
| 4 | 1 | Ref | ref | - | - | - | ||
| Income | Low/Middle Income | 90 | 1.12 (0.75–1.66) | 0.584 | 0.583 | 2.70 (0.92–7.97) | 0.072 | 0.072 |
| High Income | 142 | Ref | ref | ref | ref | |||
| Insurance | Government Insurance | 165 | 0.84 (0.55–1.29) | 0.434 | 0.432 | 0.08 (0.01–0.56) | 0.01 | 0.01 |
| Private | 67 | Ref | ref | ref | ref | |||
| Histology (NSCLC) | Non-Squamous NSCLC | 190 | 0.62 (0.39–0.99) | 0.044 | 0.042 | 2.34 (0.68–8.06) | 0.176 | 0.176 |
| Squamous NSCLC | 45 | Ref | ref | ref | ref | |||
| PD-L1 Status | <1% | 29 | 1.99 (0.95–4.20) | 0.069 | 0.11 | 2.58 (0.78–8.56) | 0.121 | 0.05 |
| 1–49% | 20 | 0.89 (0.32–2.47) | 0.818 | 0.45 (0.12–1.69) | 0.236 | |||
| >=50% | 47 | Ref | ref | ref | ref | |||
| Body Mass Index (BMI) | Underweight/Normal Weight | 132 | 0.99 (0.59–1.64) | 0.959 | 0.994 | - | - | - |
| Overweight | 73 | 1.01 (0.57–1.78) | 0.972 | - | - | |||
| Obese | 46 | Ref | ref | - | - | |||
| Brain metastasis | No | 154 | 1.14 (0.75–1.72) | 0.541 | 0.539 | - | - | - |
| Yes | 86 | Ref | ref | - | - | |||
| Liver metastasis | No | 192 | 1.16 (0.67–2.00) | 0.606 | 0.604 | - | - | - |
| Yes | 39 | Ref | ref | - | - | |||
| NLR | <5 | 122 | 0.67 (0.46–1.00) | 0.05 | 0.048 | 0.05 (0.00–0.91) | 0.043 | 0.043 |
| >=5 | 114 | Ref | ref | ref | ref | |||
| Age | 257 | 1.01 (0.99–1.03) | 0.364 | - | 1.06 (1.00–1.14) | 0.065 | 0.065 | |
Number of observations in the original data set = 257. Number of observations used = 56.
Backward selection with an alpha level of removal of .2 was used. The following variables were removed from the model: Brain Metastasis, Liver Metastasis, Race, and BMI
Table 3 displays the results of bivariate and multivariate models for PFS. PFS was significantly associated with higher PD-L1 status (Log-rank p-value=0.02), absence of liver metastasis (Log-rank p-value=0.004), and lower ECOG. The multivariate model included race, sex, ECOG, insurance type, PD-L1 status, and NLR. The risk of disease progression or death was significantly lower in females compared to males [HR 0.34 (95% CI: 0.16–0.75], in patients with NLR <5 versus NLR ≥5 [HR 0.31 (95% CI (0.14–0.69)], and in patients with ECOG 0, 1, or 2 compared to 3 (Log-rank p-value=0.022). Patients with PD-L1 less than 1% had more than 5 times the risk of progression than those with PD-L1 of 50% or higher [HR 5.38 (95% CI: 2.30–12.59), p-value<0.001]
Table 3:
Bivariate and multivariate association of progression-free survival with demographic and clinical characteristics in sample of patients with advanced or metastatic NSCLC (N=257)
| Bivariate model | Multivariate model | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Level | N | Hazard Ratio (95% CI) | HR P-value |
Log-rank P-value |
Hazard Ratio (95% CI) | HR P-value |
Log-rank P-value |
| Race | Black/African American | 75 | 1.17 (0.84–1.64) | 0.357 | 0.386 | 2.70 (1.08–6.74) | 0.033 | 0.077 |
| Other | 18 | 0.76 (0.40–1.45) | 0.402 | 0.66 (0.14–3.15) | 0.604 | |||
| White/Caucasian | 161 | - | - | - | - | |||
| Sex | Female | 130 | 0.82 (0.60–1.10) | 0.188 | 0.184 | 0.34 (0.16–0.75) | 0.007 | 0.007 |
| Male | 127 | - | - | - | - | |||
| ECOG | 0 | 18 | 0.08 (0.01–0.68) | 0.02 | 0.098 | 0.07 (0.01–0.83) | 0.035 | 0.022 |
| 1 | 84 | 0.13 (0.02–0.97) | 0.047 | 0.05 (0.00–0.53) | 0.013 | |||
| 2 | 68 | 0.12 (0.02–0.94) | 0.043 | 0.04 (0.00–0.45) | 0.009 | |||
| 3 | 21 | 0.13 (0.02–1.04) | 0.055 | 0.15 (0.01–1.84) | 0.14 | |||
| 4 | 1 | - | - | - | - | |||
| Income | Low/Middle Income | 90 | 1.07 (0.77–1.48) | 0.682 | 0.679 | - | - | - |
| High Income | 142 | - | - | - | - | |||
| Insurance | Government Insurance | 165 | 0.89 (0.63–1.27) | 0.528 | 0.525 | 0.45 (0.19–1.07) | 0.071 | 0.071 |
| Private | 67 | - | - | - | - | |||
| Histology (NSCLC) | Non-Squamous NSCLC | 190 | 0.77 (0.52–1.15) | 0.207 | 0.203 | - | - | - |
| Squamous NSCLC | 45 | - | - | - | - | |||
| PD-L1 Status | <1% | 29 | 2.25 (1.25–4.05) | 0.007 | 0.02 | 5.38 (2.30–12.59) | <.001 | <.001 |
| 1–49% | 20 | 1.61 (0.80–3.27) | 0.183 | 2.26 (0.86–5.93) | 0.098 | |||
| >=50% | 47 | - | - | - | - | |||
| BMI | Underweight/Normal Weight | 132 | 0.95 (0.62–1.45) | 0.805 | 0.34 | - | - | - |
| Overweight | 73 | 1.22 (0.77–1.94) | 0.39 | - | - | |||
| Obese | 46 | - | - | - | - | |||
| Brain metastasis | No | 154 | 1.09 (0.78–1.51) | 0.625 | 0.622 | - | - | - |
| Yes | 86 | - | - | - | - | |||
| Liver metastasis | No | 192 | 0.56 (0.37–0.84) | 0.005 | 0.004 | - | - | - |
| Yes | 39 | - | - | - | - | |||
| NLR | <5 | 122 | 0.92 (0.67–1.25) | 0.583 | 0.582 | 0.31 (0.14–0.69) | 0.004 | 0.004 |
| >=5 | 114 | - | - | - | - | |||
| Age | 257 | 1.00 (0.98–1.02) | 0.793 | - | - | - | - | |
Number of observations in the original data set = 257. Number of observations used = 62;
Backward selection with an alpha level of removal of 0.2 was used.
The following variables were removed from the model: Age, Brain Metastasis, Liver Metastasis, BMI, Histology (NSCLC), and Income
Immune-related adverse events by race and gender
Of 257 patients in our study, 72 (27.8%) had reported treatment-related adverse events, the most common organ system was pulmonary (25), followed by constitutional (18), gastrointestinal (11), and dermatological (7). Grade ≥3 adverse event was recorded in 54 of these 72 patients, where 44% (24/54) had Grade 3 and 25.9% (14/54) had Grade 4. Overall, withholding treatment for irAE was required in 47 patients (18%) with 18 of those cessations being permanent. There were no death attributed to ICB treatment. Blacks had a numerically lower incidence of irAEs on treatment than Whites (20.0% vs. 29.9%), a difference that was not statistically significant (parametric p=0.148 by Chi-square test). We also found no statistically significant difference in the rate of Grade 1 or 2 irAEs vs. Grade 3 or 4 between Blacks and Whites (parametric p=0.733 by Chi-square test). Our analysis showed no association between gender and irAEs (parametric p=0.148 on Chi-square test).
DISCUSSION
Our real-world study evaluates the impact of race and gender on ICBs outcomes in patients with advanced or metastatic NSCLC. The results show that Blacks and Whites derive similar benefit from ICBs with a comparable incidence of irAEs. This was consistent after adjusting for a large number of demographic and clinical characteristics. To our knowledge, our cohort of Black patients with advanced NSCLC treated with ICB (N=75) is the largest to date. Comparison of the retrospective results for Blacks only from our study to pivotal NSCLC Phase III trials is limited by the very low enrollment of such patients in those trials (e.g.: CheckMate 057: 16 of 533 participants were Black18; KEYNOTE 010: 13 out of 24619; OAK trial: 16 out of 59820).
The ORR in our study (20.6%) – with nivolumab second line being the most commonly used ICB - is reassuringly similar to the one reported in Checkmate 057 of 19%18. The absence of differences in outcomes between Black and White NSCLC patients treated with ICB compares favorably to known worse outcome in NSCLC chemotherapy treatment for Blacks versus Whites and generally worse cancer-related outcomes in minority patients21. Interestingly, the FDA analyzed 3,399 Asian advanced NSCLC patients (a group with generally better outcomes than non-Asians) receiving ICB in randomized trials and found no better or worse benefit for ICB when compared to chemotherapy22, raising the possibility that factors unique to Blacks might explain our findings. Investigators have previously postulated a potentially greater benefit of ICB in Blacks based on a higher prevalence of smoking and higher mutational burden23. In light of the very low enrollment of Blacks in the pivotal trials, our data provides evidence to the treating clinical that Whites and Blacks have a similar benefit to ICB in advanced NSCLC.
Blacks had no statistically significant difference in tolerability to ICB in our cohort compared to Whites while noting that the incidence of irAEs was numerically lower (20.0% in Blacks vs. 29.9% in Whites). Among the irAEs seen in Blacks, the severity of those events are similar compared to Whites. The incidence of Grade 3 or more irAEs in our study (13.3%) is comparable to a recent real-world retrospective review of Black patients on ICB by Shah et al. (N=94, Grade 3 or more toxicities incidence 8%)24 and to the pivotal clinical trials. Therefore, our findings suggest a favorable benefit-to-risk ratio of ICB for advanced or metastatic NSCLC in Blacks.
In our study, females had a trend towards better OS and PFS that did not reach statistical significance on Kaplan-Meier analysis although gender was a significant predictor of OS and PFS on the multivariate analyses. The results might be due to higher rates of censoring among females in our sample. Whether true and meaningful gender differences exist in NSCLC response to ICB remains a subject to debate. In one recent meta-analysis on sex heterogeneity of outcomes of lung cancer immunotherapy, males had better outcomes than females for anti-PD-1 alone (pooled ratio of OS-HRs in women vs. men was 0.83 (95% (0.65 to 1.06), p=0.002)25. However, another large meta-analysis of 23 ICB clinical trials in advanced cancers with 9,322 men and 4,399 women found no statistically significant difference between sexes for OS (P=0.60)26.
The study has a number of limitations. It is a retrospective review from a single cancer institute network and might not be generalizable to other settings. Potential heterogeneity of practice sites within the network and self-reported nature of race are common challenges in real-world racial disparity studies. Because not all patients were tested or had available mutational profile, the driver mutation status was not included in the multivariate analysis. The NLR analysis is limited by the absence of collected NLR 2 weeks post-treatment, which precludes calculating delta NLR15. The adverse events and comorbidities are likely underestimated given the heterogeneity in how they were documented by the provider. Larger datasets would be needed to investigate some of our non-statistically significant findings (e.g. numerically shorter median PFS in Blacks with comparable 12-month and 24-month PFS and numerically lower incidence of irAEs in Blacks compared to Whites). Furthermore, our study had a very small number of Hispanic and Asian patients.
Despite the limitations, our study has several points of strength. The real-world dataset has a large minority population (N=75 Blacks patients) that is substantially larger than the number of Black patients in the pivotal clinical trials. The patient population those trials is homogenous in terms of insurance status, socio-economics, and geographic region in the US. The bivariate and multivariate analyses adjusted for a large number of clinical and demographic factors. The previously mentioned similarities of ORR and irAEs with published studies support the validity of our methods. Further, the results of the bivariate and multivariate analyses for OS and PFS in our dataset are consistent with well-established factors of better ICB outcomes, including high PD-L1 expression, low NLR15, and better ECOG functional status.
Our work opens the door for future studies aimed at maximizing the clinical benefits of ICB in Blacks and achieving equity among all racial groups. It remains unclear whether the similar OS and PFS benefits between Blacks and Whites in our study will extend to when ICB are combined with platinum-based chemotherapy. This is important as fewer patients at our institution had received platinum-based chemotherapy plus ICB in the time duration of the study (Jan 2013-July 2018) than would today based on the results of KEYNOTE-18927, KEYNOTE 40728, and IMPower 15029. Further, elucidating whether consolidation durvalumab therapy in Stage III NSCLC is equally effective in different racial groups will provide further insight into this topic.
CONCLUSION:
Real-world analysis of our institutional experience showed no significant racial disparity of Blacks vs. Whites in OS, PFS, and safety of NSCLC patients treated with single agent ICB. Larger multi-institutional prospective studies are needed to include other US minority populations and to general data that supports the diverse patient population seen in the clinic. This would make our findings generalizable.
Supplementary Material
Acknowledgements:
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding:
The study received no specific funding.
Footnotes
Conflict of interest statement:
SSR served as consultant for Amgen, Abbvie, BMS, Genentech/Roche, Merck, AstraZeneca, and Takeda, and received grants from Tesaro, Merck, AstraZeneca, Advaxis, BMS, Amgen, Takeda. TKO has been on the paid advisory board BMS, AstraZeneca, and Merck, and served as paid consultant (data monitoring board) for Roche/Genentech and EMD Serono. The rest of the authors have no conflict of interest to disclose.
REFERENCES
- 1.Katz RV, Kegeles SS, Kressin NR, et al. The Tuskegee Legacy Project: willingness of minorities to participate in biomedical research. J Health Care Poor Underserved. 2006;17: 698–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz RV, Green BL, Kressin NR, Claudio C, Wang MQ, Russell SL. Willingness of minorities to participate in biomedical studies: confirmatory findings from a follow-up study using the Tuskegee Legacy Project Questionnaire. J Natl Med Assoc. 2007;99: 1052–1060. [PMC free article] [PubMed] [Google Scholar]
- 3.Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrero S, López-Cortés A, Indacochea A, et al. Analysis of Racial/Ethnic Representation in Select Basic and Applied Cancer Research Studies. Sci Rep. 2018;8: 13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwiatkowski K, Coe K, Bailar JC, Swanson GM. Inclusion of minorities and women in cancer clinical trials, a decade later: Have we improved? Cancer. 2013;119: 2956–2963. [DOI] [PubMed] [Google Scholar]
- 6.American Cancer Society. Cancer Facts & Figures 2018. Atlanta, GA: American Cancer Society, 2018. [Google Scholar]
- 7.Health NIo. NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research. Available from URL: https://grants.nih.gov/grants/funding/women_min/guidelines.htm [accessed January 22, 2019].
- 8.Chen MS Jr., Lara PN, Dang JH, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120 Suppl 7: 1091–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geller SE, Koch AR, Roesch P, Filut A, Hallgren E, Carnes M. The More Things Change, the More They Stay the Same: A Study to Evaluate Compliance With Inclusion and Assessment of Women and Minorities in Randomized Controlled Trials. Acad Med. 2018;93: 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA Safety and Innovation Act of 2012, 2012.
- 11.Woodcock Janet, Anagnostiadis E, Lolic M 2018. DRUG TRIALS SNAPSHOTS SUMMARY REPORT2018.
- 12.Vespa J, Armstrong DM, Medina L. Demographic Turning Points for the United States: Population Projections for 2020 to 2060. Current Population Reports. P25–1144, U.S. Census Bureau, Washington, DC, 2018. [Google Scholar]
- 13.Nazha B, Mishra M, Pentz R, Owonikoko TK. Enrollment of Racial Minorities in Clinical Trials: Old Problem Assumes New Urgency in the Age of Immunotherapy. American Society of Clinical Oncology Educational Book. 2019: 3–10. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor JM, Fessele KL, Steiner J, et al. Speed of Adoption of Immune Checkpoint Inhibitors of Programmed Cell Death 1 Protein and Comparison of Patient Ages in Clinical Practice vs Pivotal Clinical Trials. JAMA Oncol. 2018;4: e180798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park W, Lopes G. Perspectives: Neutrophil-to-lymphocyte Ratio as a Potential Biomarker in Immune Checkpoint Inhibitor for Non–Small-Cell Lung Cancer. Clinical lung cancer. 2019;20: 143–147. [DOI] [PubMed] [Google Scholar]
- 16.Bureau USC. Median household income in the past 12 months (in 2017 inflation-adjusted dollars). Available from URL: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_17_5YR_B19013&prodType=table. [accessed September 1, 2019].
- 17.Zhang YLDCNC, Kowalski JMSJ. Carrying out streamlined routine data analyses with reports for observational studies: introduction to a series of generic SAS® macros [version 2; peer review: 2 approved] F1000Research. 2019;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 20.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aizer AA, Wilhite TJ, Chen MH, et al. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014;120: 1532–1539. [DOI] [PubMed] [Google Scholar]
- 22.Chang E, Gong Y, Vallejo JJ, et al. FDA analysis of outcomes in Asian patients (pts) with metastatic non-small cell lung cancer (mNSCLC) receiving immune checkpoint inhibitors (ICI): American Society of Clinical Oncology, 2019. [Google Scholar]
- 23.Choudhury NJ, Eghtesad M, Kadri S, et al. Fewer actionable mutations but higher tumor mutational burden characterizes NSCLC in black patients at an urban academic medical center. Oncotarget. 2019;10: 5817–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah NJ, Blackburn M, Cook MR, et al. Real-world outcomes of underrepresented patient populations treated with immune checkpoint inhibitors (ICIs): African American descent, poor ECOG performance status, and chronic viral infections: American Society of Clinical Oncology, 2019. [Google Scholar]
- 25.Conforti F, Pala L, Bagnardi V, et al. Sex-based heterogeneity in response to lung cancer immunotherapy: a systematic review and meta-analysis. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallis CJD, Butaney M, Satkunasivam R, et al. Association of Patient Sex With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Advanced Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New England Journal of Medicine. 2018;378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 28.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2018;379: 2040–2051. [DOI] [PubMed] [Google Scholar]
- 29.Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. New England Journal of Medicine. 2018;378: 2288–2301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


