Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, coronavirus disease 2019, critical care transport, mechanical ventilation, prone position
Abstract
Objectives:
To assess the safety and feasibility of a new protocol for interhospital critical care transport of mechanically ventilated patients in the prone position during the coronavirus disease 2019 pandemic by nurse and paramedic critical care transport teams.
Design:
Retrospective observational study.
Setting:
Single critical care transport agency serving multiple centers in the greater Boston area.
Patients:
All transports of intubated patients in the prone position with severe hypoxemic respiratory failure secondary to coronavirus disease 2019.
Interventions:
Records were reviewed for patients transported in the prone position. Major adverse events in transport, defined as severe hypoxemia (oxygen saturation < 80% or an absolute decrease in oxygen saturation > 10%), hypotension (mean arterial pressure < 65 mm Hg) not responsive to vasopressors or inotropes, endotracheal tube or vascular catheter dislodgement, and cardiac arrest, were recorded.
Measurements and Main Results:
A total of 25 patients were transported in prone position. The mean Pao2:Fio2 ratio in the group was 101.3 mm Hg, and 76% (n = 19) were on vasopressors. Fourteen patients (56%) had hypotension with at least one episode of mean arterial pressure less than 65 mm Hg en route, and seven (28%) had an episode of oxygen desaturation less than 88%. Only one major adverse event of severe hypoxemia (oxygen saturation < 80%) was noted.
Conclusions:
Critical care transport of severe hypoxemic respiratory failure patients with coronavirus disease 2019 in the prone position is safe when performed by a dedicated team of critical care nurse and paramedics with an established protocol.
The emergence of a novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) causing the disease coronavirus disease 2019 (COVID-19) has created a worldwide health crisis. (1). The virus causes a spectrum of illness ranging from mild fevers and cough to severe acute respiratory distress syndrome (ARDS). Therapeutics to treat COVID-19 are limited, and the cornerstone of management has been supportive care with advanced hypoxemic respiratory failure management. The prone position has been shown to improve oxygenation in COVID-19 patients (2). Prone positioning offers several physiologic benefits, including improved ventilation-perfusion matching, increased recruitment of lung, and a reduction in lung stress and strain. The Prone Positioning in Severe Acute Respiratory Distress Syndrome trial demonstrated a significant reduction in mortality in severe ARDS patients with prone positioning (3). As a result, guidelines have called for adoption of prone positioning when managing COVID-19 patients with moderate to severe ARDS (4).
Early on during the spread of the virus throughout the United States, the state of Massachusetts was profoundly affected by the COVID-19 pandemic, with the third greatest number of cases in the United States as of May 2020 (5). Many patients throughout the state required transfer to tertiary care centers due to capacity issues or lack of advanced critical care therapies at the sending facilities. Many of these institutions have used prone positioning to temporize patients prior to transport. Given the lack of data and risk of equipment issues with movement, including inadvertent extubation (6), most critical care transport (CCT) agencies only transport patients in the supine position. Previously, only limited reports described the transport of mechanically ventilated patients in the prone position (7, 8). During the COVID-19 pandemic, anticipating the increased utilization of prone positioning, Boston MedFlight developed a new protocol for transporting ARDS patients prone. We sought to examine the safety and feasibility of Boston MedFlight’s transfer of prone patients during the COVID-19 pandemic, with a primary objective of assessing for adverse events during the transport.
MATERIAL AND METHODS
We performed a retrospective review of transports to tertiary care hospitals in the greater Boston area, with suspected or confirmed COVID-19, from April 1, 2020, to May 20, 2020. Inclusion criteria were all patients transported in the prone position with suspected or confirmed COVID-19. Exclusion criteria were patients being transported in the supine position and patients with no concern for COVID-19 infection. The electronic medical record database for the CCT service was queried for “COVID” and “coronavirus.” All charts were reviewed for inclusion by two authors (M.A.F., S.R.W.). This study was a quality-improvement initiative to monitor performance of a new prone transport protocol initiated during the COVID-19 pandemic and as such was deemed exempt from Institutional Review Board review. Given the restrictions of large group gatherings during the COVID-19 pandemic, most of the training of this new protocol occurred online using an already established training platform used by Boston MedFlight, which included video demonstrations and a protocol competency-based examination. The prone transport protocol was also reviewed at a live, video-based online staff meeting during its implementation.
Boston MedFlight is a state-licensed and internationally accredited CCT organization operated by a consortium of tertiary care medical centers in the Boston metropolitan area and serving all southern New England. All transports took place in ambulances specially designed to optimize patient and team safety during CCT. Features include 360° access to the patient, purpose-built hard mounts for all medical equipment, individual medical crew seating with five-point restraints and moveable bases that allow patient care while remaining restrained, and electronic stretcher lifting/loading systems. Teams have full access to all medical devices and therapeutics from the patient compartment. Transports are performed by nurse and paramedic CCT teams who undergo extensive training in management of severe hypoxemic respiratory failure through didactics, case reviews, and simulation scenarios. The ventilator strategy is optimized prior to initiation of transport (9). The transports were completed with the team in full contact and airborne precautions, with eye protection, for the entire patient encounter. The vehicle operators were segregated from the patient compartment by a physical barrier and wore surgical masks, and the transport vehicle and equipment were fully decontaminated between cases.
All decisions to transfer a subject are initiated by the physicians at sending facilities. The prone transport protocol begins with a conference call between the CCT team and Boston MedFlight physician discussing the potential fatal risks of transporting a patient in the prone position. The risks are discussed with the sending providers to reach consensus regarding prone transport. The ultimate decision to transfer the patient lies entirely with the sending physician, who obtains consent for the transfer. The transport team also obtains consent from patients with capacity, from a qualified representative, or confirms consent from the sending team. A prone transport checklist (Supplemental Digital Content 1, http://links.lww.com/CCX/A442) is completed by the CCT team to ensure that patient and provider safety is maintained throughout the duration of patient care. The CCT team pads the face, shoulders, pelvis, and lower extremities. A commercial endotracheal tube securement device is placed over existing securement methods for additional stabilization. Due to the seating arrangement of the CCT providers in the transport vehicle, the patient’s head is turned to their right side to allow for improved access to the endotracheal tube in the event of an airway emergency.
Heart rate and rhythm, noninvasive blood pressure, end-tidal Co2 waveform, oxygen saturation (Spo2), and invasive catheters are monitored continuously throughout patient care with the Zoll Propaq (ZOLL Medical Corporation, Chelmsford, MA) MD cardiac monitor. The patient is given long-acting neuromuscular blockade for the duration of the transport. The patient is then placed on 100% Fio2 and transitioned onto the transport Hamilton T1 ventilator (Hamilton Medical AG, Bonaduz, Switzerland) with endotracheal tube clamping to reduce the chance of aerosolization of viral particles. The transport stretcher is prepared with a base layer sheet to be used to flip the patient in the event that emergency supination is required during the transport. The patient is then transitioned to the transport stretcher with a minimum of five people, with one being solely dedicated to the maintenance of the endotracheal tube. This transition is done in two steps to ensure that all catheters and tubes have adequate slack. The Fio2 this is then titrated back to its previous value after the patient is placed on the transport stretcher.
The selected transport records were electronically queried for demographic data. Data reviewed from transport included all oxygen saturations and mean arterial pressures (MAPs) from time zero until the transition of care to the receiving ICU. When a patient had an arterial catheter and noninvasive blood pressure measurements, MAP values from the arterial line were used. Vital signs are captured electronically during transports utilizing the Zoll Propaq MD monitor. At the completion of the transport, all data are uploaded from the monitor to Zoll’s cloud-based storage. The data are then imported into the patient’s ImageTrend (Lakeville, MN) electronic patient care record. Per transport protocols, vital signs are recorded a minimum of once every 10 minutes but are often recorded more frequently, especially with fluctuations or instability. To account for the change in Spo2 and MAPs over time, all recorded Spo2 and MAPs were electronically downloaded for each transport. The times of vital sign collection were rounded to the nearest 5-minute intervals.
Patient follow-up data were obtained on May 31, 2020 for patients transported to consortium hospitals only. These follow-up data included rates of extubation, duration of mechanical ventilation, incidence of tracheostomy, ICU length of stay, incidence of extracorporeal membrane oxygenation (ECMO), and mortality.
The primary outcome was the occurrence of a major adverse event during the transport. We defined major adverse event as follows: severe hypoxemia (Spo2 < 80% or an absolute decrease in Spo2 > 10%), hypotension (MAP < 65 mm Hg) not responsive to vasopressors or inotropes, endotracheal tube or vascular catheter dislodgement, and cardiac arrest. We also measured the rate of minor adverse events defined as any episode of hypotension (MAP < 65 mm Hg) and hypoxemia (Spo2 < 88%). To evaluate for the most common adverse events in transport, hypoxemia, and hypotension, we assessed all SpO2s and MAPs as a function of time of transport. The secondary outcomes were inpatient mortality, the rate of successful extubation, and the rate of discharge from the hospital.
Continuous data were reported as medians and interquartile ranges (IQRs). Categorical data were reported as counts and percentages. The duration of transports was visually inspected, and the duration of transports included the mean plus two sds of the total transport time for the entire cohort. The SpO2s and MAPs were visually inspected and determined to be nonparametric in distribution. Linear regression analyses between Spo2 and duration of transport and MAP and duration of transport were performed, and leverage regression plots were generated to assess for correlations between changes in Spo2 or MAP and duration of transport. All statistical analyses were performed using JMP Pro version 14.0 (SAS Institute Inc, Cary, NC).
RESULTS
A total of 25 patients with confirmed COVID-19 were transferred in the prone position from April 14, 2020, to May 20, 2020. The median time of follow-up was 28 days after transport (IQR, 17–35 d). Patients were transported from 14 sending institutions across Massachusetts to six receiving institutions. Median transport time was 38 minutes (IQR, 28–48 mins) with a range of 25–83 minutes. The majority of patients were male (56%), with a median age of 60 years (IQR, 44–62 yr) (Table 1). Hypertension (28%), diabetes (24%), and obesity (20%) were the most common reported comorbidities. Patients were at the sending hospital a median of 8 days before transport (IQR, 5–12 d) and were intubated for a median of 5 days prior to transport (IQR, 1–6 d).
TABLE 1.
Patient Characteristics
| Patient Characteristics | Value |
|---|---|
| Age, median (IQR) | 60 (44–62) |
| Sex, n (%), male | 14 (56.0) |
| Comorbidities, n (%) | |
| Chronic obstructive pulmonary disease | 0 (0.0) |
| Asthma | 2 (8.0) |
| Obesity | 5 (20.0) |
| Hypertension | 7 (28.0) |
| Tobacco use | 4 (16.0) |
| Chronic renal disease | 1 (4.0) |
| Diabetes | 6 (24.0) |
| Timing of transport (n = 25) | |
| Days at sending before transport, median (IQR) | 8 (5–12) |
| Days intubated at sending before transport, median (IQR) | 5 (1–6) |
| Transported within 1 d of arrival, n (%) | 2 (8.0) |
| Transported within 1 d of intubation, n (%) | 9 (36.0) |
| Pao2:Fio2 prior to transport, median (IQR) | 101.3 (65.0–145.3) |
| Vasopressors or inotropes, n (%) | |
| Norepinephrine | 19 (76.0) |
| Epinephrine | 0 (0) |
| Phenylephrine | 0 (0) |
| Vasopressin | 6 (24.0) |
| Dobutamine | 0 (0) |
| Milrinone | 0 (0) |
| Other hemodynamic medication infusions, n (%) | |
| Amiodarone | 1 (4.0) |
| Sedation and analgesia, n (%) | |
| Dexmedetomidine | 3 (12.0) |
| Fentanyl | 15 (60.0) |
| Ketamine | 3 (12) |
| Lorazepam | 0 (0) |
| Midazolam | 16 (64.0) |
| Morphine | 0 (0) |
| Propofol | 12 (48.0) |
| Vital signs, median (IQR) | |
| Highest heart rate | 113 (104–144) |
| Lowest heart rate | 82 (76–97) |
| Highest mean arterial pressure | 105 (93–113) |
| Lowest mean arterial pressure | 63 (57–71) |
| Highest Spo2 | 100 (97–100) |
| Lowest Spo2 | 92 (87–95) |
| Highest Etco2 | 55 (43–59) |
| Lowest Etco2 | 34 (26–47) |
Etco2 = end-tidal Co2, IQR = interquartile range, Spo2 = oxygen saturation.
Management by CCT Team
The transport team made numerous interventions during transport, including changes to support ventilation in 56% and interventions for hemodynamics in 36% of subjects (Table 2). The most common ventilator changes were increasing Fio2 and increasing the positive end-expiratory pressure (PEEP). Table 3 describes the ventilator settings before and during transport. Inhaled epoprostenol was used in five patients (20%), initiated by the transport team in two of those five patients (8%). One patient was transported with inhaled nitric oxide. There was minimal correlation between duration of transport and increasing Spo2 (R2 = 0.009) and MAP (R2 = 0.004) (Fig. 1).
TABLE 2.
Management by the Critical Care Transport Team During Transport
| Intervention (n = 25) | Frequency of Changes Made, n (%) |
|---|---|
| Ventilator management | 14 (56.0) |
| Increase tidal volume | 0 (0) |
| Decrease tidal volume | 2 (8.0) |
| Increase respiratory rate | 1 (4.0) |
| Decrease respiratory rate | 1 (4.0) |
| Increase PEEP | 5 (20.0) |
| Decrease PEEP | 0 (0) |
| Increase Fio2 | 5 (20.0) |
| Decrease Fio2 | 2 (8.0) |
| Hemodynamic management | 9 (36.0) |
| Start new vasopressor | 3 (12.0) |
| Increase vasopressor dose | 4 (16.0) |
| Decrease vasopressor dose | 5 (20.0) |
| Start new inotrope | 0 (0) |
| Increase inotrope dose | 0 (0) |
| Decrease inotrope dose | 0 (0) |
PEEP = positive end-expiratory pressure.
TABLE 3.
Ventilator Settings and Interventions
| Ventilator Variable or Respiratory Intervention | Prior to Transport | During Transport | p |
|---|---|---|---|
| Mode of mechanical ventilation, n (%) | |||
| Volume assist control | 18 (72.0) | 16 (64.0) | 0.76 |
| Pressure assist control | 5 (20.0) | 7 (28.0) | 0.74 |
| Airway pressure release ventilation | 2 (8.0) | 2 (8.0) | 1.00 |
| Ventilator settings, median (IQR) | |||
| Tidal volume | 380 (350–450) | 380 (320–415) | 0.66 |
| Respiratory rate | 26 (20–32) | 28 (24–32) | 0.19 |
| Positive end-expiratory pressure | 12 (10–14) | 14 (12–16) | 0.04 |
| Fio2 | 90 (70–100) | 100 (90–100) | 0.15 |
| Ventilator measurements | |||
| Peak inspiratory pressure, median (IQR) | 35 (32–37) | 35 (32–42) | 0.36 |
| Plateau pressure, median (IQR) | 30 (28.0–32.5) | NA | NA |
| Resistance, median (IQR) | 10.5 (9.8–12.0) | 11.5 (10.3–14.0) | 0.43 |
| Compliance, median (IQR) | 33(25.8–37.7) | 25 (21.4–32.5) | 0.21 |
| Neuromuscular blockade, n (%) | 19 (76.0) | 25 (100.0) | 0.02 |
| Inhaled nitric oxide, n (%) | 1 (4.0) | 1 (4.0) | 1.00 |
| Inhaled epoprostenol, n (%) | 3 (12.0) | 5 (20.0) | 0.70 |
IQR = interquartile range, NA = not applicable.
Figure 1.
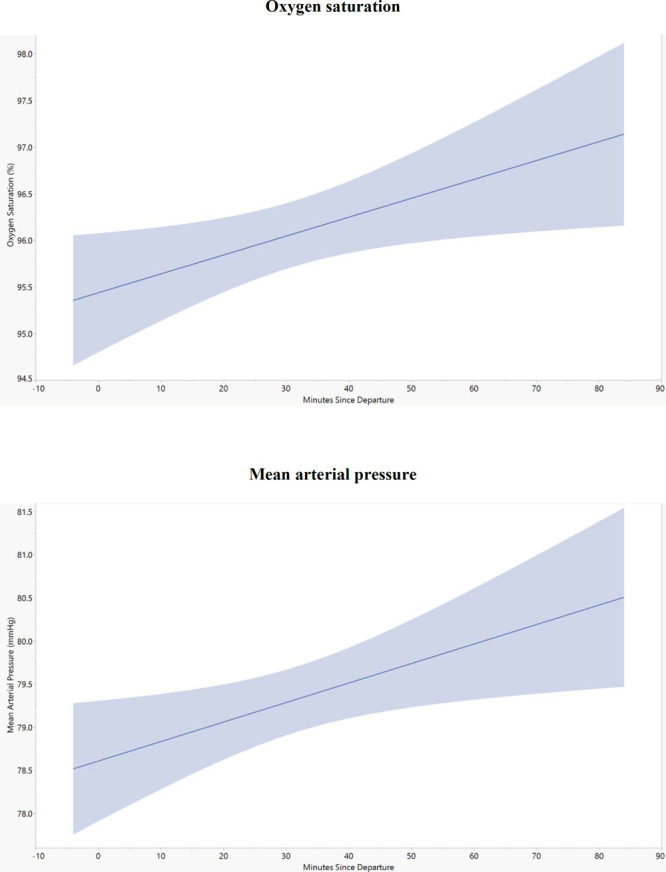
The relationship between mean arterial pressure (MAP) and oxygen saturation with duration of transport.
Adverse Events
There was one episode of major desaturation, Spo2 less than 80%, that was responsive to ventilatory management en route. There were no episodes of refractory hypotension, not responsive to vasoactive management. There were no unintended dislodgements of endotracheal tubes or vascular catheters, and there were no cardiac arrests. Fourteen patients (56%) had hypotension with at least one episode of MAP less than 65 mm Hg en route, and seven (28%) had an episode of oxygen desaturation less than 88% (Table 4).
TABLE 4.
Adverse Events and Outcomes
| Event/Outcome | Finding |
|---|---|
| Minor adverse events, n (%) | |
| Mild hypoxemia (any Spo2 < 88% or decrease in Spo2 > 5%) | 7 (28.0) |
| Any episode of MAP < 65 mm Hg | 14 (56.0) |
| Major adverse events, n (%) | |
| Severe hypoxemia (any Spo2 < 80%, or decrease in Spo2 > 10%) | 1 (4.0) |
| Endotracheal tube dislodgement | 0 |
| Refractory hypotension (MAP < 65 mm Hg) not responsive to vasopressor/inotropes | 0 |
| Cardiac arrest | 0 |
| Outcomes (n = 21) | |
| Median patient follow-up (d), median (IQR) | 28 (17–35) |
| Successful extubation, n (%) | 7 (33.3) |
| Median duration of mechanical ventilation, median (IQR) | 17 (12–21) |
| Tracheostomy, n (%) | 5 (23.8) |
| Median ICU length of stay, median (IQR) | 18 (13–21) |
| Extracorporeal membrane oxygenation, n (%) | 7 (33.3) |
| Death, n (%) | 9 (42.9) |
IQR = interquartile range, MAP = mean arterial pressure, Spo2 = oxygen saturation.
Outcomes
Hospital follow-up data were available for 21 patients (84%) transported to Boston MedFlight Consortium hospitals (Table 4). At the time of data collection, seven (33.3%) were cannulated for venovenous ECMO, nine (42.9%) had died, seven (33.3%) had been extubated alive, and three (14.3%) had been discharged alive.
DISCUSSION
To our knowledge, this report represents the largest cohort of critically ill mechanically ventilated patients transported in the prone position. Our study demonstrates that only one major adverse event occurred with interfacility ground transport of mechanically ventilated patients in the prone position. We additionally showed that with a dedicated, highly experienced CCT team, Boston MedFlight was safely able to institute this protocol with minimal hands-on training to respond to the rapidly evolving COVID-19 pandemic.
The most common complication of critically ill COVID-19 patients is respiratory failure (10). An Italian study reported 88% of patients admitted to the ICU required mechanical ventilation, with most of the patients meeting criteria for moderate ARDS (11). In that study, the median PEEP was 14 cm H2O, and 89% of the intubated patients had Fio2 greater than 50%. Many of the COVID-19 patients require therapy beyond standard lung-protective ventilation. A cohort study in Boston reported that 42% received neuromuscular blockade, 27% received inhaled pulmonary artery vasodilators, 47% were placed in prone position, and 5% received ECMO (2). Some of these advanced therapies, including inhaled pulmonary artery vasodilators and ECMO, are not available in community hospitals. Lack of these therapies or ICU bed availability has led to many interhospital patient transfers.
Prior to the COVID-19 pandemic, Boston MedFlight would not transfer patients in the prone position. Patients were returned to the supine position prior to transport, which could be detrimental for the patient’s oxygenation. Our study of 25 transports provides evidence that when carefully implemented, prone transport may be safe and facilitate otherwise impossible transfers for potentially life-saving tertiary care. With the current pandemic, we anticipate many interfacility transfers of COVID-19 patients worldwide. With the emphasis on early proning of COVID-19 patients, many hospitals will be using this technique for the first time. Our report may assist other CCT agencies in developing their own protocols to transport prone ventilated patients safely.
It is well known that interfacility transport of hypoxemic respiratory failure patients has the potential for worsening hypoxemia, arrhythmias, hemodynamic compromise, acidemia, and cardiac arrest (12–15). However, these risks are counterbalanced by data demonstrating that transferring patients to an advanced respiratory failure center capable of ECMO improves survival (16). Furthermore, data support that it is safe to transport patients with severe hypoxemia with a dedicated CCT team (17). In one report, over 60 patients with a mean Pao2/Fio2 ratio of 64 mm Hg were transported by ground without any adverse events (18). Similar to these studies, our cohort of prone patients had only one major adverse event, which was managed en route by the team. Twenty-eight percent of patients had mild desaturation events, consistent with transports of hypoxemic patients in the supine position (15). However, the cohort overall did not demonstrate desaturation as related to the duration of transport. Vasopressors and inotropes were increased in 20% of patients, with no episodes of refractory hypotension, which is similar to our experience with CCTs of COVID-19 in the supine position. As with hypoxemia, hypotension was not associated with the duration of transport. Endotracheal tube (ET) displacement is a relatively common complication that can occur with prone ventilation in the hospital, occurring in about 10–13% of patients (3, 19). Nevertheless, in our cohort, no ETs dislodged during transport.
To date, there have only been small case series and case reports describing prone transport. The largest case series reported seven prone patients over 5 years (7). Another study transported 66 respiratory failure patients over 6 years, with 14 of them prone (18). We were able transport 25 patients in the prone position over 5 weeks. Our study is unique in that, compared with prior literature, we were able to safely transport a relatively large number of patients over a short period of time. Typically, at Boston MedFlight, a new protocol like this would have undergone in-person training with a hands-on component in the simulation laboratory, but training was limited by pandemic guidelines. We used a distributed learning model with a multimedia presentation through our learning management system and in-person review from our staff education team, who were still present at the bases as part of their transport duties. With our modified training methods, experienced critical care nurses and paramedics were able to implement this protocol rapidly.
This study has several limitations. First, although this is the largest prone transport cohort reported to date, the sample size is still small. Additionally, these are the results at a single CCT agency, limiting generalizability. Additionally, the transport times in our study were relatively short, and it is unknown if these results would be generalizable to rural areas with longer transport times. The included patients were selected by the sending hospital for transfer and are at a high risk of selection bias. It is unknown if these results would apply to all patients transported in severe respiratory failure. All patients in this cohort were transported by ground; the study does not evaluate if air transport would yield similar results. Finally, all study patients were already in the prone position at the referring institution; we did not evaluate the effect of our CCT team proning patients after their arrival to improve oxygenation.
CONCLUSIONS
Highly trained CCT teams were able to rapidly implement a protocol for the transport of mechanically ventilated respiratory failure patients in the prone position with minimal adverse events.
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
This investigation was a coordinated effort out of the Boston MedFlight consortium hospitals, which include Brigham and Women’s Hospital, Massachusetts General Hospital, Beth Israel Deaconess Medical Center, Tufts Medical Center, and Lahey Medical Center.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
REFERENCES
- 1.Coronavirus disease (COVID-19). Situation Report – 133. 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200601-covid-19-sitrep-133.pdf?sfvrsn=9a56f2ac_4. Accessed June 3, 2020
- 2.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: A cohort study. Am J Respir Crit Care Med 2020;201:1560–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 4.Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA 2020; 323:1839–1841 [DOI] [PubMed] [Google Scholar]
- 5.CDC, CDC COVID Data Tracker: 2020. Available at: https://www.cdc.gov/covid-data-tracker/index.html. Accessed May 20, 2020
- 6.Gimenez FMP, de Camargo WHB, Gomes ACB, et al. Analysis of adverse events during intrahospital transportation of critically ill patients. Crit Care Res Pract 2017; 2017:6847124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DellaVolpe JD, Lovett J, Martin-Gill C, et al. Transport of mechanically ventilated patients in the prone position. Prehosp Emerg Care 2016; 20:643–647 [DOI] [PubMed] [Google Scholar]
- 8.Hersey D, Witter T, Kovacs G. Transport of a prone position acute respiratory distress syndrome patient. Air Med J 2018; 37:206–210 [DOI] [PubMed] [Google Scholar]
- 9.Wilcox SR, Saia MS, Waden H, et al. Mechanical ventilation in critical care transport. Air Med J 2016; 35:161–165 [DOI] [PubMed] [Google Scholar]
- 10.Phua J, Weng L, Ling L, et al. ; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir Med 2020; 8:506–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA 2020; 323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh JM, MacDonald RD, Bronskill SE, et al. Incidence and predictors of critical events during urgent air-medical transport. CMAJ 2009; 181:579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckmann U, Gillies DM, Berenholtz SM, et al. Incidents relating to the intra-hospital transfer of critically ill patients. An analysis of the reports submitted to the Australian incident monitoring study in intensive care. Intensive Care Med 2004; 30:1579–1585 [DOI] [PubMed] [Google Scholar]
- 14.Evans A, Winslow EH. Oxygen saturation and hemodynamic response in critically ill, mechanically ventilated adults during intrahospital transport. Am J Crit Care 1995; 4:106–111 [PubMed] [Google Scholar]
- 15.Wilcox SR, Saia MS, Waden H, et al. Improved oxygenation after transport in patients with hypoxemic respiratory failure. Air Med J 2015; 34:369–376 [DOI] [PubMed] [Google Scholar]
- 16.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009; 374:1351–1363 [DOI] [PubMed] [Google Scholar]
- 17.Wilcox SR, Richards JB, Genthon A, et al. Mortality and resource utilization after critical care transport of patients with hypoxemic respiratory failure. J Intensive Care Med 2018; 33:182–188 [DOI] [PubMed] [Google Scholar]
- 18.Uusaro A, Parviainen I, Takala J, et al. Safe long-distance interhospital ground transfer of critically ill patients with acute severe unstable respiratory and circulatory failure. Intensive Care Med 2002; 28:1122–1125 [DOI] [PubMed] [Google Scholar]
- 19.Taccone P, Pesenti A, Latini R, et al. ; Prone-Supine II Study Group. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: A randomized controlled trial. JAMA 2009; 302:1977–1984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


