Abstract
Objective
To study the effectiveness of COVID-19 convalescent plasma (CCP) therapy for patients with moderate and severe COVID-19 disease.
Methods
This non-randomized prospective cohort study was conducted from May 21 to June 30, 2020, at four major tertiary hospitals in Kuwait. CCP was administered to 135 patients. The control group comprised 233 patients who received standard treatment. All patients (N = 368, median age 54 [range 15–82]) had laboratory-confirmed SARS-CoV-2 infection and either moderate or severe COVID-19 disease.
Results
CCP treatment was associated with a higher rate of clinical improvement in patients with moderate or severe disease. Among those with moderate COVID-19 disease, time to clinical improvement was 7 days in the CCP group, versus 8 days in the control group (p = 0·006). For severe COVID-19 disease, time to clinical improvement was 7 days in the CCP group, versus 15.5 days in the control group (p = 0·003). In the adjusted analysis, patients with moderate disease treated with CCP had a significantly lower 30-day mortality rate. Compared to the control group, oxygen saturation improved within 3 days of CCP transfusion, and lymphocyte counts improved from day 7 in patients with moderate COVID-19 disease and day 11 in patients with severe disease. C-reactive protein levels declined throughout the first 14 days after CCP transfusion. None of the CCP patients developed a serious transfusion reaction.
Conclusions
The data show that administration of CCP is a safe treatment option for patients with COVID-19 disease with a favorable outcome in the rate of, and time to, clinical improvement.
Keywords: Convalescent plasma, COVID-19, SARS-CoV-2, Pneumonia, Clinical improvement, Mortality
Introduction
The novel coronavirus disease of 2019 (COVID-19) pandemic is considered one of the greatest global public health crises since the 1918 influenza pandemic (Brown and McCullough, 2020). COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a single- stranded RNA virus that belongs to family coronaviridae (Huang et al., 2020, Zhu et al., 2020). The clinical spectrum of infection is broad, ranging from asymptomatic to severe pneumonia, multiorgan failure, and death.
Due to the absence of an established antiviral treatment for COVID-19, the historical choice of therapeutic convalescent plasma (CP) was globally appealing, especially with its prior success against RNA viruses (Brown and McCullough, 2020). CP has been proposed not only to neutralize the pathogen, but also to provide passive immunomodulatory properties that allow the recipient to control the exaggerated inflammatory cascade (Garraud et al., 2016, Shakir et al., 2010). However, the mechanism of action of therapeutic CP remains unclear. Reports from open label trials and case series suggest that plasma collected from convalescent COVID-19 patients is safe to administer and may be effective in treating patients with COVID-19 (Joyner et al., 2020, Li et al., 2020, Shen et al., 2020). Therefore, the World Health Organization (WHO) and United States Food and Drug Administration (FDA) have issued guidelines for the usage of COVID-19 convalescent plasma (CCP) and standardized donor selection, which was further supported by an Emergency Use Authorization (EUA) from the FDA (Integrated Management of Adolescent and Adult Illness (IMAI) District Clinician Manual: Hospital Care for Adolescents and Adults, 2011, Investigational COVID-19 Convalescent Plasma Guidance for Industry, 2020).
A number of reports have demonstrated promising results of CCP in severe and critically ill COVID-19 patients, albeit not consistently (Shen et al., 2020, Xia et al., 2020, Zeng et al., 2020). The effectiveness of CCP in less than severe cases remains unclear. The aim of the current study was to assess the effectiveness of CCP in both moderate and severe COVID-19 cases compared to the standard treatment alone. The effectiveness of CCP was evaluated in terms of time to clinical improvement, hospital mortality, and changes in oxygen saturation and laboratory markers (lymphocytes, neutrophils counts, lactate dehydrogenase [LDH], and C-reactive protein [CRP]).
Patients and methods
Study design
This prospective multicenter interventional study was conducted in four major tertiary hospitals in Kuwait (Al-Sabah, Farwaniya, Mubarak Al-Kabeer, and Jahra) from May 21 to June 30, 2020, with the last follow-up data collected on July 12, 2020. All patients and CCP donors had confirmed SARS-CoV-2 infection by an EUA-approved real-time reverse transcriptase-polymerase chain reaction (RT-PCR) using nasopharyngeal swabs (Cobas 6800 Systems, Roche, Switzerland and Taq Path, Thermo-Fisher Scientific, USA). The CCP donors were tested for SARS-CoV-2 IgG using a CE-marked rapid test (BIOZEK COVID-19 IgG/IgM qualitative test: Inzek International Trading, Apeldoom, the Netherlands).
The study protocol was approved by the Ethics and Research Committee of Kuwait Ministry of Health (#2020/1417). Written informed consent was obtained from all patients or next of kin. If the patient was unconscious and the next of kin was not available, the healthcare proxy provided written informed consent.
Inclusion criteria
We included patients aged ≥18 with confirmed laboratory diagnosis of SARS-CoV-2 infection and admission diagnosis of COVID-19. Patients <18 years old were enrolled after thorough evaluation and discussion among an interdisciplinary team. Patients were eligible for CCP if they had moderate or severe COVID-19, determined according to the WHO classification, at admission by the treating physician (Integrated Management of Adolescent and Adult Illness (IMAI) District Clinician Manual: Hospital Care for Adolescents and Adults, 2011). Patients with moderate disease exhibited clinical signs of pneumonia (fever, cough, dyspnea, fast breathing) and had SpO2 > 90% in room air. Patients with severe disease exhibited clinical signs of pneumonia and at least one of the following: respiratory rate >30 breaths/minutes, SpO2 < 90% in room air, or admission to intensive care unit (ICU) for respiratory support (i.e., non-invasive mechanical ventilation and intubation).
Exclusion criteria
Patients were excluded if they had a contraindication to transfusion (volume overload, history of anaphylaxis to blood products), acute severe multiorgan failure, hemodynamic instability, severe disseminated intravascular coagulopathy (DIC), septic shock, or expected survival of less than 48 h.
Control sampling procedure
After screening for the inclusion and exclusion criteria, we applied a systematic stratified sampling technique to identify control patients from the COVID-19 national registry. For each patient who received CCP, we selected the first two patients with the same disease severity strata admitted on that calendar date from the same participating center.
Standard treatment
Standard treatment was based on our institutional protocol and international guidelines (World Health Organization, 2020). The majority of patients received antibiotics and low molecular weight heparin. None of our patients received antiviral therapy or hydroxychloroquine. Special therapy (i.e., steroids and/or tocilizumab) was prescribed at the discretion of treating physicians.
Data collection
Clinical and laboratory data were extracted from patient medical records using a standardized data collection sheet. We captured pre-existing medical conditions, including diabetes mellitus, hypertension, and obesity. Data on specific therapies, such as anticoagulants, antibiotics, steroids, and tocilizumab, were also collected. Complete blood counts (CBCs), CRP, D-dimer, and LDH were collected on days 1, 3, 7, 11, and 14 of hospitalization. We followed the patients’ progress daily from day 1 through day 30, or until discharge, whichever came first. Clinical improvement was defined as a two-grade decrease from hospitalization by assessing the clinical status based on the WHO ordinal scale (Integrated Management of Adolescent and Adult Illness (IMAI) District Clinician Manual: Hospital Care for Adolescents and Adults, 2011).
Selection of CCP donors
The CP was collected from individuals who had recovered from RT-PCR-confirmed COVID-19 disease and tested positive for IgG antibodies against SARS-CoV-2. All donors tested positive for SARS-CoV-2 by an EUA-approved RT-PCR test on a specimen collected by nasopharyngeal swab at the time of illness and a positive EUA-approved qualitative serological test for SARS-CoV-2 IgG antibodies after recovery. Donors who tested negative or were positive only for SARS-CoV-2 IgM were excluded. All donors were negative for HLA antibodies. Additional donor eligibility criteria and qualification were based on the American Association of Blood Banks (AABB) standards and the FDA guidance on CCP administration, including questionnaires and testing for relevant transfusion-transmitted infections (Investigational COVID-19 Convalescent Plasma Guidance for Industry, 2020, American Association of Blood Banks (AABB), 2020). CCP was only collected from individuals who met all donor eligibility requirements according to the United States Code of Federal Regulations (21 CFR 630.10 and 21 CFR 630.15). Pathogen inactivation was performed for each unit.
CCP administration
A total of 135 patients with moderate to severe COVID-19 were enrolled in this cohort (89 patients with moderate disease and 46 patients with severe disease). All patients were pre-medicated (paracetamol, antihistamine, steroids) according to an institutional protocol. Overall, 107 (79·3%) patients received 2 units of ABO-compatible CCP (each unit containing 200 ml of CCP), 12 h apart, and 28 (20·7%) received 1 unit of CCP according to the treating physician and protocol dosage range (200–400 mL). All patients received CCP within 24 h of admission. Three patients (2%) had allergic skin reactions that were completely resolved after transfusion.
Statistical analysis
Categorical variables are presented as number and percentage (%). Continuous variables are presented as median and interquartile range (IQR). Pairwise comparisons were performed using Pearson’s chi-squared test for categorical variables and Kruskal-Wallis test or Mann-Whitney U test for continuous variables. For the primary end point, time to clinical improvement between the groups before 30 days was right censored at day 30. We used the Kaplan Meier analysis to assess the time to clinical improvement and the log rank test to compare the endpoint among groups. Mixed ordinal logistic regression modeling was used to measure the effect size (odds ratio [OR] and 95% confidence interval [CI]) of CCP on the WHO ordinal scale, stratified by disease severity.
A Cox proportional hazards model was used to assess the overall 30-day clinical improvement. Hazard ratios (HRs) with 95% CIs were reported in the Cox model. For the secondary endpoint, we calculated the OR and 95% CI using logistic regression analysis. Variables included in the multivariate Cox proportional hazard and logistic regression models were age, baseline SpO2 < 88%, lymphocyte count <1 × 109, and CRP.
We also examined whether an interaction occurred between CCP and adjunct therapy (i.e., steroids and/or tocilizumab) that affected the outcomes. We tracked laboratory results, including lymphocyte and neutrophil counts, CRP, D-dimer, and LDH, in addition to oxygen saturation over time. For the analysis of repeated measures, we used a linear fixed model (regression coefficient β with 95% CI) to analyze the effect of CCP on laboratory markers and oxygen saturation at baseline and during the first 14 days of hospitalization.
Missing data were not handled in a specific manner. Statistical analyses were carried out using Stata 14 software (StataCorp, College Station, Texas). Two-sided p-values <0·05 were considered significant.
Results
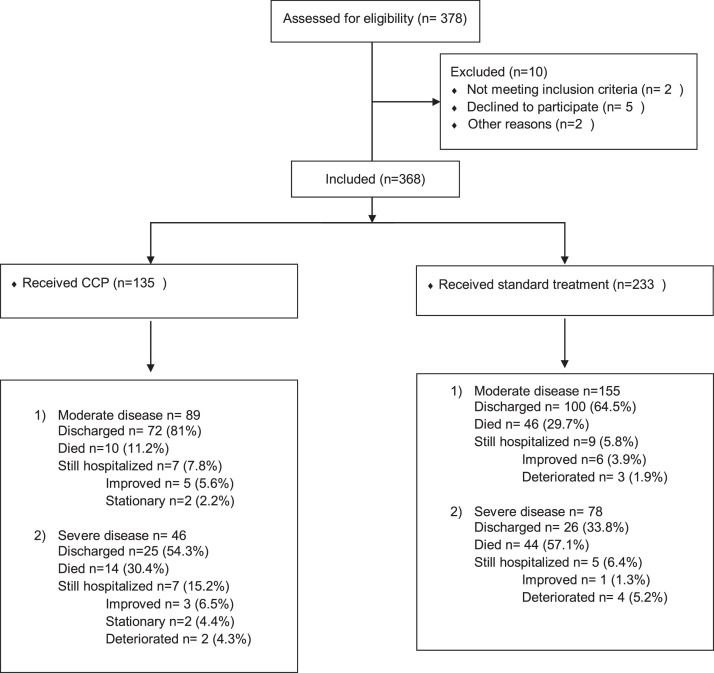
A total of 368 patients were included in the cohort; 135 received CCP and 233 were in the control group (Fig. 1 ). Demographic and baseline characteristics are shown in Table 1 . The median age was 54 (range 15–82), and the majority of patients were male (n = 304, 82·6%). We found no differences between the groups in terms of baseline characteristics or admission laboratory values, with the exception of baseline CRP, which was significantly higher in the CCP-treated patients with severe disease than other groups.
Fig. 1.
Study Flow chart.
Table 1.
Demographic and baseline characteristics by disease severity.
| Characteristic | Control |
CCP |
P-value | ||||
|---|---|---|---|---|---|---|---|
| All | Moderate | Severe | All | Moderate | Severe | ||
| N = 233 | N = 155 | N = 78 | N = 135 | N = 89 | N = 46 | ||
| Age (years) | 54 [45–62] | 52 [43–61] | 57 [51–65] | 54 [48–60] | 54 [49–60] | 53·5 [45–58] | 0·74 |
| Male | 198 (85%) | 136 (87·7%) | 62 (79·5%) | 105 (77·8%) | 63 (70·8%) | 42 (91·3%) | 0·27 |
| Diabetes | 94 (45·4%) | 58 (43·3%) | 36 (49·3%) | 57 (42·5%) | 43 (48·3%) | 14 (31·1%) | 0·21 |
| Hypertension | 80 (39%) | 44 (33·3%) | 36 (49·3%) | 60 (45%) | 43 (48·8%) | 17 (37·8%) | 0·15 |
| Obesity (BMI > 40) | 20 (8·9%) | 14 (9·5%) | 6 (8%) | 16 (12%) | 8 (9·1%) | 8 (17·8%) | 0·21 |
| Laboratory findings | |||||||
| Lymphocytes ×109 | 0·93 [0·7–1·4] | 1 [0·72–1·4] | 0·85 [0·6–1·3] | 1·06 [0·8–1·38] | 1·1 [0·9–1·4] | 1 [0·8–1·3] | 0·59 |
| Lymphocytes <1 × 109 | 101 (47%) | 68 (43·9%) | 46 (59%) | 73 (57%) | 35 (39·3%) | 20 (43·5%) | 0·63 |
| Neutrophils ×109 | 5·3 [4–7·6] | 5·2 [4–7·8] | 5·7 [4,5678910] | 5·2 [4,56789] | 5·4 [4–7·7] | 5·8 [4–7·5] | 0·68 |
| Platelets | 226 [170–292] | 226 [168–305] | 224 [186–264] | 219 [175–283] | 309 [175–284] | 229 [171–273] | 0·69 |
| CRP (mg/L) | 81·4 [34·1–136] | 82·4 [38–141] | 78 [25–121] | 96 [73·5–154] | 94 [73–148] | 98 [82·4–179] | 0·04 |
| LDH (IU/L) | 429 [333–598] | 410 [331–567] | 480 [347–710] | 391 [325–605] | 360 [289–467] | 514 [387–686] | 0·84 |
| D-dimer (ng/mL) | 459 [289–800] | 431 [288–796] | 507 [292–820] | 384 [296–588] | 345 [235–562] | 477 [348–919] | 0·96 |
| Ferritin (ng/mL) | 668 [362–1889] | 665 [360–1243] | 678 [401–1050] | 638 [334–1130] | 624 [332–940] | 824 [412–1178] | 0·63 |
| PT (seconds) | 14·1 [13–16] | 13·9 [12·6–15·3] | 15 [13·5–17] | 15·1 [14–16·8] | 15 [13·8–16·7] | 15·7 [13·7–17] | 0·36 |
| APTT (seconds) | 36·4 [32–40·8] | 36·4 [32–40·6] | 37 [32–42·4] | 35·6 [32·6–38·5] | 35·5 [33–38·4] | 36 [32–39] | 0·46 |
| Clinical characteristics | |||||||
| Baseline Spo2 (%) | 91 [88–94] | 92 [90–95] | 90 [85–92] | 91 [89–94] | 92 [90–94] | 90 [87–92] | 0·06 |
| WHO ordinal scale on admission | |||||||
| 47 (20·2%) | 46 (29·1%) | 1 (1·3%) | 13 (9·6%) | 12 (13·5%) | 1 (2·2%) | ||
| 123 (52·7%) | 94 (60·6%) | 29 (23·2%) | 72 (53·3%) | 60 (67·4%) | 12 (26·1%) | ||
| 59 (25·3%) | 15 (9·7%) | 0 | 45 (33·3%) | 17 (19·2%) | 28 (60·8%) | ||
| 4 (1·7%) | 0 | 4 (5·1%) | 5 (3·7%) | 0 | 5 (10·9%) | ||
| Treatment received | |||||||
| Steroid | 113 (48·5%) | 76 (49%) | 37 (47·4%) | 77 (57%) | 46 (51·7%) | 31 (67·4%) | 0·14 |
| Tocilizumab | 153 (66·7%) | 100 (64·5%) | 53 (67·9%) | 65 (67·7%) | 43 (63·2%) | 22 (78·5%) | 0·45 |
Data are given as median [IQR] or n (%). Abbreviations: IQR, interquartile; BMI, body mass index (as calculated in kilograms divided by height in meters squared); LDH, lactate dehydrogenase; CRP, C-reactive protein; PT, prothrombin time; APTT, activated partial prothrombin time.
SI conversion factors: to convert lactate dehydrogenase to microkatal per liter, multiply by 0·167; to convert D-dimer to nanomoles per liter, divide by 5·476; to convert CRP to nanomoles per liter, multiply by 9·524.
Moderate COVID-19: defined as the presence of clinical signs of pneumonia (fever, cough, dyspnea) and oxygen saturation (SpO2) of more than 90% in room air.
Severe COVID-19: defined as clinical signs of pneumonia plus SpO2 less than 90% in room air or admission to intensive care unit (ICU) for respiratory support (i.e., high flow nasal cannula, non-invasive mechanical ventilation and intubation).
WHO 7-category ordinal scale that ranges from 1 (discharged with normal activity) to 7 (death). Scale 2 = Not hospitalized, but unable to resume normal activities; scale 3 = hospitalized, not requiring supplemental oxygen; scale 4 = hospitalized, requiring supplemental oxygen, scale 5 = hospitalized, requiring high-flow nasal oxygen (HFNC) and/or non-invasive mechanical ventilation (IMV); scale 6 = hospitalized, requiring ECMO and/or IMV; scale 7 = death.
Bold value signifies the oxygen saturation baseline and the treatment recieved has no statistical significance.
Primary outcomes
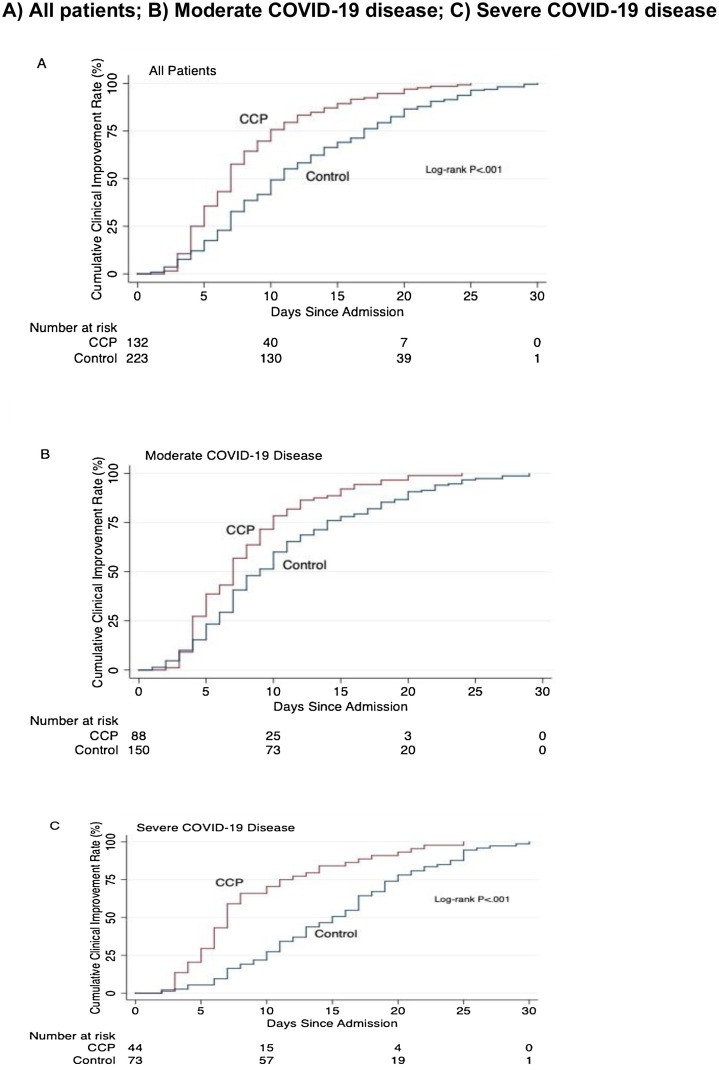
Overall 30-day clinical improvement was observed in 77 (86·5%) of the CCP-treated patients with moderate disease and 106 (68·4%) of corresponding controls (p = 0·001). Among those with severe disease, clinical improvement was observed in 28 (60·8%) of the CCP-treated patients and 27 (34·6%) of their control counterparts (p = 0·001). The median time to clinical improvement among those with moderate disease was 7 (IQR 4-9) days with CCP treatment and 8 (IQR 6–12) days in the control group (p = 0·006); among those with severe disease it was 7 (IQR 5–12) days with CCP treatment and 15.5 (IQR 10–20) days in the control group (p = 0·003, Fig. 2 ). The distribution of patients in the WHO ordinal scale categories over the 30 days of hospitalization are shown in eFigure 1 and eFigure 2 in Supplement 2. For both moderate and severe disease, CCP treatment was associated with a significantly lower score on the WHO ordinal scale compared to the control group on days 7, 14 and 30. On day 7, the score reduction was significant in both the moderate (OR, 0·25 [95% CI 0·13–0·47]) and severe disease groups (OR, 0·33 [95% CI 0·14–0·82]). We also noted a score reduction in both moderate and severe disease on day 14 (OR, 0·17 [95% CI 0·08–0·34] and OR, 0·28 [95% CI 0·11–0·73], respectively) and day 30 (OR, 0·17 [95% CI 0·07–0·36] and OR, 0·11 [95% CI 0·04–0·31], respectively).
Fig. 2.
Kaplan-Meier Estimate for Time to Clinical improvement according to disease severity.
A) All patients; B) Moderate COVID-19 disease; C) Severe COVID-19 disease.
In the adjusted Cox model, the overall CCP group had a significantly higher rate of clinical improvement than the control group (HR, 1·9 [95% CI 1·4–2·7]). The effect was seen among both the moderate (HR, 1·9 [95% CI 1·3–2·8]) and severe disease (HR, 2·5 [95% CI 1·2–5·2]) groups (Table 2 ). There were no significant effects caused by interaction between CCP and steroids (p = 0·40), tocilizumab (p = 0·12), or steroids plus tocilizumab (p = 0·44), on clinical improvement.
Table 2.
Primary and secondary outcomes.
| Primary outcome | Overall control | Overall CCP | P-value | Adjusted HR* (95% CI); p-value |
|---|---|---|---|---|
| Clinical improvement | 133 (58·6%) | 100 (80·6%) | <0·001 | 1·9 (1·4–2·7) ;<0·001 |
| Days to clinical improvements, median (IQR) | 10 (6–15) | 7 (5–9) | <0·001 |
| Primary outcome | Moderate control | Moderate CCP | P-value | Adjusted HR* (95% CI); p-value |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Clinical improvement | 106 (68·4%) | 77 (86·5%) | 0·001 | 1·9 (1·3–2·8); 0·001 |
| Days to clinical improvements, median (IQR) | 8 (6–12) | 7 (4–9) | 0·006 |
| Primary outcome | Severe control | Severe CCP | P-value | Adjusted HR* (95% CI); p-value |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Clinical improvement | 27 (34·6%) | 28 (60·8%) | 0·006 | 2·5 (1·2–5·2); 0·012 |
| Days to clinical improvements, median (IQR) | 15·5 (10–20) | 7 (5–12) | 0·003 |
| Secondary outcome | Overall control | Overall CCP | P-value | Adjusted OR* (95% CI); p-value |
|---|---|---|---|---|
| Death | 90 (38·8%) | 24 (17·8%) | <0·001 | 0·32 (0·18–0·58); 0·001 |
| Secondary outcome | Moderate control | Moderate CCP | P-value | Adjusted OR* (95% CI); p-value |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Death | 46 (29·7%) | 10 (11·4%) | 0·001 | 0·27 (0·12–0·62); 0·002 |
| Secondary outcome | Severe control | Severe CCP | P-value | Adjusted OR* (95% CI); p-value |
|---|---|---|---|---|
| Death | 44 (57·1%) | 14 (30·4%) | 0·006 | 0·38 (0·14–1·02); 0·06 |
Adjusted for age, baseline oxygen saturation <88%, lymphocytes <1 × 109, and C-reactive protein.
Secondary outcome
Effect of CCP on mortality
In univariate analysis, the CCP-treated patients in both severity groups had lower mortality rates than the control groups (n = 10, 11·4% and n = 14, 30·4% versus n = 46, 29·7% and n = 44, 57·1%; p = 0·001 and p = 0·006, respectively). In the adjusted model, the risk of mortality was significantly lower in the CCP-treated patients with moderate disease (adjusted OR, 0·27 [95% CI 0·12–0·62]) compared to the control group, but not significantly different in the CCP-treated patients with severe disease (adjusted OR, 0·38 [95% CI 0·14–1·02]) compared to the control group. No significant effects on mortality rate were observed due to interaction between CCP and steroids (p = 0·36 for interaction), CCP and tocilizumab (p = 0·56 for interaction), or CCP and steroids plus tocilizumab (p = 0·75 for interaction).
Effect of CCP on oxygen saturation and laboratory value kinetics
The effects of CCP on patients’ oxygen saturation and laboratory markers over the first 14 days of the hospital stay are shown in Table 3 . Relative to baseline, CCP treatment improved oxygen saturation by 5·4% [95% CI 3·3–7·4] on day 1 and 4·1% [95% CI 2·3–5·9] on day 3 in patients with moderate disease, but not among those with severe disease (eFigure 3 in Supplement). Lymphocyte counts increased significantly on days 7 (β = 0·46 [95% CI 0·15–0·78]) and 14 (β = 0·59 [95% CI 0·11–1·1]) post-hospital admission in the moderate disease CCP group. Similarly, lymphocyte counts increased on days 11 (β = 0·76 [95% CI 0·03–1·5]) and 14 (β = 0·98 [95% CI 0·04–1·9]) in the severe disease CCP group (eFigure 4 in Supplement). Total neutrophil counts were not significantly different among the groups at baseline or any other time point (eFigure 5 in Supplement). Relative to baseline, CRP significantly decreased in both the moderate and severe groups at every time point after CCP treatment (p = 0·001; eFigure 6 in Supplement). D-dimer was not significantly different between the groups at baseline, or in the first 11 days, but the levels were lower 14 days post-admission after CCP in the moderate disease group (eFigure 7 in Supplement). Furthermore, LDH was not significantly different among moderate and severe disease groups at baseline or in the first 14 days, but was lower at day 14 in the overall CCP group (eFigure 8 in Supplement).
Table 3.
Changes in oxygen saturation and laboratory markers.
| Oxygen saturation | Day 1 | Day 3 | Day 7 | Day 11 | Day 14 |
|---|---|---|---|---|---|
| All patients | 4·4 (2·2 to 6·6)** | 3·6 (1·8 to 5·3)** | 2·1 (-0·22 to 4·5) | −0·2 (-4·1 to 3·6) | 0·98 (-1·3 to 3·3) |
| Moderate patients | 5·4 (3·3 to 7·4) ** | 4·1 (2·3 to 5·9)** | 1·9 (-0·55 to 4·4) | 0·27 (-3·9 to 4·5) | 1·2 (-1·1 to 3·4) |
| Severe patients | 5·3 (-0·8 to 11·4) | 2·8 (-1·1 to 6·7) | 0·50 (-4·4 to 5·4) | −1·02 (-7·8 to 5·7) | 0·92 (-5·9 to 7·7) |
| Lymphocytes counts | Day 1 | Day 3 | Day 7 | Day 11 | Day 14 |
| All patients | 0·18 (-0·27 to 0·63) | 042 (-0·26 to 1·04) | 0·55 (0·22 to 0·88)** | 0·68 (0·21 to 1·1)* | 0·92 (0·41 to 1·4) ** |
| Moderate disease | 0·06 (-0·37 to 0·48) | 0·21 (-0·23 to 0·66) | 0·46 (0·15 to 0·78)* | 0·52 (-0·11 to 1·2) | 0·59 (0·11 to 1·1) * |
| Severe disease | 0·49 (-0·59 to 1·5) | 0·85 (-0·58 to 2·2) | 0·57 (-0·05 to 1·2) | 0·76 (0·03 to 1·5)* | 0·98 (0·04 to 1·9) * |
| Neutrophils count | Day 1 | Day 3 | Day 7 | Day 11 | Day 14 |
| All patients | 4·3 (-8·6 to 17·4) | 1·6 (-0·81 to 3·9) | 1·9 (-0·19 to 4·1) | −1·8 (-6·04 to 2·3) | −3·8 (-7·9 to 0·17) |
| Moderate disease | 0 | 0·85 (-1·87 to 3·5) | 2·2 (-0·5 to 4·8) | - 7·9 (-13·7 to 2·1) | −3·8 (-8·04 to 0·39) |
| Severe disease | 8·5 (-5·04 to 22·1) | 3·6 (-0·34 to 6·8) | 1·7 (-1·7 to 5·1) | 4·5 (-1·4 to 10·4) | −1·8 (-9·2 to 5·6) |
| C-Reactive Protein | Day 1 | Day 3 | Day 7 | Day 11 | Day 14 |
| All patients | −128·8 (-160·9 to -96·7) ** | −125 (-161·4 to -88·7) ** | −86·6 (-113·5 to -59·7) ** | −115 (-162 to -68·1) ** | −90·7 (-134·6 to -46·8) ** |
| Moderate disease | −112·2 (-147·8 to -76·6) ** | −143·9 (-188·5 to -99·4) ** | −76·1 (-109·7 to -42·5) ** | −99·3 (-141·4 to -57·2) ** | −57·7 (-87·7 to -27·7) ** |
| Severe disease | −176·5 [-234 to -119] ** | −91·3 [-173 to -9·5] * | −104·2 [-163·2 to -45·3] ** | −171 [-265·8 to -76·3] ** | −99·8 [-190·4 to -9·3] * |
| D-Dimer | Day 1 | Day 3 | Day 7 | Day 11 | Day 14 |
| All patients | NA | 109 (-557 to 775·4);0·749 | −253·1 (-938·5 to 432·4);0·469 | −327·3 (-2578·2 to 1923·6);0·776 | −718·3 (-1580·5 to 143·9);0·103 |
| Moderate disease | NA | 240·3 (-572·5 to 1053) | −348·7 (-1441·1 to 743) | 0 | −535·7 (-981·7 to -89·7)* |
| Severe disease | NA | 297 (-1805·6 to 2399·5) | −541·9 (-1064·4 to 980·5) | 1777·5 (-1625 to 5180) | −1162·1 (-3565·7 to 1241·5) |
| Lactate dehydrogenase | Day 1 | Day 3 | Day 7 | Day 1 1 | Day 14 |
| All patients | NA | −77·2 (-361·4 to 207·1) | 61·8 (-109·5 to 233·1) | NA | −393·4(-637·4 to -149·5)* |
| Moderate disease | NA | −89·2 (-789·4 to 610·9) | 7·04 (-212·9 to 226·9) | NA | −272·7 (-641·6 to 96·2) |
| Severe disease | NA | 122·4 (-870·2 to 1115) | −78·4 (-568·2 to 411·4) | NA | −272·2 (-989·2 to 444·9) |
All values are compared to laboratory values at baseline (before CCP or at admission in control group), CCP compared to control NA = not available.
Values are expressed as β coefficient (95% CI) *p-value < 0·05 **p-value < 0·001.
Discussion
Currently, no vaccine or specific antiviral treatments are approved for COVID-19 patients. CP is a widely available, non-pharmaceutical treatment that has been used with varying degrees of success in prior pandemics, including SARS, MERS, and Ebola (Soo et al., 2004, Arabi et al., 2015, Ko et al., 2018, van Griensven et al., 2016). In our study, we investigated the effect of CCP on time to clinical improvement, 30-day mortality, changes in oxygen saturation, and laboratory values within 14 days of hospital admission, in both moderate and severe COVID-19 patients. Many prior studies focused on severe and critically ill patients whereas we included severe cases as well as moderate cases where the risk benefit balance is more delicate. Improved clinical symptoms and mortality among patients with severe and critical COVID-19 after CCP transfusion has been previously reported (Shen et al., 2020, Xia et al., 2020). In a randomized clinical trial of CCP in severe and life-threatening COVID-19, Li et al could not detect a significant reduction in time to clinical improvement within 28 days after CCP treatment (Li et al., 2020). However, the study was terminated early after enrollment of 52 patients who received CCP and 51 in the control group, and may have been underpowered to detect significant differences. In contrast, our study included 135 patients in the CCP arm and 233 in the control group. Another report, concluded that CCP treatment did not reduce mortality and suggested that earlier administration of CCP might be beneficial (Zeng et al., 2020). The fact that patients in our study received CCP within 24 h of hospital admission may have contributed to improving outcomes. In our study, CCP administration was associated with both a higher rate of, and faster time to, clinical improvement among patients with moderate and severe disease. Clinical improvement was observed in 80·6% of the CCP group compared to 58·6% of the control group. In addition, median time to clinical improvement in the CCP group was 7 days compared to 10 days in the control group. This is consistent with a prior case series of 10 patients, in which symptoms resolved within the first 72 h with complete improvement on radiological examination within 7 days post-CCP administration (Ruan et al., 2020).
In the H1N1 pandemic, CP was associated with a significant reduction in mortality, especially if administered early (Hung et al., 2011). An exploratory post hoc meta-analysis revealed a significant reduction in the pooled odds of mortality following CP treatment of SARS coronavirus infection and severe influenza compared to placebo or no therapy (Mair-Jenkins et al., 2015). Li et al did not detect a significant reduction in 28-day mortality among CCP-treated patients (Li et al., 2020). In our study, 30-day mortality was significantly reduced in moderate, but not severe, COVID-19 cases. This may imply that CCP is more effective in reducing 30-day mortality in moderate disease, or may be attributed to inclusion of a relatively small number of severe cases. Our results are also consistent with those of a single arm study of 46 patients with moderate to severe disease where 7-day mortality was 6.5% in patients who received CCP compared to the 15% average in national statistics and 30% in a concurrent cohort of 23 patients (Perotti et al., 2020).
CCP in our study was also associated with significantly improved oxygen saturation and recovery in lymphocyte counts and CRP levels within 14 days post-CCP treatment. These findings are consistent with recent reports on the effect of CCP (Shen et al., 2020, Xia et al., 2020, Duan et al., 2020).
Prior studies have associated lower lymphocyte counts and high CRP levels with adverse outcomes in COVID-19 patients (Brown and McCullough, 2020, Ruan et al., 2020). In our study, lymphocyte counts started to increase 1 day after CCP transfusion, with a significant increase 7 days’ post-admission in the CCP group compared to the control group. Furthermore, CRP was substantially decreased from day 1 in the CCP group. The improvement of both lymphocyte counts and CRP levels in our study may be attributed in part to a previously postulated immunomodulatory effect of CCP such as providing passive immunity by blocking inflammatory cytokines, autoantibodies, and complement pathways (Lunemann et al., 2015). CCP may also inhibit inflammatory cascades and immune cell infiltration within the lungs (Gralinski et al., 2018). These effects can clinically be seen as improvement in oxygen saturation and pulmonary radiological lesions (Ruan et al., 2020).
In our study, oxygen saturation significantly increased in CCP-treated patients during the first 72 h. This finding is consistent with earlier studies reporting associations between CCP and improved oxygen saturation (Shen et al., 2020, Duan et al., 2020).
All CCP recipients tolerated the transfusion except for 3 (2%) who had minor allergic skin reactions, which resolved completely without preventing administration of the full CCP dose. A recent study of early safety indicators for CCP reported 36 cases (0·72%) with serious adverse effects (SAEs) (Eckhardt et al., 2020). The incidence of severe allergic reaction in that study was 0·06%, and the incidence of less than severe allergic reactions was not reported.
Our study has a number of limitations, including a lack of randomization. However, randomization was not feasible during the pandemic in our region. The administration of CCP within 24 h of hospital admission was intended to limit selection bias. Another limitation is that at the time of analysis some patients were still hospitalized which may have biased the results. The clinical management of a potentially life-threatening illness with an unpredictable clinical course was the main contributor to this limitation. In addition, there was not an approved standard protocol for the treatment during our study, especially regarding steroid and tocilizumab use. However, this stems from the lack of universally established treatments for COVID-19. We did not observe an interaction between the administration of either or both of the drugs and the observed effect of CCP in our patients. Finally, we were not able to determine the titer of SARS-CoV-2 IgG antibodies in CCP donors, or whether the detected antibodies were neutralizing antibodies, due to a lack of these tests in Kuwait at the time of the study. We did exclude donors who were negative for IgG antibodies. Not performing additional antibody characterizing tests could only lead to underestimation of the benefit of this therapeutic modality due to potential inclusion of CCP units with low IgG levels or antibodies of limited neutralizing capacity. In addition, in many parts of the world, including some developed countries, there is a striking shortage of testing for COVID-19 in general, and particularly for more specialized tests, such as neutralizing antibodies.
To the best of our knowledge, this study is the first to investigate the effectiveness and feasibility of CCP in COVID-19 patients in the Middle East. Although we did not perform an a priori sample size calculation, 135 patients in the CCP group demonstrated significant differences. The involvement of multiple centers supports the generalizability of the results. Throughout the worldwide lockdown, the supply of CCP was consistently available in Kuwait, which offers free access to this therapeutic intervention. This contrasts with pharmaceuticals subject to supply chain limitations. Availability is crucial in the Middle East, which relies on pharmaceutical manufacturing that is currently concentrated in Western countries.
Conclusion
In our prospective interventional study including patients with moderate and severe COVID-19, CCP administration was significantly associated with improved clinical outcomes. Thirty-day survival was significantly improved in the moderate group. In addition, administration of CCP in both moderate and severe cases was also associated with improved oxygen saturation, and recovery of lymphocytes and CRP levels. Larger multicenter controlled randomized trials to further evaluate the effectiveness of CCP in COVID-19 patients with particular emphasis on CCP donor qualification based on neutralizing antibody levels are warranted.
Contributors
SA conceptualized and planned the study design, planned data collection, oversaw data collection with MA, literature review, performed the text mining analysis, drafted and revised the final version of the manuscript. MA also performed the statistical analysis. MZA and RA conducted literature review, contributed to the writing and reviewing of the manuscript. All other co-authors contributed to data collection and oversaw the manuscript.
Funding
Kuwait Ministry of Health.
Trial registration
Clinicaltrials.gov NCT04474340.
Declaration of interests
We declare no competing interests.
Acknowledgments
This work was supported by Ministry of Health in Kuwait. We thank Jeethu Anu Geo and Jibi Roshan for the technical support and reviewing the data, Kuwait University. We also thank Dr Mohammed Buhamra, for the technical support from the COVID-19 dispatch team, Ministry of Health, Kuwait.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.11.198.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- American Association of Blood Banks (AABB) American Association of Blood Banks (AABB); 2020. Donor eligibility (screening and testing) [Google Scholar]
- Arabi Y., Balkhy H., Hajeer A.H., Bouchama A., Hayden F.G., Al-Omari A., et al. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. SpringerPlus. 2015;4:709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B.L., McCullough J. Treatment for emerging viruses: convalescent plasma and COVID-19. Transfus Apher Sci. 2020;59(3) doi: 10.1016/j.transci.2020.102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Huang J., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt C.M., Cummings M.J., Rajagopalan K.N., Borden S., Bitan Z.C., Wolf A., et al. Evaluating the efficacy and safety of human anti-SARS-CoV-2 convalescent plasma in severely ill adults with COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2020;21(1):499. doi: 10.1186/s13063-020-04422-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraud O., Heshmati F., Pozzetto B., Lefrere F., Girot R., Saillol A., et al. Plasma therapy against infectious pathogens, as of yesterday, today and tomorrow. Transfus Clin Biol. 2016;23(1):39–44. doi: 10.1016/j.tracli.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9(5) doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F., To K.K., Lee C.K., Lee K.-L., Yan W.-W., Liu R., et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52(4):447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integrated Management of Adolescent and Adult Illness (IMAI) District Clinician Manual: Hospital Care for Adolescents and Adults . World Health Organization; Geneva, Switzerland: 2011. Guidelines for the management of common illnesses with limited resources. [Google Scholar]
- Investigational COVID-19 Convalescent Plasma Guidance for Industry . 2020. Food and drug administration center for biologics evaluation and research. [Google Scholar]
- Joyner M.J., Wright R.S., Fairweather D., Senfeld J.W., Burno K.A., Klassen S.A., et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J Clin Invest. 2020;130(9):4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.H., Seok H., Cho S.Y., Ha Y.E., Beak J.Y., Kim S.H., et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23(7):617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- Li L., Zhang W., Hu Y., Ton X., Zheng S., Yang J., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324(5):1–11. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunemann J.D., Nimmerjahn F., Dalakas M.C. Intravenous immunoglobulin in neurology—mode of action and clinical efficacy. Nat Rev Neurol. 2015;11(2):80–89. doi: 10.1038/nrneurol.2014.253. [DOI] [PubMed] [Google Scholar]
- Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.-M., Lim W.S., et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perotti C., Baldanti F., Bruno R., Del Fante C., Seminari E., Casari S., et al. Mortality reduction in 46 severe Covid-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter trial. Haematologica. 2020;105(12):2834–2840. doi: 10.3324/haematol.2020.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakir E.M., Cheung D.S., Grayson M.H. Mechanisms of immunotherapy: a historical perspective. Ann Allergy Asthma Immunol. 2010;105(5):340–347. doi: 10.1016/j.anai.2010.09.012. quiz 8, 68. [DOI] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo Y.O., Cheng Y., Wong R., Hui D.S., Lee C.K., Tsang K.K.S., et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10(7):676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Griensven J., Edwards T., de Lamballerie X., Malcom G.S., Gallian P., Baize S., et al. Evaluation of convalescent plasma for ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2020. R&D blueprint and COVID-19. [Google Scholar]
- Xia X., Li K., Wu L., Wang Z., Zhu M., Huang B., et al. Improved clinical symptoms and mortality among patients with severe or critical COVID-19 after convalescent plasma transfusion. Blood. 2020;136(6):755–759. doi: 10.1182/blood.2020007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q.L., Yu Z.J., Gou J.J., Li G., Ma S.-H., Zhang G.-F., et al. Effect of convalescent plasma therapy on viral shedding and survival in patients with coronavirus disease 2019. J Infect Dis. 2020;222(1):38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Sony J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.