Abstract
Background:
The CAregiver Perceptions About CommunIcaTion with Clinical Team members (CAPACITY) instrument measures how care partners perceive themselves to be supported by the patient’s health care team and their experiences communicating with the team.
Objectives:
The objective of this study was to assess the measurement properties (ie, structural validity of the construct and internal consistency) of the CAPACITY instrument in care partners of patients with cognitive impairment, and to examine whether care partner health literacy and patient cognitive impairment are associated with a higher or lower CAPACITY score.
Research Design:
This was a retrospective cohort study.
Subjects:
A total of 1746 dyads of community-dwelling care partners and older adults in the United States with cognitive impairment who obtained an amyloid positron emission tomography scan.
Measures:
The CAPACITY instrument comprises 12 items that can be combined as a total score or examined as subdomain scores about communication with the team and care partner capacity-assessment by the team. The 2 covariates of primary interest in the regression model are health literacy and level of cognitive impairment of the patient (Modified Telephone Interview Cognitive Status).
Results:
Confirmatory factor analysis showed the CAPACITY items fit the expected 2-factor structure (communication and capacity). Higher cognitive functioning of patients and higher health literacy among care partners was associated with lower communication domain scores, lower capacity domain scores, and lower overall CAPACITY scores.
Conclusions:
The strong psychometric validity of the CAPACITY measure indicates it could have utility in other family caregivers or care partner studies assessing the quality of interactions with clinical teams. Knowing that CAPACITY differs by care partner health literacy and patient impairment level may help health care teams employ tailored strategies to achieve high-quality care partner interactions.
Keywords: care partners, communication, health care team cognitive impairment, health literacy
An estimated 5.8 million Americans have Alzheimer disease and ∼15%–20% of people aged 65 years or older have mild cognitive impairment (MCI) from any cause.1 Care partners, that is, those identified by patients as the person they consider to be the most involved with decisions and support related to their health and health care, play a key role in the information exchange between patients and medical providers on the health care team.2–4
Understanding perceptions about the quality of communication with the health care team from the care partner’s perspective is important because care partners commonly help implement treatment plans2 and support preferences of persons with cognitive impairment. Perceptions of poor communication could hinder the ability of care partners to contribute their expertise when interacting with the patient’s health care team, thus inhibiting optimization of the health care choices and treatment plan. In other settings, poor patient communication has been found to be associated with worse outcomes among patients with dementia.5,6 In addition, little is known about shared decision-making and health communication when care partners are involved7–11 or the extent to which care partners perceive their interactions with the health care team to be productive or satisfying. A better understanding of care partner perceptions of their interactions with the health care team may facilitate the development of person-centered and family-centered strategies to engage in shared decision-making with persons with memory problems, their care partners, and health care teams.
In this paper, we seek to understand care partner perceptions of quality in interactions with the health care team. The CAregiver Perceptions About CommunIcaTion with Clinical Team members (CAPACITY) instrument was originally developed using a sample of 929 caregivers of young, primarily male, injured US veterans. It was found to represent 2 distinct domains that measure caregivers’ perceptions of how well they are supported by the patient’s health care team (capacity domain) and their experiences communicating with the health care team (communication domain).12 There are concerns specific to care partners of older adults with cognitive impairment that warrant an assessment of measurement properties of the CAPACITY instrument among this particular group: for example, they are older and may have different preferences for team interaction, thus there is a need to confirm the underlying 2-domain factor structure. Thus, the first research question is whether CAPACITY has acceptable measurement properties among care partners of older adults with cognitive impairment.
The second research question is to examine how cognitive impairment of the patient and health literacy of care partners are associated with CAPACITY. A priori we did not have a strong directional hypothesis because there could be countervailing effects; thus, this second research question is exploratory. With greater cognitive impairment patients may not be able to successfully navigate the health care encounter alone. Thus, we expect that care partners will have a more active role in representing patient preferences, relaying symptoms and concerns to the team, and treatment planning. Care partners with low health literacy may perceive communication with the health care team to be of different quality compared with care partners with high health literacy. For example, patients with low health literacy have had lower satisfaction with health care teams and one pathway has been poorer communication with the team,13 yet it is unclear how health literacy is associated with care partner perception of the quality of communication.
METHODS
Study Setting and Participants
The participants in this study are from the CARE-IDEAS study, a supplemental survey study to the Imaging Dementia—Evidence for Amyloid Scanning or IDEAS study. Details of the IDEAS Study are reported elsewhere.14,15 In short, the IDEAS Study recruited 18,295 Medicare beneficiaries aged 65 years and older with progressive MCI and/or dementia of uncertain cause from 592 dementia practices over 22 months.16 Referring doctors believed that an amyloid positron emission tomography scan could help to guide their patients’ care.
The IDEAS Study transferred the contact information of 3717 IDEAS patients who agreed to be contacted for the CARE-IDEAS supplemental study. Of these, 2228 of patients and 1872 of their care partners (dyads) completed the baseline telephone interview. To be included in this study, the following criteria was met: Patient and care partner completed a survey; care partner reported attending primary care check-ups or specialty care visits “1 = Rarely,” “2 = Sometimes,” “3 = Most of the time,” or “4 = Always”; and care partner had < 2 individual missing values on the CAPACITY measure (n = 1746).
Measures
Dependent Variables
Care partners answered 12 Likert scale items from the CAPACITY instrument.12 The overall CAPACITY score is calculated as the mean of all 12 items (range: 1–4), where a higher score indicates perceptions of better quality communication and capacity-assessment from the care team. Scores for the subscales capturing the “communication” and “capacity/preferences” domains were also calculated as the mean of each respective set of 6 domain-specific items.
Independent Variables
Cognitive status was assessed using an abbreviated version of the Telephone Interview Cognitive Status (TICS-M) administered to patients, one of the most frequently used telephone cognitive screening instruments to detect cognitive change and dementia.17,18 The possible scores range from 0 to 41, and the instrument includes items of immediate and delayed 10-noun free recall; serial 7 subtraction; counting backwards; recall of the date, naming the president and the vice-president; and naming 2 common items. A higher score for the TICS-M indicates better cognitive functioning. For regression analyses, we divided the score by 5 so as to model the average effect of a 5-U increment in TICS-M total score. We also report on the patient’s level of cognitive impairment using a diagnosis of MCI versus dementia reported by the IDEAS physician; this diagnosis variable was not used in the regression models due to collinearity with the TICS-M. We also administered the TICS-M to care partners, which has been a shortcoming of some prior studies focusing on care partner or caregiver-patient dyads.
Health literacy of the care partners was measured with a 5-point Likert scale response to the following question: “How often do you have someone (like a family member, friend, hospital or clinic worker, or caregiver) help you read medical forms or hospital materials?”19 Responses included “1 = Always,” “2 = Often,” “3 = Sometimes,” “4 = Occasionally,” or “5 = Never.” A higher value on this measure indicates higher health literacy.
Sociodemographic characteristics were obtained for both members of the patient/care partner dyad; however, due to high correlation within dyads, we included only care partners’ age (grouped for analysis as < 75, 75–84, or ≥ 85), sex, and race (grouped as “White” or “Other races”) in the analysis. We included educational attainment for each dyad member (grouped as “High school graduate or less,” “Some college,” “Bachelor’s degree,” or “Graduate degree”). The relationship of the patient to the care partner was characterized as either “Spouse or significant other” or “Parent/other.” Each respondent was also asked to self-assess their general health status on a 5-point Likert scale, with response options of “1 = Excellent,” “2 = Very good,” “3 = Good,” “4 = Fair,” or “5 = Poor.”20
Care partner employment was measured by categorizing work for pay across all jobs into “Part-time (> 0–< 40 h/wk),” “Full-time (≥ 40 h/wk),” or “Not working for pay, don’t know, or refused.” Objective caregiving burden was measured as the number of hours the care partner provided care to the patient weekly, with response options including “5 hours or fewer a week,” “6–19 hours a week,” “20–39 hours a week,” “40 or more hours a week” or “Not providing care.” Subjective caregiver burden was defined using the 12-item Zarit burden measure, described as the level of stress felt by a caregiver.21–23 Responses to each item range from 0 to 4, with 0 = “Never” and 4 = “Nearly always.” The Zarit scale total score is calculated as the sum of the 12 responses and can range from 0 to 48. A score > 16 suggests a clinically significant caregiver burden.21,24
Care partner depressive symptoms were captured by selecting the PHQ-2 items from the Patient Health Questionnaire 8 (PHQ-8). The PHQ-2 is a screening instrument for further assessment for clinical depression.25 Care partners responded how often during the past 2 weeks that they have been bothered by either of these problems: “Little interest or pleasure in doings things” and “Feeling down, depressed, or hopeless.” Responses range from 0 indicating “Not at all” to 3 indicating “Nearly every day.” The sum of the 2 response values was calculated and a sum of ≥ 3 was considered positive for this screening instrument for depression.25
Statistical Analysis
We first described the total and domain-specific scores on the CAPACITY scale by reporting univariate statistics and plotting histograms. We then classified the population into tertiles of the total CAPACITY score and described the characteristics of the patients and care partners, overall and by CAPACITY tertile, using proportions for categorical variables and means with SDs for continuous variables. We tested for differences between tertile groups using Pearson χ2 tests for categorical variables and Kruskal-Wallis tests for continuous variables.
We first assess the structural validity of the CAPACITY instrument in our sample, which the COnsensus-based Standards for the selection of health status Measurement INstruments (COSMIN)26 defines as one aspect of construct validity concerning specifically the adequate reflection of the dimensionality of a construct. We, therefore, estimated confirmatory factor analysis (CFA) to determine whether the scale yields the expected construct dimensionality and then checked reliability using tests for internal consistency, that is, the degree of interrelatedness among the items. We performed weighted least square CFA for ordinal items, testing both a 1-dimensional solution and the 2-factor structure for separate communication and capacity domains that was obtained in previous validation efforts among veterans.12 We examined standardized factor loadings for each domain, whereby a value closer to one represents evidence that the majority of the variance in individual items is captured by the latent factor. High standardized factor loadings (above 0.50) constitute important evidence of a valid unobserved latent factor that drives shared variance in observed items.27 The adequacy of a 2-factor model was examined using the following model fit statistics: χ2, root mean square error of approximation (RMSEA), the Comparative Fit Index (CFI), and Tucker Lewis Index (TLI). A lower and nonsignificant χ2 indicates better fit, although this statistic is sensitive to sample size and considered too stringent among large samples. A lower value on the RMSEA indicates a better fit(≤ 0.05), while larger values (≥ 0.95) for CFI and TLI indicate a good model fit.27 MPLUS was used for all CFA. As part of sensitivity analyses, we explored whether any modification indices proposed by the software might suggest conceptual improvements in this population, and checked the factor structure among different sub-groups for meaningful differences, namely among care partners who reported always attending appointments with patients and across education levels. Internal consistency was examined using the omega for standardized factor loadings of the 1-factor solution, and for the separate subscales. Omega is interpreted the same way as Cronbach α, with values above 0.90 considered excellent, but has fewer limitations in the context of scales that are composed of ordinal items.28
We fit 3 separate multiple regression models with normal distributions and log links (ie, log-normal models) to estimate the associations between the primary variables of interest, patient cognitive impairment and care partner health literacy, and either: (1) the communication domain; (2) the capacity domain; or (3) the total CAPACITY score. Categorical control variables, such as age group and relationship to the patient, were transformed into binary or multilevel indicator variables. Multicollinearity was assessed using the SAS VARCLUS procedure; the variables included in the final analysis and described here were not considered collinear. Parameter estimates for the associations between each variable and the CAPACITY total or domain score are presented as ratio measures and interpreted as the percent change per unit increment of the independent variable.
In addition to the 3 main regressions, we performed 2 sensitivity analyses. We reran these models among the 1557 care partners who reported attending the patient’s medical visits “Most of the time” or “Always,” because we hypothesized that the associations may be even stronger with CAPACITY. We also reran the models including the care partner’s cognitive status, to ensure that covariates of interest were robust to the inclusion of this covariate.
RESULTS
The care partners in the 1746 dyads that met the study eligibility criteria were predominantly the spouses and significant others of the patient, though about 10% of them had other types of relationships, such as parent-child (Table 1). Most care partners were female (68%), White (96%), retired or not working (76%), and younger than 75 years (66%, mean age = 70.3 y). Educational attainment was high, with the majority of both patients and care partners having Bachelor’s or Graduate degrees. While the majority of patients had MCI, 482 (27.6%) were classified as having dementia. Nearly half of care partners reported spending between 1 and 19 hours per week caring for the patient (44%), and another 10% of care partners reported spending over 20 hours per week providing such care. Care partner cognitive status, as indicated by the TICS-M, was in the normal range with an average of 27.9 (Table 1).
TABLE 1.
Characteristics of the Study Sample Overall and by Tertiles of the Overall CAPACITY Scale Score
| Variables | n (%) |
||||
|---|---|---|---|---|---|
| Overall | Tertile 1 (lowest) | Tertile 2 | Tertile 3 (highest) | P* | |
| N | 1746 | 604 | 563 | 579 | |
| CAPACITY scale | |||||
| Total score [mean (SD)] | 2.4 (0.6) | 1.7 (0.2) | 2.3 (0.1) | 3.1 (0.4) | NA |
| Median (Q1, Q3) | 2.3 (1.9, 2.8) | 1.8 (1.6, 1.9) | 2.3 (2.2, 2.4) | 3.0 (2.8, 3.4) | |
| Communication domain [mean (SD)] | 3.1 (0.7) | 2.3 (0.5) | 3.2 (0.3) | 3.7 (0.3) | NA |
| Median (Q1, Q3) | 3.2 (2.7, 3.7) | 2.3 (2.0, 2.7) | 3.2 (3.0, 3.5) | 3.7 (3.5, 4.0) | |
| Capacity domain [mean (SD)] | 1.6 (0.8) | 1.1 (0.1) | 1.3 (0.3) | 2.5 (0.8) | NA |
| Median (Q1, Q3) | 1.3 (1.0, 2.0) | 1.0 (1.0, 1.2) | 1.2 (1.0, 1.5) | 2.3 (1.8, 3.0) | |
| Patient characteristics | |||||
| Cognitive status (TICS-M) [mean (SD)] | 20.4 (6.2) | 21.4 (6.2) | 20.8 (5.7) | 18.9 (6.5) | < 0.001 |
| Impairment level | < 0.001 | ||||
| Mild cognitive impairment | 1264 (72.4) | 469 (77.6) | 423 (75.1) | 372 (64.2) | |
| Dementia | 482 (27.6) | 135 (22.4) | 140 (24.9) | 207 (35.8) | |
| General health status (self-assessed) [mean (SD)] | 2.5 (1.0) | 2.5 (1.0) | 2.6 (1.0) | 2.5 (1.0) | 0.66 |
| Education | 0.03 | ||||
| High school graduate or less | 278 (15.9) | 79 (13.1) | 82 (14.6) | 117 (20.2) | |
| Some college | 439 (25.1) | 156 (25.8) | 139 (24.7) | 144 (24.9) | |
| Bachelor’s degree | 409 (23.4) | 139 (23.0) | 138 (24.5) | 132 (22.8) | |
| Graduate degree | 620 (35.5) | 230 (38.1) | 204 (36.2) | 186 (32.1) | |
| Care partner characteristics | |||||
| Health literacy [mean (SD)] | 4.5 (0.9) | 4.6 (0.9) | 4.6 (0.9) | 4.5 (0.9) | 0.19 |
| Age group | 0.07 | ||||
| < 75 | 1152 (66.0) | 375 (62.1) | 380 (67.5) | 397 (68.6) | |
| 75–84 | 539 (30.9) | 203 (33.6) | 167 (29.7) | 169 (29.2) | |
| 85+ | 55 (3.2) | 26 (4.3) | 16 (2.8) | 13 (2.2) | |
| Age [mean (SD)] | 70.3 (9.6) | 71.0 (9.9) | 70.2 (9.0) | 69.5 (9.7) | 0.01 |
| Male | 565 (32.4) | 213 (35.3) | 154 (27.4) | 198 (34.2) | 0.008 |
| Race, White | 1673 (95.8) | 582 (96.4) | 543 (96.4) | 548 (94.6) | 0.23 |
| Education | 0.41 | ||||
| High school graduate or less | 246 (14.1) | 93 (15.4) | 72 (12.8) | 81 (14.0) | |
| Some college | 502 (28.8) | 171 (28.3) | 150 (26.6) | 181 (31.3) | |
| Bachelor’s degree | 478 (27.4) | 169 (28.0) | 159 (28.2) | 150 (25.9) | |
| Graduate degree | 520 (29.8) | 171 (28.3) | 182 (32.3) | 167 (28.8) | |
| Patient is spouse/significant other | 1556 (89.1) | 545 (90.2) | 510 (90.6) | 501 (86.5) | 0.049 |
| Employment, hours per wk | 0.88 | ||||
| Part-time | 260 (14.9) | 87 (14.4) | 89 (15.8) | 84 (14.5) | |
| Full-time | 156 (8.9) | 50 (8.3) | 51 (9.1) | 55 (9.5) | |
| Not working or don’t know/refused | 1330 (76.2) | 467 (77.3) | 423 (75.1) | 440 (76.0) | |
| Time providing care for the patient | < 0.001 | ||||
| 05 h or fewer a wk | 521 (29.8) | 214 (35.4) | 170 (30.2) | 137 (23.7) | |
| 06–19 h a wk | 241 (13.8) | 64 (10.6) | 81 (14.4) | 96 (16.6) | |
| 20–39 h a wk | 98 (5.6) | 25 (4.1) | 33 (5.9) | 40 (6.9) | |
| 40 or more hours a wk | 82 (4.7) | 13 (2.2) | 32 (5.7) | 37 (6.4) | |
| Not providing care or don’t know/refused | 804 (46.0) | 288 (47.7) | 247 (43.9) | 269 (46.5) | |
| General health status (self-assessed) [mean (SD)] | 2.4 (0.9) | 2.5 (1.0) | 2.3 (0.9) | 2.4 (0.9) | 0.08 |
| Depression (PHQ-2) | 127 (7.3) | 56 (9.3) | 41 (7.3) | 30 (5.2) | 0.03 |
| Subjective burden (Zarit) [mean (SD)] | 10.8 (7.9) | 10.6 (7.8) | 11.0 (7.8) | 10.8 (8.0) | 0.55 |
| Cognitive status (TICS-M) [mean (SD)] | 27.9 (5.0) | 27.8 (4.6) | 28.0 (5.4) | 27.9 (5.0) | 0.25 |
| Accompanies patient to PCP or specialist appointments most of the time or always | 1557 (89.2) | 483 (80.0) | 515 (91.5) | 559 (96.5) | < 0.001 |
Generated from Pearson x2 tests for categorical variables and from Kruskal-Wallis tests for continuous variables.
CAPACITY indicates CAregiver Perceptions About Commun/ca7ion with Clinical Team members; NA, not available; PCP, primary care provider; PHQ-2, Patient Health Questionnaire 2; Q, quartile; TICS-M, Modified Telephone Interview Cognitive Status.
The results of the CFA revealed good standardized factor loadings in both the 1-factor and 2-factor solutions, with values above 0.50 throughout, and many above 0.75 (Table 2). The 2-factor model, with separate communication and capacity subscales, yielded better model fit statistics than the 1-factor solution, with an acceptable RMSEA of 0.09 and CFI and TLI values above 0.95, consistent with previous validation study findings.2 The 2 factors had a correlation of 0.72. None of the potential modifications that were explored, such as dropping items or switching which scale they load on, improved the model fit. Sensitivity analyses among different groups based on the level of education or reporting always attending patients’ health care visits did not modify the findings substantively. The subscales also yielded omega values indicative of excellent internal consistency, with 0.90 for the communication domain, and 0.94 for the capacity domain. The omega value for the full 1-dimensional scale was also excellent at 0.95. Altogether, these results support previous findings indicating the 2-dimensional structure of the CAPACITY scale and confirm its structural validity (or dimensionality) and reliability in a population of care partners to older adults with cognitive impairment.
TABLE 2.
Standardized Factor Loadings and Model Fit Statistics
| Individual Items of CAPACITY Measure | One-factor Solution | Two-factor Solution |
|---|---|---|
| Communication domain | ||
| Explain in way that is easy to understand | 0.53 | 0.58 |
| Understand the things that really matter to you | 0.61 | 0.67 |
| Ask your ideas about patient health | 0.81 | 0.86 |
| Give easy-to-understand instructions | 0.73 | 0.78 |
| Be responsive to your concerns about treatment plan | 0.76 | 0.80 |
| Ask for your ideas when developing/ adjusting treatment plan | 0.82 | 0.88 |
| Capacity domain | ||
| Ask how much help you could give | 0.84 | 0.89 |
| Ask how much help you wanted to provide | 0.83 | 0.87 |
| Ask whether you have the skills or training you need to help | 0.90 | 0.91 |
| Assess you to see what care you could successfully provide | 0.84 | 0.87 |
| Ask if you needed help at home in managing patient condition | 0.83 | 0.86 |
| Talk to you about community resources | 0.64 | 0.69 |
| Model fit indices | ||
| x2 | 1890.510 | 729.218 |
| df | 54 | 53 |
| Root mean square error of approximation | 0.140 | 0.085 |
| Confirmatory Fit Index | 0.928 | 0.973 |
| Tucker Lewis Index | 0.912 | 0.967 |
The x2 statistic and all factor loadings are significant at the P≤0.001 level.
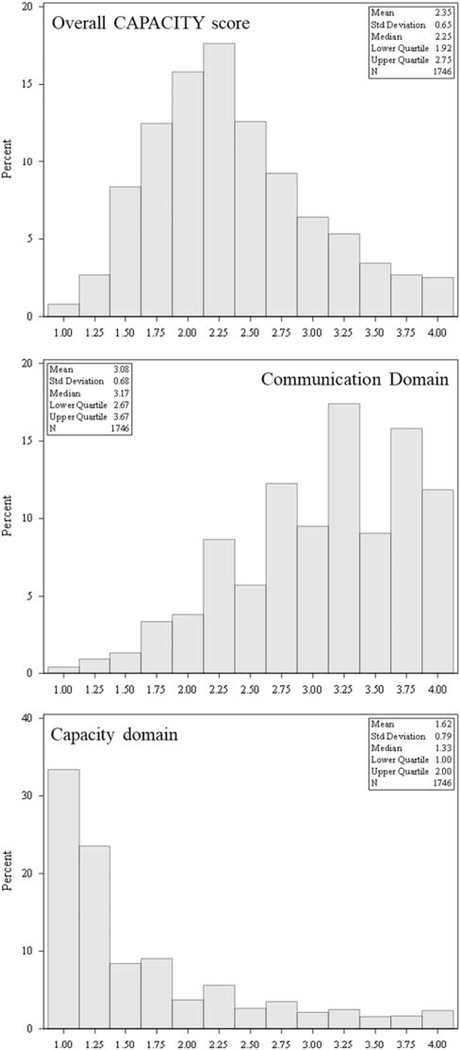
Overall CAPACITY scores had a mean of 2.35 (SD = 0.65) and median of 2.25 [quartile (Q) 1–Q3: 1.92–2.75] and had a slightly right-skewed but nearly symmetric distribution (Fig. 1). The component domains, however, were skewed in opposite directions; care partners tended to rate communication with the patient’s health care team favorably but report that those same providers rarely assessed whether the care partner felt that they had the ability, desire, skills, and resources to provide care and manage the patient’s health condition. Specifically, the communication domain scores were left-skewed, with a mean of 3.08 (SD = 0.68) and median of 3.17 (Q1–Q3: 2.67–3.67), while the capacity domain scores were right-skewed, with mean of 1.62 (SD = 0.79) and median of 1.33 (Q1–Q3: 1.00–2.00). On the basis of the factor analysis results supporting a 2-factor solution and the relatively high correlation between the factors, we present 3 regression models: the communication subscale, the capacity subscale, and an overall scale of CAPACITY. We present the overall scale to show the consistency of results between the subscales and the overall instrument.
FIGURE 1.
Histograms for the distributions in the CAPACITY scale overall and domain scores, with univariate statistics. CAPACITY indicates CAregiver Perceptions About CommunIcaTion with Clinical Team members.
The cognitive status of patients was measurably impaired, as indicated by a mean score of 20.4 (SD = 6.2) out of a possible total score of 41 on the TICS-M (Table 1). Adjusting for all other factors, a 5-point increase in the TICS-M score (ie, better patient cognition) was associated with a decrease of 2.3% [95% confidence interval (CI): 1.5%, 3.1%] on the communication domain score and a decrease of 4.5% (95% CI: 2.7%, 6.2%) on the capacity domain score (Table 3). In other words, care partners in dyads with more cognitively-intact patients tended to rate the patient’s health care providers as being less likely to communicate effectively with the care partners and less likely to assess the care partners’ capacity to care for the patient (Table 3).
TABLE 3.
Adjusted Estimates for the Associations Between the Study Variables and the CAPACITY Total Score, Communication Domain Score, and Capacity Domain Score
| Description of Included Variables | Communication Domain |
Capacity Domain |
Overall |
|||
|---|---|---|---|---|---|---|
| Ratio (95% CI) | P* | Ratio (95% CI) | P* | Ratio (95% CI) | P* | |
| Primary variables of interest | ||||||
| Cognitive impairment (TICS-M), per 5-point increase (patient) | 0.98 (0.97, 0.99) | < 0.001 | 0.95 (0.94, 0.97) | < 0.001 | 0.97 (0.96, 0.98) | < 0.001 |
| Health literacy (care partner) | 0.99 (0.98, 1.00) | 0.19 | 0.97 (0.94, 0.99) | 0.008 | 0.98 (0.97, 1.00) | 0.02 |
| Patient characteristics | ||||||
| General health status | 1.00 (0.99, 1.01) | 0.77 | 0.98 (0.95, 1.00) | 0.05 | 0.99 (0.98, 1.00) | 0.19 |
| Education | 0.10 | 0.11 | 0.049 | |||
| High school graduate or less | Reference | Reference | Reference | |||
| Some college | 0.97 (0.94, 1.00) | 0.07 | 0.94 (0.88, 1.01) | 0.10 | 0.96 (0.92, 1.00) | 0.04 |
| Bachelor’s degree | 0.97 (0.94, 1.01) | 0.14 | 0.94 (0.87, 1.00) | 0.07 | 0.96 (0.92, 1.00) | 0.05 |
| Graduate degree | 0.96 (0.93, 0.99) | 0.01 | 0.92 (0.85, 0.98) | 0.01 | 0.94 (0.91, 0.98) | 0.005 |
| Care partner characteristics | ||||||
| Age | < 0.001 | 0.007 | < 0.001 | |||
| < 75 | Reference | Reference | Reference | |||
| 75–84 | 0.97 (0.95, 0.99) | 0.008 | 0.93 (0.88, 0.98) | 0.007 | 0.96 (0.93, 0.99) | 0.003 |
| 85+ | 0.90 (0.85, 0.96) | 0.002 | 0.87 (0.76, 1.00) | 0.046 | 0.89 (0.82, 0.96) | 0.004 |
| Male | 0.96 (0.94, 0.99) | 0.002 | 1.09 (1.04, 1.14) | < 0.001 | 1.01 (0.98, 1.04) | 0.65 |
| Race | ||||||
| White | Reference | Reference | Reference | |||
| Other race | 1.00 (0.96, 1.06) | 0.85 | 1.14 (1.03, 1.25) | 0.01 | 1.05 (0.99, 1.12) | 0.10 |
| Education | 0.14 | 0.008 | 0.02 | |||
| High school graduate or less | Reference | Reference | Reference | |||
| Some college | 1.02 (0.98, 1.05) | 0.29 | 1.03 (0.96, 1.11) | 0.45 | 1.02 (0.98, 1.07) | 0.29 |
| Bachelor’ s degree | 1.02 (0.99, 1.06) | 0.26 | 0.99 (0.92, 1.07) | 0.77 | 1.01 (0.97, 1.05) | 0.64 |
| Graduate degree | 1.04 (1.00, 1.08) | 0.03 | 1.09 (1.01, 1.18) | 0.02 | 1.06 (1.01, 1.11) | 0.01 |
| Relationship to patient | ||||||
| Spouse/significant other | Reference | Reference | Reference | |||
| Parent/other | 1.00 (0.96, 1.03) | 0.89 | 1.08 (1.00, 1.17) | 0.06 | 1.03 (0.98, 1.07) | 0.27 |
| Employment | 0.95 | 0.51 | 0.69 | |||
| Not working or don’t know/refused | Reference | Reference | Reference | |||
| Part-time | 1.00 (0.97, 1.03) | 0.86 | 0.96 (0.89, 1.03) | 0.27 | 0.98 (0.94, 1.02) | 0.41 |
| Full-time | 0.99 (0.95, 1.04) | 0.75 | 0.97 (0.88, 1.06) | 0.51 | 0.99 (0.94, 1.04) | 0.63 |
| Time providing care for the patient | < 0.001 | 0.002 | < 0.001 | |||
| Not providing care or don’ t know/refused | Reference | Reference | Reference | |||
| 05 h or fewer a wk | 0.97 (0.94, 1.00) | 0.02 | 0.91 (0.86, 0.97) | 0.003 | 0.95 (0.92, 0.98) | 0.003 |
| 06–19 h a wk | 1.03 (1.00, 1.07) | 0.07 | 1.03 (0.96, 1.11) | 0.44 | 1.03 (0.99, 1.08) | 0.14 |
| 20–39 h a wk | 1.04 (0.99, 1.09) | 0.13 | 1.02 (0.92, 1.13) | 0.70 | 1.03 (0.97, 1.09) | 0.27 |
| 40 or more hours a wk | 1.07 (1.02, 1.13) | 0.004 | 1.07 (0.97, 1.19) | 0.17 | 1.07 (1.01, 1.14) | 0.02 |
| General health status | 0.98 (0.97, 0.99) | 0.002 | 0.99 (0.96, 1.01) | 0.32 | 0.98 (0.97, 1.00) | 0.03 |
| Depression (PHQ-2) | 0.93 (0.89, 0.97) | 0.001 | 0.94 (0.85, 1.03) | 0.16 | 0.93 (0.88, 0.98) | 0.01 |
| Subjective burden (Zarit), per 5-point increase | 0.99 (0.98, 1.00) | 0.003 | 0.99 (0.97, 1.00) | 0.12 | 0.99 (0.98, 1.00) | 0.01 |
As a sensitivity analysis, we reran these models among the 1557 care partners who reported attending the patient’s medical visits “most of the time” or “always,” because we hypothesized that the associations may be even stronger with CAPACITY. In the interest of brevity, these results are available upon request, showing associations between the study variables and CAPACITY scores that were consistent with the main findings. We also reran these models including care partner cognitive status (TICS-M), and results were similar. These results are also available upon request.
For categorical variables parameterized with binary indicator variables, the P-values shown on the unindented rows are for type 3 tests for the overall contribution of that variable to the model. Likelihood ratio x2 tests indicated the covariates included provided significant improvement in explaining the variance of the outcome (all P < 0.0001).
CAPACITY indicates CAregiver Perceptions About Commun/ca7ion with Clinical Team members; CI, confidence interval; PHQ-2, Patient Health Questionnaire 2; TICS-M, Modified Telephone Interview Cognitive Status.
Health literacy among care partners in this study was very high, with a mean of 4.5 (SD = 0.9) out of 5, meaning that, on average, they never or only occasionally needed help reading medical forms or hospital materials (Table 1). After adjustment, a 1-point increase in health literacy was associated with a 3.2% (95% CI: 0.9%, 5.5%) decrease in the capacity domain score, but was not significantly associated with the communication domain, and a 1.7% (95% CI: 0.3%, 3.1%) decrease in the overall CAPACITY score13 (Table 3). In plain language, care partners with higher health literacy tended to rate their interactions with patient’s medical providers slightly less favorably overall and were slightly less likely to feel that the providers adequately assessed the care partner’s capacity to care for the patient’s condition.
Several other factors were identified as post hoc as being associated with CAPACITY after adjustment. Older care partners (> 75–84 or ≥ 85 vs. ≤ 75) rated the team less favorably overall and on each domain (Table 3). While there was no association between sex and overall CAPACITY score, male care partners rated communication with the team 4% (95% CI: 1%, 6%) lower than females but rated the capacity-assessment of the providers 9% (95% CI: 4%, 14%) higher than females. Reporting a few hours of active caregiving (< 5 h a wk) was associated with lower CAPACITY overall and lower subscales. The overall and communication-specific CAPACITY scores of care partners who screened positive for depression on the PHQ-2 were 7% lower than those who were not depressed; however, depression was not statistically significantly associated with the capacity domain scores. A 5-point increment in subjective burden was associated with a 1% (95% CI: 0%, 2%) decrease in both the overall CAPACITY and communication domain scores.
Results did not change in the 2 sensitivity analyses (detailed results available upon request).
DISCUSSION
The CAPACITY instrument measures how well care partners perceive themselves to be integrated into the patient’s health care team. Specifically, it assesses how well the health care team is perceived to communicate with care partners and the extent to which care partners feel the team asks them about their capacity and preferences for involvement in the patient’s care. This study marks the first time the CAPACITY score has been tested in a sample of care partners of older adults with memory problems. CFA showed the CAPACITY items fit the expected 2-factor structure in our sample (communication and capacity). Furthermore, the 2-factor model, with separate communication and capacity subscales, yielded better model fit statistics than the 1-factor solution. We presented regression models for both subscales and the overall measure because we were interested in understanding consistency of the relationship between capacity domains and the independent variables of interest. We found some differences in the strength of the association between covariates of interest (eg, health literacy) and the individual CAPACITY domains versus the overall score, such that interpretation was aided by examining an individual domain. Given the results of the measurement properties of the instrument, other research teams should consider 2 separate domains of the CAPACITY score in their own study designs, either as separate dependent variables or using structural equation modeling for a multidimensional latent construct.
We also found that higher cognitive functioning of patients was associated with lower communication domain scores, lower capacity domain scores, and lower overall CAPACITY scores.
The negative association between higher cognition and communication merits comment. For patients with MCI, communication between the care team and the care partner may not be as essential as for more severe cognitive impairment; the patient likely can communicate directly with the team to state preferences, report concerns, and ask questions. Thus, the findings may not indicate the need for intervention. However, the communication items on the CAPACITY score may be viewed as important to care partners for future planning, so the perception that these items are neglected may represent a missed opportunity for the health care team to establish a high-quality relationship with the care partner. This would also allow teams to engage partners earlier in the disease course so that their decision-making is as consistent as possible with patient preferences.16 The inverse relationship between care partner’s perception of communication and patient’s cognitive function could also be due to patients with MCI receiving a less definitive diagnosis and prognosis from providers; this uncertainty could have been unsatisfying and reduced care partner perceived quality of interaction with the health care team. In addition, that care partners’ perceptions about interactions may track with worse quality of care more broadly is a concern given that people with cognitive impairment have been found to receive a lower quality of care.29–32 Importantly, in our sample care partners had normal cognitive status, indicated by the mean TICS-M score. Yet future studies should collect care partner cognitive status and may want to include it in their multivariable models to understand whether perceived communication with health care teams differs by the care partner’s own cognitive status (we thank a peer reviewer for making this important point). Several covariates were not significantly associated with perceived communication, such as the relationship between patients and care partners and work status. This could be due to a lack of variation in the covariate or other reasons and could be tested in other contexts.
We found that higher health literacy was associated with a lower capacity domain score on average, but had little relationship with quality of communication. Those with higher health literacy may also have higher expectations of the team,6 so this finding could be a proxy for some other quality expectation marker. Qualitative methods would be needed to fully interpret the reasons for our findings.
Our study has notable limitations, such as that it relies on cross-sectional survey data with respondents who have high access to care, as signified by them all receiving an amyloid positron emission tomography scan. Study participants were also predominantly White and more highly educated than the general US population of older adults, and care partners were predominantly spouses. In addition, we did not capture the provider’s perspective.
The finding that the CAPACITY measure is structurally valid and reliable in care partners of older adults with cognitive impairment means that it could provide an assessment of person-centered care for a broader range of care partners, including adult children or friend caregivers, or including patients with more severe memory problems, such as Alzheimer disease.33 In addition, CAPACITY affords the ability to shed light on the family experience of care for important populations of health care users, some of whom cannot report on their own. With so few measures quantifying the extent to which family members and friends feel integrated into the health care team, CAPACITY could be used more widely as a performance measure to assess the quality of health care teams.34 Assessing CAPACITY more broadly would fill a major gap in obtaining quantitative data on the experience of care from the perspective of the family or friend caregiver or care partner. Importantly, evaluating the CAPACITY measure over time could help teams monitor and evaluate systematic efforts to improve communication. A modest first step, given the relatively lower capacity domain scores compared with the communication domain scores, would be to increase interactions with care partners about their ability, desire, skills, and resources to provide care and manage the patient’s health condition. Addressing these unmet needs may then spill over to improve the communication domain. Substantially improving the perceived quality of communication and perceived support of care partners could, in turn, enable care partners to facilitate shared decision-making and person-centered care if the care partner is able to represent the preferences and wishes of the person with memory problems.35
ACKNOWLEDGMENTS
The authors acknowledge Faye Dvorchak, Kathleen Nye, and Bobbi Burwell for their support in managing the CARE-IDEAS study. They also acknowledge the caregivers and patients affiliated with the Duke Bryan Center for helping us pilot test survey questions and the participants in the CARE-IDEAS Study.
This study, entitled Caregivers’ Reactions and Experiences: Imaging Dementia—Evidence for Amyloid Scanning (CARE-IDEAS) was funded by National Institute on Aging, NIH, 1R56AG053934-01, National Institute on Aging, NIH, 5R01AG053934-02. We have deposited specific information regarding the SAS code used in the analyses in an electronic repository as a guide for investigators who obtain the requisite data use agreement for the relevant data sources and want to replicate our study (https://repository.library.brown.edu/studio/item/bdr:1114618/).
Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Number R01AG053934 and by the American College of Radiology Imaging Network and the Alzheimer’s Association. The content is solely the responsibilities of the authors and does not necessarily represent the official views of the National Institutes of Health, the American College of Radiology Imaging, or the Alzheimer’s Association.
C.H.V.H. received support from the Center for Innovation to Accelerate Discovery and Practice Transformation, at the Durham VA Healthcare System (CIN 13-410). The remaining authors declare no conflict of interest.
REFERENCES
- 1.Alzheimer’s Association. 2019 Alzheimer’s Disease Facts and Figures; 2019. Available at: www.alz.org/media/Documents/alzheimers-facts-and-figures-2019-r.pdf Accessed February 20, 2019.
- 2.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23:207–218. [DOI] [PubMed] [Google Scholar]
- 3.Laidsaar-Powell RC, Butow PN, Bu S, et al. Physician-patient-companion communication and decision-making: a systematic review of triadic medical consultations. Patient Educ Couns. 2013;91:3–13. [DOI] [PubMed] [Google Scholar]
- 4.Wolff JL, Roter DL. Family presence in routine medical visits: a meta-analytical review. Soc Sci Med. 2011;72:823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49:796–804. [PubMed] [Google Scholar]
- 6.Stewart MA. Effective physician-patient communication and health outcomes: a review. Can Med Assoc J. 1995;152:1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 7.Barr PJ, Thompson R, Walsh T, et al. The psychometric properties of CollaboRATE: a fast and frugal patient-reported measure of the shared decision-making process. J Med Internet Res. 2014;16:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garvelink MM, Ngangue PA, Adekpedjou R, et al. A synthesis of knowledge about caregiver decision making finds gaps in support for those who care for aging loved ones. Health Aff (Millwood). 2016;35: 619–626. [DOI] [PubMed] [Google Scholar]
- 9.Giguere AMC, Lawani MA, Fortier-Brochu E, et al. Tailoring and evaluating an intervention to improve shared decision-making among seniors with dementia, their caregivers, and healthcare providers: study protocol for a randomized controlled trial. Trials. 2018;19:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groen-van de Ven L, Smits C, Span M, et al. The challenges of shared decision making in dementia care networks. Int Psychogeriatr. 2016;30: 843–857. [DOI] [PubMed] [Google Scholar]
- 11.van de Pol MHJ, Fluit CRMG, Lagro J, et al. Shared decision making with frail older patients: proposed teaching framework and practice recommendations. Gerontol Geriatr Educ. 2017;38:482–495. [DOI] [PubMed] [Google Scholar]
- 12.Van Houtven C, Miller K, O’Brien E, et al. Development and initial validation of the Caregiver Perceptions About Communication With Clinical Team Members (CAPACITY). Med Care Res Rev. 2017;76: 1–23. [DOI] [PubMed] [Google Scholar]
- 13.Levy H, Janke A. Health literacy and access to care. J Health Commun. 2016;21(suppl 1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IDEAS-Study Group. IDEAS: Imaging Dementia—Evidence for Amyloid Scanning; 2018. Available at: www.ideas-study.org/ Accessed February 12, 2019.
- 16.Jutkowitz E, Van Houtven CH, Plassman BL. Willingness to undergo a risky treatment to improve cognition among persons with cognitive impairment who received an amyloid PET scan. Alzheimer Dis Assoc Disord. 2020;34:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallo JJ, Breitner JC. Alzheimer’s disease in the NAS-NRC Registry of aging twin veterans, IV. Performance characteristics of a two-stage telephone screening procedure for Alzheimer’s dementia. Psychol Med. 1995;25:1211–1219. [DOI] [PubMed] [Google Scholar]
- 18.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1993;6:103–110. [Google Scholar]
- 19.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36:588–594. [PubMed] [Google Scholar]
- 20.Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedard M, Molloy DW, Squire L, et al. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41: 652–657. [DOI] [PubMed] [Google Scholar]
- 22.Zarit SH, Orr NK, Zarit JM. The Hidden Victims of Alzheimer’s Disease: Families Under Stress. New York, NY: New York University Press; 1985. [Google Scholar]
- 23.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649–655. [DOI] [PubMed] [Google Scholar]
- 24.O’Rourke N, Tuokko HA. Psychometric properties of an abridged version of The Zarit Burden Interview within a representative Canadian caregiver sample. Gerontologist. 2003;43:121–127. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41: 1284–1292. [DOI] [PubMed] [Google Scholar]
- 26.Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19:539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kline RB. Principles and Practice of Structural Equation Modeling. New York, NY: Guilford Press; 2010. [Google Scholar]
- 28.Dunn TJ, Baguley T, Brunsden V. From alpha to omega: a practical solution to the pervasive problem of internal consistency estimation. Br J Psychol. 2014;105:399–412. [DOI] [PubMed] [Google Scholar]
- 29.Hsiao FY, Peng LN, Wen YW, et al. Care needs and clinical outcomes of older people with dementia: a population-based propensity score-matched cohort study. PLoS One. 2015;10:e0124973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mecocci P, von Strauss E, Cherubini A, et al. Cognitive impairment is the major risk factor for development of geriatric syndromes during hospitalization: results from the GIFA study. Dement Geriatr Cogn Disord. 2005;20:262–269. [DOI] [PubMed] [Google Scholar]
- 31.Mukadam N, Sampson EL. A systematic review of the prevalence, associations and outcomes of dementia in older general hospital inpatients. Int Psychogeriatr. 2011;23:344–355. [DOI] [PubMed] [Google Scholar]
- 32.Naef R, Ernst J, Burgi C, et al. Quality of acute care for persons with cognitive impairment and their families: a scoping review. Int J Nurs Stud. 2018;85:80–89. [DOI] [PubMed] [Google Scholar]
- 33.Lynn Snow A, Cook KF, Lin PS, et al. Proxies and other external raters: methodological considerations. Health Serv Res. 2005;40:1676–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Houtven CH, Hastings SN, Colon-Emeric C. A path to high-quality team-based care for people with serious illness. Health Aff (Millwood). 2019;38:934–940. [DOI] [PubMed] [Google Scholar]
- 35.Mejia AM, Smith GE, Wicklund M, et al. Shared decision making in mild cognitive impairment. Neurol Clin Pract. 2019;9:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]