Abstract
Organisms respond to various environmental stressors by modulating physiology and behavior to maintain homeostasis. Steroids and catecholamines are involved in the highly conserved signaling pathways crucial for mounting molecular and cellular events that ensure immediate or long-term survival under stress conditions. The insect dopamine/ecdysteroid receptor (DopEcR) is a dual G-protein coupled receptor for the catecholamine dopamine and the steroid hormone ecdysone. DopEcR acts in a ligand-dependent manner, mediating dopaminergic signaling and unconventional “nongenomic” ecdysteroid actions through various intracellular signaling pathways. This unique feature of DopEcR raises the interesting possibility that DopEcR may serve as an integrative hub for complex molecular cascades activated under stress conditions. Here, we review previously published studies of Drosophila DopEcR in the context of stress response and also present newly discovered DopEcR loss-of-function phenotypes under different stress conditions. These findings provide corroborating evidence that DopEcR plays vital roles in responses to various stressors, including heat, starvation, alcohol, courtship rejection, and repeated neuronal stimulation in Drosophila. We further discuss what is known about DopEcR in other insects and DopEcR orthologs in mammals, implicating their roles in stress responses. Overall, this review highlights the importance of dual GPCRs for catecholamines and steroids in modulating physiology and behavior under stress conditions. Further multidisciplinary studies of Drosophila DopEcR will contribute to our basic understanding of the functional roles and underlying mechanisms of this class of GPCRs.
Keywords: stress, GPCR, nongenomic steroid action, catecholamine, Drosophila
I. Introduction
The insect dopamine/ecdysteroid receptor, or DopEcR, is a unique G-protein-coupled dual receptor for the catecholamine dopamine and the insect ecdysteroids, ecdysone (E) and 20-hydroxyecdysone (20E) (Srivastava et al., 2005). This receptor mediates unconventional rapid nongenomic actions of steroids, as well as dopamine signaling, and initiates multiple intracellular signaling pathways including the cyclic adenosine 3′,5′-monophosphate (cAMP), phosphoinositide 3-kinase (PI3K), and mitogen-activated protein kinase (MAPK) pathways, in a ligand-dependent manner. Because DopEcR is primarily expressed in the nervous system (Srivastava et al., 2005; Graveley et al., 2011; Abrieux et al., 2013), and both steroids and catecholamines act as stress hormones in vertebrates (McEwen, 2008), it is intriguing to consider that DopEcR coordinates different signaling pathways to modulate the physiological and behavioral response to stress. This review begins by introducing stress hormones and rapid unconventional steroid responses (section II), and then discusses work on ecdysone and dopaminergic signaling in Drosophila stress responses (section III). Following a historical perspective behind the identification and characterization of Drosophila DopEcR (section IV), we describe previous studies on DopEcR and provide new evidence showing that DopEcR is required for starvation-induced sleep suppression, neuronal stability during heat stress, and ethanol-induced locomotor behavior (section V). Next, we review the role of DopEcR in other insects (section VI) and its functional orthologs in mammals (section VII). Lastly, we provide perspective on future DopEcR research (section VIII). Together, the experimental findings of DopEcR’s function in insects, and comparative mammalian orthologs, provide the foundation for understanding dual GPCRs for catecholamines and steroids in regulation of physiological and behavioral response to stress.
II. Stress hormones and unconventional actions of steroids through G-protein coupled receptors (GPCRs)
The biological term “stress” was first coined more than 80 years ago by the endocrinologist Hans Selye to describe “the non-specific response of the body to any demand for change that potentially disturbs a complex dynamic equilibrium, or homeostasis” (Selye, 1936). Diverse environmental factors and conditions can serve as stressors that trigger a range of physiological and behavioral responses. They include heat, cold, toxic chemicals, starvation, dehydration, sleep deprivation, exposure to predators, social defeat, and many others. Responses to these stressors have evolved to maintain homeostasis and better an organism’s chance of survival in hazardous, potentially life-threatening situations. The fundamental molecular and genetic mechanisms underlying stress responses are therefore expected to be conserved among diverse organisms (Singh et al., 2008; Nesse et al., 2016).
Environmental stressors induce changes in, among others, endocrine states and stimulate the secretion of stress hormones such as cortisol. Steroid hormones were originally found to act through transcriptional regulation by nuclear steroid hormone receptors that function as steroid-dependent transcription factors, eliciting biological responses with a time lag of hours or even days (Evans, 1988; Beato, 1989). However, it has been known for a long time that steroids can also act via rapid mechanisms independent of transcriptional regulation, actions referred to as “nongenomic” responses to steroids. For example, it was reported as early as 1939 that estrogen rapidly exerts effects within 3–15 minutes on peripheral blood vessels were reported as early as 1939 (Reynolds and Foster, 1939). Selye (1942) also demonstrated that steroids, including cortisol, have an anesthetic effect in rats within minutes. Following these early reports, a number of studies have shown that steroid-mediated phenomena can occur very quickly, within seconds to minutes (Blackmore et al., 1990; Winter et al., 1999) and be observed even in cells lacking a functional nucleus, such as erythrocytes, platelets, or spermatozoa (Shivaji and Jagannadham, 1992; Moro et al., 2005; Ivanova et al., 2008).
Steroid hormones use multiple cellular mechanisms to generate rapid nongenomic effects. They can change physiological properties of target cells by influencing membrane fluidity (the viscosity of the lipid bilayer that determines the mobility of lipids and membrane associated proteins) or by directly binding ion channels, including GABA receptors and NMDA receptors (Park-Chung et al., 1997; Whiting et al., 2000; Hosie et al., 2006), to allosterically regulate their function. Under certain conditions, the rapid actions of steroids can be mediated by classical nuclear hormone receptors that localize to the plasma membrane and interact with cytosolic signaling molecules (Prager et al., 2010; Groeneweg et al., 2012). In addition to these mechanisms, it is now accepted that steroids can bind GPCRs and play an important role in nongenomic activation of steroid-triggered signaling pathways. The most well-studied GPCR of this class is the G-protein-coupled estrogen receptor 1 (GPER1) (Prossnitz and Barton, 2011; Feldman and Limbird, 2017). This receptor was initially identified as an orphan GPCR (GPR30) and then renamed GPER1 when it was discovered to mediate nongenomic actions of estrogen (Filardo et al., 2002; Filardo and Thomas, 2005; Revankar et al., 2005; Vivacqua et al., 2006; Revankar et al., 2007; Wang et al., 2008). GPER1 is expressed in many tissues, including reproductive organs, pancreas, liver, heart, arteries, breast, lung, leukocytes, and neural tissue (Hasbi et al., 2005). GPER1 activity affects multiple signaling pathways, including those involving epidermal growth factor receptor (EGFR), extracellular-related kinase (ERK), and inositol-trisphosphate 3-kinase (IP3K), and plays a role in diverse estrogen-dependent processes regulating endocrine, immune, neuronal, and cardiovascular functions. Considering that aldosterone is a more potent agonist of GPER1 than estrogen (Gros et al., 2011), GPER1 may have even wider roles in nongenomic steroid signaling than previously thought. Relevant to this review, GPER1 knockout male mice display reduced anxiety under stressful conditions compared to control males, whereas female mice show altered corticosterone levels and acute stress coping behavior in an estrous cycle-dependent manner (Kastenberger and Schwarzer, 2014). As such, the roles of GPER1 in stress responses are sex- and paradigm-dependent, but the cellular mechanisms by which GPER1 mediates steroid actions in response to stress still remain elusive.
III. Involvement of ecdysone and dopamine in the stress response in Drosophila
Ecdysone.
20-hydroxyecdysone (20E), commonly known as the molting hormone, is the major steroid hormone in insects (Riddiford, 1993). Its secretion from the endocrine ring gland plays a pivotal role in coordinating major developmental transitions, including larval molting and metamorphosis via the 20E-activated nuclear hormone receptor, ecdysone receptor (EcR) (Thummel, 1996; Beckstead et al., 2005; Yamanaka et al., 2013). Although the primary focus of ecdysone research has been on the regulation of development, accumulating evidence from studies in Drosophila suggest that ecdysone signaling has important functions in mature flies as well, particularly in responses to environmental stressors (Ishimoto and Kitamoto, 2011). For example, 20E levels increase in the ovaries and hemolymph of adult females upon exposure to stressors such as starvation, heat, and dehydration (Hirashima et al., 2000a; Rauschenbach et al., 2000; Gruntenko et al., 2005). Activated ecdysone signaling in adult females triggers the arrest of oogenesis (Soller et al., 1999; Terashima and Bownes, 2004), ovulation (Knapp and Sun, 2017), and egg-laying (Gruntenko et al., 2005). Under these unfavorable environments, development-arrested eggs are reabsorbed by the female’s body, and the energy and materials derived from the eggs are reutilized for their own survival (Terashima et al., 2005). Stress-induced elevation of 20E is accompanied by decreased ecdysis triggering hormone (ETH) levels, which results in the reduction of octopaminergic input to the reproductive tract and consequently ovulation arrest (Meiselman et al., 2018). In adult males, the social stress of being constantly rejected by sexually unreceptive females increases 20E levels, and this endocrinological change is necessary for the formation of long-term memory represented as courtship suppression (Ishimoto et al., 2009).
20E in adult flies also plays major roles in the regulation of gene expression in response to immune challenge (Flatt et al., 2008). Elevated levels of 20E in response to dehydration leads to adaptive enhancement of the innate immune response, promoting the robust induction of antimicrobial peptide (AMP) genes upon immune challenge (Zheng et al., 2018). Thus, environmental stressors acutely increase 20E levels in adult flies and induce physiological and behavioral transitions that are potentially advantageous for coping with stress. Counterintuitively, flies heterozygous for EcR loss-of-function mutations are more resistant to dying from heat, starvation, and oxidative stress, and exhibit a remarkable 50% longer lifespan under controlled laboratory conditions (Simon et al., 2003). Consistent with these findings, when 20E levels are reduced in mutants of an ecdysone biosynthetic gene, a similar lifespan extension is observed and the effects are reversed by feeding of 20E (Simon et al., 2003). These results suggest that ecdysone signaling in wildtype flies may be beneficial in certain contexts but detrimental in others. EcR or ecdysone synthesis mutants with reduced ecdysone signaling may optimize physiological states and health in adult flies to extend lifespan (Simon et al., 2003). However, it is possible that flies with subnormal ecdysone signaling are more vulnerable in the wild where they are more likely to be exposed to various environmental stressors.
Dopamine.
Dopamine is a biogenic catecholamine that functions as a neurotransmitter and hormone in organisms across taxa. Dopamine is produced and released in response to various stressors, which acutely alters animal’s physiology and behavior. Dopaminergic signaling in vertebrates governs and modulates a wide range of behavioral processes including motor control, appetite, mood, and motivation (Crocker, 1997; Arias-Carrion et al., 2010; Singh, 2014). Although not the focus of this review, similar roles for dopamine have been extensively studied in flies including locomotion, courtship, sleep/arousal, circadian modulation, temperature preference, drug response, and learning and memory, to name a few (For reviews please see Kume et al., 2005; Yamamoto and Seto, 2014; Kasture et al., 2018). There are approximately 130 neurons in the adult fly brain that synthesize and release dopamine (Mao and Davis, 2009), and the fly genome encodes four GPCRs serving as dopamine receptors: two D1-like Gs-coupled, Dop1R1 and Dop1R2, one D2-like, Dop2R, which is Gi-coupled, and the non-canonical dopamine and ecdysone receptor, DopEcR (Gotzes et al., 1994; Feng et al., 1996; Brody and Cravchik, 2000; Srivastava et al., 2005). The canonical receptors have each been implicated in various behavioral responses and are required in temporal-, cell- and pre- and/or post-synaptic manners (Karam et al., 2019).
There is also a profound appreciation for the precise spatiotemporal release of dopamine within the fly central nervous system that underlies behavioral regulation and stress responses (Neckameyer and Weinstein, 2005; Kasture et al., 2018). It was found that dopamine synthesis is altered in response to nutritional, mechanical, and oxidative stress (Neckameyer and Weinstein, 2005). Starvation-induced changes in dopaminergic signaling have further been shown to drive enhanced perception of nutrients (Linford et al., 2015). Dopaminergic signaling also modulates response to high temperatures via FOXO/insulin signaling pathway and juvenile hormone signaling (Hirashima et al., 2000b; Gruntenko et al., 2004; Rauschenbach et al., 2007; Gruntenko et al., 2016). The following sections will elaborate more on the non-canonical dopamine ecdysone receptor, DopEcR.
IV. Identification and characterization of Drosophila DopEcR as a dual GPCR for ecdysteroids and dopamine
Identification.
The Drosophila melanogaster gene CG18314 was originally categorized as an “unclassified biogenic amine receptor” (Brody and Cravchik, 2000) and ”nonpeptide orphan receptor” (Hewes and Taghert, 2001). Its product was first singled out as being a prominent target of RNA-editing in the fly transcriptome (Stapleton et al., 2002; Xia et al., 2005), but was soon after recognized for its unusual function as a GPCR that mediates nongenomic actions of ecdysone (Srivastava et al., 2005). Specifically, heterologous expression experiments demonstrated that cell-surface expression of this GPCR leads to cAMP activation, a D1-like response to dopamine, but could additionally elicit rapid ERK signaling in the presence of ecdysteroids. In recognition of its unique dual ligand sensitivity, CG18314 was aptly renamed DopEcR, the dopamine/ecdysteroid receptor (Srivastava et al., 2005). DopEcR contains conserved amino acids in its transmembrane region V that are important for catecholamine binding, and the physiologically relevant concentration of 1 μM of dopamine activated the PI3K/Akt pathway in fly Sf9 and mammalian CHO cells. Competition binding assays were then performed with ecdysteroids, including ponasterone A (PoA), a plant ecdysteroid. Radiolabeled PoA showed saturable binding specificity at KD = 10.4 ± 0.38 nM, and both ecdysone (E) and 20-hydroxyecdysone (20E) were able to displace PoA. The order of affinity was PoA > E > 20E and neither makisterone A, another insect steroid that activate nuclear ecdysteroid receptors nor dopamine could displace PoA. In addition to ecdysteroid specificity, ecdysteroids dose-dependently inhibited dopaminergic responses (Srivastava et al., 2005).
Gene expression profiling in different tissues and developmental stages revealed that DopEcR is highly expressed, particularly within the nervous system, and at the end of embryogenesis, during pupation, and into adulthood (Chintapalli et al., 2007; Ishimoto et al., 2009; Graveley et al., 2011). New evidence also finds DopEcR expression in heart tissue (Zheng et al., 2019). Loss-of-function and cDNA expression studies have now confirmed that DopEcR serves many functions in adult behavior, most of which are in response to stress.
Sequence.
The DopEcR gene spans a 12,739 bp region on the left arm of the third chromosome in D. melanogaster. Three different DopEcR transcripts are currently known, which differ in 5’ and 3’ UTR sequences, but each encodes for the same 322 aa protein sequence. Interestingly, at least 25 different RNA editing sites have been identified between DopEcR cDNA and EST sequences (Brody and Cravchik, 2000), but their functional implication remains to be determined. Phylogenetic analysis of the DopEcR protein sequence indicates that it shares greater similarity to vertebrate β-adrenergic receptors than it does to the fly dopaminergic receptor family (Srivastava et al., 2005), suggesting that DopEcR may have unique non-redundant dopaminergic functions compared to the other fly dopamine receptors.
Ligands.
As mentioned in the next section, DopEcR is necessary for sensing the starvation-induced dopamine released onto primary sugar-sensing neurons (Inagaki et al., 2012). It is also likely that DopEcR mediates dopaminergic signaling in starvation-induced sleep suppression (Fig. 1) and alcohol-induced hyperactivity (Fig. 3). These findings suggest that dopaminergic activation of DopEcR ultimately enhances neuronal excitability and potentiation. They also suggest that DopEcR is poised to locally respond within primary and higher order neuronal circuits to produce generalized or specific behavioral outcomes.
Figure 1: DopEcR is required for starvation-induced sleep suppression.
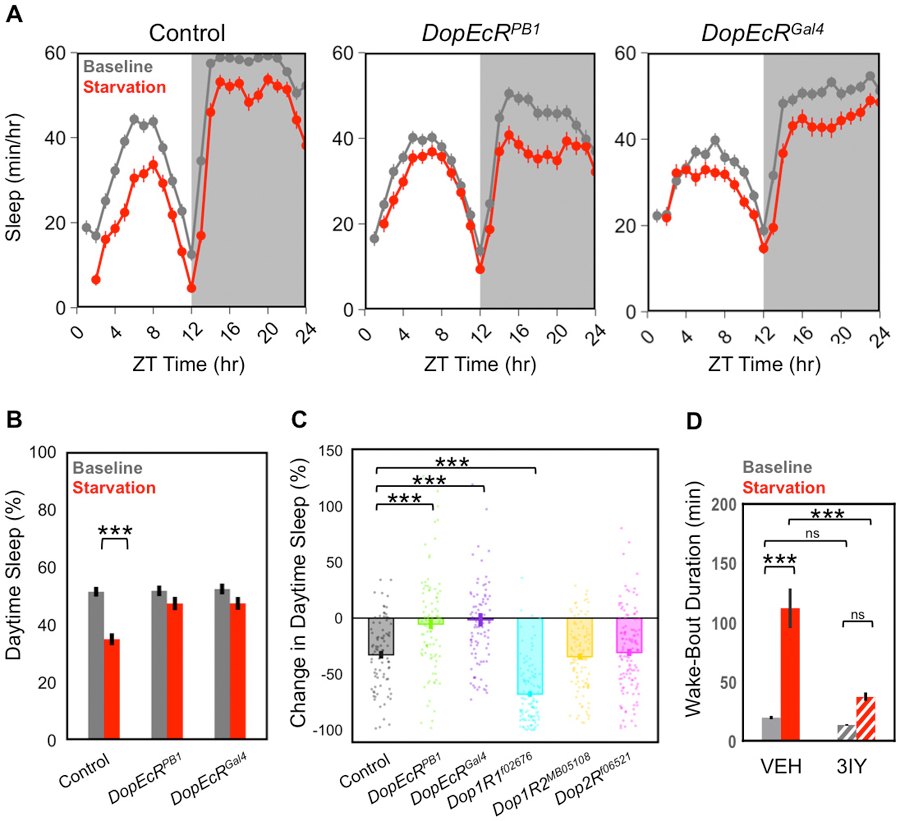
A) Sleep profiles of homozygous DopEcR hypomorphic mutants (DopEcRPB1, n = 95), null mutants (DopEcRGal4, n = 90), and control flies (w1118, n = 88) during a baseline (fed) day and the following starvation days. B) The amount of daytime (1–12 ZT hr) sleep in (A) on baseline and starvation days showed a statistically significant SISS response in w1118 controls, but not in DopEcRPB1 and DopEcRGal4 mutants. C) The percent change in sleep was determined for w1118, DopEcRPB1, DopEcRGal4, Dop1R1PL00420 (n = 115), Dop1R2MB05108 (n = 116), and Dop2Rf06521 (n = 126) flies. DopEcR mutants lacked SISS behavior, Dop1R1 mutants showed enhanced SISS, and Dop1R2 and Dop2R mutants showed normal SISS as compared to controls. D) Vehicle-treated flies (w1118, n = 82) showed a significant increase in wake-bout duration in response to starvation, whereas 6 mM 3IY treated flies (w1118, n = 105) did not display this extension in response to starvation. Experimental Methods: Flies were maintained in standardized conditions (25°C, 65% humidity, 12 hr day/night cycle) and aged to 3–5 days old. Both DopEcR mutants were backcrossed to w1118. Mated female flies were gently mouth pipetted into Drosophila activity monitor (DAM) glass vials (5 × 65 mm) containing 5% sugar 1% agar on a baseline day and then flies were manually flipped to 1% agar for a starvation day at Zeitgeber Time 0 (ZT 0 hr). For pharmacological manipulations, 3IY (Sigma-Aldrich, St. Louis, MO) or vehicle (1N HCl) was fed via addition to standard food for two days prior to experiment and throughout the experiment. Activity counts per minute were recorded and sleep was defined as 5 minutes or more of inactivity (Hendricks et al., 2000; Shaw et al., 2000). Roughly 2–3 experiments were performed for each genotype; averages and SEMs are plotted; ANOVA with Tukey posthoc tests were performed in R. A two-way ANOVA with tukey’s multiple comparison test was performed in prism for the pharmacological assay. **p<0.01,***p<0.001. DopEcRPB1 (BDSC #10847); DopEcRGal4 (Petruccelli et al., 2016); w1118 (VDRC #6000); Dop1R1PL00420 (BDSC #19491); Dop1R2MB05108 (BDSC #24743); Dop2Rf06521 (Exelixis f06521).
Figure 3. DopEcR mutants display abnormal ethanol-induced locomotion.
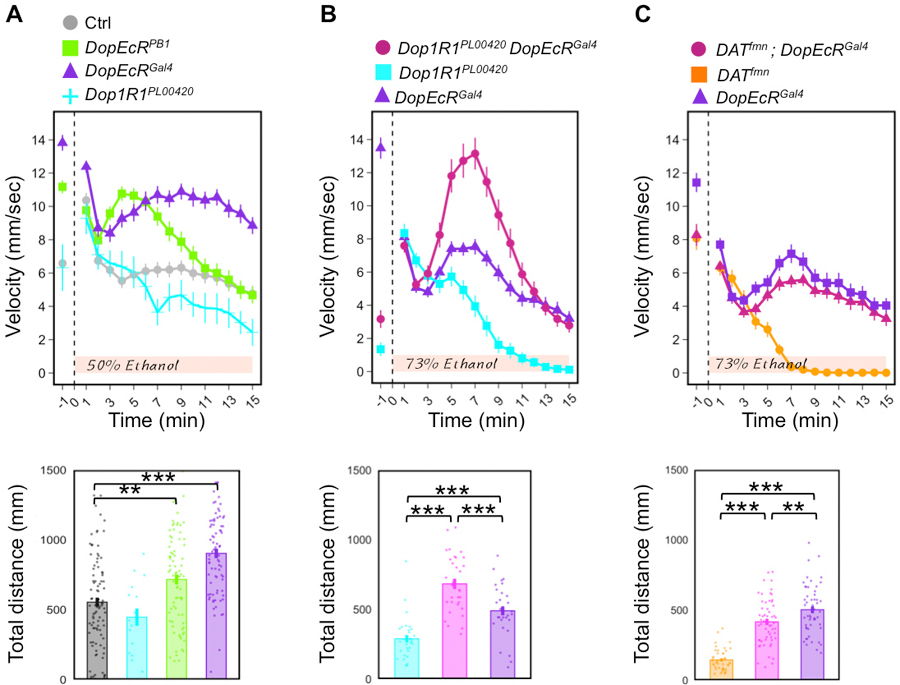
A) Homozygous hypomorphic DopEcRPB1 (n = 80) and null DopEcRGal4 (n = 80) mutants showed greater locomotor activity than control w1118 (n = 95) flies in response to 50% ethanol vapor, whereas Dop1R1PL00420 (n = 16) mutants displayer hypo-locomotor responses. B) Double mutant Dop1R1PL00420 DopEcRGal4 (n = 32) displayed a robustly higher locomotor activity than either DopEcRGal4 (n = 32) and Dop1R1PL00420 (n = 31) single mutants flies upon exposure to 73% ethanol vapor. C) Double mutant DATfmn ; DopEcRGal4 (n = 56) displayed a robustly higher locomotor activity than either DATfmn (n = 44) and Dop1R1PL00420 (n = 52) single mutants flies upon exposure to 73% ethanol vapor. Experimental Methods: Fly cultures were maintained in standardized conditions (25°C, 65% humidity, 12hr day/night cycle) and aged to 3–5-days-old. Male flies were loaded in DAM glass vials, placed in a custom-made plexiglass chamber and delivered 1 minute of humidified air and then 15 minutes of ethanol vapor. Videos were recorded using a LogiTech webcam and analyzed with pySolo tracking (Gilestro, 2012). 2–3 experiments were performed for each genotype; averages and SEMs are plotted; ANOVA repeated measures tests with Bonferroni post hoc tests were performed in R. ***p<0.001. DATfmn (obtained from Kazuhiko Kume, Nagoya City University, Japan).
In addition to dopamine, the other ligand of DopEcR, is the insect hormone ecdysone. It is generally believed that lipophilic steroid hormones, such as 20E, can enter cells through the plasma membrane by simple diffusion. However, a recent study demonstrated that Ecdysone Importer (EcI), an evolutionarily conserved solute carrier organic anion transporter, is required for 20E transport across the plasma membrane (Okamoto et al., 2018) to bind EcRs in the cytoplasm. DopEcR is currently the only non-canonical steroid GPCR identified in flies, and its activation by ecdysteroids on the plasma membrane elicits rapid (within minutes) nongenomic intracellular signaling events independent of canonical EcR (Srivastava et al., 2005; Evans et al., 2014). This function has been shown to mediate ecdysteroid responses in vivo including experience-dependent courtship suppression (Ishimoto et al., 2013) and alcohol-induced sedation (Petruccelli et al., 2016). DopEcR has also recently been implicated in mediating aldosterone steroid responses in adult nephrocytes, which filter hemolymph and have been shown to share functional, morphological, and molecular features with vertebrate podocytes (Zheng et al., 2019). Since DopEcR expression is detected, albeit at lower levels, in non-neural tissues such as the midgut, fat body, salivary gland, and testis (Leader et al., 2018), it is possible that DopEcR plays novel roles in these tissues in a dopamine- and/or ecdysone-dependent manner. Loss of DopEcR function does not appear to grossly impact D. melanogaster’s developmental processes despite detectable mRNA expression of DopEcR during development (Petruccelli et al., 2016). A recent paper, though, has thoroughly implicated DopEcR in the development of the lepidopteran agricultural pest Helicoverpa armigera (Kang et al., 2019)(see ‘Roles of DopEcR in other insects‘). It is worth reexamining a potential role of DopEcR in Drosophila development particularly under unfavorable conditions that may trigger stress responses.
Intracellular signaling.
Whether activated by dopamine, ecdysone or independent of a ligand, DopEcR can trigger rapid intracellular signaling events. Both PI3K/Akt and MAPK/ERK signaling was initiated in response to dopamine and ecdysone, respectively, in in vitro heterologous expression systems (Srivastava et al., 2005). In vivo, cAMP and ERK signaling have been functionally observed to be downstream of DopEcR (Ishimoto et al., 2009; Petruccelli et al., 2016). In vivo, bioluminescent calcium imaging revealed elevated calcium responses to nicotinic stimulation within mushroom body (MB) neurons lacking DopEcR, indicating that DopEcR signaling normally functions to negatively modulate MB excitability (Lark et al., 2017). Furthermore, dopamine signaling likely drives these inhibitory changes in the medial lobes of the MBs, whereas ecdysone enhances calcium signaling primarily in the calyx and cell body region of the MBs. Together these findings suggest that DopEcR mediates complex changes to MB excitability in an agonist-specific manner. In general, GPCRs are known to extensively interact with ion channels to influence neuronal excitability. Interactions include receptor-channel co-trafficking, channel modulation by G-α or -β/γ molecules, post-translational modifications, and changes in lipid membrane dynamics (Inanobe and Kurachi, 2014). Although the particular G-proteins coupled to DopEcR currently remain unknown, the evidence for DopEcR-ion channel interactions or crosstalk is mounting (e.g., heat-induced seizure and defective habituation in DopEcR loss-of-function mutants). Revealing these possible interactions will help elucidate DopEcR’s influence in excitable cells.
V. Functions of Drosophila DopEcR in stress responses
In Drosophila, DopEcR is dispensable for survival, at least under controlled laboratory conditions (Petruccelli et al., 2016). Loss-of-function mutants of Drosophila DopEcR exhibit no gross developmental or anatomical defects. Nonetheless, their physiological and behavioral responses to some environmental stressors are considerably different from those of genetic controls, indicating the critical function of DopEcR in various stress responses.
Starvation.
DopEcR’s first documented function in behavior was for the dopaminergic modulation of enhanced proboscis extension response (PER) following starvation (Inagaki et al., 2012). The PER assay has been used to evaluate taste sensitivity as well as the overall motivation to consume food in flies (Dethier, 1976). Inagaki and colleagues show that starvation increases dopaminergic release onto Gr5a sugar-sensing neurons, and that DopEcR is required in these sensory neurons to mediate evoked calcium in response to extra dopamine release and ultimately increase PER (Inagaki et al., 2012). Thus, DopEcR is required for starvation-dependent enhancement in sugar sensitivity and PER behavioral response. Building upon this finding, a follow up study by Inagaki and colleagues showed that DopEcR is not required for the reciprocal starvation-induced reductions in bitter compound sensitivity, which is instead mediated by sNPF (short neuropeptide F) via sNPFR (Inagaki et al., 2014). DopEcR is also required for increasing appetite in flies chronically fed sucralose. Sucralose is a non-nutritive synthetic sweetener, which is known to trigger a neuronal fasting response in control flies and increases the motivation to eat (Wang et al., 2016). Thus, DopEcR appears to be necessary in specific pathways in the nervous system for higher-order detection and/or response to starvation.
Since starvation is potentially life-threatening, many organisms under starvation conditions will forgo sleep in order to obtain food for survival. This behavior, known as starvation-induced sleep suppression (SISS), has been observed across phyla. In Drosophila, a mild starvation, or fasting state, promotes wakefulness by largely impairing sleep initiation and prolonging wake-bout lengths (Keene et al., 2010; McDonald and Keene, 2010; Melnattur and Shaw, 2019; Yurgel et al., 2019). SISS is most readily observed in the daytime hours just after flies are transferred to wet starvation conditions. Consistent with the critical role of DopEcR in enhancing feeding behavior under starvation, we found that DopEcR loss-of-function mutants were defective in SISS behavior. As shown in Fig. 1A and 1B, DopEcR hypomorphic (DopEcRPB1) and null (DopEcRGal4) mutants showed less sleep suppression than control flies. It is also important to note that baseline sleep patterns also appear to be structurally different, with mutants showing reduced sleep in the middle of the day and less overall evening sleep. Quantification of the total daytime sleep was consistent across control and DopEcR flies on the baseline day, but only controls showed a statistically significant loss of sleep upon starvation (Fig. 1B). The percent change response from baseline confirmed that DopEcR mutants displayed statistically significant differences from control flies (Fig. 1C). This suggests that DopEcR is necessary for wake-promoting behavior in response to starvation. Since dopamine is known to promote wakefulness in Drosophila under regular fed conditions (Andretic et al., 2005; Kume et al., 2005), DopEcR likely mediates SISS through a dopaminergic signal. Elimination of dopamine via application of dopamine synthesis inhibitor 3-iodotyrosine (3IY) was shown to suppress the starvation-induced lengthening of wake-bouts in control flies (Fig. 1D), indicating the critical involvement of dopamine signaling in SISS, possibly via DopEcR. Mutants for the other conventional dopamine receptors Dop1R1, Dop1R2, and Dop2R, displayed normal, or even exaggerated, SISS behavior (Fig. 1C). Thus, DopEcR serves a unique wake-promoting role in response to starvation. Ecdysone signaling can also influence sleep via EcRs (Ishimoto and Kitamoto, 2010), but whether DopEcR mediates an ecdysteroid signal to control SISS remains to be investigated.
Heat.
Heat is another commonly experienced environmental stressor. Insects are ectotherms (“cold-blooded” organisms), and thus internal body temperatures are especially vulnerable to change in the ambient temperature. Since temperature has a significant impact on conductance and gating kinetics of ion channels (Kiernan et al., 2001; Buzatu, 2009; Chowdhury et al., 2014), heat treatment may acutely induce abnormalities in neural activity and behavior. Mutations in ion channel genes often increase the sensitivity to heat and result in heat-induced behavioral phenotypes such as paralysis and seizure. parats1, a recessive loss-of-function, temperature-sensitive mutation in the fly voltage-gated sodium channel gene paralytic (para), causes an immediate, but reversible, paralysis of adult flies when they are shifted from 22°C to 29°C (Suzuki et al., 1971). paraGEFS+ is another para allele which harbors a gain-of-function mutation that mimics the human mutation underlying an epilepsy syndrome called generalized epilepsy with febrile seizures plus (GEFS+). Upon exposure to 40°C homozygous or hemizygous paraGEFS+ flies display seizure-like behavior, including leg twitches, failure to maintain standing posture, wing buzzing, and occasional abdomen curling (Sun et al., 2012). Remarkably, we found that DopEcR mutants show behavioral changes reminiscent of paraGEFS+ responses upon heat stress (Fig. 2A). Mutants displayed robust heat-induced seizure-like behavior with ~90% of homozygous DopEcRGal4 null mutants and ~40% of homozygous DopEcRPB1 hypomorphic mutants showing seizure-like activity within two minutes of 40°C heat treatment. After the onset of heat, DopEcRGal4 flies rapidly lost postural control and fell over onto their backs. They often extended or buzzed their wings and repeatedly kicked their legs or curled their abdomens up into the air. DopEcR trans-heterozygotes (DopEcRGal4/DopEcRPB1) expectantly failed to compliment, and DopEcR appeared to be haplosufficient for this trait since heterozygous mutants (DopEcRGal4/+ and DopEcRPB1/+) showed little seizure-like behavior upon heat treatment (Fig. 2B). Although DopEcR is required to maintain neuronal homeostasis under extreme heat, DopEcR mutants have normal heat place memory (Ostrowski et al., 2015), suggesting that they have intact noxious heat sensation.
Figure 2: Loss-of-function DopEcR mutants exhibit a heat-induced seizure-like phenotype.
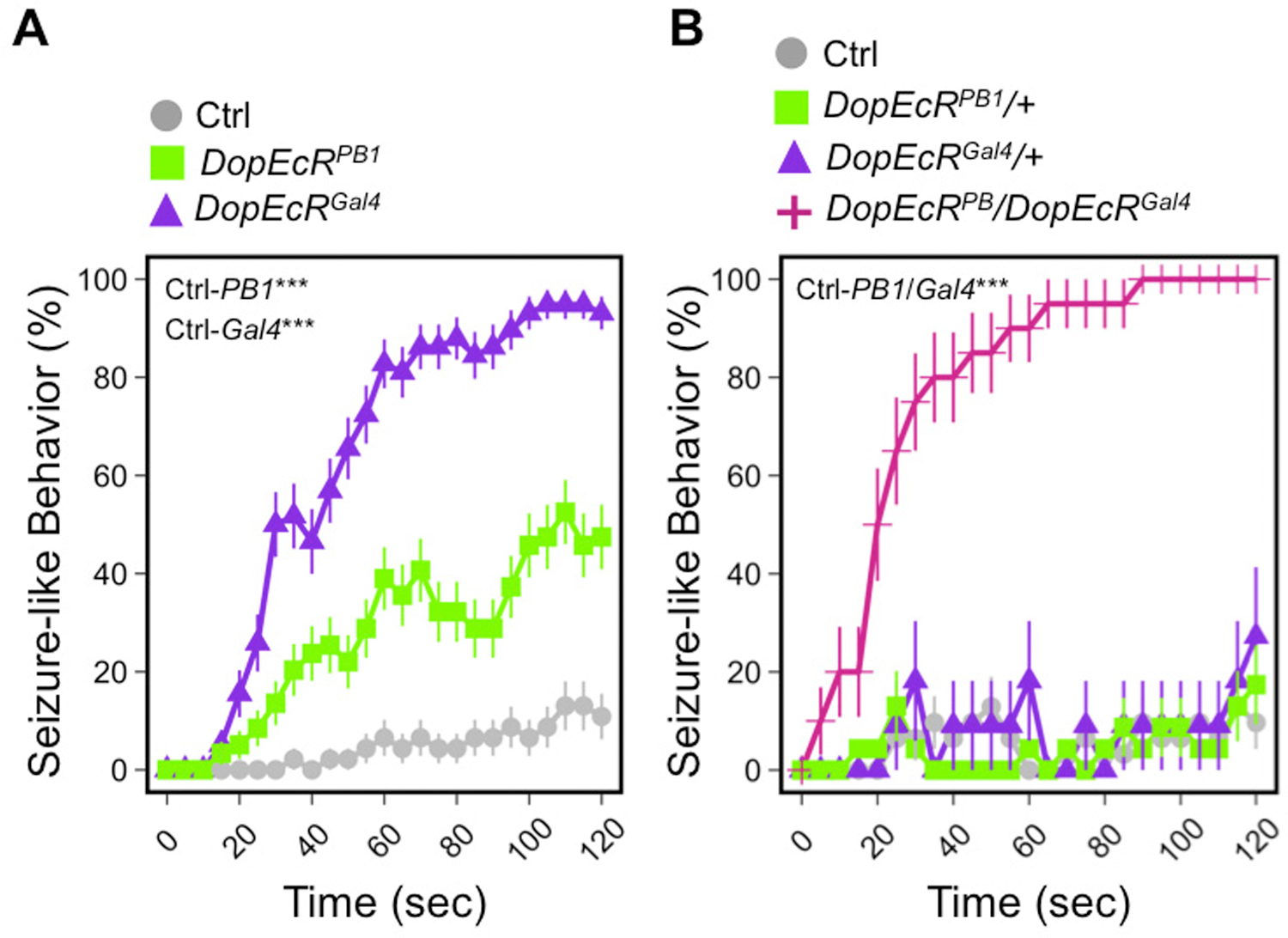
A) Homozygous hypomorphic DopEcRPB1 (n = 59) and null DopEcRGal4 (n = 46) mutants were more susceptible than w1118 (n = 58) control flies to heat-induced seizure behavior. B) Heterozygous DopEcRPB1 /+ (n = 33) and DopEcRGal4 / + (n = 20) flies behaved similarly to control w1118 / + (n = 35), suggesting haplosufficency of DopEcR, whereas trans-allelic heterozygotes DopEcRPB1 / DopEcRGal4 (n = 45) expectantly failed to compliment and showed heat-induced seizure susceptibility. Experimental Methods: Fly cultures were maintained in standardized conditions (25°C, 65% humidity, 12 hr day/night cycle) and aged to 5-days-old. Flies were then placed into small glass vials (15 mm x 45 mm), allowed to acclimate for 5–15 minutes, and then submerged in a 40°C water bath for 2 minutes. Behavior was scored at 5-second intervals. Flies were scored as having a ‘seizure’ if they had lost posture and fallen over, or exhibited abdomen curling and wing buzzing behaviors. 2–3 experiments were performed for each genotype and male and female data were combined for genotypic averages; averages and SEMs are plotted; ANOVA repeated measures tests with Tukey posthoc tests were performed in R. ***p<0.001.
Alcohol.
Alcohol is a natural by-product of fermentation, and many organisms are sensitive to its disinhibiting effects. Flies are ecologically drawn to low-levels of alcohol since it represents good food sources and egg-laying locations. Similar to mammals, as internal levels of ethanol rise, flies display characteristic loss-of-postural control and sedation behavior (Wolf et al., 2002). DopEcR has previously been found to mediate an ecdysteroid response in the context of acute ethanol exposure (Petruccelli et al., 2016). During 50% ethanol vapor exposure, DopEcR mutants lost postural control around the same time as control flies, but sustained shaking and re-righting movements long after control flies sedated. DopEcR was required in cholinergic or peptidergic neurons, but not MB neurons, to promote ethanol-induced sedation, and 20E feeding negatively modulated this function (Petruccelli et al., 2016; Aranda et al., 2017). Beyond its role in ethanol-induced sedation, we show here that DopEcR is also required for ethanol-induced locomotion (Fig. 3A). Loss-of-function DopEcR mutants displayed enhanced and prolonged hyperactivity during 50% ethanol vapor exposure compared to control flies. This phenotype is interestingly opposite of the previously published phenotype of a Dop1R1 hypomorphic allele, Dop1R1PL00420 (Kong et al., 2010), suggesting that DopEcR counteracts Dop1R1 signaling. To test the genetic interaction between these receptors, double mutant Dop1R1PL00420 DopEcRGal4 flies were created and assayed in 73% ethanol vapor. Surprisingly, double mutants showed a dramatically exaggerated hyperlocomotor response to ethanol (Fig. 3B), suggesting a complex neuronal hierarchy or cellular interaction between DopEcR and Dop1R1. Lastly, to determine if DopEcR is indeed mediating a dopaminergic signaling in this behavior, we assessed the response of dopamine transporter fumin (DATfmn) mutants (Kume et al., 2005) and DATfmn; DopEcRGal4 double mutants. Double mutants showed a similar response to that of DopEcRGal4 mutants (Fig. 3C) supporting the model where DopEcR acts downstream of synaptic dopamine. It is also important to note that baseline locomotion during air exposure may, itself, contribute to subsequent ethanol-induced responses, but to our knowledge this correlation has not yet been extensively investigated. Future studies are needed to elucidate the molecular and cellular underpinnings of interactions between DopEcR and Dop1R1, and whether DopEcR mediates ecdysteroid signaling to influence ethanol-induced locomotion.
Courtship rejection.
Drosophila males are innately able to display a stereotypical sequence of courtship rituals toward females (Hall, 1994). However, male courtship behavior is also subject to modification through social experience with females. Recently mated females are sexually unreceptive and resist male courtship advances by displaying a series of characteristic rejection behaviors, including extruding their ovipositor, kicking, or decamping (Villella and Hall, 2008). Receiving rejections from unreceptive females is considered a form of social stress for the courting male, and repeated courtship rejection ultimately suppresses male’s courting behavior, a behavior exploited for a learning and memory assay in Drosophila (Siegel and Hall, 1979). The first evidence of DopEcR mediating a nongenomic ecdysteroid response in vivo was for its role in experience-dependent courtship suppression (Ishimoto et al., 2013). Specifically, DopEcR in the MB neurons is required for 20E-induced changes in cAMP that necessitates short-term courtship memory formation and/or expression. Later, calcium imaging experiments demonstrated that ecdysone enhances nicotine-induced Ca2+ signaling in the MB calyces and somas in a DopEcR-dependent manner, supporting the possibility that DopEcR plays an important role in experience-dependent modification of the physiological properties of MB neurons (Lark et al., 2017).
Repetitive neuronal stimulations.
In adult flies, visual or mechanical stimulation activates the descending giant fiber (GF) neurons and triggers the stereotypical jump-and-flight escape response (Tanouye and Wyman, 1980; Wyman et al., 1984). Direct electrical stimulation of the brain can bypass sensory receptors and activate the GF pathways to set off the escape response. Strong electrical stimulation can directly trigger GF activation (short-latency response), whereas lower intensity stimulation indirectly activates the GF pathway through GF afferents in the brain (long-latency response) (Elkins and Ganetzky, 1990). The lowest stimulation intensities required for triggering short- and long-latency responses are not different between DopEcR loss-of-function mutants and control flies, indicating that reduced DopEcR does not significantly affect the overall neuronal sensitivity of the GF pathway. However, when the GF pathway is repetitively stimulated, DopEcR mutants show functional abnormalities; the minimum time required for the GF system to recover from the 1st stimulus and fire a response to the 2nd stimulus (the refractory period) is significantly reduced in DopEcR mutants compared to control flies (Ishimoto et al., 2013). This finding implies that neuronal circuits in DopEcR mutants are less vulnerable to activity-dependent modifications than the relevant circuits in control flies. With repeated delivery of electrical stimulation across the brain, the GF pathway undergoes habituation and the probability of a motor output significantly decreases (Engel and Wu, 1996). Analysis of habituation clearly demonstrated defects of the DopEcR mutant circuits in responses to repetitive neuronal stimulations. In contrast to control flies that became rapidly habituated to 5-Hz stimulation of the brain and failed to respond to the brain stimulation, DopEcR mutants consistently showed a significant delay in habituation (Ishimoto et al., 2013). These electrophysiological abnormalities in DopEcR mutants may underlie their defects in courtship memory and other behaviors.
VI. Roles of DopEcR in other insects
The diverse roles of DopEcR homologs have been implicated in insects other than Drosophila. These include biological processes such as phase trait polyphenism, reproduction, apoptosis, cAMP metabolism, and ecdysis-associated behavioral quiescence, some of which are related to stress response (Elmogy et al., 2004; Elmogy et al., 2006; Chen et al., 2010; Abrieux et al., 2013; Kang et al., 2019).
Gregarious transformation in Locusta migratoria.
Phase polyphenism is a phenomenon where animals of a single species undergo great phenotypic changes in response to environmental cues (Applebaum and Heifetz, 1999). In the case of the migratory locust Locusta migratoria, increased population density drives a transformation from the solitarious to gregarious phase within a generation with marked metabolic and neurobiological changes, resulting in their dark coloration, swarming, and dramatic mass migrations which can lead to terrible agricultural losses (Gray et al., 2009). This transformation involves changes in both transcription of catecholamine-related genes (Ma et al., 2011) and ecdysteroids titer (Tawfik and Sehnal, 2003). DopEcR was upregulated in gregarious-phase locusts compared to solitarious-phase locusts (Chen et al., 2010). While the exact role of DopEcR in the phase transformation is unclear, its upregulation in the gregarious phase together with changes in ecdysteroid levels indicates that ecdysone signaling through DopEcR may play a role in helping locusts properly manage their phenotypic responses to a high-density environment, which is considered socially stressful for locust (Roessingh et al., 1998; Simpson et al., 2001).
Age-dependent courtship plasticity in Agrotis ipsilon.
As previously mentioned DopEcR is required for experience-dependent courtship suppression in D. melanogaster males. A similar situation is found in the moth Agrotis ipsilon, where DopEcR was shown to modulate age-dependent behavioral plasticity (Abrieux et al., 2013). A. ipsilon males eclose from pupal cases sexually immature and over the course of several days, gain competence to be attracted to female-derived sex pheromones. This age-dependent plasticity is partially dependent upon ecdysteroids and dopaminergic signaling through DopEcR, as injection of DopEcR dsRNA in male moths inhibited behavioral attraction to female-derived sex pheromones (Abrieux et al., 2013). Sex pheromones activate neurons in the male antennal lobes in an age-dependent fashion and concentrations of the sex pheromone necessary to evoke neuronal responses decrease with age (Anton and Gadenne, 1999). Behavioral response to sex pheromone is augmented by treating male moths with either dopamine or 20E, and this response requires DopEcR expression (Abrieux et al., 2013). Together these findings highlight the role of DopEcR-mediated dopamine and ecdysone actions in the plasticity of sexual behavior in A. ipsilon.
Larval feeding and pupation in Helicoverpa armigera.
In the agricultural pest Helicoverpa armigera, DopEcR has been shown to function as a switch of sorts that integrates dopamine and 20E signals to decide whether a larva will continue to actively feed and grow or enter behavioral quiescence and begin pupation (Kang et al., 2019). This is accomplished through the antagonistic effects of 20E upon DopEcR-dependent dopamine signals. The 20E titer is low during larval feeding and high during wandering stages, while the dopamine titer is opposite to it; high during larval feeding and low during wandering stages. When dopamine is the dominant DopEcR ligand during larval feeding, protein kinase B (Akt) is phosphorylated and activated, thus promoting cell-cycle progression, cell proliferation, and the continuation of larval development. In the hours preceding pupation, hemolymph dopamine titer decreases while 20E titer increases, thus biasing the action of DopEcR towards 20E-dependent effects and life-stage progression. Increased levels of 20E antagonizes phosphorylation of Akt and instead promotes 20E-responsive gene transcription indirectly by promoting phosphorylation of USP1, which enhances EcR-B1/USP1 binding to ecdysone response elements. 20E signals lead to behavioral quiescence, apoptosis, and eventual metamorphosis. This study by Kang et al. (2019) revealed that DopEcR is necessary for coordinating cellular and behavioral events to complete pupation in Helicoverpa armigera and demonstrated, for the first time, the functional interaction between genomic and nongenomic ecdysone signaling pathways.
Ecdysteroid-induced rapid increase in cAMP levels in Bombyx mori.
In the silkworm Bombyx mori, a population of membrane ecdysteroid receptors was identified within cells of the anterior silk glands (ASGs) using tritiated steroid ligands (Elmogy et al., 2004). At pupariation the silkworm uses ASGs to spin their cocoons, but shortly after pupariation the ASGs undergo programmed cell death in response to high ecdysteroid levels. Initially, these ecdysteroids act genomically to begin the sequence of ASG apoptosis, but the sequence also requires nongenomic action of steroids to complete the lysis (Terashima et al., 2000). Ecdysteroid treatment of ASGs triggers an increase of intracellular cAMP, a critical second messenger, that is not abolished by inhibition of transcription, indicating that ASG cAMP induction is likely due to the nongenomic effects. Whether this cellular response requires DopEcR to mediate these effects has yet to be determined (Elmogy et al., 2006). Similar increases in cAMP in response to ecdysteroids have been observed in other insects (Applebaum and Gilbert, 1972; Sass et al., 1983), and the rapid nature of cAMP induction makes it an ideal mechanism to mediate an animal’s responses to a dynamic stressful environment.
VII. Functional counterparts and potential orthologs of DopEcR in vertebrates.
Although there is little sequence similarity between insect DopEcR and the aforementioned mammalian GPER1, these two GPCRs are considered functional counterparts because they share several key characteristics in terms of their intracellular localization, ligands, secondary signaling properties and pharmacology (Srivastava et al., 2005; Evans et al., 2014). When expressed in heterologous cell lines, both DopEcR and GPER1 are localized to the plasma membrane but are rapidly internalized into endosomal compartments. Similar to DopEcR, GPER1 is also activated by steroids (i.e., 17β-estradiol, and aldosterone) and dopamine. These ligands can differentially trigger distinct downstream cascades through both receptors, thus providing unique molecular hubs for steroid and dopamine actions.
Sequence analysis with the DIOPT (Drosophila RNAi Screening Center Integrative Ortholog Prediction Tool, http://www.flyrnai.org/diopt) predicts two putative mammalian orthologs of DopEcR, GPR52 (amino acid sequence identity: 26%, similarity: 42%) and GPR21 (identity: 24%, similarity: 42%). Both are orphan GPCRs – receptors whose endogenous ligands have yet to be identified. A study showed that reduced GPR52 function suppresses Huntington’s disease (HD) phenotypes in both patient iPS-derived neurons and in an in vivo Drosophila HD model (Yao et al., 2015). In the latter study, DopEcR was referred to as “Drosophila GPR52” and examined as the functional equivalent of mammalian GPR52. More recent experiments found that expression levels of mutant huntingtin (HTT) were reduced and HD-associated behavioral phenotypes were mitigated in a mouse model of HD when an additional GPR52 mutation was introduced (Huang and Gitler, 2018; Song et al., 2018). Since chronic stress has been implicated in the onset and progression of HD (Mo et al., 2014), DopEcR’s correlation to stress-induced responses and the disease severity is intriguing to consider.
GPR21, another potential mammalian ortholog of DopEcR, is preferentially expressed in neurons and responds to plasmalogens (Pls), lipid ligands, albeit not steroids (Hossain et al., 2016). PIs are the special glycerophospholipids that are enriched in the brain and can protect neuronal cells against the damaging effects of stress (e.g., oxidative stress) by enhancing phosphorylation of Akt and ERK (Nagan and Zoeller, 2001). The Pls-mediated phosphorylation of ERK was inhibited in GPR21 knockdown cells, and conversely, GPR21 overexpression enhanced Pls-mediated phosphorylation of ERK and Akt. These results indicate that GPR21 is involved in stress response through modulating phosphorylation (Hossain et al., 2016). To the best of our knowledge, the response of DopEcR to PIs has not yet been investigated.
VIII. Future perspectives
A number of interesting questions regarding DopEcR’s function remain to be answered. First, the intracellular distribution of DopEcR in various cell types, particularly in neurons, is still unclear. DopEcR is expected to be present on the plasma membrane, but whether the receptor is enriched around the cell soma, lining axons, or found in dendrites has yet to be addressed. Furthermore, whether DopEcR acts pre- or post-synaptically to modulate specific behaviors will also need to be determined.
Another unanswered question is how DopEcR is activated by endogenous ligands, particularly ecdysteroids. Adult Drosophila can synthesize ecdysteroids within the female ovary (Gaziova et al., 2004, Domanitskaya et al., 2014) and from Malpighian tubules in response to desiccation stress (Zheng et al., 2018), but other adult tissues that produce and release ecdysteroids requires further investigation. The developmental release of ecdysone from the steroidogenic prothoracic gland is similar to that of neurotransmitters from neurons, mediated by calcium-regulated vesicular trafficking (Yamanaka et al., 2015). This raises the interesting possibility that ecdysteroids are synthesized and stored in neurosecretory cells, and released as neurosteroids in an activity-dependent manner.
Equally important is the question of how DopEcR-mediated dopamine and ecdysone signaling interact under various stress conditions. While Srivastava et al. (2005) showed that ecdysone inhibited dopamine-associated downstream signaling in culture, this characteristic bias toward ecdysone may differ in vivo and even vary by tissues and brain regions. Additionally, a growing body of work has shown that allosteric modulation of GPCRs may confer bias on or differentially modulate multiple downstream signaling pathways (Foster and Conn, 2017). In the context of DopEcR, this may suggest that the downstream signaling associated with dopamine and ecdysone is regulated dynamically and flexibly. Equally intriguing still, as suggested by the work of Kang et al. (2019) in Helicoverpa armigera, the dual ligand capacity of DopEcR may serve as a sort of switch or coincidence detector alone or in league with other dopamine receptors to initiate selective responses to stressors. Clearly there remains much to explore in regard to the specificity and spatiotemporal nature of DopEcR’s signaling mechanisms.
An additional question left to be addressed concerns the possible role of DopEcR in Drosophila development. Ecdysis (molting) is arguably a stressful event. Many arthropods become behaviorally quiescent before a molt, suggesting that ecdysteroid changes muscle excitability. In support of this, ecdysteroid application to the larval neuromuscular junction (NMJ) acutely reduced the size of postsynaptic currents by reducing the number of synaptic vesicles released by motor neurons, but not changing quantal content (Cooper and Ruffner, 1998; Ruffner et al., 1999). To the best of our knowledge, the role of DopEcR in this context has not yet been investigated.
The findings presented here provide a novel framework for dissecting GPCR-mediated steroid signaling at the molecular and cellular levels. Furthermore, future analysis of the functional interplay between genomic and nongenomic steroid signaling pathways is expected to reveal novel mechanisms through which steroid hormones regulate responses to stressors and other biological phenomena.
Acknowledgements
We thank the late Dr. Troy Zars (University of Missouri Columbia) for his long-term consistent friendship and fruitful discussions about the DopEcR project and other scientific issues. We dedicate this paper to Dr. Zars. We also thank members of the Kitamoto lab for technical assistance and the Bloomington Stock Center and VDRC for fly stocks. This study was supported by the following grants and fellowships: NIH R03AA021968 (TK), NIH R01MH085081 (TK), NSF IOS1352882 (TK), NIH F31AA021625 (EP), NIH T32NS045549 (EP, AL), NIH T32NS007421 (AL), Chateaubriand Fellowship (AL), University of Iowa Graduate College Dean’s Graduate Fellowship (AL), NIH T32GM008629 (JM), and NIH T32NS045549 (JM).
Abbreviations
- Akt
protein kinase B
- AMP
antimicrobial peptide
- ASG
anterior silk gland
- cAMP
cyclic adenosine monophosphate
- DopEcR
dopamine/ecdysteroid receptor
- E
ecdysone
- 20E
20-hydroxyecdysone
- EcI
Ecdysone Importer
- EcR
ecdysone receptor
- EGFR
epidermal growth factor receptor
- ERK
extracellular-related kinase
- ETH
ecdysis triggering hormone
- FOXO
forkhead box subfamily O transcription factors (FOXO)
- fmn
fumin
- GF
giant fiber
- GPER1
G-protein-coupled estrogen receptor 1
- GABA
γ-aminobutyric acid
- GPCR
G-protein coupled receptor
- HD
Huntington’s disease
- HTT
Huntingtin
- IP3K
inositol-trisphosphate 3-kinase
- 3IY
3-Iodotyrosine
- MB
mushroom body
- NMDA
N-methyl-D-aspartate receptor
- PoA
ponesterone A
- SISS
starvation-induced sleep suppression
- USP
Ultraspiracle
References
- Abrieux A, Debernard S, Maria A, Gaertner C, Anton S, Gadenne C, Duportets L (2013) Involvement of the G-protein-coupled dopamine/ecdysteroid receptor DopEcR in the behavioral response to sex pheromone in an insect. PLoS One 8:e72785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, van Swinderen B, Greenspan RJ (2005) Dopaminergic modulation of arousal in Drosophila. Curr Biol 15:1165–1175. [DOI] [PubMed] [Google Scholar]
- Anton S, Gadenne C (1999) Effect of juvenile hormone on the central nervous processing of sex pheromone in an insect. Proceedings of the National Academy of Sciences 96:5764–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebaum SW, Gilbert LI (1972) Stimulation of adenyl cyclase in pupal wing epidermis by -ecdysone. Dev Biol 27:165–175. [DOI] [PubMed] [Google Scholar]
- Applebaum SW, Heifetz Y (1999) DENSITY-DEPENDENT PHYSIOLOGICAL PHASE IN INSECTS. Annu Rev Entomol 44:317–341. [DOI] [PubMed] [Google Scholar]
- Aranda GP, Hinojos SJ, Sabandal PR, Evans PD, Han KA (2017) Behavioral Sensitization to the Disinhibition Effect of Ethanol Requires the Dopamine/Ecdysone Receptor in Drosophila. Front Syst Neurosci 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Carrion O, Stamelou M, Murillo-Rodriguez E, Menendez-Gonzalez M, Poppel E (2010) Dopaminergic reward system: a short integrative review. Int Arch Med 3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M (1989) Gene regulation by steroid hormones. Cell 56:335–344. [DOI] [PubMed] [Google Scholar]
- Beckstead RB, Lam G, Thummel CS (2005) The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol 6:R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore PF, Beebe SJ, Danforth DR, Alexander N (1990) Progesterone and 17 alpha-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J Biol Chem 265:1376–1380. [PubMed] [Google Scholar]
- Brody T, Cravchik A (2000) Drosophila melanogaster G protein-coupled receptors. J Cell Biol 150:F83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzatu S (2009) The temperature-induced changes in membrane potential. Riv Biol 102:199–217. [PubMed] [Google Scholar]
- Chen S, Yang P, Jiang F, Wei Y, Ma Z, Kang L (2010) De novo analysis of transcriptome dynamics in the migratory locust during the development of phase traits. PLoS One 5:e15633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA (2007) Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39:715–720. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Jarecki BW, Chanda B (2014) A molecular framework for temperature-dependent gating of ion channels. Cell 158:1148–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RL, Ruffner ME (1998) Depression of synaptic efficacy at intermolt in crayfish neuromuscular junctions by 20-hydroxyecdysone, a molting hormone. J Neurophysiol 79:1931–1941. [DOI] [PubMed] [Google Scholar]
- Crocker AD (1997) The regulation of motor control: an evaluation of the role of dopamine receptors in the substantia nigra. Rev Neurosci 8:55–76. [DOI] [PubMed] [Google Scholar]
- Dethier VG (1976) The hungry fly: A physiological study of the behavior associated with feeding. Oxford, England: Harvard U Press. [Google Scholar]
- Elkins T, Ganetzky B (1990) Conduction in the giant nerve fiber pathway in temperature-sensitive paralytic mutants of Drosophila. J Neurogenet 6:207–219. [DOI] [PubMed] [Google Scholar]
- Elmogy M, Iwami M, Sakurai S (2004) Presence of membrane ecdysone receptor in the anterior silk gland of the silkworm Bombyx mori. Eur J Biochem 271:3171–3179. [DOI] [PubMed] [Google Scholar]
- Elmogy M, Terashima J, Iga M, Iwami M, Sakurai S (2006) A rapid increase in cAMP in response to 20-hydroxyecdysone in the anterior silk glands of the silkworm, Bombyx mori. Zoological science 23:715–719. [DOI] [PubMed] [Google Scholar]
- Engel JE, Wu CF (1996) Altered habituation of an identified escape circuit in Drosophila memory mutants. J Neurosci 16:3486–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PD, Bayliss A, Reale V (2014) GPCR-mediated rapid, non-genomic actions of steroids: comparisons between DmDopEcR and GPER1 (GPR30). General and comparative endocrinology 195:157–163. [DOI] [PubMed] [Google Scholar]
- Evans RM (1988) The steroid and thyroid hormone receptor superfamily. Science 240:889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman RD, Limbird LE (2017) GPER (GPR30): A Nongenomic Receptor (GPCR) for Steroid Hormones with Implications for Cardiovascular Disease and Cancer. Annu Rev Pharmacol Toxicol 57:567–584. [DOI] [PubMed] [Google Scholar]
- Feng G, Hannan F, Reale V, Hon YY, Kousky CT, Evans PD, Hall LM (1996) Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J Neurosci 16:3925–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P (2005) GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab 16:362–367. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR Jr., Bland KI (2002) Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol 16:70–84. [DOI] [PubMed] [Google Scholar]
- Flatt T, Heyland A, Rus F, Porpiglia E, Sherlock C, Yamamoto R, Garbuzov A, Palli SR, Tatar M, Silverman N (2008) Hormonal regulation of the humoral innate immune response in Drosophila melanogaster. J Exp Biol 211:2712–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Conn PJ (2017) Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron 94:431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilestro GF (2012) Video tracking and analysis of sleep in Drosophila melanogaster. Nat Protoc 7:995–1007. [DOI] [PubMed] [Google Scholar]
- Gotzes F, Balfanz S, Baumann A (1994) Primary structure and functional characterization of a Drosophila dopamine receptor with high homology to human D1/5 receptors. Receptors Channels 2:131–141. [PubMed] [Google Scholar]
- Graveley BR et al. (2011) The developmental transcriptome of Drosophila melanogaster. Nature 471:473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LJ, Sword GA, Anstey ML, Clissold FJ, Simpson SJ (2009) Behavioural phase polyphenism in the Australian plague locust (Chortoicetes terminifera). Biol Lett 5:306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg FL, Karst H, de Kloet ER, Joels M (2012) Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Molecular and cellular endocrinology 350:299–309. [DOI] [PubMed] [Google Scholar]
- Gros R, Ding Q, Sklar LA, Prossnitz EE, Arterburn JB, Chorazyczewski J, Feldman RD (2011) GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension 57:442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruntenko N, Chentsova NA, Bogomolova EV, Karpova EK, Glazko GV, Faddeeva NV, Monastirioti M, Rauschenbach IY (2004) The effect of mutations altering biogenic amine metabolism in Drosophila on viability and the response to environmental stresses. Arch Insect Biochem Physiol 55:55–67. [DOI] [PubMed] [Google Scholar]
- Gruntenko NE, Karpova EK, Adonyeva NV, Chentsova NA, Faddeeva NV, Alekseev AA, Rauschenbach IY (2005) Juvenile hormone, 20-hydroxyecdysone and dopamine interaction in Drosophila virilis reproduction under normal and nutritional stress conditions. J Insect Physiol 51:417–425. [DOI] [PubMed] [Google Scholar]
- Gruntenko NE, Adonyeva NV, Burdina EV, Karpova EK, Andreenkova OV, Gladkikh DV, Ilinsky YY, Rauschenbach IY (2016) The impact of FOXO on dopamine and octopamine metabolism in Drosophila under normal and heat stress conditions. Biol Open 5:1706–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC (1994) The mating of a fly. Science 264:1702–1714. [DOI] [PubMed] [Google Scholar]
- Hasbi A, O’Dowd BF, George SR (2005) A G protein-coupled receptor for estrogen: the end of the search? Mol Interv 5:158–161. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI (2000) Rest in Drosophila is a sleep-like state. Neuron 25:129–138. [DOI] [PubMed] [Google Scholar]
- Hewes RS, Taghert PH (2001) Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res 11:1126–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima A, Rauschenbach I, Sukhanova M (2000a) Ecdysteroids in stress responsive and nonresponsive Drosophila virilis lines under stress conditions. Bioscience, biotechnology, and biochemistry 64:2657–2662. [DOI] [PubMed] [Google Scholar]
- Hirashima A, Sukhanova M, Rauschenbach I (2000b) Genetic control of biogenic-amine systems in Drosophila under normal and stress conditions. Biochem Genet 38:167–180. [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG (2006) Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444:486–489. [DOI] [PubMed] [Google Scholar]
- Hossain MS, Mineno K, Katafuchi T (2016) Neuronal Orphan G-Protein Coupled Receptor Proteins Mediate Plasmalogens-Induced Activation of ERK and Akt Signaling. PLoS One 11:e0150846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Gitler AD (2018) Hunting the G-unit in Huntington’s. Brain 141:1586–1589. [DOI] [PubMed] [Google Scholar]
- Inagaki HK, Panse KM, Anderson DJ (2014) Independent, reciprocal neuromodulatory control of sweet and bitter taste sensitivity during starvation in Drosophila. Neuron 84:806–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki HK, Ben-Tabou de-Leon S, Wong AM, Jagadish S, Ishimoto H, Barnea G, Kitamoto T, Axel R, Anderson DJ (2012) Visualizing neuromodulation in vivo: TANGO-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell 148:583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inanobe A, Kurachi Y (2014) Membrane channels as integrators of G-protein-mediated signaling. Biochim Biophys Acta 1838:521–531. [DOI] [PubMed] [Google Scholar]
- Ishimoto H, Kitamoto T (2010) The steroid molting hormone Ecdysone regulates sleep in adult Drosophila melanogaster. Genetics 185:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Kitamoto T (2011) Beyond molting--roles of the steroid molting hormone ecdysone in regulation of memory and sleep in adult Drosophila. Fly 5:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Sakai T, Kitamoto T (2009) Ecdysone signaling regulates the formation of long-term courtship memory in adult Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America 106:6381–6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Wang Z, Rao Y, Wu CF, Kitamoto T (2013) A novel role for ecdysone in Drosophila conditioned behavior: linking GPCR-mediated non-canonical steroid action to cAMP signaling in the adult brain. PLoS Genet 9:e1003843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova L, Bernhardt R, Bernhardt I (2008) Nongenomic effect of aldosterone on ion transport pathways of red blood cells. Cell Physiol Biochem 22:269–278. [DOI] [PubMed] [Google Scholar]
- Kang XL, Zhang JY, Wang D, Zhao YM, Han XL, Wang JX, Zhao XF (2019) The steroid hormone 20-hydroxyecdysone binds to dopamine receptor to repress lepidopteran insect feeding and promote pupation. PLoS Genet 15:e1008331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam CS, Jones SK, Javitch JA (2019) Come Fly with Me: An overview of dopamine receptors in Drosophila melanogaster. Basic & clinical pharmacology & toxicology. [DOI] [PMC free article] [PubMed]
- Kastenberger I, Schwarzer C (2014) GPER1 (GPR30) knockout mice display reduced anxiety and altered stress response in a sex and paradigm dependent manner. Horm Behav 66:628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasture AS, Hummel T, Sucic S, Freissmuth M (2018) Big Lessons from Tiny Flies: Drosophila melanogaster as a Model to Explore Dysfunction of Dopaminergic and Serotonergic Neurotransmitter Systems. Int J Mol Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Duboue ER, McDonald DM, Dus M, Suh GS, Waddell S, Blau J (2010) Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr Biol 20:1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Cikurel K, Bostock H (2001) Effects of temperature on the excitability properties of human motor axons. Brain 124:816–825. [DOI] [PubMed] [Google Scholar]
- Knapp E, Sun J (2017) Steroid signaling in mature follicles is important for Drosophila ovulation. Proceedings of the National Academy of Sciences of the United States of America 114:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong EC, Woo K, Li H, Lebestky T, Mayer N, Sniffen MR, Heberlein U, Bainton RJ, Hirsh J, Wolf FW (2010) A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS One 5:e9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Kume S, Park SK, Hirsh J, Jackson FR (2005) Dopamine is a regulator of arousal in the fruit fly. J Neurosci 25:7377–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark A, Kitamoto T, Martin JR (2017) Modulation of neuronal activity in the Drosophila mushroom body by DopEcR, a unique dual receptor for ecdysone and dopamine. Biochim Biophys Acta 1864:1578–1588. [DOI] [PubMed] [Google Scholar]
- Leader DP, Krause SA, Pandit A, Davies SA, Dow JAT (2018) FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic acids research 46:D809–D815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linford NJ, Ro J, Chung BY, Pletcher SD (2015) Gustatory and metabolic perception of nutrient stress in Drosophila. Proc Natl Acad Sci U S A 112:2587–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Guo W, Guo X, Wang X, Kang L (2011) Modulation of behavioral phase changes of the migratory locust by the catecholamine metabolic pathway. Proceedings of the National Academy of Sciences 108:3882–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Davis RL (2009) Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits 3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM, Keene AC (2010) The sleep-feeding conflict: Understanding behavioral integration through genetic analysis in Drosophila. Aging (Albany NY) 2:519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2008) Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583:174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiselman MR, Kingan TG, Adams ME (2018) Stress-induced reproductive arrest in Drosophila occurs through ETH deficiency-mediated suppression of oogenesis and ovulation. Bmc Biol 16:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnattur K, Shaw P (2019) Staying awake to stay alive: A circuit controlling starvation-induced waking. PLoS Biol 17:e3000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo C, Renoir T, Hannan AJ (2014) Effects of chronic stress on the onset and progression of Huntington’s disease in transgenic mice. Neurobiol Dis 71:81–94. [DOI] [PubMed] [Google Scholar]
- Moro L, Reineri S, Piranda D, Pietrapiana D, Lova P, Bertoni A, Graziani A, Defilippi P, Canobbio I, Torti M, Sinigaglia F (2005) Nongenomic effects of 17beta-estradiol in human platelets: potentiation of thrombin-induced aggregation through estrogen receptor beta and Src kinase. Blood 105:115–121. [DOI] [PubMed] [Google Scholar]
- Nagan N, Zoeller RA (2001) Plasmalogens: biosynthesis and functions. Progress in lipid research 40:199–229. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, Weinstein JS (2005) Stress affects dopaminergic signaling pathways in Drosophila melanogaster. Stress 8:117–131. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Bhatnagar S, Ellis B (2016) Evolutionary Origins and Functions of the Stress Response System In: Stress: Concepts, Cognition, Emotion, and Behavior (Fink G, ed), pp 95–101. Cambridge, Massachusetts: Academic Press. [Google Scholar]
- Okamoto N, Viswanatha R, Bittar R, Li Z, Haga-Yamanaka S, Perrimon N, Yamanaka N (2018) A Membrane Transporter Is Required for Steroid Hormone Uptake in Drosophila. Developmental cell 47:294–305 e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski D, Kahsai L, Kramer EF, Knutson P, Zars T (2015) Place memory retention in Drosophila. Neurobiol Learn Mem 123:217–224. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Wu FS, Purdy RH, Malayev AA, Gibbs TT, Farb DH (1997) Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol Pharmacol 52:1113–1123. [DOI] [PubMed] [Google Scholar]
- Petruccelli E, Li Q, Rao Y, Kitamoto T (2016) The Unique Dopamine/Ecdysteroid Receptor Modulates Ethanol-Induced Sedation in Drosophila. J Neurosci 36:4647–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager EM, Brielmaier J, Bergstrom HC, McGuire J, Johnson LR (2010) Localization of mineralocorticoid receptors at mammalian synapses. PLoS One 5:e14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M (2011) The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 7:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschenbach IY, Sukhanova MZ, Hirashima A, Sutsugu E, Kuano E (2000) Role of the ecdysteroid system in the regulation of Drosophila reproduction under environmental stress. Dokl Biol Sci 375:641–643. [DOI] [PubMed] [Google Scholar]
- Rauschenbach IY, Bogomolova EV, Gruntenko NE, Adonyeva NV, Chentsova NA (2007) Effects of juvenile hormone and 20-hydroxyecdysone on alkaline phosphatase activity in Drosophila under normal and heat stress conditions. J Insect Physiol 53:587–591. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–1630. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Mitchell HD, Field AS, Burai R, Corona C, Ramesh C, Sklar LA, Arterburn JB, Prossnitz ER (2007) Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem Biol 2:536–544. [DOI] [PubMed] [Google Scholar]
- Reynolds SR, Foster FI (1939) Peripheral Vascular Action of Estrogen in the Human Male. J Clin Invest 18:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddiford LM (1993) Hormones and Drosophila Development In: The Development of Drosophila melanogaster (Bate M, Arias AM, ed), pp 899–929. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Roessingh P, BouaïChi A, Simpson SJ (1998) Effects of sensory stimuli on the behavioural phase state of the desert locust, Schistocerca gregaria. J Insect Physiol 44:883–893. [DOI] [PubMed] [Google Scholar]
- Ruffner ME, Cromarty SI, Cooper RL (1999) Depression of synaptic efficacy in high- and low-output Drosophila neuromuscular junctions by the molting hormone (20-HE). J Neurophysiol 81:788–794. [DOI] [PubMed] [Google Scholar]
- Sass M, Csikos G, Komuves L, Kovacs J (1983) Cyclic AMP in the fat body of Mamestra brassicae during the last instar and its possible involvement in the cellular autophagocytosis induced by 20-Hydroxyecdysone. General and comparative endocrinology 50:116–123. [DOI] [PubMed] [Google Scholar]
- Selye H (1936) A Syndrome produced by Diverse Nocuous Agents. Nature 138:32–32. [DOI] [PubMed] [Google Scholar]
- Selye H (1942) Correlations between the chemical structure and the pharmacological actions of the steroids. Endocrinology 30:437–453. [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G (2000) Correlates of sleep and waking in Drosophila melanogaster. Science 287:1834–1837. [DOI] [PubMed] [Google Scholar]
- Shivaji S, Jagannadham MV (1992) Steroid-induced perturbations of membranes and its relevance to sperm acrosome reaction. Biochim Biophys Acta 1108:99–109. [DOI] [PubMed] [Google Scholar]
- Siegel RW, Hall JC (1979) Conditioned responses in courtship behavior of normal and mutant Drosophila. Proceedings of the National Academy of Sciences of the United States of America 76:3430–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AF, Shih C, Mack A, Benzer S (2003) Steroid control of longevity in Drosophila melanogaster. Science 299:1407–1410. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Despland E, Hagele BF, Dodgson T (2001) Gregarious behavior in desert locusts is evoked by touching their back legs. Proceedings of the National Academy of Sciences 98:3895–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AH, Wolf DM, Wang P, Arkin AP (2008) Modularity of stress response evolution. Proceedings of the National Academy of Sciences of the United States of America 105:7500–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M (2014) Mood, food, and obesity. Front Psychol 5:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller M, Bownes M, Kubli E (1999) Control of oocyte maturation in sexually mature Drosophila females. Dev Biol 208:337–351. [DOI] [PubMed] [Google Scholar]
- Song H, Li H, Guo S, Pan Y, Fu Y, Zhou Z, Li Z, Wen X, Sun X, He B, Gu H, Zhao Q, Wang C, An P, Luo S, Hu Y, Xie X, Lu B (2018) Targeting Gpr52 lowers mutant HTT levels and rescues Huntington’s disease-associated phenotypes. Brain 141:1782–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, Smith T, Evans PD (2005) Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. J Neurosci 25:6145–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton M, Carlson J, Brokstein P, Yu C, Champe M, George R, Guarin H, Kronmiller B, Pacleb J, Park S, Wan K, Rubin GM, Celniker SE (2002) A Drosophila full-length cDNA resource. Genome Biol 3:RESEARCH0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Gilligan J, Staber C, Schutte RJ, Nguyen V, O’Dowd DK, Reenan R (2012) A knock-in model of human epilepsy in Drosophila reveals a novel cellular mechanism associated with heat-induced seizure. J Neurosci 32:14145–14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki DT, Grigliatti T, Williamson R (1971) Temperature-sensitive mutations in Drosophila melanogaster. VII. A mutation (para-ts) causing reversible adult paralysis. Proceedings of the National Academy of Sciences of the United States of America 68:890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye MA, Wyman RJ (1980) Motor outputs of giant nerve fiber in Drosophila. J Neurophysiol 44:405–421. [DOI] [PubMed] [Google Scholar]
- Tawfik AI, Sehnal F (2003) A role for ecdysteroids in the phase polymorphism of the desert locust. Physiol Entomol 28:19–24. [Google Scholar]
- Terashima J, Bownes M (2004) Translating available food into the number of eggs laid by Drosophila melanogaster. Genetics 167:1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima J, Takaki K, Sakurai S, Bownes M (2005) Nutritional status affects 20-hydroxyecdysone concentration and progression of oogenesis in Drosophila melanogaster. J Endocrinol 187:69–79. [DOI] [PubMed] [Google Scholar]
- Terashima J, Yasuhara N, Iwami M, Sakurai S, Sakurai S (2000) Programmed cell death triggered by insect steroid hormone, 20-hydroxyecdysone, in the anterior silk gland of the silkworm, Bombyx mori. Dev Genes Evol 210:545–558. [DOI] [PubMed] [Google Scholar]
- Thummel CS (1996) Flies on steroids--Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet 12:306–310. [DOI] [PubMed] [Google Scholar]
- Villella A, Hall JC (2008) Neurogenetics of courtship and mating in Drosophila. Adv Genet 62:67–184. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M (2006) The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol 20:631–646. [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H (2008) GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol 22:636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QP, Lin YQ, Zhang L, Wilson YA, Oyston LJ, Cotterell J, Qi Y, Khuong TM, Bakhshi N, Planchenault Y, Browman DT, Lau MT, Cole TA, Wong AC, Simpson SJ, Cole AR, Penninger JM, Herzog H, Neely GG (2016) Sucralose Promotes Food Intake through NPY and a Neuronal Fasting Response. Cell metabolism 24:75–90. [DOI] [PubMed] [Google Scholar]
- Whiting KP, Restall CJ, Brain PF (2000) Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms. Life Sci 67:743–757. [DOI] [PubMed] [Google Scholar]
- Winter DC, Schneider MF, O’Sullivan GC, Harvey BJ, Geibel JP (1999) Rapid effects of aldosterone on sodium-hydrogen exchange in isolated colonic crypts. J Membr Biol 170:17–26. [DOI] [PubMed] [Google Scholar]
- Wolf FW, Rodan AR, Tsai LT, Heberlein U (2002) High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci 22:11035–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman RJ, Thomas JB, Salkoff L, King DG (1984) The Drosopohila giant fiber system. New York: Plenum. [Google Scholar]
- Xia S, Yang J, Su Y, Qian J, Ma E, Haddad GG (2005) Identification of new targets of Drosophila pre-mRNA adenosine deaminase. Physiol Genomics 20:195–202. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Seto ES (2014) Dopamine dynamics and signaling in Drosophila: an overview of genes, drugs and behavioral paradigms. Exp Anim 63:107–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N, Rewitz KF, O’Connor MB (2013) Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol 58:497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka N, Marques G, O’Connor MB (2015) Vesicle-Mediated Steroid Hormone Secretion in Drosophila melanogaster. Cell 163:907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Cui X, Al-Ramahi I, Sun X, Li B, Hou J, Difiglia M, Palacino J, Wu ZY, Ma L, Botas J, Lu B (2015) A striatal-enriched intronic GPCR modulates huntingtin levels and toxicity. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgel ME, Kakad P, Zandawala M, Nassel DR, Godenschwege TA, Keene AC (2019) A single pair of leucokinin neurons are modulated by feeding state and regulate sleep-metabolism interactions. PLoS Biol 17:e2006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Ocorr K, Tatar M (2019) Extra-cellular matrix induced by steroids through a G-protein coupled receptor in a Drosophila model of renal fibrosis. bioRxiv:653329. [DOI] [PMC free article] [PubMed]
- Zheng W, Rus F, Hernandez A, Kang P, Goldman W, Silverman N, Tatar M (2018) Dehydration triggers ecdysone-mediated recognition-protein priming and elevated anti-bacterial immune responses in Drosophila Malpighian tubule renal cells. Bmc Biol 16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]


