Abstract
Background
Severe combined immunodeficient (SCID) pigs are an emerging animal model being developed for biomedical and regenerative medicine research. SCID pigs can successfully engraft human induced pluripotent stem cells and cancer cell lines. The development of a humanized SCID pig through xenotransplantation of human hematopoietic stem cells (HSCs) would be a further demonstration of the value of such a large animal SCID model.
Xenotransplantation success with HSCs into non-obese diabetic (NOD)-derived SCID mice is dependent on the ability of NOD mouse signal regulatory protein alpha (SIRPA) to bind human CD47, inducing higher phagocytic tolerance than other mouse strains. Therefore, we investigated whether porcine SIRPA binds human CD47 in the context of developing a humanized SCID pig.
Methods
Peripheral blood mononuclear cells (PBMCs) were collected from SCID and non-SCID pigs. Flow cytometry was used to assess if porcine monocytes could bind to human CD47. Porcine monocytes were isolated from PBMCs and were subjected to phagocytosis assays with pig, human, and mouse red blood cell (RBC) targets. Blocking phagocytosis assays were performed by incubating human RBCs with anti-human CD47 blocking antibody B6H12, non-blocking antibody 2D3, and non-specific IgG1antibody and exposing to human or porcine monocytes.
Results
We found that porcine SIRPA binds to human CD47 in vitro by flow cytometric assays. Additionally, phagocytosis assays were performed, and we found that porcine monocytes phagocytose human and porcine RBCs at significantly lower levels than mouse RBCs. When human RBCs were pre-incubated with CD47 antibodies B6H12 or 2D3, phagocytosis was induced only after B6H12 incubation, indicating the lower phagocytic activity of porcine monocytes with human cells requires interaction between porcine SIRPA and human CD47.
Conclusions
We have shown the first evidence that porcine monocytes can bind to human CD47 and are phagocytically tolerant to human cells, suggesting that porcine SCID models have the potential to support engraftment of human HSCs.
Keywords: Porcine, Severe combined immunodeficiency, SIRPA, CD47, phagocytosis
Introduction
Mice are currently the most common animal model used in biomedical research. However, it has become apparent that results pertaining to human disease states and drug responses in mice may not be directly translatable to humans [1,2]. Therefore, progress is being made to develop larger animal models to provide a more accurate representation of human health. One such species that is increasing in research versatility is the domestic pig. Porcine models are advantageous over other rodent models due to their similarities in anatomy, physiology, immunology, and gene expression to humans [3,4,5,6,7]. Although primate models exist, pigs are preferable for biomedical research due to fewer ethical issues. Pigs also have a shorter gestation period and larger litter sizes than primates, which better facilitate experimental groups. Therefore, further development of porcine models is needed for translational biomedical research.
We have previously characterized naturally occurring T- B- NK+ severe combined immunodeficient (SCID) pigs with mutations within the Artemis gene [8]. Other SCID pig models that were artificially created include IL2Rγ−/− [9,10], RAG2−/− [11], and RAG2−/− IL2Rγ−/− double knockouts [12]. One way to utilize and improve the SCID pig as a model is the humanization through efficient engraftment of human hematopoietic stem cells (HSCs). Currently, the only humanized SCID animal model is the mouse. Humanized mice have allowed researchers to study human immune cell function in the context of HIV, cancer, transplantation, and other important diseases[13,14].
The mouse strain used for the most efficient human cell engraftment is the non-obese diabetic (NOD) SCID IL2Rγnull (NSG) mouse, which has a T- B- NK- cellular phenotype. One mechanism for success of human cell engraftment within the NSG background is due to polymorphisms within the NOD signal regulatory protein alpha (SIRPA) gene [15], which allows it to intrinsically bind to human CD47 and signaling leads to phagocytic tolerance of human cells. SIRPA is expressed on the surface of monocytes, macrophages, and other immune cells and binds to the “self” protein CD47 [16]. SIRPA binding to CD47 results in an inhibitory “don’t eat me” signal within SIRPA expressing cells, resulting in the inhibition of phagocytosis [17,18]. Upon binding of CD47, intracellular tyrosine based inhibitory motifs on the intracellular domain of SIRPA are phosphorylated, leading to a dephosphorylation cascade mediated by SHP1, leading to phagocytic inhibition [19]. The SIRPA protein in C57BL/6 and BALB/c both have low affinity to human CD47 that is not sufficient for inhibitory signaling, which explains the lack of successful human cell engraftment in these mouse strains [20], and further demonstrates the importance of SIRPA-CD47 recognition for humanization of these rodent models [21]. Certain rats and mice of mixed 129/BALB/c background are genetically modified to express human SIRPA, making these animals candidates for humanization if crossed onto a SCID genetic background [22,23]. Interestingly, past studies have investigated the interaction between porcine CD47 and human SIRPA, the opposite interaction to that described here, in relation to transplantation of pig organs into human patients. Results found in one study showed that porcine CD47 was able to bind to human SIRPA in vitro [24], while another showed that phagocytic inhibition was not induced between human macrophages and pig cells [25]. Expression of human CD47 on porcine cells was required to inhibit phagocytosis by human macrophages [25,26]. Together, these studies show evidence that human SIRPA and pig CD47 can bind, although binding is not sufficient for phagocytic inhibition. However, the ability of swine SIRPA to bind to human CD47 has not been explored.
Humanized SCID pigs would be an excellent biomedical model for the study of human disease and cancer. To identify potential cellular barriers to humanization of SCID pigs related to SIRPA/CD47 interactions, we investigated if porcine SIRPA binds to human CD47 and if binding is sufficient to inhibit phagocytosis of human cells. Flow cytometry assays with porcine cells and recombinant human CD47 showed that porcine SIRPA can bind to human CD47. Additionally, porcine monocytes phagocytosed pig and human cells at very low levels, while readily phagocytosing murine cells. Antibody blocking assays showed that the inhibition of phagocytosis of human cells by porcine monocytes was dependent on the SIRPA-CD47 interaction. The natural ability of porcine SIRPA to bind to human CD47 suggests that SCID pigs have the potential to be used for humanization and serve as a valuable biomedical model.
Materials and Methods
Generation of piglets and sample collections from pig, mouse and human subjects
Piglets were derived as described [8]. Piglets from all litters were co-housed with their littermates. All animal protocols and procedures were approved by Iowa State University’s Institutional Animal Care and Use Committee. Informed consent was obtained from human subjects and was approved by the Iowa State University’s Institutional Review Board. Blood was collected into a heparinized vacutainer tube (BD, Franklin Lanes, NJ, USA). Peripheral blood mononuclear cells (PBMCs) were isolated as described [27].
Flow cytometry for hCD47 binding to PBMCs
PBMCs from pig and human were washed in HBSS to remove excess biotin from complete RPMI media (10% FBS, 2 mM glutamine, 50 µg/mL gentamicin, 10 mM HEPES) and incubated with biotinylated human CD47-Fc (hCD47-Fc) (BPS Biosciences, San Diego, CA, USA) followed by PE-Cy5 Streptavidin (BD Biosciences). Human cells were stained with anti-human SIRPA (R&D, Minneapolis, MN, USA, clone #602411), while porcine cells were stained with anti-pig monocyte/granulocyte (SWC3a/SIRPA) (BD Biosciences, clone 74–22-15A). A mouse IgG2b isotype control antibody (BD Biosciences) and goat anti-mouse IgG2b FITC (Southern Biotech, Birmingham, AL, USA) were used as controls. Human and porcine monocytes were singly stained with SIRPA and hCD47-Fc. We stained from the same stock of cells and gated monocytes the same way for each set of stained cells. In order to prevent non-specific Fc binding, Human TruStain FcX (BioLegend) and heat inactivated swine serum were also added to human and pig cells, respectively. Data was acquired on a BD FACSCanto II flow cytometer and analyzed with FlowJo (Tree Star). Cells from a total of six pigs (3 SCID and 3 non-SCID) and four humans were used in three independent experiments. The three experimental groups contained piglets from two separate litters.
Sequence alignment of SIRPA and CD47
Sequences of SIRPA from human (CAA71403.1, NP_542970), porcine (NP_001011508.1), BALB/C [20], C57BL/6 [28], and NOD [28] mouse, as well as CD47 from human (NP_001768.1), porcine (ABW24514.1), and mouse (BAA25401.1, XP_006521871.1) were obtained from the NCBI database and aligned with Clustal Omega alignment tool [29]. BLASTP was used to determine percent identity for CD47 amino acid sequences between species [30].
Preparation of labeled red blood cells for phagocytosis assays
To serve as a source of cells in phagocytosis assays, red blood cells (RBCs) were isolated from pigs, humans, and mice. Whole blood was collected into a heparinized vacutainer tube (BD) and was diluted 1:100 into complete RPMI media and was used as a source of RBCs. The RBCs were then counted using BD counting beads and a flow cytometer. After counting, RBCs were stained with PKH67 fluorescent dye and counted again to verify positive PKH67 staining.
Monocyte preparation for phagocytosis assays
PBMCs were plated into tissue culture treated flasks and monocytes were allowed to adhere overnight at 37°C. Non-adherent PBMCs were removed, and remaining monocytes were washed with HBSS. Monocytes were then trypsinized with 0.25% HyClone trypsin (Fisher Scientific, Hampton, NH, USA) with 0.02% EDTA and were released, washed, enumerated by flow cytometry with BD counting beads, and seeded at 105 cells per well in a 24 well plate. Monocytes were incubated over night at 37°C in complete RPMI to adhere. After incubation, additional fresh media was added to each well.
Labeled target RBCs were added to 105 monocytes at ratios of 1:20, 1:40, and 1:60 and incubated at 37° C for 6 hours. Media with RBCs was removed, and remaining RBCs were lysed with ammonium chloride lysing solution; therefore removing any non-phagocytosed RBCs from the surface of the monocyte. Monocytes were trypsinized, fixed, and fluorescence was acquired on a BD Biosciences FACSCanto II flow cytometer to evaluate internalized RBCs. The percentage of PKH67 positive monocytes was determined for each sample. A total of six pigs (3 SCID and 3 non-SCID) and were used in two independent experiments. The two experimental groups contained piglets from three separate litters.
CD47 blocking assay
PKH67-stained human RBCs were incubated with either 0.5 µg of anti-CD47 clone B6H12.2 (eBioscience, San Diego, CA, USA), anti-CD47 clone 2D3 (eBioscience), or mouse IgG1 isotype control antibody (eBioscience) per 106 RBCs for 30 minutes at room temperature. The B6H12 binds to human CD47 on an epitope which blocks its ability to bind to human SIRPA [31], while 2D3 binds to human CD47, but does not block the interaction with human SIRPA. The 2D3 antibody acts as an opsonization control to show that the presence of the antibody on the surface of the cell does not contribute increases in phagocytosis. After the incubation period, washed RBCs were then added to adhered monocytes at an effector to target ratio of 1:10. Similar binding between B6H12 and 2D3 was verified with a goat anti-mouse IgG1 PE secondary (Southern Biotech) (Supplemental Figure 2). A total of seven pigs (2 SCID and 5 non-SCID) and two humans were used in three independent experiments. The three experimental groups contained piglets from two separate litters.
Statistical Analysis
To compare levels of phagocytosis between RBCs from human, pig, and mouse (Figure 3) and to evaluate the ability of different monoclonal antibodies to human CD47 to inhibit phagocytosis of human RBCs (Figure 4 A,B), a multiple linear regression model was assessed. P values are reported from a type III analysis of variance (ANOVA) with fixed effects of target RBC species (Figure 3) or antibody treatment (Figure 4) using R [32].
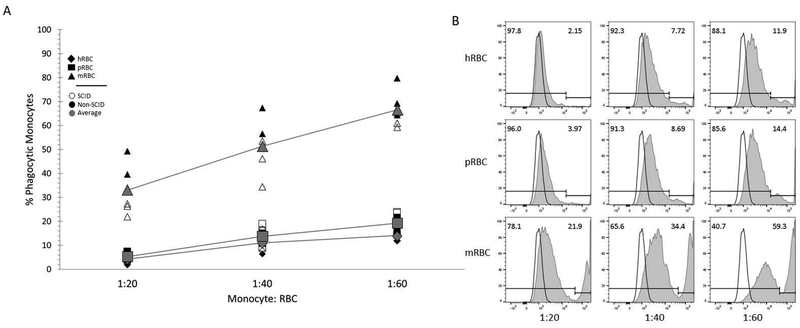
Figure 3. Porcine monocytes phagocytose human and pig RBCs at low levels.
(A) Percent phagocytic monocytes from SCID (S) (open symbol) and non-SCID (NS) (filled symbol) pigs incubated with fluorescently labeled human (diamond), pig (square), and mouse (triangle) RBCs at ratios of 1:20, 1:40, and 1:60 are shown. The average phagocytosis for both SCID and non-SCID pigs are shown in grey. Results from two independent experiments are shown (n= 3 S pigs, 3 NS pigs). * p <0.001.
(B) Fluorescence intensity within monocytes was assessed by flow cytometry as a measure for phagocytosis. Phagocytic monocytes were characterized by positive fluorescent signal by flow cytometry for each animal. Fluorescence intensity for monocytes not incubated with any RBC is shown as the black outlined histogram; the same control histogram is on all nine plots. Histograms shaded in grey are monocytes after exposure to the different species and amounts of RBC. Images were generated in FlowJo.
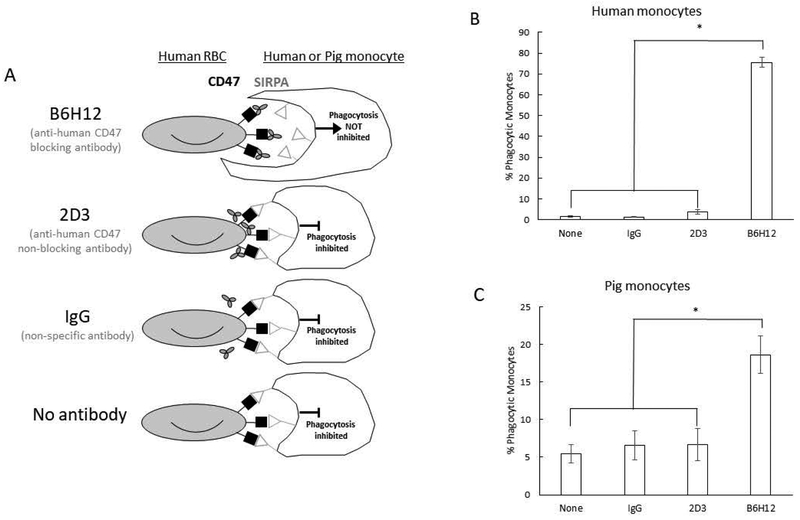
Figure 4. Porcine monocyte phagocytosis of human red blood cells is inhibited by SIRPA-CD47 interaction.
(A) Schematic of cells and antibodies used. Phagocytosis was only induced between human RBC and porcine monocytes when human RBC were incubated with anti-human CD47 blocking antibody B6H12, showing that human CD47 binds to porcine SIRPA to inhibit phagocytosis.
(B) Human monocyte phagocytosis of human RBCs incubated with B6H12, 2D3, or mouse IgG control antibodies (n=2).
(C) Porcine monocyte phagocytosis of human RBCs (n=2 S pigs, 5 NS pigs) incubated with the same antibodies. Percent phagocytic monocytes are shown for both human and porcine monocytes. Results from four independent experiments shown. Error bars represent standard error. * p < 0.001
Results
Porcine SIRPA and CD47 share high degree of amino acid sequence homology with human
The extracellular IgV domain of SIRPA takes part in binding to the IgSF domain of CD47 [33]. CD47 IgSF amino acids from human, pig, and mouse were aligned (Figure 1A). In comparing amino acids of the IgSF domain among the different species, mouse shares less homology with porcine (53–62%) or human (53–63%) than is shared between porcine and human (72%). The higher degree of similarity between human and porcine CD47 suggests that human CD47 may be similar enough to porcine CD47 to be recognized by porcine SIRPA.
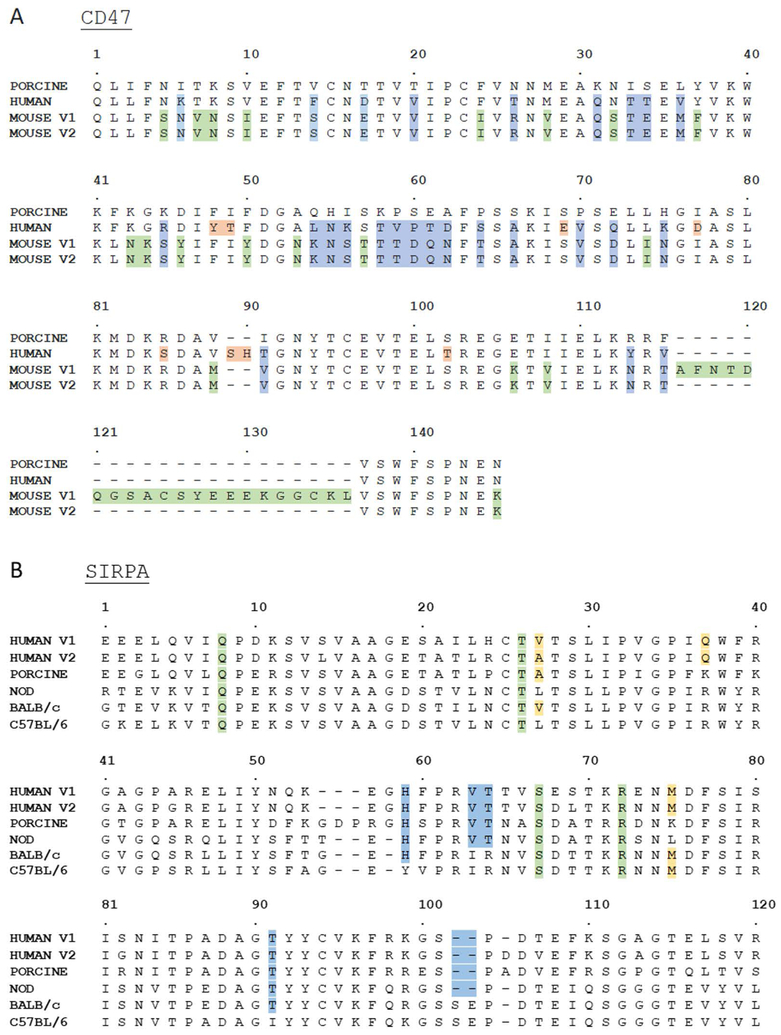
Figure 1. Multi-species alignment of SIRPA and CD47.
(A) Sequence alignment of human (NP_001768.1), porcine (ABW24514.1), and mouse (BAA25401.1, XP_006521871.1) CD47 IgSF domain. Blue highlighted residues indicate amino acids in human and mouse sequences that are different from pig. Green and red highlighted residues are amino acids that are different from pig in mouse and human sequences, respectively.
(B) Sequence alignment of human (CAA71403.1, NP_542970), porcine (NP_001011508.1), BALB/c [20], C57BL/6 [28], and NOD [28] mouse SIRPA IgV domain. Green and yellow highlighted residues indicate amino acids on human SIRPA that are deemed critical for binding human CD47 through mutagenesis experiments [34,35,36] with green highlighted showing conserved and yellow showing species specific amino acids. Residues in blue are those that differ between C57BL/6 and NOD that both human, porcine, BALB/c (only H59 and T91) SIRPA contain. Most importantly the SE residues only present in BALB/c and C57BL/6 are shown to block human CD47 binding [28].
To clarify the level of amino acid conservation of SIRPA across human, pig and mouse, SIRPA IgV amino acids were aligned from human, pig, C57BL/6, BALB/C, and NOD mouse backgrounds (Figure 1B). Previously, mutagenesis experiments have identified critical residues in human SIRPA that are required for successful binding of human CD47, including Ala/Val 27 and Gln 37 [34,35,36]. Swine SIRPA contains Ala 27, suggesting that it could interact with human CD47 at this position. Additionally, porcine SIRPA contains residues present in NOD SIRPA that are shared with human, but not in C57BL/6 mice, including His 59, Val 63, Thr 64, and Thr 91. Importantly, NOD, pig, and human SIRPA all lack Ser 102 and Glu 103 present in the C57BL/6 and BALB/c derived protein; the re-insertion of these residues into NOD SIRPA in vitro dramatically decreased human CD47 binding [28]. Ser 102 and Glu 103 are found on the surface of the binding interface of SIRPA and are near residues shown to physically interact with CD47 including Ser 98 and Asp 100 (depicted as S101 and D106 in Figure 1) [33]. Other critical residues, including Gln 8, Thr 26, Ser 66, and Arg 69 (depicted as R72 in Figure 1) are conserved across human, pig, and mouse, indicating that these residues do not participate in the species-specific differences in the CD47-SIRPA interaction [34,35,36]. Taken together, porcine SIRPA shares specific amino acid similarities to human and NOD SIRPA that suggested it may bind to human CD47.
Porcine monocytes bind to recombinant hCD47-Fc
Previous studies have utilized flow cytometry and recombinant human CD47 (hCD47-Fc) to assess binding to mouse SIRPA [20], and thus we used a similar approach to determine if porcine SIRPA was capable of binding human CD47. PBMCs were isolated from human, SCID, and non-SCID pig blood and analyzed for surface expression of SIRPA and binding capability of hCD47-Fc. When staining with hCD47-Fc and anti-human SIRPA on human monocytes, we found that the anti-human SIRPA antibody we used inhibited binding of the hCD47-Fc protein. The anti-pig SIRPA antibody we used also appeared to block hCD47-Fc binding (Supplemental Figure 1). Therefore, cells were stained with SIRPA antibodies and hCD47-Fc separately as to avoid the potential for SIRPA antibodies to block SIRPA-hCD47-Fc interactions.
Monocytes from SCID and non-SCID pigs both showed positive binding to hCD47-Fc (Figure 2A). The percent SIRPA+ positive monocytes in SCID and non-SCID pigs did not differ, nor did the percent of hCD47-Fc (Figure 2B). However, in cells from both types of pigs, as well as humans, there was variability in binding capability to hCD47-Fc (Figure 2B,), with some binding at high levels (Figure 2A, top row), or low levels (Figure 2A, bottom row). Monocytes from all pigs were shown to bind hCD47-Fc at higher levels than fluorophore controls (Figure 2B). In summary, we interpreted these results to indicate that porcine SIRPA+ cells bound to human CD47-Fc in vitro.
Figure 2. Porcine monocytes bind to recombinant hCD47 in vitro.
(A) PBMCs from human subjects (H), non-SCID (NS), and SCID (S) pigs were isolated from whole blood and stained for SIRPA expression and hCD47-Fc binding. PBMCs were gated on monocyte populations and analyzed. Grey histograms shows isotype or fluorophore controls. The top row shows samples with high binding to hCD47-Fc, and the bottom row shows low binding for each sample type. Three independent experiments were run (n= 3 S pigs, 3 NS pigs, 4 humans). Images were generated in FlowJo.
(B) Percentage and MFI values for SIRPA and hCD47-Fc staining are shown for all stained samples.
Porcine blood-derived monocytes phagocytose human RBCs at very low levels
We next investigated if porcine monocytes phagocytose human cells in an in vitro system. The SIRPA/CD47 interaction is critical for RBC clearance from the blood [37,38], and failure to express CD47 on the surface of a RBCs results in immediate phagocytosis [39]. Additionally, RBCs are an easily obtainable and comparable small cell type that can be collected from humans, pigs, and mice. Therefore, in this study, we utilized RBCs as a model to study the interaction between porcine SIRPA and human CD47.
In comparing CD47 amino acid sequences among species, mouse shares less homology with porcine CD47 than human (Figure 1A), indicating that mouse CD47 may not be recognized by porcine SIRPA. Based on sequence differences between pig and mouse CD47, we expected that porcine monocytes would phagocytose mouse RBCs due to lack of pig SIRPA- mouse CD47 binding. SCID and non-SCID pig SIRPA+ monocytes were incubated with fluorescently labeled human, pig, and mouse RBCs, and phagocytosis was assessed by the percentage of fluorescent monocytes, indicating phagocytosis of labeled RBCs. SCID and non-SCID pigs did not differ in their ability to phagocytose the RBC targets. As predicted, porcine monocytes phagocytosed mouse RBC at very high levels (Figure 3A), which indicates that the porcine monocytes have the potential to be phagocytic. Due to the high levels of mRBC phagocytosis, a slightly different gating strategy was used for mRBC phagocytosis since the phagocytic population had a higher fluorescence intensity than when incubated with hRBC or pRBC (Figure 3B). The brighter monocyte population (to the right of the gate) are indicated as the phagocytic populations. The levels of phagocytosis of human and porcine RBCs by porcine monocytes were very similar, and significantly lower, than mouse RBCs (p < 0.001) (Figure 3A, B). Collectively, low levels of human RBC phagocytosis by porcine monocytes suggested that CD47 expressed on human RBCs bound to porcine SIRPA to subsequently inhibit phagocytosis.
Blocking porcine SIRPA- human CD47 interaction induces phagocytosis
To demonstrate that human RBCs were not phagocytosed by porcine monocytes due to inhibitory effects of porcine SIRPA-human CD47 binding, we utilized an antibody blocking assay used in a variety of cancer therapy studies [40,41,42,43,44]. The B6H12 clone of anti-human CD47 antibody binds to an epitope of human CD47 that prevents it from binding to human SIRPA, while the 2D3 anti-human CD47 clone does not block the interaction. The 2D3 antibody acts as an opsonization control in these experiments to show that it is the blocking activity of the B6H12 anti-CD47 clone, and not the presence of the antibody on the cellular surface, that allows for phagocytosis to be induced (not inhibited). In human studies, cancer cells that express high levels of CD47 that are incubated with B6H12 are phagocytosed at higher levels than those incubated with 2D3 or non-specific antibodies [40,41,42,43,44]. Thus, The B6H12 and 2D3 antibodies were used with human RBCs in an assay with porcine and human monocytes to interrogate if human CD47 binds to porcine SIRPA to inhibit phagocytosis
Since we have previously shown that SCID and non-SCID pigs do not differ in their ability to bind to human CD47 or phagocytose human RBC targets, they were analyzed as a single group. Human RBCs were incubated with B6H12, 2D3, and IgG control antibodies prior to incubation with either human or porcine monocytes. A schematic of cells and antibodies used are shown in Figure 4A. Only pretreatment with B6H12 increased RBC phagocytosis for both human (Figure 4B) and pig (Figure 4C) monocytes (p < 0.001). Importantly, B6H12 and 2D3 bound to human RBCs similarly (Supplemental Figure 2). On average, 80% of human monocytes phagocytosed B6H12 treated hRBCs, compared to 1–2% for controls. We used human monocytes as a proof of concept to show that blocking the interaction between SIRPA/CD47 within the same species increases phagocytosis. Of porcine monocytes, 20% of monocytes phagocytosed B6H12 treated hRBC, compared to 5% for controls. One hypothesis for the difference in B6H12 phagocytosis induction between human and pig monocytes is that the protein interacting surface between human SIRPA-human CD47 and porcine SIRPA-human CD47 may slightly differ. The B6H12 antibody may not be as efficient at blocking the porcine SIRPA-human CD47 interaction, which would explain the lower levels of phagocytosis induction. While the increase in phagocytosis was not as high for porcine monocytes as human monocytes, a significant increase in the level of phagocytosis was induced upon blocking pig SIRPA-human CD47 binding. This result indicates that the SIRPA-CD47 interaction between porcine monocytes and human RBCs is critical for limiting phagocytosis of human RBCs.
Discussion
We present the first evidence that porcine SIRPA can bind and recognize human CD47 to inhibit phagocytosis of human cells by porcine phagocytes in an in vitro system. Importantly, monocytes specifically from SCID pigs were capable of binding human CD47 to inhibit phagocytosis of human RBCs. Specifically, porcine monocytes uniformly failed to phagocytose hRBC and blocking the porcine SIRPA-human CD47 axis induced phagocytosis of hRBC. Together, these results show that the porcine SIRPA-human CD47 binding interaction was sufficient to inhibit phagocytosis of human cells. Mouse and rat models have previously required genetic modification to express human SIRPA to allow engraftment of human cells [22,23]. Due to the fact that porcine SIRPA binds to human CD47 intrinsically, we do not believe that pigs would require introduction of the human SIRPA gene to allow for HSC engraftment. In moving forward with the development of a humanized SCID pig model, it is necessary to characterize interactions that are required for successful engraftment.
There are few studies that have investigated how porcine immune cells interact with human cells, particularly phagocytosis of human cells by pig myeloid lineage cells. Porcine liver sinusoidal endothelial cells phagocytose human platelets perfused through the liver [45]; however, the involvement of porcine SIRPA and human CD47 in the phagocytosis of human platelets was not assessed in this study. A follow-up report suggests that differences in Galβ and βGlcNAc oligosaccharides on human and porcine platelets contributed to the phagocytosis of human platelets [46]. Previous reports in mouse models indicate NOD mouse macrophages phagocytose human RBC despite the ability of NOD SIRPA to bind to human CD47. The phagocytosis of human RBC by NOD macrophages is hypothesized to be attributed to either mouse complement [47] or xenoantigens on the human RBCs [48]. In our study we were able to show low levels of in vitro phagocytosis of human RBC by porcine monocytes; enhanced phagocytosis only occurred when the porcine SIRPA-human CD47 axis was blocked with the anti-human CD47 B6H12 antibody. One method that could be utilized in future studies would be generating a crystal structure of the human CD47- porcine SIRPA complex to study this interaction in more detail. Additionally, it would be insightful to determine if there are unidentified polymorphisms in the IgV domain of swine SIRPA that could contribute to different binding affinities to human CD47. Porcine SCID models have already been utilized for different facets of biomedical research including cancer [49,50], influenza [51], and norovirus research [12]. In addition, human cancer cells lines [49] and human induced pluripotent stem cells [52] can survive and persist in SCID pigs. The ability of these cell types to survive within SCID pigs suggests that other human cell types, including HSCs could also survive in SCID pigs. Humanized SCID pig models could aid in the development and testing of vaccines, cancer immunotherapies, stem cell therapies, as well as other important biomedical advancements [53]. Development of such a humanized SCID pig requires clean housing and personnel protocols to ensure minimal exposure of these animals to pathogens. There are protocols developed to raise SCID pigs in clean, positive-pressure bubble environments to limit disease and increase lifespan so these pigs can be used in long term studies [54], including development of methods to introduce and maintain a human immune system in SCID pigs.
As of this writing, very few studies have been conducted in relation to introducing human HSCs into non-murine models. Non-SCID lambs and piglets can support the development of human T, B, NK, and myeloid lineages when HSC cells were injected in the fetal intraperitoneal space via in utero injections with human HSCs [55,56]. Additionally, the pig thymus can support the development of a wide range of human T cells [57,58]. The development of human immune cell subsets suggests that porcine cytokines can support the development of human immune cells. The differentiation of human cells in pig models is consistent with existing literature that state porcine immune genes are highly similar to human [3,59]. In considering past studies, it is expected that human HSCs would engraft and differentiate in SCID pigs at least as well as they have in non-SCID pigs. Showing that porcine phagocytes tolerate human cells in vitro though the SIRPA-CD47 pathway is important evidence that this interaction would not be a barrier to human HSC engraftment in SCID pigs.
Supplementary Material
Acknowledgements
This work was supported by grant 1R24OD19813–02 from the National Institutes of Health. We thank Shawn Rigby from Iowa State University’s Flow Cytometry facility for advice. We thank Iowa State University’s Laboratory Animal Research Facility staff for care of the SCID pig colonies and for assisting with mouse blood procurement. We also thank Iowa State University’s Occupational Medicine staff for help with human sample collections.
Abbreviations used in this article:
- NOD
non-obese diabetic
- SCID
severe combined immunodeficiency
- SIRPA
signal regulatory protein alpha
- hCD47-Fc
recombinant human CD47
- RBC
red blood cell
- HSC
hematopoietic stem cell
- NSG
NOD SCID IL2Rγnull
- PBMC
peripheral blood mononuclear cell
- ANOVA
analysis of variance
Footnotes
Conflict of Interest Disclosure
The authors declare no competing financial interests.
References
- 1.Seok J, Warren HS, Cuenca AG et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013; 110: 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musther H, Olivares-Morales A, Hatley OJD, Liu B, Rostami Hodjegan A. Animal versus human oral drug bioavailability: Do they correlate? Eur J Pharm Sci 2014; 57: 280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson HD, Loveland JE, Pascal G et al. Structural and functional annotation of the porcine immunome. BMC Genomics 2013; 14: 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson AL, Carlson DF, Largaespada DA, Hackett PB, Fahrenkrug SC. Engineered Swine Models of Cancer. Front Genet 2016; 7: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth JA, Tuggle CK. Livestock models in translational medicine. ILAR journal 56 2015. 1–6. [DOI] [PubMed] [Google Scholar]
- 6.Fairbairn L, Kapetanovic R, Sester DP, Hume DA. The mononuclear phagocyte system of the pig as a model for understanding human innate immunity and disease. J Leukoc Biol 2011; 89: 855–871. [DOI] [PubMed] [Google Scholar]
- 7.Meurens F, Summerfield A, Nauwynck H, Saif L, Gerdts V. The pig: a model for human infectious diseases. Trends Microbiol 2012; 20: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waide EH, Dekkers JCM, Ross JW et al. Not All SCID Pigs Are Created Equally: Two Independent Mutations in theArtemisGene Cause SCID in Pigs. J Immunol 2015; 195: 3171 LP–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki S, Iwamoto M, Saito Y et al. Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell 2012; 10: 753–758. [DOI] [PubMed] [Google Scholar]
- 10.Kang J-T, Cho B, Ryu J et al. Biallelic modification of IL2RG leads to severe combined immunodeficiency in pigs. Reprod Biol Endocrinol 2016; 14: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki S, Iwamoto M, Hashimoto M et al. Generation and characterization of RAG2 knockout pigs as animal model for severe combined immunodeficiency. Vet Immunol Immunopathol 2016; 178: 37–49. [DOI] [PubMed] [Google Scholar]
- 12.Lei S, Ryu J, Wen K et al. Increased and prolonged human norovirus infection in RAG2/IL2RG deficient gnotobiotic pigs with severe combined immunodeficiency. Sci Rep 2016; 6: 25222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brehm MA, Shultz LD, Greiner DL. Humanized Mouse Models to Study Human Diseases. Curr Opin Endocrinol Diabetes Obes 2010; 17: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh N, Kenney L, Jangalwe S et al. Humanized mouse models of clinical disease. Annu Rev Pathol 2017; 12: 187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takenaka K, Prasolava TK, Wang JCY et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol 2007; 8: 1313–1323. [DOI] [PubMed] [Google Scholar]
- 16.Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J Biol Chem 1999; 274: 559–562. [DOI] [PubMed] [Google Scholar]
- 17.Barclay AN. Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr Opin Immunol 2009; 21: 47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barclay AN, Van den Berg TK. The interaction between signal regulatory protein alpha (SIRPalpha) and CD47: structure, function, and therapeutic target. Annu Rev Immunol 2014; 32: 25–50. [DOI] [PubMed] [Google Scholar]
- 19.Takizawa H, Manz MG. Macrophage tolerance: CD47-SIRP-alpha-mediated signals matter. Nature immunology 8 2007. 1287–1289. [DOI] [PubMed] [Google Scholar]
- 20.Iwamoto C, Takenaka K, Urata S et al. The BALB/c-specific polymorphic SIRPA enhances its affinity for human CD47, inhibiting phagocytosis against human cells to promote xenogeneic engraftment. Exp Hematol 2014; 42: 163–171.e1. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T, Takenaka K, Urata S et al. Polymorphic Sirpa is the genetic determinant for NOD-based mouse lines to achieve efficient human cell engraftment. Blood 2013; 121: 1316–1325. [DOI] [PubMed] [Google Scholar]
- 22.Jung CJ, Menoret S, Brusselle L et al. Comparative Analysis of piggyBac, CRISPR/Cas9 and TALEN Mediated BAC Transgenesis in the Zygote for the Generation of Humanized SIRPA Rats. Sci Rep 2016; 6: 31455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strowig T, Rongvaux A, Rathinam C et al. Transgenic expression of human signal regulatory protein alpha in Rag2−/−gamma(c)−/− mice improves engraftment of human hematopoietic cells in humanized mice. Proc Natl Acad Sci U S A 2011; 108: 13218–13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian S, Parthasarathy R, Sen S, Boder ET, Discher DE. Species- and cell type-specific interactions between CD47 and human SIRPalpha. Blood 2006; 107: 2548–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ide K, Wang H, Tahara H et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A 2007; 104: 5062–5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan J-J, Koo TY, Lee H-S et al. Role of Human CD200 Overexpression in Pig-to-Human Xenogeneic Immune Response Compared With Human CD47 Overexpression. Transplantation 2018; 102: 406–416. [DOI] [PubMed] [Google Scholar]
- 27.Powell EJ, Cunnick JE, Knetter SM et al. NK cells are intrinsically functional in pigs with Severe Combined Immunodeficiency (SCID) caused by spontaneous mutations in the Artemis gene. Vet Immunol Immunopathol 2016; 175: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwong LS, Brown MH, Barclay AN, Hatherley D. Signal-regulatory protein α from the NOD mouse binds human CD47 with an exceptionally high affinity – implications for engraftment of human cells. Immunology 2014; 143: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sievers F, Wilm A, Dineen D et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 2011; 7: 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Altschul SF, Madden TL, Schaffer AA et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997; 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pietsch EC, Dong J, Cardoso R et al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J 2017; 7: e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rs Team. Integrated development for R. 2015; [Google Scholar]
- 33.Hatherley D, Graham SC, Turner J et al. Paired receptor specificity explained by structures of signal regulatory proteins alone and complexed with CD47. Mol Cell 2008; 31: 266–277. [DOI] [PubMed] [Google Scholar]
- 34.Lee WY, Weber DA, Laur O et al. Novel structural determinants on SIRP alpha that mediate binding to CD47. J Immunol 2007; 179: 7741–7750. [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Tong Q, Zhou Y et al. Functional elements on SIRPalpha IgV domain mediate cell surface binding to CD47. J Mol Biol 2007; 365: 680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatherley D, Harlos K, Dunlop DC, Stuart DI, Barclay AN. The structure of the macrophage signal regulatory protein alpha (SIRPalpha) inhibitory receptor reveals a binding face reminiscent of that used by T cell receptors. J Biol Chem 2007; 282: 14567–14575. [DOI] [PubMed] [Google Scholar]
- 37.Burger P, Hilarius-Stokman P, de Korte D, van den Berg TK, van Bruggen R. CD47 functions as a molecular switch for erythrocyte phagocytosis. Blood 2012; 119: 5512–5521. [DOI] [PubMed] [Google Scholar]
- 38.Sosale NG, Rouhiparkouhi T, Bradshaw AM et al. Cell rigidity and shape override CD47’s “self”-signaling in phagocytosis by hyperactivating myosin-II. Blood 2015; 125: 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oldenborg PA, Zheleznyak A, Fang YF et al. Role of CD47 as a marker of self on red blood cells. Science 2000; 288: 2051–2054. [DOI] [PubMed] [Google Scholar]
- 40.Tseng D, Volkmer J-P, Willingham SB et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A 2013; 110: 11103–11108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majeti R, Chao MP, Alizadeh AA et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 2009; 138: 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chao MP, Alizadeh AA, Tang C et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res 2011; 71: 1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chao MP, Alizadeh AA, Tang C et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 2010; 142: 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willingham SB, Volkmer J-P, Gentles AJ et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A 2012; 109: 6662–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burlak C, Paris LL, Chihara RK et al. The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation 2010; 17: 350–361. [DOI] [PubMed] [Google Scholar]
- 46.Paris LL, Chihara RK, Sidner RA, Tector AJ, Burlak C. Differences in human and porcine platelet oligosaccharides may influence phagocytosis by liver sinusoidal cells in vitro. Xenotransplantation 2012; 19: 31–39. [DOI] [PubMed] [Google Scholar]
- 47.Chen B, Fan W, Zou J et al. Complement Depletion Improves Human Red Blood Cell Reconstitution in Immunodeficient Mice. Stem cell reports 2017; 9: 1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu Z, Van Rooijen N, Yang Y-G. Macrophages prevent human red blood cell reconstitution in immunodeficient mice. Blood 2011; 118: 5938–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basel MT, Balivada S, Beck AP et al. Human Xenografts Are Not Rejected in a Naturally Occurring Immunodeficient Porcine Line: A Human Tumor Model in Pigs. Biores Open Access 2012; 1: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powell EJ, Graham J, Ellinwood NM et al. T Cell Lymphoma and Leukemia in Severe Combined Immunodeficiency Pigs following Bone Marrow Transplantation: A Case Report. Front Immunol 2017; 8: 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajao DS, Loving CL, Waide EH et al. Pigs with Severe Combined Immunodeficiency Are Impaired in Controlling Influenza A Virus Infection. J Innate Immun 2017; 9: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee K, Kwon D-N, Ezashi T et al. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated <em>RAG2</em> and accompanying severe combined immunodeficiency. Proc Natl Acad Sci 2014; 111: 7260 LP–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powell EJ, Cunnick JE, Tuggle CK. SCID pigs: An emerging large animal NK model. J rare Dis Res Treat 2017; 2: 1–6. [PMC free article] [PubMed] [Google Scholar]
- 54.Powell EJ, Charley S, Boettcher AN et al. Creating effective biocontainment facilities and maintenance protocols for raising specific pathogen-free, severe combined immunodeficient (SCID) pigs. Lab Anim 2018; 23677217750691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujiki Y, Fukawa K, Kameyama K et al. Successful multilineage engraftment of human cord blood cells in pigs after in utero transplantation. Transplantation 2003; 75: 916–922. [DOI] [PubMed] [Google Scholar]
- 56.Goodrich AD, Varain NM, Jeanblanc CM et al. Influence of a dual-injection regimen, plerixafor and CXCR4 on in utero hematopoietic stem cell transplantation and engraftment with use of the sheep model. Cytotherapy 2014; 16: 1280–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogle BM, Knudsen BE, Nishitai R, Ogata K, Platt JL. Toward development and production of human T cells in swine for potential use in adoptive T cell immunotherapy. Tissue Eng Part A 2009; 15: 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalscheuer H, Onoe T, Dahmani A et al. Xenograft tolerance and immune function of human T cells developing in pig thymus xenografts. J Immunol 2014; 192: 3442–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dawson H Comparative assesment of the pig, mouse, and human genomes: A structural and functional analysis of genes involved in immunity. minipig Biomed Res 2011; 321–341. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.