Abstract
COVID-19 is the infectious disease caused by the recently discovered coronavirus, SARS-CoV-2, unknown before the outbreak in Wuhan, China, in December 2019. COVID-19 is a pandemic, infectious disease that has simultaneously affected many countries globally. The leading cause of dead in patients with COVID-19 is hypoxic respiratory failure from acute respiratory distress syndrome (ARDS). Diffuse alveolar damage (DAD) is the histopathological pattern commonly described in all the postmortem series up to date. DAD is divided into two phases, and depending on the length of the disease, the morphological features seen in the specimens vary. There is an acute/exudative phase, which occurs during the first week after the pulmonary injury, following by the organizing/proliferative phase. Additional features detailed include vascular thrombosis, endothelialitis and angiogenesis. Interestingly, there is an ongoing discussion about the specificity of these changes, as diffuse alveolar damage seen in other viral infections show similar features.
Keywords: CoV-2, COVID-19, diffuse alveolar damage, lung pathology, SARS
Introduction
The main histopathology findings in the lung of COVID-19 patients described in post-mortem studies concur. Diffuse alveolar damage is the most consistent finding which could be organised depending on the length of the clinical course. Additional findings described with some variability are thrombosis and microvascular injury. The aim of this review is to summarise the histological findings described in lungs from patients with COVID-19 disease.
Methods
We have searched PubMed, rcpath.org, www.who.int and ecdc europa.eu using the terms COVID-19 and pathology for articles, studies and reports published until August 31st, 2020. Additionally, we have manually retrieved references from published articles that we considered relevant for this review.
Background
Coronavirus are a large family of viruses which may affect animals and humans. In humans, several coronaviruses cause respiratory infections spanning from the common cold to severe diseases as Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) caused by SARS-CoV.1
COVID-19 is the infectious disease caused by the recently discovered coronavirus, SARS-CoV-2, unknown before the outbreak in Wuhan, China, in December 2019. COVID-19 is a pandemic, infectious disease that has simultaneously affected many countries globally.1
Coronavirus are large, enveloped, single stranded RNA virus, 60–140 nm in diameter. It shows distinctive spikes giving the appearance of a solar corona.
In early infection, SARS-CoV-2 target cells are nasal, bronchial epithelial cells and pneumocytes. The virus spikes protein (S) binds the angiotensin-converting enzyme 2 (ACE2) receptor along the type 2 transmembrane serine protease (TMPRSS2) from the host cells. This binding activates the virus spike S protein, which mediates the virus entry into the host cells. These receptors are expressed particularly in type 2 pneumocytes. In later stages of infection, the virus infects the pulmonary capillary endothelial cells and pericytes. This process then triggers the inflammatory reaction and the stream of monocytes and neutrophils, which evolves to diffuse alveolar damage. In severe infection, there is a fulminant activation of the coagulation cascade, with microthrombus formation, pulmonary embolism and variable size lung infarcts2 , 3 in critically ill patients.
COVID-19 autopsies
In February 2020, the Royal College of Pathologists released guidance on postmortem examinations for mortuary workers in suspected COVID-19 cases.4
Pathogens are categorized according to their risk to humans by the advisory committee on dangerous pathogens (ACDP) within the Health and Safety Executive in the UK, and into four risk groups in the WHO. These hazard groups (HG1-4) are assigned according to the risk of human infection, the likelihood spread and access to treatment or prophylaxis. The SARS and MERS-related Coronavirus are both consider HG3 pathogens. HG3 organisms require risk assessment, previous understanding of the pathology and universal standard precautions and procedures for specific HG3 pathogens.5
Post-mortems should be undertaken in specific premises with adequate ventilation or down-drafts at the workstations and performed under well stablished specifications and special personal protective equipment. The Royal College of Pathologists’ guidelines with an excellent review with the summary and interpretation about how to perform autopsies have recently been published.4
Diffuse alveolar damage, morphological features
Autopsy studies of deceased patients with severe COVID-19 always describe the presence of diffuse alveolar damage (DAD). The lung parenchyma morphologically responds always in a similar way to acute lung injury (ALI). Acute lung injury may occur secondary to a large number of direct or indirect insults and clinically evolves to acute hypoxemia.
DAD is the histopathological pattern seen in patients with acute lung injury or acute respiratory distress syndrome (ARDS), who will require mechanical ventilation. Clinically, these two entities were defined by the American-European Consensus Conference on ARDS. The consensus defined ARDS as the presence of acute hypoxemia with a ratio of partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2:FiO2) of 200 mmHg or less, whilst acute lung injury is a less severe entity defined with the same criteria, but with a PaO2:FiO2 of 300 mg Hg.6 The mortality rate for ARDS is 50%–60%, and it increases with age. On a morphological basis, most cases of clinical ALI and ARDS will have DAD. Other histological patterns seen in a clinical setting of ALI/ARDS include acute eosinophilic pneumonia (AEP) and acute fibrinous and organizing pneumonia (AFOP).7
The histological features of DAD vary depending on when a biopsy is obtained during the course of the disease. On radiology typically it shows diffuse bilateral pulmonary infiltrates (’‘white out’‘) by conventional chest X-ray, while computed tomography scans, often reveal non-homogenous distribution, more marked in the lower lobes of the lungs.8
The histological features have been nicely described in an article by Beasley.6 The knowledge of the histology of DAD is fundamental to avoid misinterpretations.
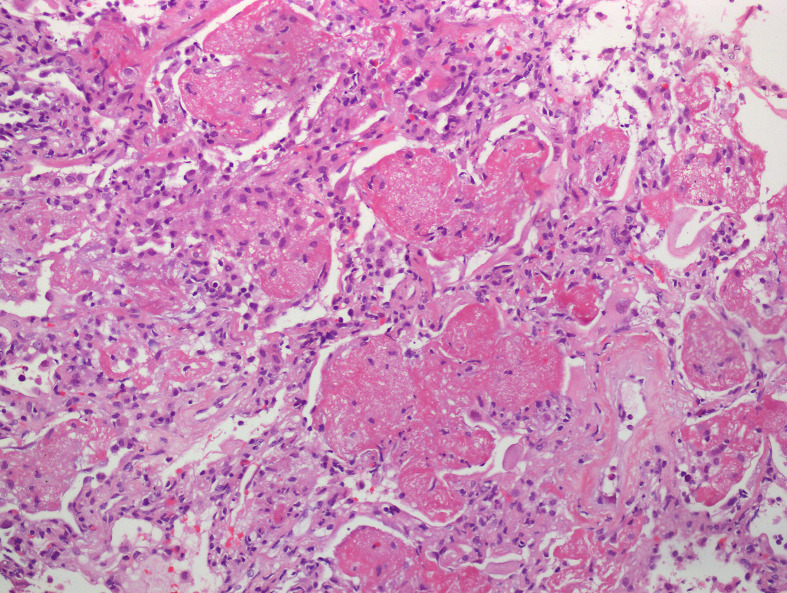
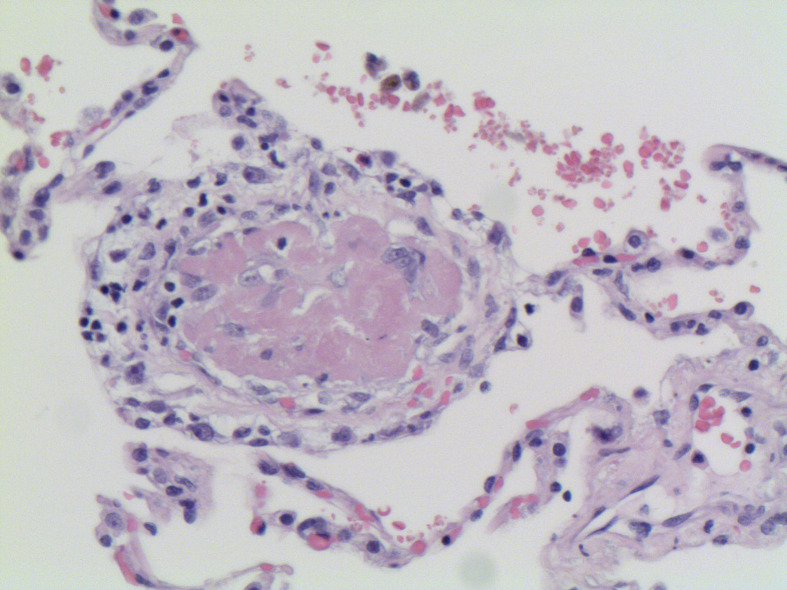
DAD is divided into 2 phases: the acute/exudative phase, which occurs during the first week after the pulmonary injury, following by the organizing/proliferative phase. Some authors additionally include a final proliferative/fibrotic stage.9 The acute/exudative phase by day 2 shows intra-alveolar edema and interstitial widening. Hyaline membranes may be seen at this point and may reach a peak 4–5 days after the initial insult (Figure 1 ). Hyaline membranes are generally diffuse but may also be seen focally (Figure 2 ). The inflammation is generally low, unless DAD is the result of previous pneumonia. Thrombi may be present and is the result of the local alteration of the coagulation pathway. Thrombi in DAD should not be considered evidence of an underlying thromboembolic disorder in the patient.10 , 11
Figure 1.
Acute lung injury with intra-alveolar hyaline membranes, fibrin plugs and interstitial inflammation.
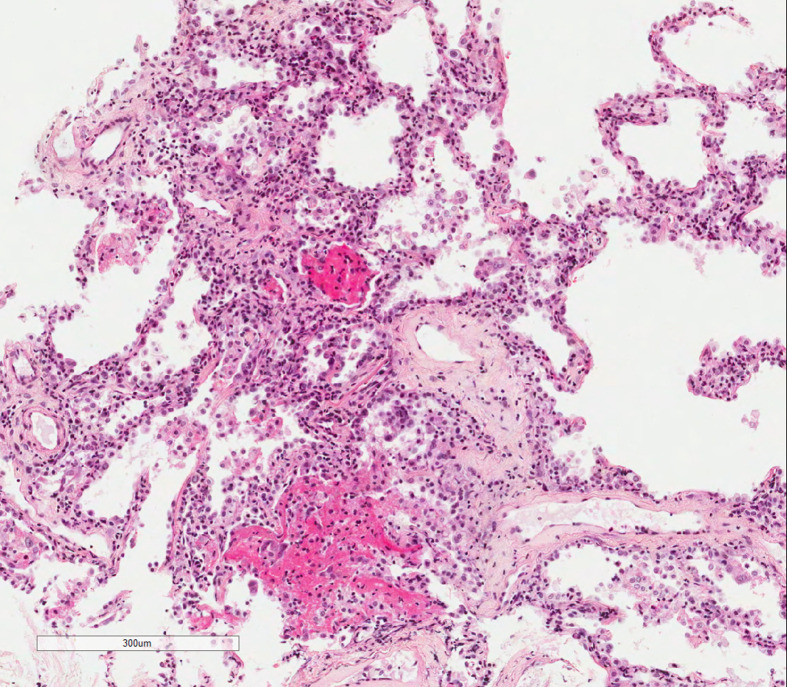
Figure 2.
Patchy intra-alveolar plugs of fibrin and interstitial inflammation.
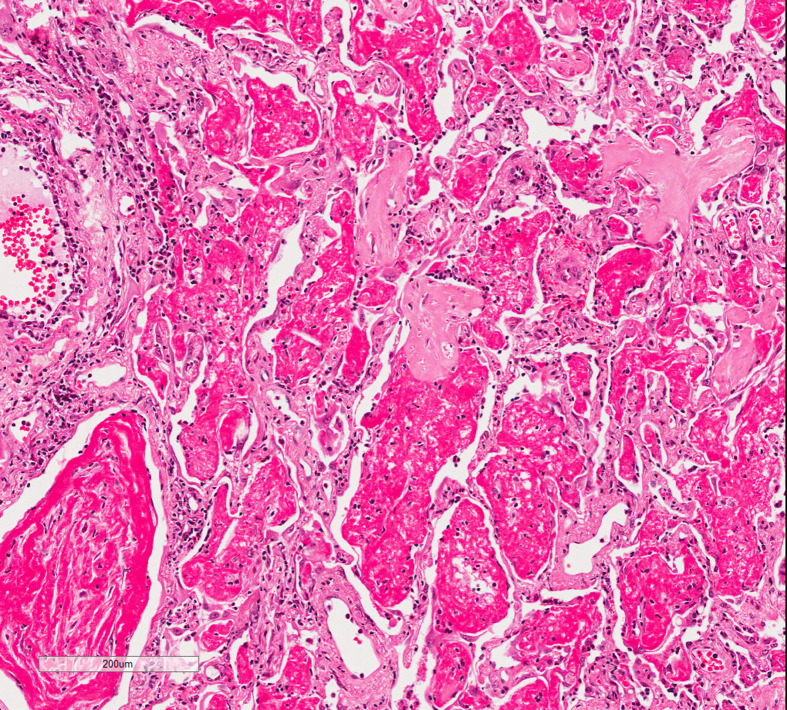
The organizing phase is characterized by cellular fibroblastic proliferation, which displays a loose and myxoid appearance in the haematoxylin-eosin section (Figure 3 ). This is also associated with type 2 pneumocyte hyperplasia and squamous metaplasia, which occasionally is quite pronounced. The hyaline membranes disappear and become integrated into the alveolar septa.7 Residual fibrin rests can be identified. At this stage, the cytological atypia may be quite marked in the type 2 pneumocytes as well in the squamous metaplasia along with mitotic figures, which can cause misinterpretation of malignancy or even viral inclusions.
Figure 3.
Intra-alveolar fibrin and patchy areas of organisation.
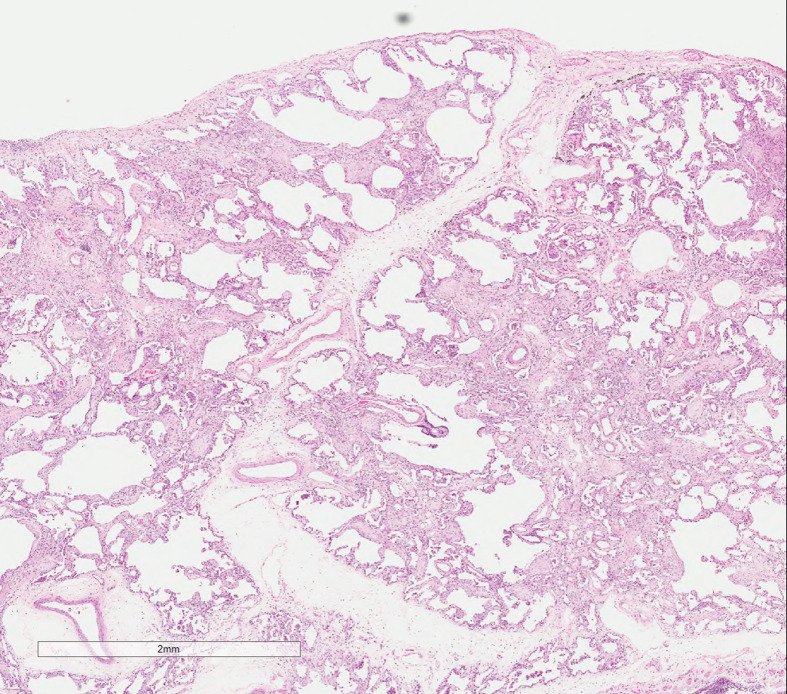
After the organizing phase, some cases of DAD resolve gradually while others develop interstitial fibrosis with interstitial remodeling (Figure 4 )7; however, most survivors experience some kind of residual functional impairment.
Figure 4.
Organizing acute lung injury with interstitial remodeling.
The aetiology of DAD is varied and include infection, drug reactions, connective tissue or immune-mediated disorders, inhalant or ingested exposure, shock, sepsis, disseminated intravascular coagulation or oxygen toxicity. When the origin is not identifiable clinically is referred as acute interstitial pneumonia, the historically referred Hamman-Rich syndrome.7 , 12
Diffuse alveolar damage in mechanical ventilation
The purpose of mechanical ventilation is to rest the respiratory muscles while providing adequate gas exchange. However, many patients eventually die after the initiation of the mechanical ventilation due to ventilation complications as barotrauma, oxygen toxicity and hemodynamic compromise. In 1967, the term “respirator lung” was coined to describe the diffuse alveolar infiltrates and hyaline membranes that were found on postmortem examination of patients who had undergone mechanical ventilation. Postmortem examinations of patients who had undergone mechanical ventilation showed inflammatory cell infiltrates, hyaline membranes and pulmonary edema. This damage has been termed ventilator-induced lung injury.13
Diffuse alveolar damage in patients with COVID-19
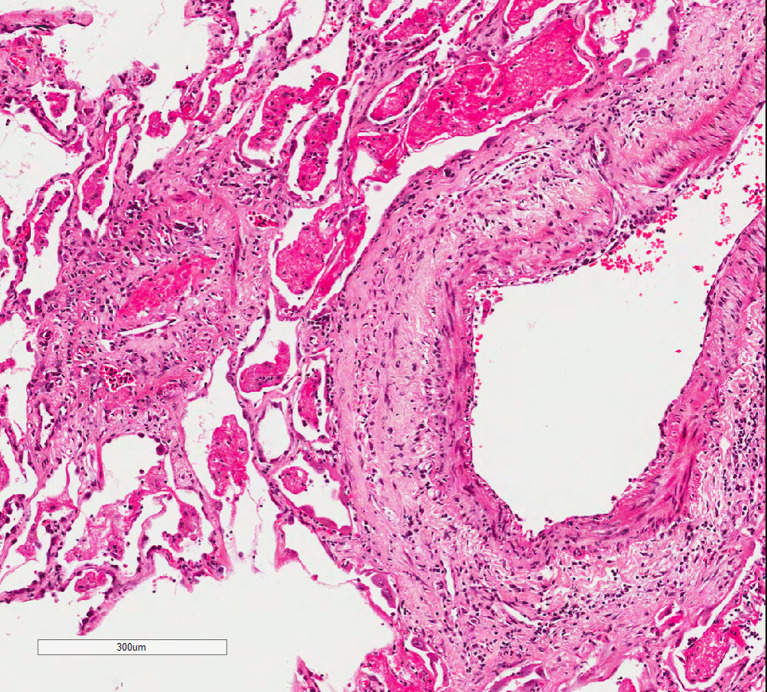
Since the beginning of the pandemic, descriptions of the histological findings in deceased patients with COVID-19 showed predominantly diffuse alveolar damage, which comprises in some cases a predominance of fibrin while in others the predominant pattern was an organizing acute lung injury, depending in the length of the disease. However, some papers stressed the presence of microvascular involvement. Buja et al14 assembled the pathological findings from initial published autopsies reported in 23 patients from 5 centers in the United States of America. All deceased showed one or several background conditions – mainly obesity, hypertension and type 2 diabetes. They divided the disease into three stages: early infection, pulmonary and severe hyperinflammation. The autopsy findings seen in the early phase were those of interstitial pneumonia with DAD pattern. This pneumonia could be accompanied by small vessel thrombi (Figure 5 ) with associated hemorrhage in the lung periphery, and in some cases complicated by multiple pulmonary thromboemboli. Additional patterns of DAD and organizing pneumonia (ON) are also described, mainly ON and bronchiolitis obliterans and cryptogenic organizing pneumonia as well as a variant of DAD known acute fibrinous and organizing pneumonia (AFOP), which comprise patchy distribution of intra-alveolar fibrin aggregates.
Figure 5.
Thrombus within a small vessel in a lung resection of a COVID-19 patient.
Recently, a post-mortem study describing the histopathological findings and viral tropism in UK patients has been published.15 The most consistent lung finding in all deceased patients was DAD. The authors detailed the inflammation in the lung, which comprised a predominance of interstitial macrophages along-with scattered plasma cells and mild to moderate CD4 positive T-cell lymphocytic inflammation. Most patients also showed chronic bronchiolitis. Thromboembolism was also a frequent finding in all the patients. Thrombi were found variably in alveolar septal capillaries, small and medium sized vessels; however, no evidence of endothelialitis or capillaritis was noted in their patients.
Pulmonary vascular damage: endothelialitis, thrombosis and angiogenesis
More recent papers highlight the presence of pulmonary vascular endothelialitis (Figure 6 ), thrombosis and angiogenesis in COVID-19. Ackerman et al described the post-mortem lung features on 7 deceased and compared them with the lung features of patients deceased after influenza-associated respiratory failure. Both viral infections induced DAD with perivascular T-cell infiltration. However, the authors described features consistent with severe endothelial injury due to intracellular virus, alveolar capillary microthrombi and intussusceptive angiogenesis more prevalent in patients with COVID-19 than with influenza virus. Additionally, the authors pointed out that these alterations increased significantly with increasing length of hospitalization in patients with COVID-19 conversely to those with influenza, which remained stable.16
Figure 6.
Endothelialitis and acute lung injury.
Interestingly, Burel-Vandebos et al have stressed the importance of the pericytes. Pericytes are perivascular cells responsible of the maintenance of microvessel integrity and show high expression of ACE2 receptor. The authors described pericyte loss coexisting with preserved endothelial cells in alveolar capillaries of the lungs from patients with COVID-19.17
The authors hypothesized that the decrease in pericytes and apoptosis due to direct effect of SARS-COV-2 observed on their studies could be the initial trigger of the microvasculopathy.18
Summary
COVID-19 is the infectious disease caused by SARS-COV-2 virus, and the new pandemic in 2020. The leading cause of death is hypoxic respiratory failure from acute respiratory distress syndrome, and the histological features are those of a diffuse alveolar damage with extensive thrombosis and vascular damage. Additional studies are needed to establish if the morphological features are specific for COVID-19 infection.
Practice points
-
•
COVID-19 is the infectious disease caused by coronavirus, SARS-CoV-2.
-
•
DAD is the histological pattern seen in patients with COVID-19, who may require mechanical ventilation.
-
•
The histology features of DAD vary depending on when a biopsy is obtained during the course of the disease.
-
•
Pulmonary vascular endothelialitis, thrombosis and angiogenesis are also features seen in COVID-19.
-
•
The specificity of these histological features is a matter of discussion, as vascular lesions and thrombosis are also seen in DAD.
Multiple choice questions
1 The most consistent histopathological finding in the lung of COVID-19 patients is:
-
A
Vascular thrombosis
-
B
Endothelialitis
-
C
Angiogenesis
-
D
Diffuse alveolar damage
-
E
Microvascular injury
Answer D.- Diffuse alveolar damage
2 Pathogens are categorized according to their risk to humans by the advisory committee on dangerous pathogens (ACDP) within the Health and Safety Executive in the UK, and into four risk groups in the WHO. To which group belongs COVID -19
-
A
HG1
-
B
HG2
-
C
HG3
-
D
HG4
-
E
Any of them
Answer C.- HG3
3 Related to the acute/exudative phase of diffuse alveolar damage:
-
A
It occurs during the second week after the pulmonary injury
-
B
Hyaline membranes are the first change detected
-
C
Last changes detected are intra-alveolar edema and interstitial widening
-
D
When Diffuse Alveolar Damage is the result of previous pneumonia inflammation is low
-
E
Thrombi is the result of the local alteration of the coagulation pathway
Answer E.- Thrombi are the result of the local alteration of the coagulation pathway
References
- 1.www.who.int/home/healthtopic/coronavirus.
- 2.Wiersinga W., Rhodes A., Cheng A., Peacock S., Prescott H. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J Am Med Assoc. 2019;2020:1–13. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 3.Magro C., Mulvey J.J., Berlin D. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osborn M., Lucas S., Stewart R., Swift B., Youd E. 2020. Autopsy practice relating to possible cases of COVID-19 (2019- nCov, novel coronavirus from China 2019/2020) secondary autopsy practice relating to possible cases of COVID-19 (2019- nCov, novel coronavirus from China 2019/2020)https://www.rcpath.org Available: [Google Scholar]
- 5.Hanley B., Lucas S.B., Youd E., Swift B., Osborn M. Autopsy in suspected COVID-19 cases. J Clin Pathol. 2020;73:239–242. doi: 10.1136/jclinpath-2020-206522. [DOI] [PubMed] [Google Scholar]
- 6.Bernard G.R., Artigas A., Brigham K.L. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 7.Beasley M.B., Franks T.J., Galvin J.R., Gochuico B., Travis W.D. Acute fibrinous and organizing pneumonia: a histologic pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126:1064–1070. doi: 10.5858/2002-126-1064-AFAOP. [DOI] [PubMed] [Google Scholar]
- 8.Caironi P., Carlesso E., Gattinoni L. Radiological imaging in acute lung injury and acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:404–415. doi: 10.1055/s-2006-948294. [DOI] [PubMed] [Google Scholar]
- 9.Tomashefski J.F., Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000;21:435–466. doi: 10.1016/s0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 10.Tomashefski J.F., Jr., Davies P., Boggis C., Greene R., Zapol W.M., Reid L.M. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983 Jul;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- 11.Sapru A., Wiemels J.L., Witte J.S., Ware L.B., Matthay M.A. Acute lung injury and the coagulation pathway: potential role of gene polymorphisms in the protein C and fibrinolytic pathways. Intensive Care Med. 2006;32:1293–1303. doi: 10.1007/s00134-006-0223-5. [DOI] [PubMed] [Google Scholar]
- 12.Parambil J.G.1, Myers J.L., Aubry M.C., Ryu J.H. Causes and prognosis of diffuse alveolar damage diagnosed on surgical lung biopsy. Chest. 2007 Jul;132:50–57. doi: 10.1378/chest.07-0104. [DOI] [PubMed] [Google Scholar]
- 13.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. New Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. 28. [DOI] [PubMed] [Google Scholar]
- 14.Buja L.M., Wolf D., Zhao B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanley B., Naresh K.N., Roufosse C. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. The Lancet Microbe. 2020 Oct;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burel-Vandenbos F., Cardot-Leccia N., Passeron T. Pulmonary vascular pathology in Covid-19. N Engl J Med. 2020 Aug 27;383:886–887. doi: 10.1056/NEJMc2022068. [DOI] [PubMed] [Google Scholar]
- 17.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardot-Leccia N., Hibuche T., Dellamonica J. Pericyte alteration sheds light on microvasculopathy in COVID-19 infection. Intensive Care Med. 2020;46:1777–1778. doi: 10.1007/s00134-020-06147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]