Abstract
Motor inhibition is a key control mechanism that allows humans to rapidly adapt their actions in response to environmental events. One of the hallmark signatures of rapidly exerted, reactive motor inhibition is the non-selective suppression of cortico-spinal excitability (CSE): unexpected sensory stimuli lead to a suppression of CSE across the entire motor system, even in muscles that are inactive. Theories suggest that this reflects a fast, automatic, and broad engagement of inhibitory control, which facilitates behavioral adaptations to unexpected changes in the sensory environment. However, it is an open question whether such non-selective CSE suppression is truly due to the unexpected nature of the sensory event, or whether it is sufficient for an event to be merely infrequent (but not unexpected). Here, we report data from two experiments in which human subjects experienced both unexpected and expected infrequent events during a two-alternative forced-choice reaction time task while CSE was measured from a task-unrelated muscle. We found that expected infrequent events can indeed produce non-selective CSE suppression – but only when they occur during movement initiation. In contrast, unexpected infrequent events produce non-selective CSE suppression relative to frequent, expected events even in the absence of movement initiation. Moreover, CSE suppression due to unexpected events occurs at shorter latencies compared to expected infrequent events. These findings demonstrate that unexpectedness and stimulus infrequency have qualitatively different suppressive effects on the motor system. They also have key implications for studies that seek to disentangle neural and psychological processes related to motor inhibition and stimulus detection.
Keywords: Motor inhibition, motor evoked potentials, cortico-motor excitability, surprise, oddball
Introduction
Motor inhibition is a core component of controlled and flexible human behavior. The rapid interruption of active motor representations allows humans to momentarily cancel ongoing movements and movement plans, which in turn allows them to reevaluate whether those movements are still appropriate when environmental circumstances suddenly change. In the laboratory, motor inhibition is usually assessed in tasks like the stop-signal task (Logan, Cowan, and Davis, 1984), where it allows humans to rapidly stop actions even after their initiation. In such tasks, subjects are explicitly instructed to stop an action following a previously instructed infrequent signal, which follows the response prompt on a minority of trials (Verbruggen et al., 2019). Because subjects in tasks like the stop-signal task expect that these infrequent stop-signals will occur on a subset of trials, successful action-stopping in such tasks results from the implementation of both proactive and reactive inhibitory control mechanisms (Aron, 2011, Kenemans, 2015). Proactive inhibition denotes the anticipatory implementation of control processes during the expectation of a stop-signal, while reactive inhibition denotes the cascade of processes that is triggered by the stop-signal itself (Verbruggen et al., 2009; Chikazoe et al., 2009; Jaffard et al., 2008).
Within the stop-signal task, the signals that instruct participants to cancel an action are infrequent events. However, since stop-signals are explicitly part of the task instruction, their occurrence is also expected. Notably, however, in recent years, work on tasks that involve unexpected sensory events (e.g., the novelty-oddball paradigm or the cross-modal oddball task; Courchesne et al., 1975, Parmentier et al., 2008) has shown that such events automatically induce motor inhibition, even when there is no instruction to ever stop an action. In other words, unexpected sensory events induce a reflexive engagement of motor inhibition, and they can do so even in the absence of proactive control (i.e., when the task does not involve an instruction to exert inhibitory control; Wessel, 2018).
This automatic recruitment of reactive motor inhibition after unexpected events is evident on many levels of observation, including behavior, brain activity, and physiological changes of the motor system (cf. Wessel & Aron, 2017, for a review). In behavior, this engagement of motor inhibition is suggested by the fact that unexpected events presented during forced-choice reaction time tasks lead to a slowing of the prompted motor responses (Dawson et al., 1982, Ljungberg et al., 2012). Concomitantly, in the brain, unexpected events activate some of the same cortical and subcortical circuitry that is involved in stopping actions in tasks like the stop-signal task (Bockova et al., 2011; Wessel et al., 2016; Fife et al., 2017).
However, the inhibitory effects that unexpected events exert on the motor system are perhaps most evident from physiological measurements of cortico-spinal excitability (CSE). CSE can be non-invasively measured using transcranial magnetic stimulation (TMS) and electromyography (Barker et al., 1985; Rothwell et al., 1999; Bestmann & Krakauer, 2015). By applying single pulses of TMS to the contralateral motor cortex representation of a specific muscle, a motor evoked potential is produced in the electromyogram of that muscle. The amplitude of this motor evoked potential provides a proxy for the net-CSE of the underlying corticomotor tract. In tasks like the stop-signal task, CSE of the muscles involved in the action is suppressed when a stop-signal occurs (Coxon et al., 2006, 2007). In addition, several studies have shown that this suppression of the motor system extends even beyond the muscle group that is targeted for stopping (Badry et al., 2009; Cai, Oldenkamp, and Aron, 2011; Majid et al., 2013; Wessel et al., 2013, 2016). Subsequent studies have found that the proactive-reactive control balance is a key factor in determining this non-selective property of motor inhibition: the more proactive control is exerted, the more selectively it can be applied. In turn, the more stopping relies on reactive mechanisms, the greater the non-selective suppression of CSE (Greenhouse, Oldenkamp, and Aron, 2012, Duque et al., 2017). In other words, non-selective CSE suppression is a hallmark signature of the reactive implementation of motor inhibition. Consequently, consistent with the proposal that unexpected sensory events lead to an automatic recruitment of the brain’s reactive inhibition circuity even when stopping is not explicitly required (i.e., in the absence of proactive control), such events do indeed also lead to a non-selective suppression of CSE (Wessel & Aron, 2013). In that particular study, subjects performed a verbal reaction time task, in which unexpected sounds were infrequently presented prior to the imperative stimulus. This led to CSE suppression at a task-unrelated hand muscle, specifically at 150ms following sound onset. The same is true when a task is performed with the legs and CSE is measured at the hand (Dutra et al., 2018).
Such studies of unexpected sensory events (see also Novembre et al., 2018, 2019) have led us to propose that unexpected events automatically activate the same reactive inhibitory control systems that are recruited during outright action-stopping the stop-signal task. Specifically, we propose that the purpose of this automatically engaged inhibitory control effort is to rapidly interrupt ongoing behavior, thereby purchasing time for the cognitive system to resolve the surprise produced by the unexpected event. This additional processing time can be used to evaluate whether ongoing motor plans are still appropriate in light of the sudden unexpected change in environmental regularity (Wessel & Aron, 2017).
However, there is a notable alternative to this surprise-inhibition theory. Specifically, while the two classes of psychological events that are known to result in non-selective CSE suppression (stop-signals and unexpected events) differ in the degree to which they produce surprise (stop-signals are expected, unexpected events are not), they also have a notable commonality: they are both infrequent events within the context of their respective tasks. Stop-signals typically occur in around 25–33% of trials in the stop-signal task (Verbruggen et al., 2019; though see Dykstra et al., 2020 for a recent exception). Similarly, in studies of unexpected events, only about 10–20% of trials typically involve an unexpected event. Therefore, it is possible that the infrequency of a stimulus alone can account for the presence of non-selective CSE suppression after both stop-signals and unexpected events. If that is the case, surprise itself is not necessary to explain the presence of non-selective CSE suppression, and may in fact not uniquely engage motor inhibition at all. Indeed, while surprise and infrequency are often confounded, they are meaningfully different cognitive constructs. For example, infrequent events can be entirely expected (hearing a fire alarm during a previously announced drill), or entirely unexpected (hearing the same fire alarm without prior warning), with fundamentally different cognitive and behavioral implications.
Therefore, the goal of the current study was to investigate whether infrequent events can produce reactive motor inhibition, as indexed by non-selective CSE suppression, even when they are not surprising and they do not involve a stopping-instruction.
Notably, this question is not just relevant to test the proposed theoretical link between surprise and motor inhibition. Indeed, if expected infrequent events can recruit reactive motor inhibition without any instruction to stop an ongoing action, this would be highly relevant for the study of motor inhibition in the stop-signal task. In fact, the question of which exact neural or psychological processes following stop-signals are related to the attentional detection of the infrequent stop-signal, and which are related to the actual implementation of motor inhibition has been one of the most controversial debates in the recent stop-signal literature (Verbruggen et al., 2010; Hampshire et al., 2010; Matzke et al., 2013). To address this question, many studies have utilized control tasks whose stimulus layout matches the stop-signal task (i.e., a go-signal is followed by an infrequent second signal) but with an instruction that does not involve outright action stopping to the second, infrequent signal (e.g., to press a second button after the original go-response or to ignore the second signal entirely, Hampshire et al., 2010; Dodds et al., 2011; Chatham et al., 2012; Erika-Florence et al., 2014; Waller et al., 2019). If such expected infrequent stimuli presented outside of a stop-signal task produced the same type of reactive, non-selective motor inhibition that is found after unexpected infrequent stimuli, it would invalidate the assumption that a contrast between a stop-signal task and an infrequent-signal control task would cleanly isolate the inhibitory process that is found in the stop-signal task.
Therefore, in sum, we here aimed to explicitly test whether expected infrequent events produce the same type of non-selective suppression of the motor system that is found after unexpected infrequent events. We tested this possibility using tasks that presented such infrequent events both before and during action initiation. Experiment 1 mirrored existing work with unexpected infrequent events - i.e., sounds were presented before the imperative stimulus (as is the case in the common cross-modal oddball paradigm; cf. Parmentier 2008; Wessel & Aron, 2013). Experiment 2 was designed to match the “expected infrequent” control conditions that are often used in conjunction with stop-signal tasks – i.e., infrequent events were presented after the initial signal to initiate an action.
Methods
Participants
In Experiment 1, participants were twenty young, healthy adults (all right-handed, 17 female, mean age 18.65, SD: .9). In Experiment 2, participants were twenty-one young, healthy adults (all right-handed, 14 female, mean age: 20.76, SD: 4.2). All participants were recruited via a University of Iowa research-dedicated email list or via the University of Iowa Department of Psychological Brain and Sciences’ online recruitment tool and compensated in correspondence to their recruitment means, either by an hourly rate of $15 or by receiving course credit. The participants were all screened using a safety questionnaire (Rossi et al., 2011) to ensure it was safe for them to undergo TMS. Experimental procedures were approved by the University of Iowa Institutional Review Board (#201711750).
Experimental task
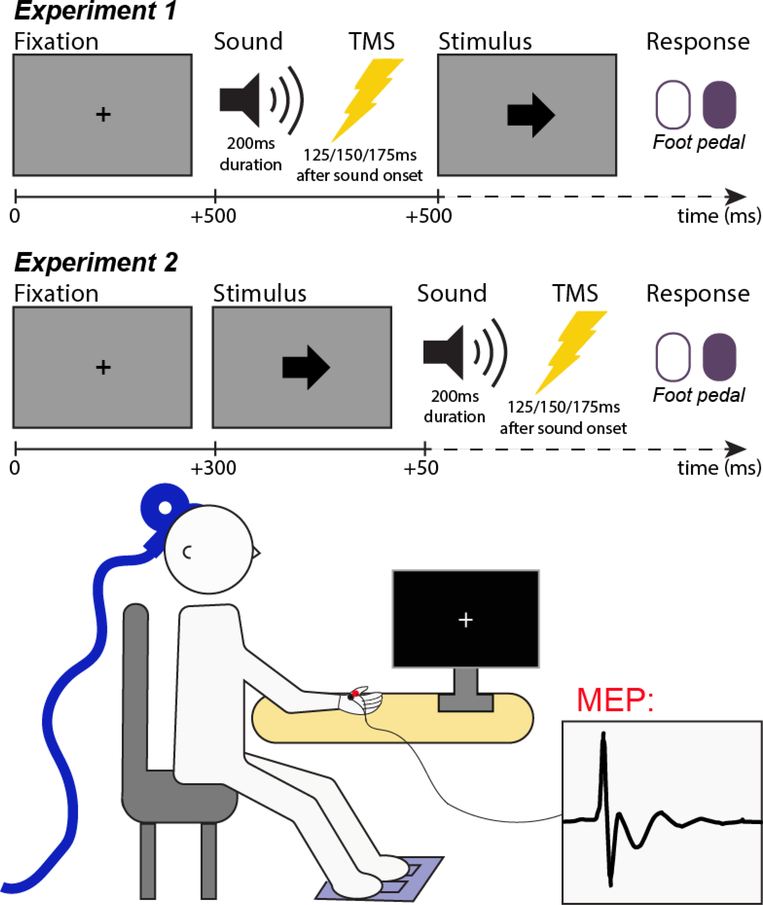
The stimuli for the behavioral paradigms for both Experiment 1 and Experiment 2 were presented using Psychtoolbox (Brainard et al., 1997) and MATLAB 2015b (TheMathWorks, Natick, MA) on a desktop computer running Ubuntu Linux. Participants responded to the stimuli on the screen using their feet by pushing Kinesis Savant Elite 2 foot pedals (left or right; see Figure 1 for visualization of task setup). In Experiment 1, at the beginning of every trial, a black fixation cross was displayed in the center of a gray screen background. After 500ms, a sound stimulus was played for 200ms, which could be of one of the following conditions: STANDARD (frequent), EXPECTED (infrequent), UNEXPECTED (infrequent). The STANDARD and EXPECTED sounds were sine wave tones of either 600 or 800Hz frequency, counterbalanced across participants. The participant was introduced to the STANDARD and EXPECTED sounds in a practice block prior to the recorded experiment. In the practice block, the EXPECTED sound occurred during 20% of trials, with the remainder being STANDARD sounds. In the actual experiment, the EXPECTED sound occurred on 10% of trials. The UNEXPECTED sounds occurred on 10% of trials and were only introduced during the main experiment, without prior instruction. These novel sounds were 90 bird song samples from European starlings (recorded by Jordan A. Comins), which were matched in amplitude envelope and duration to the sine wave tones. Each unique bird song sample only occurred once per experiment run, ensuring that each UNEXPECTED tone trial included a truly novel stimulus. After the sound, on each trial, a single pulse of TMS was delivered with a delay of 125, 150, or 175ms (i.e., centered around 150ms, which was the time point at which the CSE suppression after unexpected sounds was observed in Wessel & Aron, 2013). Subjects were instructed that the sound would cue them to the timing of the appearance of the imperative stimulus. The imperative stimulus was a black arrow pointing left or pointing right and appeared 500ms after the onset of the sound. Participants responded according to the direction of the arrow by pressing the left or right foot pedal (deadline: 1,000ms). If no response was made in time, “Too Slow!” was displayed on screen in red. After an inter-trial interval of 150, 175, 200, 225, or 250ms (during which the fixation cross was displayed), the next trial began. The practice block lasted 30 trials. During the main block, participants completed a total of 810 trials (648 STANDARD, 81 EXPECTED, 81 UNEXPECTED), divided into 9 blocks separated by self-timed breaks.
Figure 1.
Diagrams of speeded response tasks participants completed in Experiments 1 and 2. In Experiment 1, participants heard the sound before an imperative stimulus (the arrow) was shown. In Experiment 2, participants heard the sound immediately following the arrow. Below the task diagrams is a diagram of the experimental setup: TMS to elicit a MEP is delivered over motor cortex contralateral to the hand muscle with EMG electrodes, while participants respond with the feet.
The task used in Experiment 2 was the same as in Experiment 1, except for the order and relative timing of the sound relative to the imperative stimulus. In Experiment 2, the sound played 50ms after the onset of the imperative stimulus. Again, TMS stimulation occurred 125, 150, or 175ms after the sound.
All task code, analysis code, and data can be found on the Open Science Framework (OSF) at [link will be added at time of publication].
TMS protocol
Cortico-spinal excitability (CSE) was measured via motor-evoked potentials elicited by TMS. TMS stimulation was performed with a MagStim 200–2 system (MagStim, Whitland, UK) using a 70-mm figure-of-eight coil. Hotspotting was performed to identify the first dorsal interosseous muscle (FDI) stimulation locus and correct intensity. The coil was first placed 5 cm lateral and 2 cm anterior to the vertex and repositioned to where the largest MEPs were observed consistently. Resting motor threshold (RMT) was then defined as the minimum intensity required to induce MEPs of amplitudes exceeding .1 mV peak to peak in 5 of 10 consecutive probes (threshold chosen based on recommendations from Rossini et al., 1994). This MEP threshold is the same as used by Dutra et al., 2018 and Wessel, Waller, and Greenlee, 2019. TMS stimulation intensity was then adjusted to 115% of RMT (Experiment 1: mean intensity: 52.7% of maximum stimulator output; range: 40–68%; Experiment 2: mean intensity: 56.9% of maximum stimulator output; range: 47–67%) for stimulation during the experimental task. In both experiments, TMS pulses occurred with a delay of 125, 150, or 175ms after sound onset (uniform distribution). A passive baseline for MEP normalization was collected by delivery of 10 single TMS pulses at the end of each experimental task block. One baseline pulse was delivered every 3 seconds during baseline collection. During passive baseline collection, the participant was instructed to relax and saw a blank screen with the text “Collecting baseline, Please relax”.
EMG recordings
An EMG sweep was triggered 90ms before each TMS pulse. EMG was recorded using a bipolar belly-tendon montage over the FDI muscle of the right hand using adhesive electrodes (H124SG, Covidien Ltd., Dublin, Ireland), with a ground electrode placed over distal end of ulna. Electrodes were connected to a Grass P511 amplifier (Grass Products, West Warwick, RI; 1000 Hz sampling rate, filters: 30 Hz high-pass, 1000Hz low-pass, 60Hz notch). The amplified EMG data were sampled via a CED Micro 1401–3 sampler (Cambridge Electronic Design Ltd., Cambridge, UK) and recorded to the disc using CED Signal software (Version 6).
Behavioral analysis
Behavioral performance in Experiments 1 and 2 was analyzed using custom scripts in MATLAB. Trials were excluded from further analysis if participants did not respond within the 1s response deadline or if participants responded with the wrong button. In both experiments, we calculated the mean accuracy and reaction time for each condition of interest (SOUND: STANDARD, EXPECTED, UNEXPECTED). Accuracy was computed using trials that contained a response made before the deadline. RT means were tested for differences using a 1×3 ANOVA with the factor SOUND.
Motor evoked potential analysis
MEPs were identified from the EMG trace via in-house software developed in MATLAB (TheMathWorks, Natick, MA). Trials were excluded if the root mean square power of the EMG trace 90ms before the TMS pulse exceeded .01 mV or if the MEP amplitude did not exceed .01 mV. MEP amplitude was quantified with a peak-to-peak rationale, measuring the difference between maximum and minimum amplitude within a time period of 10–50 ms after the pulse. Both automated artifact rejection and MEP amplitude quantification were visually checked for accuracy on each individual trial for every data set by a rater who was blind to the specific trial type. Before statistical analysis, we trimmed the MEP data to account for the high variability and potential for outliers inherent in MEPs. We ranked the trials within each condition by MEP amplitude and removed the bottom and top 5% of trials. We then normalized by dividing amplitudes by the mean baseline MEP estimate and calculated the mean MEP amplitudes for each condition of interest (SOUND: STANDARD, EXPECTED, UNEXPECTED; TMS TIMING: 125ms, 150ms, 175ms; non-normalized results are reported in the Supplementary Materials to this manuscript). After artifact correction, MEP amplitudes were tested for differences using a 3×3 ANOVA with the factors SOUND and TMS TIMING. To rule out any systematic contamination of the pre-TMS baseline, we also conducted the same ANOVA on the mean root mean square of the EMG signal in the 90ms period prior to the TMS pulse.
When appropriate, follow-up pairwise t-tests were used to compare different SOUND conditions following findings of main effects from ANOVAs. When pairwise t-tests were used, we corrected for multiple comparisons using a Bonferroni-Holm procedure. For Experiment 1, the mean number of trials per condition were 170 (standard, 125ms), 174 (standard, 150ms), 173 (standard, 175ms), 22 (expected, 125ms), 22 (expected, 150ms), 22 (expected, 175ms), 22 (unexpected, 125ms), 20 (unexpected, 150ms), and 22 (unexpected, 175ms), respectively. For Experiment 2, the mean number of trials per condition were 176 (standard, 125ms), 176 (standard, 150ms), 172 (standard, 175ms), 22 (infrequent expected, 125ms), 22 (infrequent expected, 150ms), 21 (infrequent expected, 175ms), 22 (unexpected novel, 125ms), 21 (unexpected novel, 150ms) and 22 (unexpected novel, 175ms), respectively.
Results
Behavior
Condition-wise mean RT and accuracy results for both Experiment 1 and 2 can be found in Table 1. Of note, error trials (wrong button presses) were rare, and average accuracy was nearly perfect in both experiments. Trials during which no response was made or during which a response was made (miss trials) after the 1s were not included in analysis. Miss trials accounted for 1% of trials on average in Experiment 1 and .4% of trials on average in Experiment 2.
Table 1.
Behavioral results for each sound type from Experiments 1 and 2. Results denote mean +/− standard deviation.
| Reaction times | Accuracy | ||||
|---|---|---|---|---|---|
| Standard | Expected | Unexpected | Standard | Expected | Unexpected |
| Experiment 1 | |||||
| 468.65 ± 28.67 | 460.76 ± 27.28 | 465.80 ± 30.50 | 0.99 ± .01 | 0.99 ± .01 | 0.99 ± .01 |
| Experiment 2 | |||||
| 421.89 ± 34.44 | 420.19 ± 38.28 | 427.98 ± 37.72 | 0.97 ± .03 | 0.99 ± .02 | 0.98 ± .02 |
For Experiment 1, we conducted an ANOVA (repeated measures, 1-way/factor) on RT to assess the effects of SOUND type. An overall main effect of SOUND type was found (F(2,19) = 4.47, p = .02, η2 = .19). Pairwise t-tests were conducted to evaluate which sound (EXPECTED or UNEXPECTED) resulted in mean RT that differed significantly from RT during the STANDARD trials. Reaction time for UNEXPECTED trials was not significantly different from RT during STANDARD trials (t(19) = 1.56, p = .14, d = .09) but RT for EXPECTED trials was significantly faster than RT on STANDARD trials (t(19) = 3.09, p = .006, d = .28).
In Experiment 2, we presented the sound stimulus following the target arrow to assess the effects of infrequent stimuli on an already-initiated movement. For Experiment 2, we conducted an ANOVA (repeated measures, 1-way/factor) on RT to assess the effects of SOUND type. An overall main effect of SOUND type was found (F(2,19) = 5.22, p < .01, η2 = .21). Pairwise t-tests were conducted to evaluate which infrequent sound (EXPECTED or UNEXPECTED) resulted in mean RT that differed significantly from RT during the STANDARD trials. Reaction time for UNEXPECTED trials was significantly slower than RT during STANDARD trials (t(20) = −2.77, p = .01, d = .17), but RT for EXPECTED trials was not significantly different from RT on STANDARD trials (t(20) = 0.83, p = .42, d = .05).
We also conducted the behavioral analyses described above after trimming reaction times by 10% (similarly to how MEPs were trimmed prior to analysis). Though implementation of this procedure changed the exact values of our results, it did not change the statistical significance of any of our tests. Because of this, we only present the behavioral results found using the standard procedure without RT trimming.
Cortico-spinal excitability
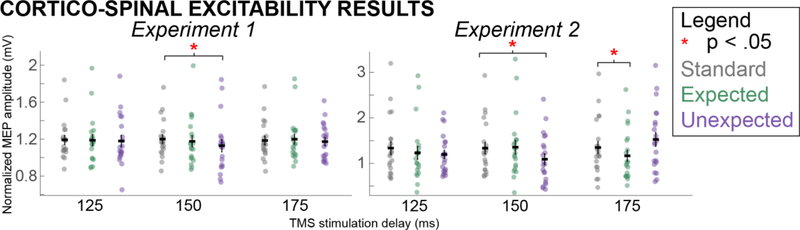
In Experiment 1 (sound prior to imperative stimulus), no significant main effects of SOUND (F(2,19) = 2.11, p = .14, η2 = .05) or TMS TIMING (F(2,19) = .57, p = .57, η2 = .01) were found, and there was no significant interaction of those factors (F(4,19) = .95, p = .44, η2 = .05). Though this ANOVA revealed no omnibus results of SOUND, TMS TIMING, or an interaction, we still computed a pairwise t-test between MEP amplitudes on STANDARD and UNEXPECTED sounds, specifically at the 150ms delay. This was done in an attempt to replicate the previous finding of CSE suppression on UNEXPECTED sounds compared to STANDARD at that exact time point (Wessel & Aron, 2013). Indeed, MEPs for UNEXPECTED sounds at 150ms were significantly smaller than MEPs from STANDARD sounds (t(19) = 2.32, p = .016, d = .38, Figure 2). In contrast, the STANDARD vs. EXPECTED comparison showed no significance at any time point (125ms: t(19) = 0.60, p = .54, d = .06; 150ms: t(19) = 0.54, p = .60, d = .07; 175ms: t(19) = −0.86, p = .40, d = .11). For the sake of completion, we also report the results from a 1×3 ANOVA of MEPs across all three SOUND conditions at the 150ms delay (F = 3.08, p = .06, eta squared = .15). In terms of RMS baseline EMG effects, no significant main effects of SOUND (F(2,19) = 0.69, p = .51, η2 = .02), TMS TIMING (F(2,19) = 1.51, p = .23, η2 = .10), or an interaction between the two (F(2,19) = 0.61, p = .65, η2 = .03) were found, suggesting that baseline EMG activity did not account for the observed effects.
Figure 2.
MEP results from Experiments 1 and 2, separated into trial averages (+/− standard error) by SOUND and TMS TIMING conditions. Statistically significant comparisons are noted.
In Experiment 2 (sound after imperative stimulus), no significant main effects of SOUND (F(2,20) = 1.40, p = .26, η2 = .04) or TMS TIMING (F(2,20) = 1.55, p = .22, η2 = .03) were found, but a there was a significant interaction (F(4,20) = 4.78, p < .01, η2 = .24). Follow-up pairwise t-tests revealed that UNEXPECTED MEPs were suppressed compared to STANDARD MEPs at the 150ms delay (t(20) = 2.59, p = .02, d = .30), replicating our previous findings. In addition, the EXPECTED MEP was suppressed compared to the STANDARD trial MEP at the 175ms delay (t(20) = 2.28, p = .03, d = .30, Figure 2).
In terms of RMS baseline, no significant main effects of SOUND (F(2,19) = 0.64, p = .53, η2 < .01), TMS TIMING (F(2,19) = 0.45, p = .64, η2 < .01), nor an interaction between the two (F(2,19) = 1.88, p = .12, η2 = .10) were found, suggesting that baseline EMG activity did not account for the observed effects.
Discussion
Across two experiments, we investigated whether infrequent but expected events induce a non-selective suppression of the motor system, similar to what has been reported for unexpected infrequent events and stop-signals. Using single-pulse TMS combined with EMG of task-unrelated muscles during a forced-choice reaction time task, we found that infrequent expected sounds are indeed followed by a non-selective suppression of task-unrelated motor effectors. However, we found that this is only the case when a movement is currently being initiated (i.e., when the infrequent event follows the imperative stimulus that cues movement initiation). In contrast, unexpected infrequent events non-selectively suppress CSE compared to expected, frequent events even in the absence of movement initiation (i.e., when presented before any imperative stimulus). The latter finding presents a direct replication of our previous report of non-selective CSE suppression when unexpected sounds precede an imperative stimulus, compared to when the stimulus is preceded by a frequent, standard stimulus (Wessel & Aron, 2013; here, this was performed using a direct comparison between the two respective conditions in Experiment 1). Notably, the timing of the non-selective suppression of CSE after unexpected infrequent events was also in line with our prior work, in that it took place at 150ms following the onset of the sound (Wessel & Aron, 2013; Dutra et al., 2018). In turn, it is notable that non-selective CSE suppression after expected infrequent events that followed the imperative stimulus did not occur until 175ms after the event (cf., Experiment 2). These findings have two primary implications, which we will now discuss in turn.
First, the results suggest that there is a qualitative difference in the non-selective suppression of the motor system that takes place after infrequent events, depending on whether these events were unexpected or expected. Specifically, unexpected events induce CSE suppression compared to expected, frequent events even in the absence of motor initiation, which suggests a more drastic type of inhibitory control that is not evoked by expected infrequent events. Moreover, the latency difference in CSE suppression between unexpected and expected infrequent events suggests a more rapid engagement of inhibitory control when infrequent events are unexpected. In that respect, it is interesting to observe that the respective suppressive effects of expected and unexpected infrequent events do not seem to be additive. This is evident from the fact that while expected infrequent sounds produced CSE suppression compared to standard sounds at 175ms following sound onset in Experiment 2, no such suppression was observed at that time point for unexpected sounds (which resulted in CSE suppression at 150ms in both experiments). If the effects of surprise and infrequency were additive, unexpected sounds should have produced suppression at both 150ms (due to the unexpectedness) and at 175ms (due to the infrequency). Instead, infrequency and unexpectedness appear to independently engage the same inhibitory process, but with different latency. This supports the theory that surprise is accompanied by a unique pattern of automatically engaged inhibitory control (Wessel & Aron, 2017).
Beyond these implications for the processing of unexpected and infrequent events, the current findings also have very important implications for the study of motor inhibition in the context of the stop-signal task. As mentioned in the introduction, recent years have seen a controversial discussion regarding the exact psychological and neural mechanisms that contribute to action-stopping in the stop-signal task. Specifically, there has been a particular emphasis on the notion that the ability to stop an action is not solely dependent on the efficacy of the inhibitory process itself, but also depends on the initial attentional detection of the (infrequent) stop-signal and the associated triggering of the inhibitory process (Levy & Wagner, 2011; Verbruggen et al., 2014; Erika-Florence et al., 2014; Matzke et al., 2013, 2017). This notion has spurred a fundamental discussion about which aspects of the neural cascade after stop-signals reflect the attentional detection of an infrequent instructed signal to stop, and which reflect the motor inhibition process itself (Aron et al., 2014; Hampshire & Sharp, 2015; Swick & Chatham, 2014). In many studies that address this question, an inferential contrast is used in which brain activity following stop-signals is compared to brain activity following perceptually identical, infrequent, expected events that do not convey a ‘stopping’ instruction (Schmajuk et al., 2006; Dimoska & Johnstone, 2008; Hampshire et al., 2010; Boehler et al., 2010; Tabu et al., 2011; Dodds et al., 2011; Chatham et al., 2012; Erika-Florence et al., 2014; Bissett & Logan, 2014; Elchlepp et al., 2015; Lawrence et al., 2015; Verbruggen et al., 2010; Waller, Hazeltine, and Wessel, 2019). In other words, those studies employ a purportedly ‘non-inhibitory’ control condition that resembles the design of our current Experiment 2, where a go-signal is followed by an infrequent expected event. The current results clearly show that presenting such infrequent, expected events after go-signals lead to an automatic engagement of non-selective motor inhibition. This is in line with our other recent work, which has shown that expected infrequent events after a go-signal lead to an incidental slowing of reaction times and elicit scalp-recordable neurophysiological activity from the same neural generator that is active after stop-signals (Waller et al., 2019). Together, these findings suggests that the ‘inhibition-free’ control conditions that are used in studies to isolate attentional from inhibitory processes are not, in fact, free of inhibitory activity. Consequently, a subtraction contrast between stop-trials and such control conditions will likely cancel out (at least parts of) the inhibitory process, instead of isolating it. Therefore, these subtractive contrasts might operationalize other condition differences between stop-trials and control trials with infrequent signals (such as the fact that stop-trials do not include a motor response).
The current study has three shortcomings, largely owing to methodological limitations associated with recordings of CSE via TMS. First, we did not find the behavioral effects of unexpected and expected infrequent events (reaction time slowing) that are usually found in studies that use similar experimental paradigms (e.g., Dawson et al., 1982, Parmentier et al., 2008, Waller et al., 2019). This is likely due to the presence of the TMS pulses, which tend to eliminate such behavioral effects. Indeed, TMS of motor cortex interferes with ongoing behavior itself by interrupting the underlying motor processes (Hadipour-Niktarash et al., 2007; Cohen et al., 2009). Moreover, TMS pulses produce a stereotypic auditory and haptic sensation that occurs on every trial. Prior research has shown that when infrequent or surprising sounds are immediately followed by stereotypic, non-surprising sounds, the effect of infrequency or surprise on behavior is greatly reduced (Parmentier, 2014; Parmentier et al., 2008). This issue is unavoidable in studies that use TMS to probe the effects of unexpected events on motor excitability. A second shortcoming of the study is that it has been demonstrated that single-pulse TMS itself may affect CSE when delivered every in repetitive intervals of around four seconds (Pellicciari et al., 2016). We cannot rule such out additive effects of single pulses on CSE, though we believe that it is reasonable to assume that these effects impact all conditions equally, and hence average out of the condition comparisons. A third shortcoming of the current study is we did not use an active baseline in the inter-trial interval during the task (unlike e.g., Wessel & Aron, 2013). The introduction of such baseline trials would have further elongated an already tedious and tiring task for the subjects, who had to respond to more than 800 very simple stimuli for more than 45 minutes to provide a sufficient number of trials in all three conditions. Therefore, it is – strictly speaking – not possible to ascertain whether infrequent events study lead to a suppression of the MEP below a task-baseline based on the current data, or whether they merely suppress CSE relative to frequent events. However, since our previous study (Wessel & Aron, 2013) has shown that unexpected infrequent events indeed suppress CSE below active baseline, one could extrapolate that the same would be true for the expected infrequent events in the current study (as the CSE suppression that occurred at 175ms after expected sounds was similar in amplitude from the CSE suppression that occurred at 150 following unexpected sounds). Nevertheless, this hypothesis would necessitate independent validation.
Finally, the differential timing of CSE suppression found for unexpected and expected infrequent events provides some interesting aspects for future study. There are several potential explanations for this difference in timing. It is widely believed that non-selective CSE suppression is due to the engagement of a specific fronto-basal ganglia inhibitory pathway (Aron, 2011; Jahanshahi et al., 2015; Neubart et al., 2010; Wiecki & Frank 2013; Wessel et al., 2016; Wessel & Aron, 2017; Kelley et al., 2018; Wessel et al., 2019). It is unclear whether the timing difference between unexpected and expected infrequent events found here is mechanistically attributable to differences in subcortical processing in the basal ganglia, or to differences in the ‘up-stream’ cortical processes that trigger those the basal ganglia processes. One property of the proposed fronto-basal ganglia pathway underlying non-selective CSE suppression is its ostensible hyper-direct, mono-synaptic connection from the cortical areas that trigger the inhibitory process into the basal ganglia structures that implement the actual inhibition (Nambu et al., 2002; Parent and Hazrati, 1995; Kelley et al., 2018; Chen et al., 2020). If this circuit is indeed as hard-wired and low-level as believed, differences in cortical processing related to the triggering of the inhibitory process are perhaps more likely to account for the differences in timing of the CSE suppression between expected and unexpected infrequent events. Indeed, classic EEG studies of such events do indicate that while unexpected infrequent events evoke a fronto-central P3a waveform, expected frequent events evoke a slower-latency, more posterior P3b (Courchesne et al., 1975; Friedman et al., 2001; Comerchero & Polich, 1999), which could suggest differences in cortical processing depending on whether an infrequent event is surprising or not. Future studies could test whether both of these potentials reflect the activity of different cortical pathways that detect infrequent events depending on their expectedness, but ultimately converge to produce inhibition via the same downstream basal ganglia circuit.
In summary, we here found that infrequent events produce a non-selective suppression of the motor system, even when they are expected. Notably, however, this suppression of the motor system is qualitatively different than the suppression observed after unexpected events, which manifests with lower latency and is also observable in the absence of motor preparation. The presence of such frequency-related inhibitory effects poses an important challenge for studies of motor inhibition that seek to produce conditions that do not include inhibitory activity. Furthermore, the current results show that surprise caused by unexpectedness has unique effects on the motor system that are not attributable to the relative frequency of an event alone.
Supplementary Material
Acknowledgements:
The authors would like to thank Nathan Chalkley, Kylie Dolan, and Brynne Dochtermann for their help with data collection.
Funding: National Institutes of Health NINDS R01 102201 and National Science Foundation CAREER 1752355 (JRW), National Institutes of Health T32GM108540 (DAD), Iowa Center for Research by Undergraduates (CI).
Footnotes
COI: The authors report no conflict of interest.
Ethics approval: University of Iowa Institutional Review Board (#201711750)
Code/data availability: All data and code will be made publicly available on the OSF.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Aron AR (2011). From reactive to proactive and selective control: developing a richer model for stopping inappropriate responses. Biological Psychiatry, 69(12), e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, & Poldrack RA (2014). Inhibition and the right inferior frontal cortex: one decade on. Trends in Cognitive Sciences, 18(4), 177–185. [DOI] [PubMed] [Google Scholar]
- Badry R, Mima T, Aso T, Nakatsuka M, Abe M, Fathi D, … & Fukuyama H (2009). Suppression of human cortico-motoneuronal excitability during the Stop-signal task. Clinical Neurophysiology, 120(9), 1717–1723. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, & Freeston IL (1985). Non-invasive magnetic stimulation of human motor cortex. The Lancet, 325(8437), 1106–1107. [DOI] [PubMed] [Google Scholar]
- Bestmann S, & Krakauer JW (2015). The uses and interpretations of the motor-evoked potential for understanding behaviour. Experimental Brain Research, 233(3), 679–689. [DOI] [PubMed] [Google Scholar]
- Bissett PG, & Logan GD (2014). Selective stopping? Maybe not. Journal of Experimental Psychology: General, 143(1), 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bočková M, Chládek J, Jurák P, Halámek J, Baláž M, & Rektor I (2011). Involvement of the subthalamic nucleus and globus pallidus internus in attention. Journal of Neural Transmission, 118(8), 1235–1245. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, & Woldorff MG (2010). Pinning down response inhibition in the brain—conjunction analyses of the stop-signal task. Neuroimage, 52(4), 1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997). The psychophysics toolbox. Spatial vision, 10(4), 433–436. [PubMed] [Google Scholar]
- Cai W, Oldenkamp CL, & Aron AR (2011). A proactive mechanism for selective suppression of response tendencies. Journal of Neuroscience, 31(16), 5965–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Claus ED, Kim A, Curran T, Banich MT, & Munakata Y (2012). Cognitive control reflects context monitoring, not motoric stopping, in response inhibition. PloS one, 7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, de Hemptinne C, Miller AM, Leibbrand M, Little SJ, Lim DA, … & Starr PA (2020). Prefrontal-Subthalamic Hyperdirect Pathway Modulates Movement Inhibition in Humans. Neuron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Hirose S, Yamashita KI, Miyashita Y, & Konishi S (2009). Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. Journal of Neuroscience, 29(50), 15870–15877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NR, Cross ES, Tunik E, Grafton ST, & Culham JC (2009). Ventral and dorsal stream contributions to the online control of immediate and delayed grasping: a TMS approach. Neuropsychologia, 47(6), 1553–1562. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, & Polich J (1999). P3a and P3b from typical auditory and visual stimuli. Clinical Neurophysiology, 110(1), 24–30. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, & Galambos R (1975). Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography and Clinical Neurophysiology, 39(2), 131–143. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, & Byblow WD (2006). Intracortical inhibition during volitional inhibition of prepared action. Journal of Neurophysiology, 95(6), 3371–3383. [DOI] [PubMed] [Google Scholar]
- Coxon JP, Stinear CM, & Byblow WD (2007). Selective inhibition of movement. Journal of Neurophysiology, 97(3), 2480–2489. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Beers JR, & Kelly A (1982). Allocation of cognitive processing capacity during human autonomic classical conditioning. Journal of Experimental Psychology: General, 111(3), 273. [DOI] [PubMed] [Google Scholar]
- Dimoska A, & Johnstone SJ (2008). Effects of varying stop-signal probability on ERPs in the stop-signal task: do they reflect variations in inhibitory processing or simply novelty effects?. Biological Psychology, 77(3), 324–336. [DOI] [PubMed] [Google Scholar]
- Dodds CM, Morein-Zamir S, & Robbins TW (2011). Dissociating inhibition, attention, and response control in the frontoparietal network using functional magnetic resonance imaging. Cerebral Cortex, 21(5), 1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Greenhouse I, Labruna L, & Ivry RB (2017). Physiological markers of motor inhibition during human behavior. Trends in Neurosciences, 40(4), 219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra IC, Waller DA, & Wessel JR (2018). Perceptual surprise improves action stopping by nonselectively suppressing motor activity via a neural mechanism for motor inhibition. Journal of Neuroscience, 38(6), 1482–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra T, Waller DA, Hazeltine E, & Wessel JR (2020). Leveling the field for a fairer race between going and stopping: neural evidence for the race model of motor inhibition from a new version of the stop signal task. Journal of Cognitive Neuroscience, 32(4), 590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchlepp H, Lavric A, Chambers CD, & Verbruggen F (2016). Proactive inhibitory control: A general biasing account. Cognitive Psychology, 86, 27–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erika-Florence M, Leech R, & Hampshire A (2014). A functional network perspective on response inhibition and attentional control. Nature Communications, 5, 4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife KH, Gutierrez-Reed NA, Zell V, Bailly J, Lewis CM, Aron AR, & Hnasko TS (2017). Causal role for the subthalamic nucleus in interrupting behavior. Elife, 6, e27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, & Gaeta H (2001). The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience & Biobehavioral Reviews, 25(4), 355–373. [DOI] [PubMed] [Google Scholar]
- Greenhouse I, Oldenkamp CL, & Aron AR (2012). Stopping a response has global or nonglobal effects on the motor system depending on preparation. Journal of Neurophysiology, 107(1), 384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, & Shadmehr R (2007). Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. Journal of Neuroscience, 27(49), 13413–13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, & Owen AM (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage, 50(3), 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, & Sharp DJ (2015). Contrasting network and modular perspectives on inhibitory control. Trends in Cognitive Sciences, 19(8), 445–452. [DOI] [PubMed] [Google Scholar]
- Jaffard M, Longcamp M, Velay JL, Anton JL, Roth M, Nazarian B, & Boulinguez P (2008). Proactive inhibitory control of movement assessed by event-related fMRI. Neuroimage, 42(3), 1196–1206. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Obeso I, Rothwell JC, & Obeso JA (2015). A fronto–striato–subthalamic–pallidal network for goal-directed and habitual inhibition. Nature Reviews Neuroscience, 16(12), 719–732. [DOI] [PubMed] [Google Scholar]
- Kelley R, Flouty O, Emmons EB, Kim Y, Kingyon J, Wessel JR, … & Narayanan NS (2018). A human prefrontal-subthalamic circuit for cognitive control. Brain, 141(1), 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenemans JL (2015). Specific proactive and generic reactive inhibition. Neuroscience & Biobehavioral Reviews, 56, 115–126. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Verbruggen F, Morrison S, Adams RC, & Chambers CD (2015). Stopping to food can reduce intake. Effects of stimulus-specificity and individual differences in dietary restraint. Appetite, 85, 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, & Wagner AD (2011). Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences, 1224(1), 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, & Davis KA (1984). On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal of Experimental Psychology: Human Perception and Performance, 10(2), 276. [DOI] [PubMed] [Google Scholar]
- Ljungberg JK, Parmentier FB, Leiva A, & Vega N (2012). The informational constraints of behavioral distraction by unexpected sounds: The role of event information. Journal of experimental psychology: learning, memory, and cognition, 38(5), 1461. [DOI] [PubMed] [Google Scholar]
- Majid DA, Cai W, Corey-Bloom J, & Aron AR (2013). Proactive selective response suppression is implemented via the basal ganglia. Journal of Neuroscience, 33(33), 13259–13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke D, Love J, Wiecki TV, Brown SD, Logan GD, & Wagenmakers EJ (2013). Release the BEESTS: Bayesian estimation of ex-Gaussian stop-signal reaction time distributions. Frontiers in Psychology, 4, 918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke D, Love J, & Heathcote A (2017). A Bayesian approach for estimating the probability of trigger failures in the stop-signal paradigm. Behavior Research Methods, 49(1), 267–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, & Takada M (2002). Functional significance of the cortico–subthalamo–pallidal ‘hyperdirect’pathway. Neuroscience Research, 43(2), 111–117. [DOI] [PubMed] [Google Scholar]
- Neubert FX, Mars RB, Buch ER, Olivier E, & Rushworth MF (2010). Cortical and subcortical interactions during action reprogramming and their related white matter pathways. Proceedings of the National Academy of Sciences, 107(30), 13240–13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre G, Pawar VM, Bufacchi RJ, Kilintari M, Srinivasan M, Rothwell JC, … & Iannetti GD (2018). Saliency detection as a reactive process: unexpected sensory events evoke corticomuscular coupling. Journal of Neuroscience, 38(9), 2385–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novembre G, Pawar VM, Kilintari M, Bufacchi RJ, Guo Y, Rothwell JC, & Iannetti GD (2019). The effect of salient stimuli on neural oscillations, isometric force, and their coupling. NeuroImage, 198, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, & Hazrati LN (1995). Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Research Reviews, 20(1), 91–127. [DOI] [PubMed] [Google Scholar]
- Parmentier FB (2008). Towards a cognitive model of distraction by auditory novelty: The role of involuntary attention capture and semantic processing. Cognition, 109(3), 345–362. [DOI] [PubMed] [Google Scholar]
- Parmentier FB (2014). The cognitive determinants of behavioral distraction by deviant auditory stimuli: a review. Psychological Research, 78(3), 321–338. [DOI] [PubMed] [Google Scholar]
- Pellicciari MC, Miniussi C, Ferrari C, Koch G, & Bortoletto M (2016). Ongoing cumulative effects of single TMS pulses on corticospinal excitability: an intra-and inter-block investigation. Clinical Neurophysiology, 127(1), 621–628. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, … & De Noordhout AM (1994). Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography and Clinical Neurophysiology, 91(2), 79–92. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Hallett M, Berardelli A, Eisen A, Rossini P, & Paulus W (1999). Magnetic stimulation: motor evoked potentials. Electroencephalogr Clin Neurophysiol Suppl, 52, 97–103. [PubMed] [Google Scholar]
- Schmajuk M, Liotti M, Busse L, & Woldorff MG (2006). Electrophysiological activity underlying inhibitory control processes in normal adults. Neuropsychologia, 44(3), 384–395. [DOI] [PubMed] [Google Scholar]
- Swick D, & Chatham CH (2014). Ten years of inhibition revisited. Frontiers in Human Neuroscience, 8, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabu H, Mima T, Aso T, Takahashi R, & Fukuyama H (2011). Functional relevance of pre-supplementary motor areas for the choice to stop during Stop signal task. Neuroscience research, 70(3), 277–284. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, & Logan GD (2009). Proactive adjustments of response strategies in the stop-signal paradigm. Journal of Experimental Psychology: Human Perception and Performance, 35(3), 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, & Chambers CD (2010). Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proceedings of the National Academy of Sciences, 107(31), 13966–13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Stevens T, & Chambers CD (2014). Proactive and reactive stopping when distracted: An attentional account. Journal of Experimental Psychology: Human Perception and Performance, 40(4), 1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Band GP, Beste C, Bissett PG, Brockett AT, … & Colzato LS (2019). A consensus guide to capturing the ability to inhibit actions and impulsive behaviors in the stop-signal task. Elife, 8, e46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller DA, Hazeltine E, & Wessel JR (2019). Common neural processes during action-stopping and infrequent stimulus detection: The frontocentral P3 as an index of generic motor inhibition. International Journal of Psychophysiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, & Aron AR (2013). Unexpected events induce motor slowing via a brain mechanism for action-stopping with global suppressive effects. Journal of Neuroscience, 33(47), 18481–18491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Jenkinson N, Brittain JS, Voets SH, Aziz TZ, & Aron AR (2016). Surprise disrupts cognition via a fronto-basal ganglia suppressive mechanism. Nature Communications, 7, 11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, & Aron AR (2017). On the globality of motor suppression: unexpected events and their influence on behavior and cognition. Neuron, 93(2), 259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR (2018). Surprise: a more realistic framework for studying action stopping?. Trends in cognitive sciences, 22(9), 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel JR, Waller DA, & Greenlee JD (2019). Non-selective inhibition of inappropriate motor-tendencies during response-conflict by a fronto-subthalamic mechanism. Elife, 8, e42959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, & Frank MJ (2013). A computational model of inhibitory control in frontal cortex and basal ganglia. Psychological Review, 120(2), 329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.