Abstract
Background:
The correlation between programmed cell death-ligand 1 (PD-L1) which may affect T cell to form the immune tolerance and breast cancer (BC) still maintains to be uncovered. This meta-analysis was about to explore PD-L1 expression as well as its prognostic role in BC.
Methods:
First of all, we performed 3 databases: PubMed, Embase, and Web of Science to explore publications between January of 2015 and January of 2020. Strict inclusion and exclusion criteria were conducted: immunohistochemistry shall be used to detect target molecule expression and at least 1 survival indicator and related data we need should be included. The hazard ratio and 95% confidence interval were pooled related with survival as well as clinicopathological parameters. The effects of PD-L1 in differed aspects like sample size and age of each cohort were demonstrated by subgroup analyses as well as sensitivity analyses which may complain the potential source of heterogeneity. P < .05 indicates factors were charge of the heterogeneity of prognosis. Begg and Egger tests were used to identify publication bias.
Results:
We identified 12 studies containing a blanket of 4336 patients with BC for whom PD-L1 positive tumor cells were related with higher tumor stage, lymph node metastasis, estrogen receptor negativity, human epidermal growth factor receptor 2 positivity, luminal B and triple negative BC molecular subtype and high nuclear-associated antigen Ki- 67 expression. Meanwhile, compared to patients with PD-L1 negative expression, PD-L1 positivity associated with worse overall survival (Hazard ratio [HR]:1.43; 95% CI:0.98–2.10; P < .001) and might have no obvious tight connection with disease free survival (HR:1.40; 95% CI:1.11–1.78; P = .101) and recurrence free survival (HR:2.36; 95% CI:1.04–5.34; P = .145). The outcome of the meta-analysis was confirmed to be credible by sensitivity analysis. Publication bias was not existed indicated (P = .640).
Conclusion:
Positive PD-L1 expression has a worse clinical outcome in patients with BC demonstrated by our meta-analysis. Being urgent to catch attention to the role of PD-L1 in BC, it may be considered as prognostic marker of immune microenvironment for improving therapy efficacy.
Keywords: breast cancer, disease free survival, meta-analysis, overall survival, programmed cell death ligand-1
1. Introduction
Breast cancer (BC) is still the leading type of malignant tumors that affects the prognosis of women with high mortality rate so far. Treatment guidelines have been followed up and improved by various researchers, especially in terms of aspect like tumor microenvironment. Recently, survival benefits have been suggested to relate with the performance of chemotherapy, adjuvant therapies, and receptor target therapies among various clinical trials, in which researchers still acclaimed that there maintain metastasis and recurrence resulting in death.[1] More effective treatment is about to be conducted, while tumor progression and consequently death have been reported to keep increasing among BC patients so far.[2] Reports show us that hormone receptor positive BC carried out the increasing tendency of that, while the incidence rate of hormone receptor negative BC goes reverse in whole text.[3] The prognostic elements in BC have been investigated for decades; however, prognostic factors other than stage and performance status are still controversial. Previous studies have inferred clinicopathological features such as larger tumor size, hormone receptor status, the presence of lymph node involvement, staining extent of nuclear-associated antigen Ki- 67 (Ki-67), as adverse distinguished prognostic traits in BC.
ln theory, heterogeneity always exists when individuals in specific ethic are identified as patients who have received similar treatment. Meanwhile, the presented anatomic staging system is not comprehensive enough which also can carry out the differed clinical outcome of those selected patients.
Except for TNM stage, these factors only can be evaluated after surgical operation. Therefore, there is significant attraction in investigating noninvasive and readily accessible pretreatment variables to evaluate survival outcome in BC. Inflammatory treatment might play an attention-catching role in tumor progression demonstrated by latest reports.
Many complicated mechanisms of immune microenvironment and tumor could be responsible by the immune tolerance causing by programmed cell death ligand-1 (PD-L1) and its related B7 family. Also, benefit has been approached for patients with BC with PD-L1 immune blockades, leaving a message that PD-L1 shall be indicative of prognostic molecule.[4,5]
In the only reported phase 3 trial, the combination of atezolizumab and nabpaclitaxel conferred a nonstatistically significant overall survival (OS) benefit compared to nab-paclitaxel alone in unselected triple negative breast cancer (TNBC) patients. Intriguingly, the diversity in OS was significant in the PD-L1+ subgroup of patients, suggesting that the potential clinical utility of PD-L1 expression.[6,7] In BC, quite a few studies have currently suggested that positive PD-L1 BC was related with poorer OS,[8–10] but other studies could not verify this finding.[11,12] However, according to provided results, this eye on the value of PD-L1 in BC still did not reach a consensus that further validation is urgently awaited.
As the spread knowing, to overcome the limitation of various variates causing the heterogeneity from cohorts, meta-analysis is a powerful statistic tool, which also may generate the comprehensive and convincible data to explain the ultimate clinical relationship.
2. Materials and methods
2.1. Ethics statement
The present study was conducted guiding by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). Due to based literature, ethical clearance is not required.[13]
2.2. Search strategy
On February 16 of 2020, a literature selection of studies was performed within recent 5 years limitation through well-known databases: PubMed, Embase, Web of science. All terms used were as follows: “breast neoplasms or BC or breast carcinoma or Breast Tumor or Mammary Cancer or Malignant Neoplasm of Breast or Human Mammary Neoplasm or Cancer of Breast,” and “Programmed Cell Death 1 Ligand 1 or B7-H1 Immune Costimulatory Protein or B7 H1 Immune Costimulatory Protein or PD-L1 Costimulatory Protein or Costimulatory Protein, PD-L1 or PD L1 Costimulatory Protein or CD274 Antigen.” Two independent authors reviewed the search results, respectively.
2.3. Inclusion and exclusion criteria
Inclusion criteria followed by:
-
(1)
selected patients with BC were confirmed by pathological standard method;
-
(2)
studies in which anti-PD-L1 antibody for the immunohistochemistry was collected;
-
(3)
studies in which one or more clinical survival outcomes were reported;
-
(4)
core needle biopsy method was used or specimens directly were resected after surgery.
Exclusion criteria followed by:
-
(1)
comments, reviews, abstracts or non-BC cohorts;
-
(2)
non-English articles;
-
(3)
incomplete data for survival with hazard ratio and 95% confidence interval;
-
(4)
duplicate studies;
-
(5)
patients who had received neoadjuvant or adjuvant treatment or enrolled PD-L1 inhibitor clinical trial.
Criteria were strictly carried out. EndNote software (version X9) and manual screening method were used to achieve the most suitable and complete studies for analysis. To achieve the consensus, final case was triggered by a third author in terms of disagreement.
2.4. Data extraction and quality assessment
Our research included author of cohort, year, nation, sample size, patient age, PD-L1 antibody information, PD-L1 positivity, detection standard, follow-up, Hazard ratio (HR) and 95% CI and clinical endpoints. Positive or high expression is defined as PD-L1+, left was classified as PD-L1-. All eligible studies were retrospective. Newcastle-Ottawa Scale (NOS) system consists of three parts: selection (0–4 points), comparability (0–2 points), and outcome assessment (0–3 points). The Newcastle-Ottawa Scale (NOS) score of 6 were assigned as high-quality studies.
2.5. Statistical analysis
OS, disease free survival (DFS) and recurrence free survival (RFS) were defined as endpoints of survival outcomes in this study. The time from the first diagnosis to death for any reason is defined for OS. DFS is defined as the interval from the date of surgery to the first observation of recurrence. RFS, the time from treatment initiation to recurrence at any site. Mantel–Haenszel method was performed to analyze pooled HR with 95% CI to all extent. The pooled results of those clinical prognostic outcomes were indicated by forest plots. Post this, to address heterogeneity, tests were conducted by Cochran Q and Higgins I-squared method. A fixed-effect model might be chosen to obtain precise results with insignificant heterogeneities, a random-effect model was utilized otherwise. I2 < 25%, I2 = 25% to 50%, and I2 > 50% denote no heterogeneity, low heterogeneity, and extreme heterogeneity separately. P < .1 or I-squared >50% was indicative of remarkable heterogeneity[14]; Sensitivity analysis is a tool that analyzes whether the combined effect amount after the exclusion of the included studies varied from the previous total effect amount. If the 2 HRs are very different, it means that the excluded study may explain the resource of heterogeneous.[15] Subgroup analyses were applied for the same purpose.[14] Publication bias was assessed by visual inspection of Begg plot and the possibility of publication bias was conducted by Egger test,[16,17] which should be taken into considered when P < .05. Review Manager 5.3, STATA version 15.0, and IBM Statistics 23 were used for analyses. McNemar and Pearson Chi-square tests were adopted to identify association between clinical parameter and expression of PD-L1. A 2-sides P < .05 was considered statistically significant.
3. Results
3.1. Study characteristics
Four hundred thirty-three literature were researched in total and the selection process was summarized in Figure 1. After manual screening by 2 authors, 368 articles were ruled out because they were letters, duplicates, reviews, abstracts, studies on other tumors, or no data related HRs and 95%CIs. After screening 65 complete records, we also eliminated 53 articles because patients were not eligible for inclusion in those studies who had already received neoadjuvant or adjuvant chemotherapy or enrolled any clinical trial before surgery, or not using IHC method to evaluate PD-L1 expression. At last, 12 articles meeting criteria were included for this meta-analysis.[9,10,12,18–26]Table 1 shows the basic characteristics like year of cohort, sample size, follow-up, NOS score et al. Table 2 demonstrated the correlation of PD-L1 expression among various clinicopathological features, like hormone status and tumor-related classification and so on. Including reports were published between 2014 and 2018. In this study 4336 patients with BC included, we identified 1279 cases as luminal A BC, 1044 patients were classified as luminal B, 360 individuals suffered HER-2 rich, 1236 women belonged to TNBC type. Multivariate analyses were performed in these included studies to address prognostic outcomes including DFS and OS in most of cohorts. And immunohistochemistry method was conducted shown in Table 1. In total, 12 studies had estimated in tumor cells expression of PD-L1[9,10,12,18–26] and 5 studies evaluated expression of PD-L1 in both tumor and immune cells.[19,22–24,26] Also, different study owns different criteria for PD-L1 cut-off values which were summarized in Table 1.
Figure 1.
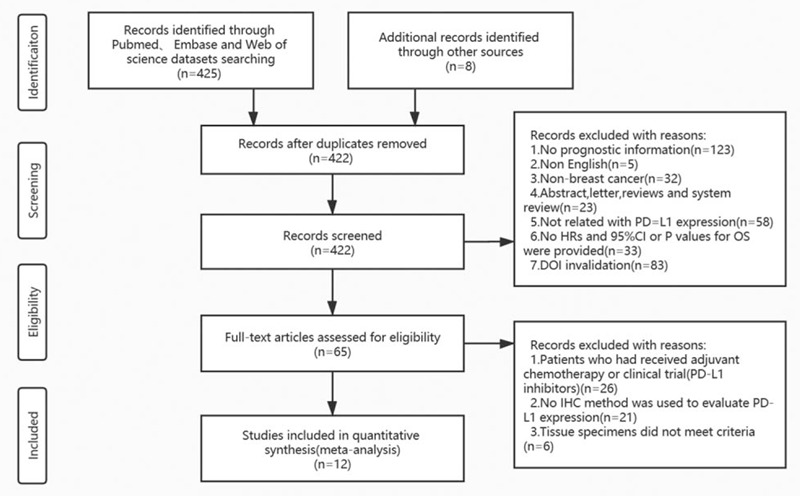
Flow chart of the included studies.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Study cohort | Year | Country | N | Age (yrs; median and range) | Type of study | PD-L1 Antibody used | PD-L1+(%) | Detection standard | Study end-points | HR | Follow-up, median in months | NOS score |
| António Polónia | 2017 | Spain | 440 | 60.0(28–92) | Retrospective | clone SP142(R) | 28 (6.4%) | membranous/cytopl-asmic staining≥1% | OS | R (M) | 120 (1–120) | 6 |
| Xiaoxian Li | 2016 | USA | 136 | NA | Retrospective | clone NAT105 (M) | 14 (10.3%) | H-score≥5 | OS/DFS | R (M) | 36-144 | 6 |
| Julia Y. S. Tsang | 2017 | China | 1091 | 54.5 ± 12.7 (22–94) | Retrospective | NA | 295 (27.0%) | Mean immunoscore (staining intensity) | OS/DFS | R (M) | 63 (1–210) | 7 |
| Jing He | 2018 | USA | 68 | 48.0 (23–75) | Retrospective | clone 28–8 (R) | 25 (36.8%) | Mean immunoscore (staining intensity) | OS/DFS | R (M/U) | 48 (23–75) | 8 |
| Ming Li | 2018 | China | 101 | 51 (27–74) | Retrospective | CST, 13,684 (R) | 39 (38.61%) | H-score≥5 | DFS | R (M/U) | 49.03 (10.97–94.27) | 7 |
| Hitomi Mori | 2017 | Japan | 284 | 59.6 | Retrospective | E1L3N (R) | 103 (41.5%) | PD-L1 expression≥50% | OS/RFS | R (M/U) | 68 (2–150) | 6 |
| Quirine F. Manson | 2018 | Netherlands | 106 | 53 | Retrospective | clone sp263 (R) | 14 (13.2%) | H-scores>0 | OS | R (M/U) | 61.2 (15.6–310.8) | 6 |
| Sang Byung Bae | 2016 | Korea | 465 | 52.3 (24–81) | Retrospective | E1L3N (R) | 63 (13.5%) | H-score ≥100 | OS/DFS | R (M) | 41 (1–158) | 8 |
| In Hae Park | 2015 | Korea | 333 | 47 (28–78) | Retrospective | Abcam (R) | 163 (48.9%) | H-score≥2+-3+ | OS/DFS | R (U) | 117.6 (4.8–153.6) | 8 |
| Rhiannon K Beckers | 2015 | Australia | 161 | 57 (28–89) | Retrospective | E1L3N (R) | 123 (76.4) | H-score≥100 | OS/CSS | R (U) | 55 (0–213) | 7 |
| S. Muenst | 2014 | Switzerland | 650 | 64 (27–101) | Retrospective | Abcam (R) | 152 (23.4%) | H-score ≥100 | OS | R (M/U) | 65 (1–174) | 6 |
| Zhenhua Li | 2016 | China | 501 | 53 (29–83) | Retrospective | ab58810 (R) | 231 (46.1%) | H-score ≥100 | OS/RFS | R (M/U) | 64 (1–80) | 8 |
USA = United States of America, N = number of patients, NA = not applicable, R/M = rabbit/mouse, OS = overall survival, DFS = disease free survival, RFS = recurrence free survival, TNBC = triple negative breast cancer, R(M) = the HR come from multivariate analysis, R(U) = the HR comes from univariate analysis, NOS score = The Newcastle-Ottawa Scale (NOS) score.
Table 2.
Correlation between PD-L1 expression and clinicopathological parameters.
| PD-L1(-)% | PD-L1(+)% | P value | |
| Age (yrs) | 0.051 | ||
| ≤50 | 333 (57.3) | 248 (42.7) | |
| >50 | 433 (62.4) | 260 (37.6) | |
| tumor size (cm) | 0.466 | ||
| ≤2 | 246 (50.8) | 238 (49.2) | |
| >2 | 322 (53.0) | 285 (47) | |
| Histologic grade | 0.779 | ||
| I | 424 (74.1) | 148 (25.9) | |
| II | 1110 (73.4) | 402 (26.6) | |
| III | 977 (72.6) | 368 (27.4) | |
| Tumor stage | 0.015 | ||
| PT1 | 662 (78.3) | 184 (21.7) | |
| PT2 | 875 (78.1) | 246 (21.9) | |
| PT3 | 84 (71.8) | 33 (28.2) | |
| PT4 | 70 (66.0) | 36 (34.0) | |
| Lymph node metastasis | 0.007 | ||
| (–) | 1007 (72.1) | 390 (27.9) | |
| (+) | 802 (67.2) | 391 (32.8) | |
| ER status | .000 | ||
| (–) | 897 (73.2) | 328 (26.8) | |
| (+) | 1741 (80.2) | 429 (19.8) | |
| PR status | .167 | ||
| (–) | 950 (75.5) | 309 (24.5) | |
| (+) | 972 (73.1) | 358 (26.9) | |
| HER2 status | 0.030 | ||
| (–) | 2122 (73.7) | 758 (26.3) | |
| (+) | 508 (69.7) | 221 (30.3) | |
| Molecular subtype | 0.043 | ||
| Luminal A | 762 (75.3) | 250 (24.7) | |
| Luminal B | 570 (70.3) | 241 (29.7) | |
| Her2 rich | 209 (77.4) | 61 (22.6) | |
| TNBC | 346 (73.2) | 127 (26.8) | |
| Ki-67 expression | 0.000 | ||
| Low | 1283 (78.5) | 352 (21.5) | |
| High | 951 (69.0) | 428 (31.0) |
ER = estrogen receptor, PR = progesterone receptor, T = tumor, P < .05: statistically significant.
3.2. PD-L1 expression and clinicopathological features
Table 2 clearly summarized the correlation between PD-L1 positive in tumor cells and clinicopathological parameters. Ten (83.3%) studies[9,10,12,18,20–25] had reported PD-L1 positivity in different ways. PD-L1 + associated with high tumor stage (stage 1 vs 2 vs 3 vs 4, 21.7% vs 21.9% vs 28.2% vs 34.0%, P = .015), lymph node metastasis (positive vs negative, 32.8% vs 27.9%, P = .007), estrogen receptor (ER) negativity (ER positive vs ER negative, 19.8% vs 26.8%, P = .000), human epidermal growth factor receptor 2 (HER2) positivity (HER2+ vs HER2, 30.3% vs 26.3%, P = .03), luminal B and TNBC molecular subtype (luminal A 24.7%, luminal B 29.7%, HER2-rich 22.6%, TNBC 26.8%, P = .043) and higher Ki-67 expression (low expression vs high expression, 21.5% vs 31.0%, P = .000). Meanwhile, we were not given the strong hint with the association between expression of PD-L1 and age (P = .051), tumor size (P = .466), histologic grade (P = .779) as well as progesterone receptor status (P = .167).
3.3. PD-L1 expression and patient survival
Related data in present study were carried out from the collected publications including figures and table numeric data to calculate total HRs and 95% CIs about PD-L1 expressed in tumor cells.[27]
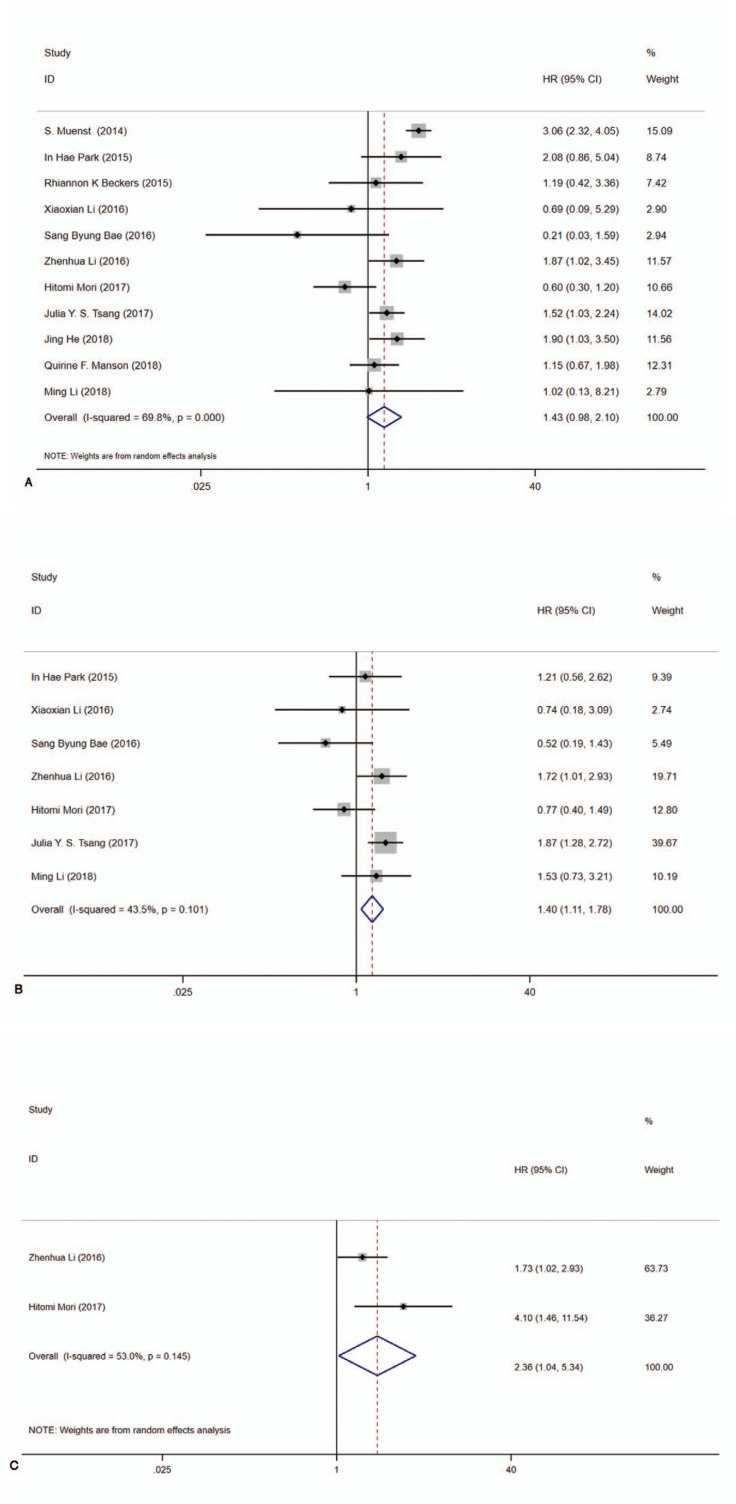
For OS, altogether 11 studies[9,10,12,19–26] reported OS data with precise HR and 95%CIs. Significant heterogeneity existed (I2 = 69.8%, Cochrane Q) among extracted studies. As a result, pooled data suggested that PD-L1 expression was implicated in shorter OS with a random model (pooled HR:1.43, 95%CI:0.98–2.10, P < .001) (Fig. 2A). For DFS, there were seven studies in which data we need were displayed[10,12,19,20,22,23,25] and low heterogeneity was existed (I2 = 43.5%, Cochrane Q). Pooled result by fixed model showed that PD-L1 expression had no profound impact on DFS in PD-L1 positive patients with BC (pooled HR:1.40, 95% CI:1.11–1.78, P = .101) (Fig. 2B). Only 2 studies[10,23] provided RFS data and obviously significant heterogeneity existed (I2 = 53.0%, Cochrane Q). According to the given explicit data, statistical difference was not observed between PD-L1 expression and RFS in PD-L1 positive BC patients (pooled HR:2.36, 95% CI:1.04–5.34, P = .145) (Fig. 2C).
Figure 2.
Forest plots of hazard ratios (HR) for survival based on PD-L1 expression. A, OS (pooled HR 1.43, 95%CI = 0.98–2.10, I2 = 69.8%, Cochrane Q, P < .000). B, DFS (pooled HR 1.40, 95% CI = 1.11–1.78, I2 = 43.5%, Cochrane Q, P = .101). C RFS (pooled HR 2.36, 95% CI = 1.04–5.34, I2 = 53.0%, Cochrane Q, P = .145). CI = confidence interval, OS = overall survival, DFS = disease-free survival, RFS = recurrence-free survival.
3.4. Sensitivity analysis
Sensitivity analysis was selected to determine whether omitting every study turning out a significant difference. Accordingly, pooled results were achieved after leaving out each study in turn, no specific study significantly changed the overall HRs, which means that the credible outcomes were indicated (Supplemental Digital Content, Fig. S1).
3.5. Subgroup analysis
Subgroup analyses evaluated OS by a random-effects model and results were summarized in Table 3. Our results indicated the pooled HR was 1.13 (95%CI:0.75–1.71, I2 = 34.30%, P = .154) for sample size ≤500 and 2.11 (95%CI: 1.28–3.46, I2 = 77.40%, P = .012) for sample size >500. In addition, subgroup analysis was performed by age (≤50 and >50), Univariate analysis and multivariate analysis (U/M), NOS score (Fig. 3). Meantime, a fixed-effects model was adopted to estimate in terms of DFS. The pooled HR was 0.96 (95%CI: 0.66–1.39, I2 = 0.000%, P = .428) for sample size ≤500 and 1.82 (95%CI: 1.34–2.47, I2 = 0.000%, P = .806) for sample size >500. Also, subgroup analysis was performed by age (≤50 and >50), Univariate analysis and Multivariate analysis (U/M), NOS score (Supplemental Digital Content, Fig. S2). Besides, we used meta regression to identify triggers of heterogeneity that contributed to differences in clinical outcomes, but unfortunately, several factors we examined were not the main causes of the heterogeneity (Supplemental Digital Content, Fig. S3), in which P > .05.
Table 3.
Summary of the meta-analysis results.
| Random-effects model | Fixed-effects model | Heterogeneity | ||||||
| Analysis | N | References | HR (95%CI) | P | HR (95%CI) | P | I2 | Ph |
| OS | 11 | 9,10,12,19,20,21,22,23,24,25,26 | 1.43 (0.98–2.10) | .067 | 1.86 (1.56–2.21) | .000 | 69.80% | 0.000 |
| Subgroup1:sample size <500 | 8 | 12,19,21,22,23,24,25,26 | 1.13 (0.75–1.71) | .551 | 1.17 (0.87–1.58) | .301 | 34.30% | 0.154 |
| sample size ≥500 | 3 | 9,10,20 | 2.11 (1.28–3.46) | .003 | 2.34 (1.89–2.90) | .000 | 77.40% | 0.012 |
| Subgroup2: age <50 | 2 | 12,21 | 1.96 (1.18–3.24) | .009 | 1.96 (1.18–3.24) | .009 | 0.00% | 0.869 |
| Age ≥50 | 7 | 9,10,20,22,23,25,26 | 1.32 (0.81–2.14) | .269 | 1.86 (1.55–2.24) | .000 | 78.20% | 0.000 |
| Subgroup3: univariate analysis | 3 | 12,23,24 | 1.08 (0.58–2.03) | .803 | 1.05 (0.72–1.55) | .795 | 59.20% | 0.000 |
| Multivariate analysis | 8 | 9,10,19,20,21,22,25,26 | 1.70 (1.13–2.54) | .01 | 2.15 (1.77–2.61) | .000 | 60.30% | 0.014 |
| Subgroup4: NOS score =6 | 4 | 9,19,23,24 | 1.23 (0.50–3.05) | .655 | 2.10 (1.66–2.64) | .000 | 88.30% | 0.000 |
| NOS score= 7 | 3 | 20,22,26 | 1.46 (1.02–2.09) | .04 | 1.46 (1.02–2.09) | .040 | 69.80% | 0.000 |
| NOS score= 8 | 4 | 10,12,21,25 | 1.70 (1.03–2.81) | .037 | 1.78 (1.21–2.60) | .003 | 33.10% | 0.214 |
| DFS | 7 | 10,12,19,20,22,23,25 | 1.26 (0.89–1.78) | .195 | 1.40 (1.11–1.78) | .005 | 43.50% | 0.101 |
| Subgroup1:sample size <500 | 5 | 12,19,22,23,25 | 0.96 (0.66–1.39) | .832 | 0.96 (0.66–1.39) | .832 | 0.00% | 0.428 |
| sample size ≥500 | 2 | 10,20 | 1.82 (1.34–2.47) | 0 | 1.82 (1.34–2.47) | .000 | 0.00% | 0.806 |
| Subgroup2: age <50 | 1 | 12 | 1.21 (0.56–2.62) | .628 | 1.21 (0.56–2.62) | .628 | NA | NA |
| Age ≥50 | 5 | 10,20,22,23,25 | 1.28 (0.83–1.96) | .259 | 1.45 (1.13–1.87) | .004 | 58.50% | 0.047 |
| Subgroup3: univariate analysis | 2 | 12,19 | 1.08 (0.55–2.13) | .818 | 1.08 (0.55–2.13) | .818 | 0.00% | 0.553 |
| Multivariate analysis | 5 | 10,20,22,23,25 | 1.28 (0.83–1.96) | .259 | 1.45 (1.13–1.87) | .040 | 58.50% | 0.047 |
| Subgroup4: NOS score =6 | 2 | 19,23 | 0.76 (0.42–1.39) | .38 | 0.76 (0.42–1.39) | .380 | 0.00% | 0.961 |
| NOS score =7 | 2 | 20,22 | 1.79 (1.28–2.50) | .001 | 1.79 (1.28–2.50) | .001 | 0.00% | 0.639 |
| NOS score =8 | 3 | 10,12,25 | 1.15 (0.61–2.16) | .665 | 1.29 (0.87–1.93) | .210 | 53.10% | 0.119 |
N = number of studies, HR = hazard ratio, 95% CI = 95% confidence interval, Ph = p values of Q test for heterogeneity test, OS = Overall survival, DFS = Disease-free survival, NOS score = The Newcastle-Ottawa Scale (NOS) score; NA = not applicable.
Figure 3.
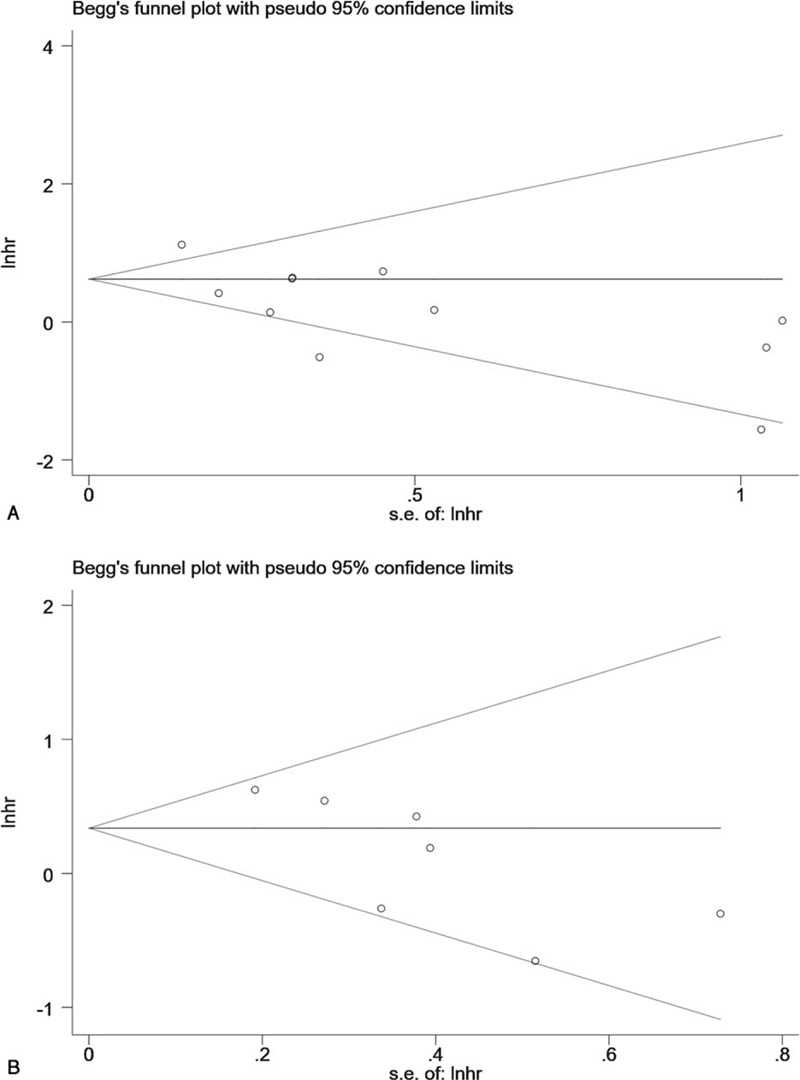
Begg test for all included studies. A, Overall survival (P = .640) B, Disease free survival (P = .072).
3.6. Publication bias
Publication bias was not detected implied by Begg plots in this meta-analysis for OS (P = .640), also the same result for DFS (P = .072) (Fig. 4 ). Egger test also was used, and the results were shown in Supplemental Digital Content, Fig. S4, in which the evidence of publication bias was indicated for OS (P = .001) and DFS (P = .014) intriguingly.
Figure 4.
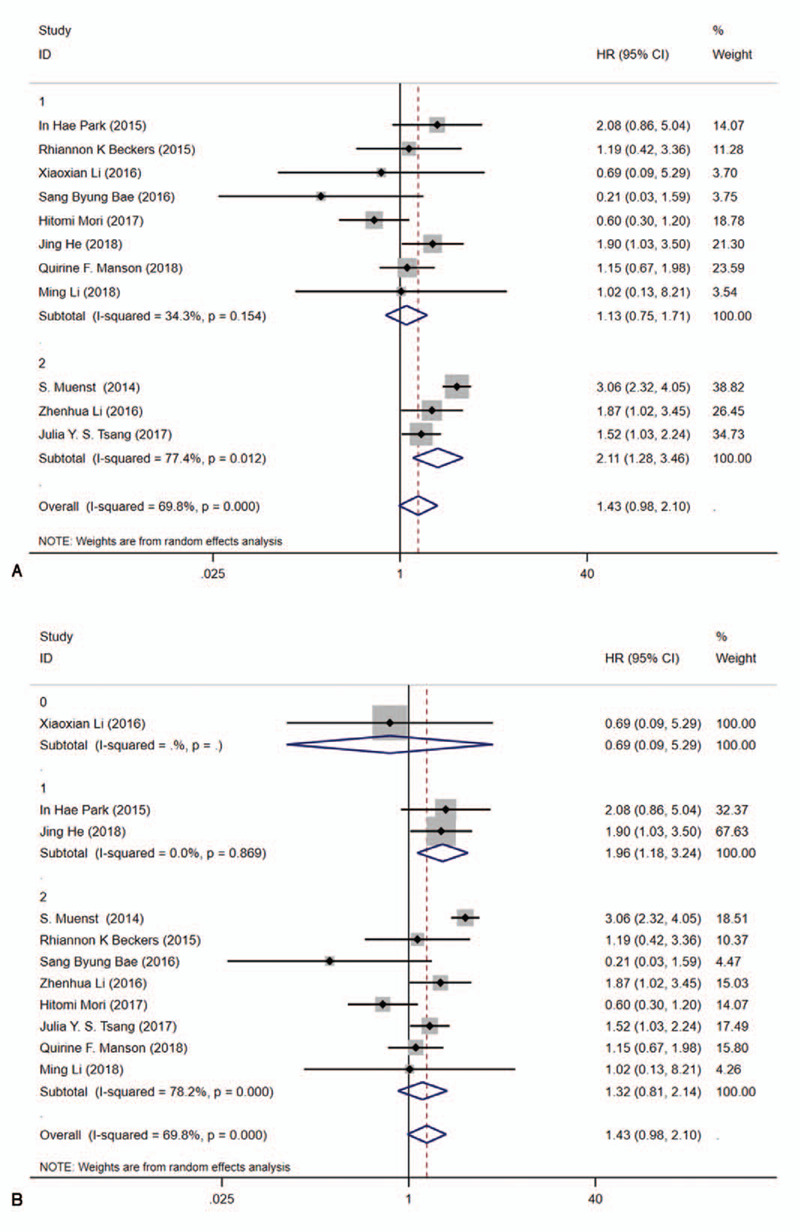
Forest plots for the association between PD-L1 expression and literature heterogeneity factors of OS with a random model. A, sample size (≤500 and >500). B age (≤50 and >50). C, Univariate analysis and multivariate analysis(U/M). D, NOS score.[6–8]
Figure 4 (Continued).
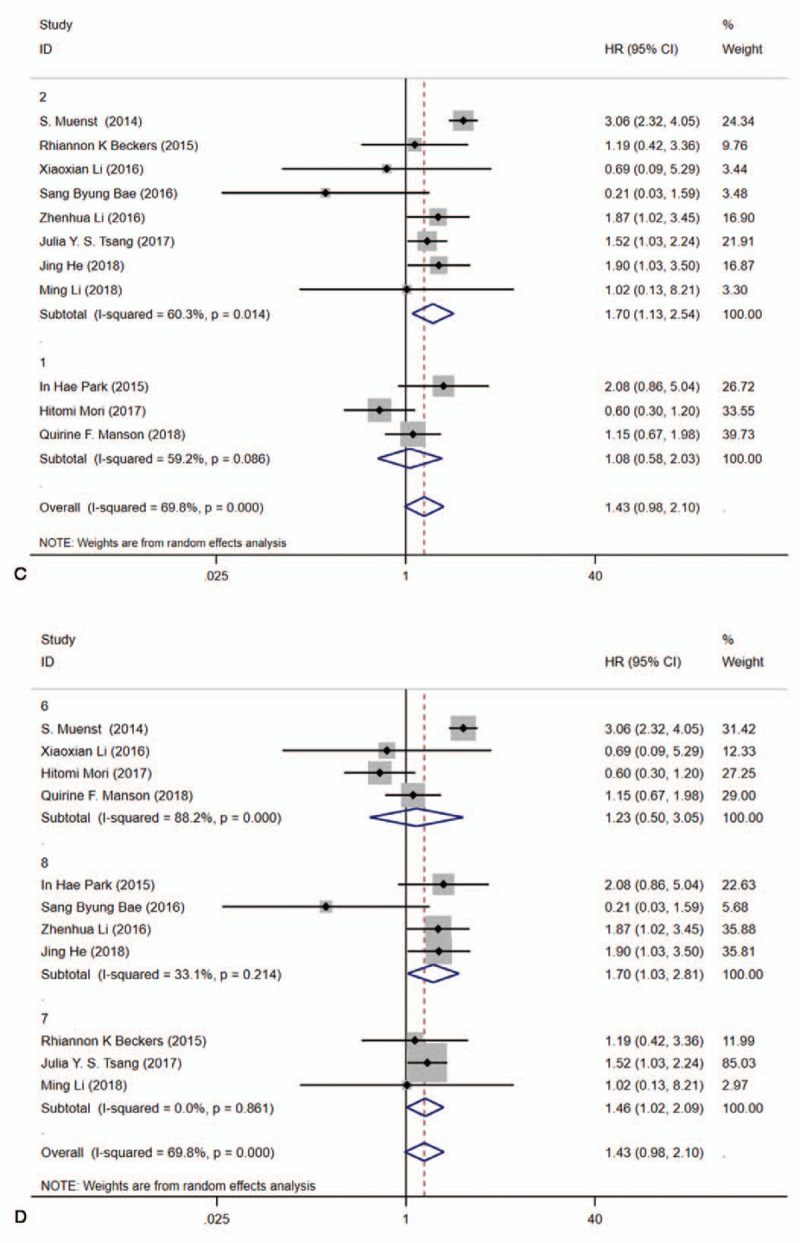
Forest plots for the association between PD-L1 expression and literature heterogeneity factors of OS with a random model. A, sample size (≤500 and >500). B age (≤50 and >50). C, Univariate analysis and multivariate analysis(U/M). D, NOS score.[6–8]
4. Discussion
Although some clinical studies have reported the effect of PD-L1 expression on BC patients, its role is still uncertain.
With the advancement of technologies, tumor identifying and treatment have been improved and benefit has been achieved for patients with BC, meanwhile, the immunotherapy also helps the therapy progression and drug regulation in terms of patient survival.[28] Recently, research indeed discovered quite a few molecules as prognosis biomarkers for BC. Within the great performance of biomarkers inhibitors, properties such as tumor invasion and aggressiveness can be eased to some extent, which may also associate with survival features or events that are exactly analyzed in this study.[29] That PD-L1 expression related to poor prognosis was confirmed by some previous researches.[30] PD-1 and PD-L1 pathway not only contributes a lot to immune microenvironment, also meaningfully effects on various tumors like lung cancer and colon cancer,[31–35] malignant melanoma,[36] and tumors of gynecology,[37] urinary malevolent tumors.[38]
PD-L1 expression also has been analyzed by several previous cohorts in patients with BC, which found that the correlation is still controversial. Meanwhile, clinical trials demonstrated that the PD-L1 inhibitors are able to improve the prognostic events for patients.[33,39] Our data and results showed that it indeed meets the founding we expect. The mechanism about the binding of PD-L1 in tumor cells and PD-1 on T cells was confirmed to facilitate tumor immune escape, resulting in cancer immune tolerance.[40]
High tumor stage, lymph node metastasis, ER negativity, HER2 positivity, luminal B and TNBC molecular subtype, and Ki-67 high expression were found to catch eyes considering prognostic factor as for survival with 12 studies including 4336 patients. Meanwhile, these results were in agreement with several lines of evidence that support the immunogenicity of TNBC: than other subtypes, expression of PD-L1 in level of mRNA in TNBC is evidently higher.[41] The correlation between PD-L1 and these clinical prognostic molecules had also been mentioned in other studies,[42–44] although the results are not exactly the same, suggesting the close relationship between PD-L1 and BC clinical patients. However, our study strictly formulated inclusion and exclusion criteria, screened out all BC patients treated with radiotherapy and chemotherapy or other drugs before and after surgical treatment in which including different and novel studies, and more accurately described the prognostic role of PD-L1 in BC patients.
In this meta-analysis, compared to patients with PD-L1 negative expression, PD-L1 positivity associated with worse OS (HR:1.43; 95% CI:0.98–2.10; P < .001)and might have no obvious tight connection with DFS (HR:1.40; 95% CI:1.11–1.78; P = .101) and RFS (HR:2.36; 95% CI:1.04–5.34; P = .145). Subgroup analyses revealed that sample size of individual study may explain the heterogeneity of the shorter DFS by PD-L1 expression, and factors such as age, Univariate/multivariate, and literature quality might be not responsible for heterogeneous root of prognosis. Besides, the lack of standardization for detection could also be responsible for the discrepant turnout due to the multiple using of TMAs within IHC for PD-L1 in nearly half of studies.[9,10,18–21,24–26] To overcome the above shortcomings, whole-tissue sections would be a good option.
Resulting from PD-L1 positive linked with shorter survival events considering various epithelial-originated cancers, various studies tended to treat it as a novel prognostic marker.[45] However, there also appeared conflicting result unsurprisingly.[46,47] The exact mechanism between immune microenvironment and tumor was undefined as we know. Thus, the correlation between immune microenvironment and tumor development has been increasingly urgent to explore and summary, especially about survival events. Meta-analysis is common tool to collect huge data and summary controversial events whereas there were still several limitations. Due to retrospective studies, some bias would be considered. Besides, this analysis was constrained to pure English studies, causing some specific ethics data group were excluded subconsciously. the origin of heterogeneity cannot be fully traced still even the sensitivity and subgroup analyses were conducted. Meanwhile, even though Begg plots showed that publication bias did not exist, however, classic funnel plot showed obvious asymmetry (Supplemental Digital Content Fig. S5), which was further confirmed with the Egger test (P < .05). After using the trim-and-fill method, for OS random effects pooled HRs was adjusted to 1.430 (95%CI:0.976–2.096) and for DFS pooled HRs was adjusted to 1.402 (95%CI 1.107–1.776). Although this study owns strict inclusion and exclusion criteria, it also loses a lot of valuable literature, resulting in relatively small eligible studies in the establishment of prognostic values, which should be poured attention into.
5. Conclusions
Higher tumor stage, lymph node metastasis, ER negativity, HER2 positivity, luminal B and TNBC molecular subtype, and Ki-67 high expression were found to be related to poorer OS in BC. Positive PD-L1 expression may be meaningful to some degree for predicting prognosis events in BC, which needs to be explored and verified by other large-scale researches.
Acknowledgments
The authors would like to acknowledge the efforts of each individual that enrolled in the same team of professor Liu of Department of Endocrine Breast Surgery, The First Affiliated Hospital of Chongqing Medical University.
Author contributions
Conceptualization: Yingzi Zhang.
Data curation: Yingzi Zhang, Jiao Tian, Chi Qu.
Methodology and Formal analysis: Zhenrong Tang, Yu Wang, Kang Li.
Supervision: Yuan Yang.
Writing – original draft: Yingzi Zhang, Yuan Yang.
Writing – review & editing: Yingzi Zhang, Jiao Tian, Chi Qu.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence interval, BC = breast cancer, DFS = disease free survival, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, HR = Hazard ratio, Ki-67 = nuclear-associated antigen Ki- 67, NOS score = The Newcastle-Ottawa Scale (NOS) score, OS = overall survival, PD-L1 = programmed cell death ligand-1, RFS = recurrence free survival, TNBC = triple negative breast cancer.
How to cite this article: Zhang Y, Tian J, Qu C, Tang Z, Wang Y, Li K, Yang Y, Liu S. Prognostic value of programmed cell death ligand-1 expression in breast cancer: A meta-analysis. Medicine. 2020;99:49(e23359).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005;353:1784–92. [DOI] [PubMed] [Google Scholar]
- [2].Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015;6:5449–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].DeSantis CE, Ma J, Goding Sauer A, et al. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin 2017;67:439–48. [DOI] [PubMed] [Google Scholar]
- [4].Wang X, Bao Z, Zhang X, et al. Effectiveness and safety of PD-1/PD-L1 inhibitors in the treatment of solid tumors: a systematic review and meta-analysis. Oncotarget 2017;8:59901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li J, Gu J. Efficacy and safety of ipilimumab for treating advanced melanoma: a systematic review and meta-analysis. J Clin Pharm Ther [Meta-Analysis Systematic Review] 2019;44:420–9. [DOI] [PubMed] [Google Scholar]
- [6].Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. [DOI] [PubMed] [Google Scholar]
- [7].Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol [Clinical Trial, Phase I Multicenter Study Research Support, Non-U S Gov’t] 2019;5:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Qin T, Zeng Y D, Qin G, et al. High PD-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget [Research Support, Non-U S Gov’t] 2015;6:33972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat [Research Support, N I H, Extramural] 2014;146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li Z, Dong P, Ren M, et al. PD-L1 expression is associated with tumor FOXP3(+) regulatory T-cell infiltration of breast cancer and poor prognosis of patient. J Cancer 2016;7:784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Baptista MZ, Sarian LO, Derchain SF, et al. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol 2016;47:78–84. [DOI] [PubMed] [Google Scholar]
- [12].Park IH, Kong SY, Ro JY, et al. Prognostic implications of tumor-infiltrating lymphocytes in association with programmed death ligand 1 expression in early-stage breast cancer. Clin Breast Cancer 2016;16:51–8. [DOI] [PubMed] [Google Scholar]
- [13].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 2008;37:1148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics [Comparative Study Research Support, U S Gov’t, P H S] 1994;50:1088–101. [PubMed] [Google Scholar]
- [18].Polonia A, Pinto R, Cameselle-Teijeiro JF, et al. Prognostic value of stromal tumour infiltrating lymphocytes and programmed cell death-ligand 1 expression in breast cancer. J Clin Pathol 2017;70:860–7. [DOI] [PubMed] [Google Scholar]
- [19].Li X, Wetherilt CS, Krishnamurti U, et al. Stromal PD-L1 expression is associated with better disease-free survival in triple-negative breast cancer. Am J Clin Pathol 2016;146:496–502. [DOI] [PubMed] [Google Scholar]
- [20].Tsang JY, Au WL, Lo KY, et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat 2017;162:19–30. [DOI] [PubMed] [Google Scholar]
- [21].He J, Huo L, Ma J, et al. Expression of programmed death ligand 1 (PD-L1) in posttreatment primary inflammatory breast cancers and clinical implications. Am J Clin Pathol 2018;149:253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li M, Li A, Zhou S, et al. Heterogeneity of PD-L1 expression in primary tumors and paired lymph node metastases of triple negative breast cancer. BMC Cancer [Research Support, Non-U S Gov’t] 2018;18:017–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mori H, Kubo M, Yamaguchi R, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget 2017;8:15584–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Manson QF, Schrijver W, Ter Hoeve ND, et al. Frequent discordance in PD-1 and PD-L1 expression between primary breast tumors and their matched distant metastases. Clin Exp Metastasis [Research Support, Non-U S Gov’t] 2019;36:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bae SB, Cho HD, Oh MH, et al. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Cancer 2016;19:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Beckers RK, Selinger CI, Vilain R, et al. Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 2016;69:25–34. [DOI] [PubMed] [Google Scholar]
- [27].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:1745–6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].De Palma M, Hanahan D. The biology of personalized cancer medicine: facing individual complexities underlying hallmark capabilities. Mol Oncol [Research Support, Non-U S Gov’t Review] 2012;6:111–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Paredes J, Correia AL, Ribeiro AS, et al. Breast carcinomas that co-express E- and P-cadherin are associated with p120-catenin cytoplasmic localisation and poor patient survival. J Clin Pathol [Research Support, Non-U S Gov’t] 2008;61:856–62. [DOI] [PubMed] [Google Scholar]
- [30].Wu P, Wu D, Li L, et al. PD-L1 and survival in solid tumors: a meta-analysis. PLoS One 2015;10:e0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med [Research Support, U S Gov’t, P H S] 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- [32].D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer [Research Support, Non-U S Gov’t] 2015;112:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature [Clinical Trial Research Support, N I H, Extramural] 2014;515:563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med [Clinical Trial, Phase I Research Support, Non-U S Gov’t Validation Study] 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- [35].Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004;10:5094–100. [DOI] [PubMed] [Google Scholar]
- [36].Thierauf J, Veit JA, Affolter A, et al. Identification and clinical relevance of PD-L1 expression in primary mucosal malignant melanoma of the head and neck. Melanoma Res 2015;25:503–9. [DOI] [PubMed] [Google Scholar]
- [37].Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A [Comparative Study Research Support, Non-U S Gov’t] 2007;104:3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–62. [DOI] [PubMed] [Google Scholar]
- [39].Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med [Clinical Trial, Phase I Multicenter Study Research Support, N I H, Extramural Research Support, Non-U S Gov’t] 2012;366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer [Research Support, N I H, Extramural Research Support, Non-U S Gov’t Review] 2015;112:1421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res [Research Support, N I H, Extramural Research Support, Non-U S Gov’t] 2014;2:361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li S, Chen L, Jiang J. Role of programmed cell death ligand-1 expression on prognostic and overall survival of breast cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang M, Sun H, Zhao S, et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget 2017;8:31347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kim HM, Lee J, Koo JS. Clinicopathological and prognostic significance of programmed death ligand-1 expression in breast cancer: a meta-analysis. BMC Cancer 2017;17:690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang Y, Kang S, Shen J, et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) expression in epithelial-originated cancer: a meta-analysis. Medicine [Meta-Analysis Research Support, Non-U S Gov’t] 2015;94:e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer [Research Support, Non-U S Gov’t] 2016;19:42–52. [DOI] [PubMed] [Google Scholar]
- [47].Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 2015;10:426–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.