Abstract
Great value in the early identification and treatment of adenomatous polyps or early canceration using colonoscopy has been recognized. A clear colonoscopic vision brought by good intestinal preparation will become crucial. Several studies have completed using the low-residue diet (LRD) versus a clear liquid diet (CLD) the day before colonoscopy that presenting contradictory results. Therefore, a more comprehensive and updated meta-analysis is needed to summarize the findings on the effects of LRD and CLD on intestinal preparation and the quality of coloscopy.
The comprehensive search was performed in PubMed/MEDLINE, Scopus, Cochrane databases (February 2020). LRD vs CLD before colonoscopy were included in this study. Mantel-Haenszel or DerSimonian and Laird models with the relative risk (RR) to evaluate differences in intestinal preparation, tolerance, readiness to repeat preparation, detected of a polyp, and overall adverse reactions.
Total 16 studies (N = 3413) were eligible. Patients with LRD compared with CLD indicated significantly better of tolerability (RR 0.92;95% CI,0.85–0.99; P < .05) and willingness to repeat intestinal preparation (RR 0.86; 95% CI 0.79–0.93; P < .05), but no differences with adequate intestinal preparations, detected polyp or overall adverse reactions (all P > .05).
Patients with LRD the day before colonoscopy show better tolerance and willingness to repeat intestinal preparation, and no difference with adequate intestinal preparations compared with CLD, but the recommended level of evidence is weak. However, in terms of the detection rate of intestinal adenomas, the LRD group is not weaker than the CLD group, for its evidence level is high, and can significantly reduce the hunger experience of patients.
Keywords: clear liquid diet, colonoscopy, low-residue diet, meta-analysis
1. Introduction
Colorectal cancer (CRC) is the second most common cancer among women and the third most common cancer among men.[1] However, the morbidity and mortality of CRC in some developed countries, especially the United States and Japan, have been decreasing in recent years.[2–4] In 2016, United States Preventive Services Task Force released new guidelines for CRC screening, which strongly recommends that general risk adults start screening at the age of 50.[5] Therefore, this is partly due to the benefits of early screening by colonoscopy, which is of great value in the early identification and treatment of adenomatous polyps or early canceration.[6] However, there is a certain rate of missed diagnosis in colonoscopy.[7] One of the main factors resulting in the rate of missed diagnosis is the quality of intestinal preparation. Inadequate intestinal preparation can obviously limit the operator's field of vision and reduce the ability to identify lesions. Improper intestinal preparation is often an important cause of inadequate intestinal preparation, such as consuming a large amount of fluid, eating only clear fluid the day before the colonoscopy, and so on. These reasons may reduce the patient's compliance and lead to poor intestinal preparation.[8]
A diet consisting of exclusively clear liquids is a clear liquid diet (CLD). Solid food on this diet is not allowed and any food including water, broth, which is considered as liquid is allowed.[9] With a CLD diet before intestinal preparation, colonoscopy is obviously unlikely to contain food residue, but at the same time, it is difficult for patients to strike a perfect balance between diet orders and the intake of large doses of laxatives, which may affect the effectiveness of colonoscopy. Many prospective studies evaluated the quality of intestinal preparation and colonoscopy results as well as overall adverse reactions to the use of a low-residue diet (LRD) and a CLD diet the day before the colonoscopy. A meta-analysis of randomized, controlled trials in 2016 showed that there was no difference in adequate intestinal preparation or adverse reactions compared with CLD, but there was a significant increase in tolerance and willingness to repeat preparation in patients with a LRD.[10]
However, since the release of the meta-analysis, seven clinical trials have been published, and some of the conclusions in these clinical trials are still contradictory.[11–17] In addition, effects of LRD and CLD on the percentage of adenomas detected under colonoscopy were not analyzed in this meta-analysis in 2016.Therefore, a more comprehensive and updated meta-analysis is needed to summarize the initial findings. The current meta-analysis is used to summarize the results of existing studies on the effects of LRD and CLD on intestinal preparation and the quality of colonoscopy.
2. Methods
2.1. Literature search strategy
Systematic and comprehensive literature review of the low-residue versus CLD before colonoscopy: randomized controlled trials were performed based on multiple databases which included PubMed/Medline, Scopus, Cochrane databases in February 2020. Search terms were described as “low-residue diet and colonoscopy,” “diet liberalization and colonoscopy” and “fiber-free diet and colonoscopy.” Detailed terms were listed below: diet [All Fields] AND Low-residue [All Fields] AND colonoscopy [All Fields], liberalization [All Fields] AND diet [All Fields] AND colonoscopy [All Fields], and fiber-free [All Fields] AND diet [All Fields] AND colonoscopy [All Fields]. Then, all references of retrieved articles are reviewed again to ensure that the relevant literature will not be missed. Incomplete data or indistinct data was got accessing to the corresponding author if necessary. Two authors (L.C and W.Z) reviewed all the titles and abstracts based on the literature inclusion criteria. Any divergences on study selection were resolved by a third author (E.G.C).
2.2. Data inclusion
This study included studies of LRD vs. CLD preparation in adult patients who needed intestinal preparation before colonoscopy. In each study, patients had to eat at least 1 low-residual meal the day before the colonoscopy, and both diet groups used the same intestinal preparation. The required data is independently extracted by 2 reviewers (L.C and W.Z) and passed by a third party (E.G.C) to resolve the disagreements in the extraction process.
2.3. Study quality assessment
The Cochrane Risk of Bias Tool was performed by 2 independent authors (F.W and W.Z) to assess the risk bias of included studies. The funnel plot was constructed to evaluate the publication bias.
2.4. Institutional review board
This manuscript is dispensed from Institutional Review Board Given for the results in this meta-analysis from published meta-analysis of randomized controlled trials.
2.5. Statistical analysis
We used a meta-analysis to compare the differences in intestinal preparation, tolerance, readiness to repeat preparation, detected of polyp and overall adverse reactions between adults who ingested LRD the day before the colonoscopy and those who ingested CLD. In this study, pooled estimates analyses were conducted in these five aspects. Better gut preparation is generally defined as a score of good or above on the Aronchick scale, greater than 6 on the Boston bowel preparation scale, or A or B on the Harefield Cleansing scale. The tolerance of the intestinal preparation was summarized and analyzed. The mantel-haenszel method with fixed effects was used in the outcome model without heterogeneity, and the DerSimonian and Laird method with random effects was used in the outcome model with heterogeneity, and the results were presented as relative risk. If there is statistically significant heterogeneity, a separate sensitivity analysis of the results will be carried out. Significant heterogeneity was identified as P < .10 or I2 > 50%. The standardized mean difference (SMD) can be selected as the effective variable for meta-analysis of measurement data. The statistical analysis of this study was conducted by the Stata 12.0 (Stata Corporation College Station, TX). The existence of publication bias was analysed by funnellingplot.
3. Results
3.1. Literature search
411 studies were initially identified after the literature search (Fig. 1). Of these articles, 24 studies were selected for further review. Of these chosen, 16 studies (N = 3413) were included in this meta-analysis, which met the inclusion criteria. Details of studying inclusion listed in the supplementary document.
Figure 1.
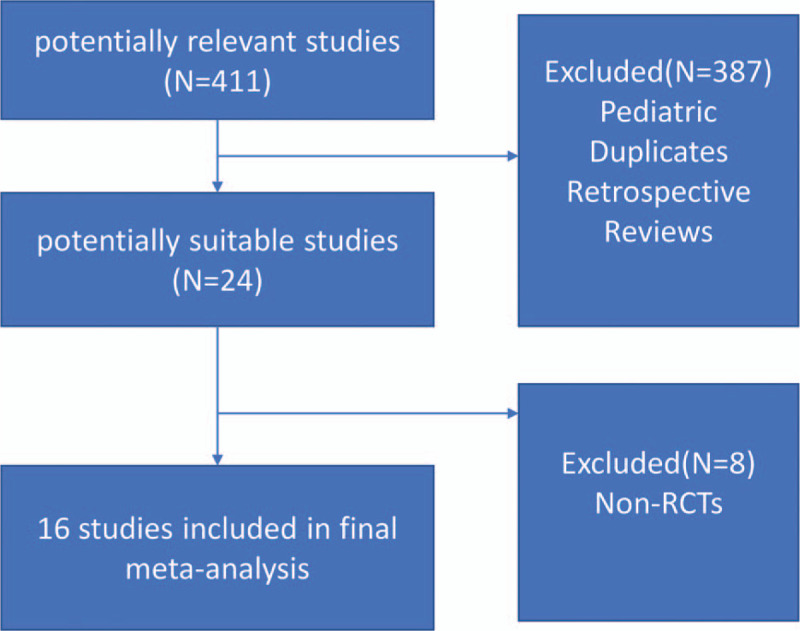
Flow chart of studies’ enrollment.
3.2. Characteristics and qualities of selected studies
Table 1 summarized the characteristics of selected 16 studies. The assessment of study quality was shown in Table 2 according to Cochrane risk of bias tool for controlled trials. In these randomized controlled trials (RCT) studies, the risk of bias was similar within higher score in the selection and outcome parts than in other parts of the study. No significant detection, attrition and reporting bias was found but majority of studies did not blind participants or personnel.
Table 1.
Characteristics of selected studies.
| Author | Study Type | Region | No. Of Patients | Demographics | Definition of Adequate Bowel Preparation | Diet During Bowel Preparation Phase | Type Bowel Preparation Solution |
| Park et al 2009 | RCT | South Korea | 214 | Male: 120 (56.1%) Female: 94 (43.9%) Mean Age: 53.1–55.2 yr | Ottawa Scale No reported cut off for adequate preparation | Prepackaged low- residue diet all day vs Clear liquid diet all day | 4L PEG with electrolytes on day of colonoscopy |
| Rapier et al 2006 | RCT | USA | 75 | Male: 44 (58.7%) Female: 31 (41.3%) Mean Age: 61.0 yr | Aronchick Scale Adequate bowel preparation was excellent or good | Prepackaged low- residue diet all day vs Clear liquid diet all day | Magnesium citrate and bisacodyl (oral and rectal) |
| Scott et al 2004 | RCT | USA | 185 | Male: 82 (44.3%) Female: 103 (55.7%) Mean Age: 56.9–57.0 yr | Aronchick Scale Adequate bowel preparation was excellent or good | Regular breakfast then low-residue diet lunch, then clear liquids rest of day vs Light breakfast then clear liquid rest of day | Sodium phosphates oral solutions split-dose |
| Sipe et al 2013 | RCT | USA | 196 | Male: 93 (47.4%) Female: 103 (52.6%) Mean Age: 56.9–57.8 yr | Boston Bowel Preparation Scale No reported cut off for adequate preparation | Low-residue diet for breakfast, lunch, snack, then clear liquids rest of day vs Clear liquid diet all day | Oral sulfate solution split-dose |
| Soweid et al 2010 | RCT | Lebanon | 200 | Male: 105 (52.5%) Female: 95 (47.5%) Mean Age: 55.5–56.6 yr | Aronchick Scale Adequate bowel preparation was excellent or good | Low-residue diet for breakfast, lunch, dinner vs Clear liquid diet all day | 4L PEG with electrolytes the evening prior |
| Melicharkova et al 2013 | RCT | Canada | 213 | Male: 109 (51.2%) Female: 104 (48.8%) Mean Age: 56.5–57.1 yr | Ottawa and Aronchick Scales Adequate bowel preparation were excellent or good | Low-residue diet for breakfast, then clear liquids the rest of day vs Clear liquid diet all day | Sodium picosulfate + magnesium citrate + bisacodyl evening prior for morning procedures and day of for afternoon procedures |
| Stolpman et al 2014 | RCT | USA | 201 | Male: 114 (56.7%) Female: 87 (43.3%) Mean Age: 60 yr | Boston Bowel Preparation Scale Adequate bowel preparation was score ≥ 6 | Low-residue diet for breakfast and lunch, then clear liquids rest of day vs Clear liquid diet all day | Oral sulfate solution split-dose |
| Butt et al 2016 | RCT | Australia | 226 | Male: 117 (51.8%) Female: 109 (48.2%) Mean Age: 52 yr | Harefield Cleansing Scale Adequate bowel preparation was score of A or B | Low-residue diet all day (white diet) vs Clear liquid diet all day | 2L PEG + ascorbic acid evening prior for morning procedures and split-dose for afternoon procedures |
| Walter et al 2017 | RCT | USA | 140 | Male: 60 (42.9%) Female: 80 (57.1%) Mean Age: NA | Boston Bowel Preparation Scale Adequate bowel preparation was score ≥ 6 | Low-residue diet for breakfast and lunch, then clear liquids rest of day vs Clear liquid diet all day | 2L PEG + ascorbic acid split-dose |
| Delegge et al 2005 | RCT | USA | 506 | Male: 184 (36.4%) Female: 322 (63.6%) Mean Age: 54.3 yr | Custom standard, Adequate bowel preparation was excellent or good | Prepackaged low-residue diet all day vs Clear liquid diet all day | sodium phosphates (2 × 45-mL, split dose.) vs low volume dose of magnesium citrate and bisacodyl |
| Flemming et al 2015 | RCT | Canada | 214 | Male: 86 (40.2%) Female: 128 (63.6%) Mean Age: 62–65 yr | Ottawa scale; Aronchick scale, Adequate bowel preparation was excellent or good | Low-residue diet for breakfast, then clear liquids rest of day vs Clear liquid diet all day | 4L PEG-ELS solution, split-dosing or traditional dosing depend on colonoscopy time |
| Dwyer et al 2017 | RCT | Australia | 250 | Male: 135 (54%) Female: 115 (46%) Mean Age: 54–54.5 yr | Ottawa scale, Adequate bowel preparation was score ≤ 6 | White Diet vs Light breakfast, then clear fluids only | 1L PEG or split-dose Picosalax + SPMC |
| Thukral et al 2017 | RCT | USA | 215 | Male: 112 (52.3%) Female: 103 (47.7%) Mean Age: 55.8–57 yr | Boston Scale, Adequate bowel preparation was score > 5 | Specific instructions on acceptable foods for breakfast, lunch, and evening snacks vs Clear liquid diet all day | Split dose magnesium citrate |
| Tikfu et al 2018 | RCT | Malaysia | 97 | Male: 51 (53%) Female: 46 (47%) Age: 16–73 years old | The modified Aronchick bowel preparation quality scale. | Low-residue, lactose-free semi-elemental enteral formula vs Clear liquid diet | Sodium phosphate (PEG, 3 L in total instead if contraindicated) |
| Marco et al 2019 | RCT | Spain | 276 | Male: 144 (52%) Female: 132 (48%) Mean Age: 59.9 yr | Boston bowel preparation scale | Low-fiber diet vs Clear liquid diet | 4 L of polyethylene glycol in a split-dose regimen |
| Elisa et al 2019 | RCT | Mexico | 205 | Male: 72 (35.1%) Female: 133 (64.9%) Mean Age: 55.6 yr | Boston bowel preparation scale | Low-residue diet vs Clear liquid diet | 4-L preparation of single-dose PEG |
RCT = randomized controlled trials.
Table 2.
Cochrane risk of bias tool for controlled trials.
| Selection bias | Performance bias | Detection bias | Attrition bias | Reporting bias | |||
| Author, year | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| Scott et al 2004 | + | + | − | + | + | + | + |
| Delegge et al 2005 | + | + | − | + | − | − | − |
| Rapier et al 2006 | + | + | − | + | N | + | + |
| Park et al 2009 | + | + | − | + | + | + | + |
| Soweid et al 2010 | + | + | − | + | N | − | − |
| Melicharkova et al 2013 | + | + | − | + | + | + | + |
| Sipe et al 2013 | + | − | − | + | − | − | + |
| Stolpman et al 2014 | + | + | − | + | N | + | + |
| Flemming et al 2015 | − | − | − | + | + | + | + |
| Butt et al 2016 | + | + | − | + | + | + | − |
| Walter et al 2017 | + | + | − | + | + | + | + |
| Dwyer et al 2017 | + | + | − | + | + | + | + |
| Thukral et al 2017 | + | + | − | + | + | N | + |
| Iikfu et al 2018 | + | + | − | + | + | N | − |
| Marco et al 2019 | + | + | − | + | − | + | + |
| Elisa et al 2019 | + | + | − | + | + | − | + |
+ = performed, − = not performed, N = not mentioned.
3.3. Adequate intestinal preparations
Of the 16 studies, 13 assessed the adequacy of intestinal preparation in 2 groups (LDR group and CLD group).[11–23] The study of meta-analysis published in 2016 had discussed the lack of relevant records of Park et al, but we carefully analyzed the study by Siped et al and found that this study did not specify the number of intestinal preparations in the CLD group and the LRD group, but scored specific measurement data. Therefore, in this study, research involving measurement data will be re-analyzed and studied according to the scoring criteria, and then compared with the existing results.
Of the 13 studies, 73.7% (1000/1356) of the CLD group was well prepared for the intestine while the percentage of the LRD group was 73.2% (1034/1413). After pooled analysis, it was found that there was no significant statistical difference in the adequacy of intestinal preparation between the 2 groups (RR, 0.99,95 CI%,0.94–1.04; P > .05; I2 = 61.5%, P = .002). (Fig. 2)
Figure 2.
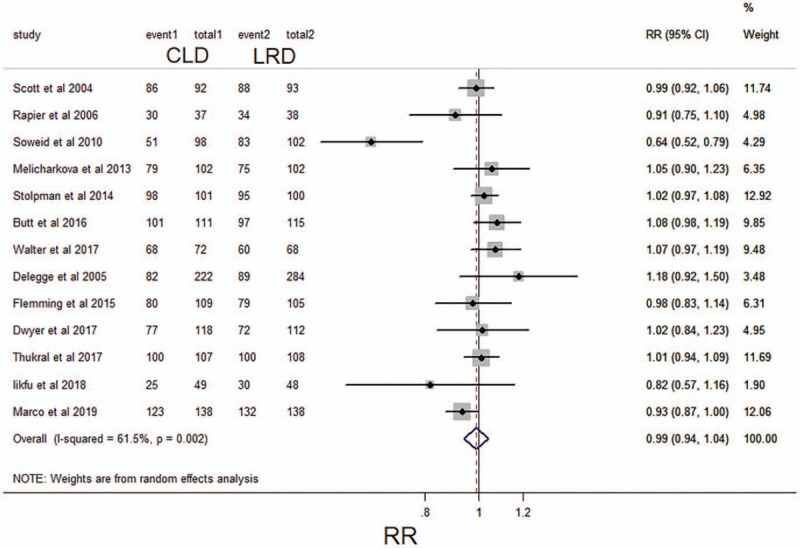
Forest plot comparing the frequency of adequate intestinal preparations between a low-residue diet and clear liquid diet before colonoscopy for categorical data. CI, confidence interval; RR, relative risk.
According to the research on the measurement data involved in the articles, it was found that there were 3 studies using the Ottawa scale scoring criteria, in which the average score (± standard deviation) of the CLD group was 4.05 ± 2.65 and the average score (± standard deviation) of the LRD group was 4.09 ± 2.55.[12,20,24] The standardized mean difference (SMD) was 0.00 (95% CI: -0.15–0.16; I2 = 68.1%, P = .044), so it could not be considered that there was a difference in intestinal preparation between the 2 groups. (Fig. 3). In the 2 studies based on the Boston scale scoring criteria, the average score (± standard deviation) of the CLD group was 7.77 ± 1.61, and the average score (± standard deviation) of the LRD group was 7.68 ± 1.55.[23,25] The SMD was 0.06 (95% CI: -0.16–0.27; I2 = 61.6%, P = .107). Similarly, it could not be considered that there was any difference in intestinal preparation between the 2 groups. After the pooled of the 2 sub-analysis, it was found that the SMD was 0.03 (95% CI: -0.16–0.22). It was still not considered that there was a difference in intestinal preparation between the 2 groups.
Figure 3.
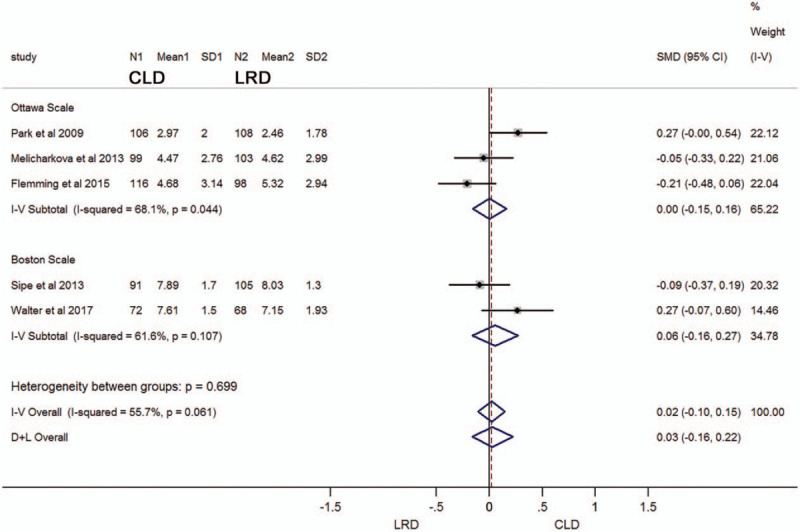
Forest plot comparing the scale scores of adequate intestinal preparations between a low-residue diet and clear liquid diet before colonoscopy for metrological data. CI, confidence interval; SMD, standardized mean difference.
3.4. Tolerance of intestinal preparations of specified diet
Of the 16 studies, 9 (N = 2096) recorded the tolerance of intestinal preparation in patients who received a specified diet.[11,12,14,17,18,20,21,24,26] 81.3% of the patients in the CLD group showed good tolerance, while in the LRD group, 72% of the patients were tolerated. The pooled analysis indicated that the intestinal preparation tolerance rate of the CLD group was slightly lower than that of the LDR group (RR 0.92;95% CI,0.85–0.99; P < .05; I2 = 77.5%, P <.001) (Fig. 4). For attributes data used in these studies, the level of evidence for this result should be considered moderate.
Figure 4.
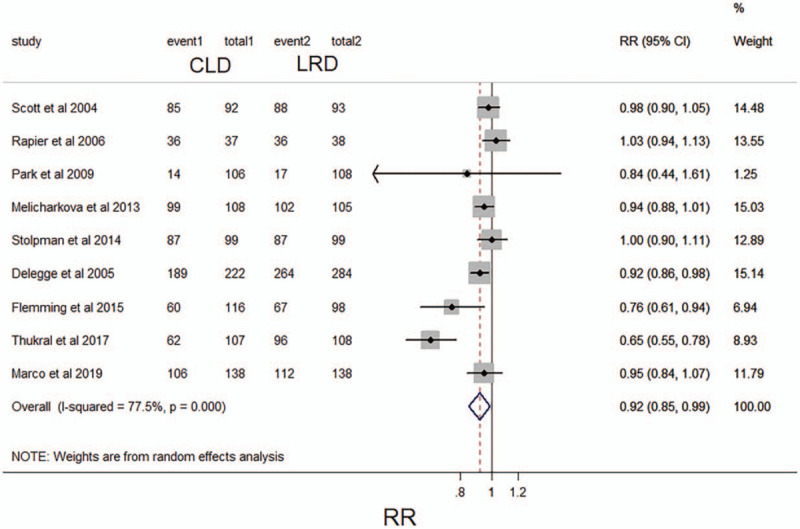
Forest plot comparing reported tolerability of intestinal preparations between a low-residue diet and clear liquid diet before colonoscopy. CI, confidence interval; RR, relative risk.
3.5. Willingness to repeat intestinal preparation with a specified diet
Six of the 16 studies provided the report that the willingness of patients with repeated intestinal preparations in a specified diet.[11,17,19,21,24,26] 88.6% (697/787) showed willingness to repeat intestinal preparation and diet in the LRD group, but only 74.9% (536/716) in the CLD group had the willingness. The pooled analysis of these 6 studies indicated that compared with CLD, patients with LRD were more likely to repeat the same intestinal preparation (RR 0.86; 95% CI 0.79–0.93; P < .05) (Fig. 5). No significant with heterogeneity was found (I2, 63.7%, P = .017). The level of evidence for this result is considered to be moderate.
Figure 5.
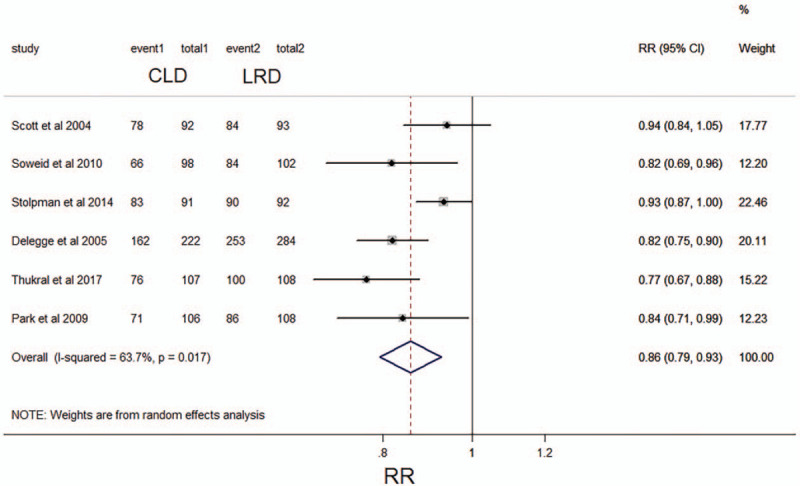
Forest plot comparing willingness to repeat intestinal preparation between a low-residue diet and clear liquid diet before colonoscopy. CI, confidence interval; RR, relative risk.
3.6. Detected polyp with a specified diet
Half of these studies (N = 1727) reported the data of polyp detected with a specified diet.[12–14,16,17,21–23] 45.2% (389/859) showed polyp detected in the LRD group, and 44.3% (385/868) in the CLD group. The pooled analysis of these eight studies showed that the detect rate of polyp in LRD group was similar with in CLD group (RR 0.98; 95% CI 0.87–1.10; P > .05; I2, 28.1%, P = .204) (Fig. 6). The level of evidence for this result is deemed as high level.
Figure 6.
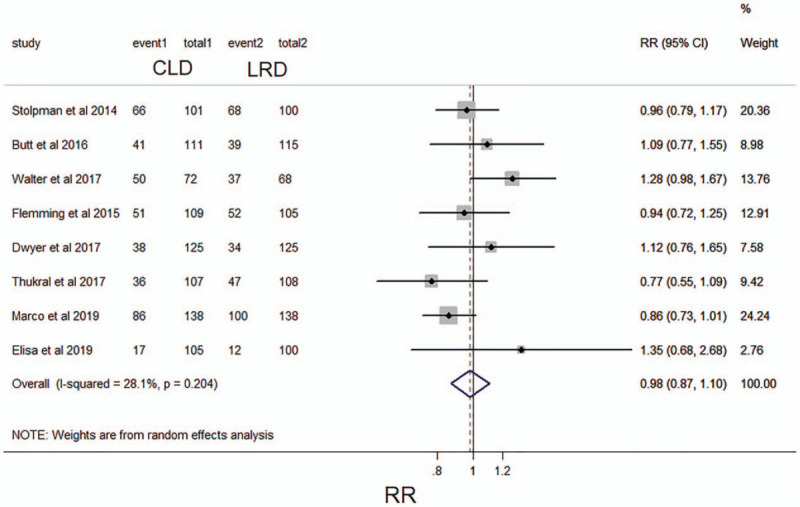
Forest plot comparing the frequency of detecting polyp between a low-residue diet and clear liquid diet before colonoscopy. CI, confidence interval; RR, relative risk.
3.7. Overall adverse reactions
First, we analyzed studies involving overall adverse reactions (including cramping, bloating, vomiting, headaches, nausea, dizziness, and so on) in this meta-analysis, and a total of 4 studies (N = 980) assessed the overall adverse reactions associated with the 2 dietary strategies used in intestinal preparation.[11,18,24,26] In these 4 studies, adverse reactions were measured using general discomfort. There was no statistical difference between LRD group (251/523, 48.0%) and CLD group (221/457, 48.4%) in terms of adverse reactions (RR, 1.04; 95%CI, 0.92–1.17; P > .05; I2 = 0.0%, P = .772) in the pooled analysis (Fig. 7A). The Grading of Recommendations Assessment, Development, and Evaluation evidence for this result was considered moderate because only 4 studies reported on adverse reactions that could be collected. Furthermore, we analyzed hunger separately as an indicator, because the feeling of hunger was not so friendly and can significantly affect the willingness of colonoscopy, if repeated intestinal preparation was needed. The analysis showed that a total of 5 studies (N = 933) involved the indicator of hunger.[13,15,19,21,26] In the pooled analysis, the hunger sensation in the CLD group (52.5%, 244/465) was significantly more severe than that in the LRD group (36.8%, 172/468). (RR, 1.64, 95% CI, 1.12–2.39; P < .05; I2 = 81.4%, P < .01) (Fig. 7B). The Grading of Recommendations Assessment, Development, and Evaluation evidence for this result can be considered high.
Figure 7.
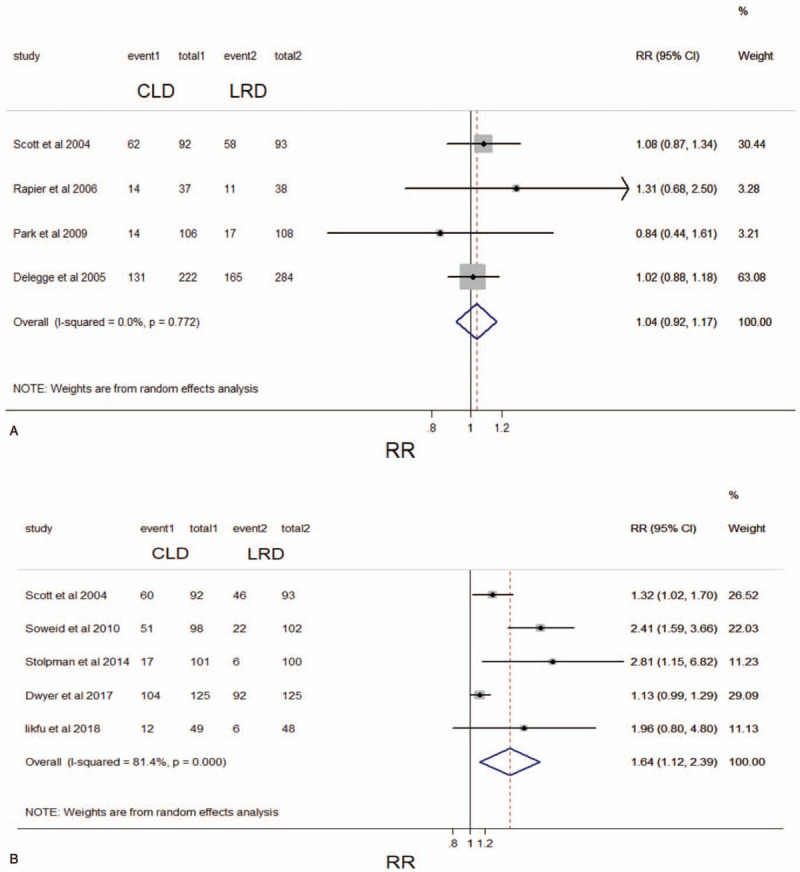
Forest plot comparing the frequency of (A) overall adverse reactions and (B) hunger between a low-residue diet and clear liquid diet before colonoscopy. CI, confidence interval; RR, relative risk.
3.8. Publication bias
No publication bias was found in the case of asymmetry of the funnel plot. (Fig. 8)
Figure 8.
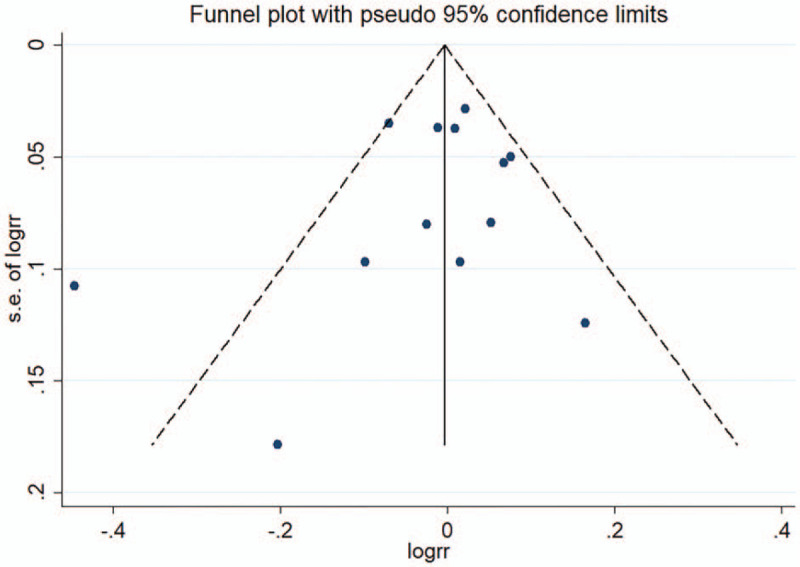
Funnel plot showing no publication bias. RR, relative risk; SE, standard error.
4. Discussions
Adequate intestinal preparation is very beneficial in all aspects of colonoscopy, especially in terms of accuracy. A prospective study of repeated colonoscopy found that when intestinal preparation was inadequate, the missed rate of adenomas larger than 5 mm was 3 times higher.[27] Current international guidelines are not entirely consistent. The US Multi-Society Task Force on CRC recommends that both CLD and LRD are acceptable until the night before colonoscopy.[28] However, Europe's latest 2019 version of European Society of Gastrointestinal Endoscopy Guideline still recommends a LRD the day before the colonoscopy.[29] The meta-analysis from Nguyen et al[10] in 2016 has done some work in this field, and its results show that the LRD the day before colonoscopy seems to be equally effective for the quality of intestinal preparation, but shows higher tolerance and willingness to repeat intestinal preparation, suggesting that a LRD should be used instead of a CLD before colonoscopy. Since then, 7 new RCT studies have been released, and there are still some contradictory conclusions in these clinical studies.[11–17] Moreover, based on these recent studies, we analyzed and compared the detection of adenomas under colonoscopy in patients who received a LRD and a clear fluid diet. Obviously, it is extremely important for colonoscopy screening for early cancer and precancerous lesions. Therefore, we immediately analyzed, updated and summarized the findings of these studies, hoping to make some contribution in this field.
The purpose of this study was to compare the intestinal preparation, tolerance, willingness to repeat intestinal preparation, detection of adenomas and overall adverse reactions in patients who received low residue diet and clear fluid diet respectively the day before the colonoscopy. These results of this study may provide some constructive reference for endoscopists.
In terms of the adequacy of intestinal preparation, 13 articles included in this study used categorical data to compare intestinal preparation between the LRD group and the CLD group. In addition, in view of the fact that some studies used metrological data to show intestinal preparation in the LRD group and the CLD group, we also conducted a separate subgroup analysis, of which 3 RCT studies[12,20,24] were conducted with Ottawa scale scoring criteria, 2 RCT studies[23,25] based on Boston scale scoring criteria. The pooled analysis showed that there was no significant difference in intestinal preparation between the 2 groups, whether expressed by RR or SMD (RR, 0.99, 95 CI%, 0.94–1.04; P > .05; SMD, 0.03, 95% CI: -0.16–0.22). However, there is a significant heterogeneity in the analysis, which is different from the meta-analysis results of Nguyen et al., which is in line with the US guidelines and makes it a recommendation level of weak. In terms of the tolerance of intestinal preparation and the willingness to repeat intestinal preparation, the pooled analysis of LDR group and CLD group showed that the performance of LRD group was significantly better than that of CLD group (P < .05) in these 2 aspects. However, it is worth noting that they have significant heterogeneity, which is also distinct from the results of Nguyen et al, so the recommendation evidence is still weak.
Intriguingly, in terms of an indicator that has not been analyzed before, a vital indicator, namely the detection of adenomas, there are 8 RCT studies involving this indicator. Pooled analysis showed that the detection rate of intestinal adenomas in the LRD group was not weaker than that in the CLD group (P > .05), and there was no significant heterogeneity (I2, 28.1%, P = .204), so the recommended evidence level should be considered to be high. In terms of overall adverse reactions, there was no significant difference in meta-analysis between LRD group and CLD group, and there was no significant heterogeneity. In the past, endoscopists often worried that low residual diet would lead to insufficient intestinal preparation, resulting in a decrease in the detection rate of intestinal precancerous lesions or adenomas. The results of this study showed clear evidence that the detection rate of intestinal lesions in the LRD group was not lower than that in the CLD group. At the same time, we separately listed the index of hunger for subgroup analysis, because the hunger before intestinal preparation brought the most intuitive feeling, the analysis showed that hunger in the CLD group was significantly greater than that in the LRD group. When it is already known that it does not affect these important indicators, such as the detection rate of intestinal adenomas, using LRD to reduce hunger can improve patients’ subjective experience of colonoscopy.
Any meta-analysis has its advantages and limitations. There is no doubt that this study only included the RCT studies, which dramatically increased the credibility of the level of evidence. Second, this study uses RR as the result analysis index, which is more commonly used in prospective cohort studies than OR, and its clinical significance is clear.[30] In addition, compared with the previous meta-analysis, this study added a new comparative study on the detection rate of intestinal adenomas, and the recommended level of evidence is high. Finally, this study was widely included RCT studies from all the world, and the same intestinal preparations were used in the same study, which was comparable. At the same time, different intestinal preparations were used in different studies, indicating that the results of the study can be extrapolated to different intestinal preparations.
Certainly, the limitations of the study are also inevitable. First of all, the analysis results of several indicators show significant heterogeneity, although the random effect model has been used, which makes the evidence recommendation level of these conclusions is not so high. However, there is no significant heterogeneity in the detection of intestinal adenomas, so that endoscopists do not have to worry about affecting the detection rate of intestinal lesions while taking care of patients to avoid the subjective experience of hunger. Secondly, it was noted that in the study of adequate intestinal preparation, several studies were represented by metrological data and were not merged into a collection of 13 categorical data, but we analyzed them separately according to their scoring criteria, and the results were similar to those of categorical data.
5. Conclusions
In summary, based on the meta-analysis of existing RCT studies, LRD the day before colonoscopy seems to be equally effective for the quality of intestinal preparation, but patients show better tolerance and willingness to repeat intestinal preparation, but the recommended level of evidence is weak. However, in terms of the detection rate of intestinal adenomas, the LRD group is not weaker than the CLD group, for its evidence level is high, and can significantly reduce the hunger experience of patients.
Author contributions
E.G.C designed the study, analyzed and interpreted the data, and wrote the manuscript. L.C and F.W acquisition of data. W.Z and X.L.C statistical analysis. G.Y.C study supervision. All authors have vouched for the data analysis, approved the final version, and agreed to publish the manuscript.
Conceptualization: Engeng Chen, Gaoyang Cao.
Data curation: Li Chen, Fei Wang, Wei Zhang.
Formal analysis: Engeng Chen, Li Chen, Fei Wang, Wei Zhang.
Investigation: Xianlei Cai.
Methodology: Fei Wang, Wei Zhang, Xianlei Cai.
Resources: Fei Wang, Wei Zhang, Xianlei Cai.
Supervision: Gaoyang Cao.
Writing – original draft: Engeng Chen.
Writing – review & editing: Engeng Chen.
Supplementary Material
Footnotes
Abbreviations: CLD = clear liquid diet, CRC = colorectal cancer, LRD = low-residue diet, RCT = randomized controlled trials, RR = relative risk, SMD = standardized mean difference.
How to cite this article: Chen E, Chen L, Wang F, Zhang W, Cai X, Cao G. Low-residue versus clear liquid diet before colonoscopy: an updated meta-analysis of randomized, controlled trials. Medicine. 2020;99:49(e23541).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files.
Supplemental digital content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- [2].Vital signs: Colorectal cancer screening, incidence, and mortality--United States, 2002-2010. MMWR, Morb Mortal Wkly, Rep 2011;60:884–9. [PubMed] [Google Scholar]
- [3].Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst 2013;105:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang DX, Gross CP, Soulos PR, et al. Estimating the magnitude of colorectal cancers prevented during the era of screening: 1976 to 2009. Cancer 2014;120:2893–901. [DOI] [PubMed] [Google Scholar]
- [5].Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. Jama 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- [6].Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Anderson R, Burr NE, Valori R. Causes of post-colonoscopy colorectal cancers based on world endoscopy organization system of analysis. Gastroenterology 2020;158:1287–99e2. [DOI] [PubMed] [Google Scholar]
- [8].Lebwohl B, Kastrinos F, Glick M, et al. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc 2011;73:1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Oates JR, Sharma S. Clear Liquid Diet. StatPearls. Treasure Island (FL): StatPearls Publishing StatPearls Publishing LLC; 2020. [PubMed] [Google Scholar]
- [10].Nguyen DL, Jamal MM, Nguyen ET, et al. Low-residue versus clear liquid diet before colonoscopy: a meta-analysis of randomized, controlled trials. Gastrointest Endosc 2016;83:499–507.e1. [DOI] [PubMed] [Google Scholar]
- [11].Delegge M, Kaplan R. Efficacy of bowel preparation with the use of a prepackaged, low fibre diet with a low sodium, magnesium citrate cathartic vs a clear liquid diet with a standard sodium phosphate cathartic. Alimentary pharmacology & therapeutics. Aliment Pharmacol Ther 2005;21:1491–5. [DOI] [PubMed] [Google Scholar]
- [12].Flemming JA, Green J, Melicharkova A, et al. Low-residue breakfast during the preparation for colonoscopy using a polyethylene glycol electrolyte solution: a randomised non-inferiority trial. BMJ Open Gastroenterol 2015;2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dwyer JP, Tan JYC, Paul E, et al. White Diet with split-dose Picosalax is preferred, better tolerated, and non-inferior to day-before clear fluids with polyethylene glycol plus sodium picosulfate-magnesium citrate for morning colonoscopy: a randomized, non-inferiority trial. JGH open [Journal: Article] 2017;1:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alvarez-Gonzalez MA, Pantaleon MA, Flores-Le Roux JA, et al. Randomized clinical trial: a normocaloric low-fiber diet the day before colonoscopy is the most effective approach to bowel preparation in colorectal cancer screening colonoscopy. Dis Colon Rectum 2019;62:491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gee T, Lee L, Liew NC, et al. Efficacy of low residue enteral formula versus clear liquid diet during bowel preparation for colonoscopy: a randomised controlled pilot trial. J Coloproctol 2019;39:62–6. [Google Scholar]
- [16]. Gomez-Reyes E, Tepox-Padron A, Cano-Manrique G, Vilchis-Valadez NJ, Mora-Bulnes S, Medrano-Duarte G, et al. A low-residue diet before colonoscopy tends to improve tolerability by patients with no differences in preparation quality: a randomized trial. Surgical endoscopy. [Journal: Article in Press]. 2019. [DOI] [PubMed] [Google Scholar]
- [17].Thukral C, Tewani SK, Lake AJ, et al. Results of a community-based, randomized study comparing a clear liquid diet with a low-residue diet using a magnesium citrate preparation for screening and surveillance colonoscopies. J Clin Gastroenterol 2019;53:34–9. [DOI] [PubMed] [Google Scholar]
- [18].Rapier R, Houston C. A prospective study to assess the efficacy and patient tolerance of three bowel preparations for colonoscopy. Gastroenterol Nurs 2006;29:305–8. [DOI] [PubMed] [Google Scholar]
- [19].Soweid AM, Kobeissy AA, Jamali FR, et al. A randomized single-blind trial of standard diet versus fiber-free diet with polyethylene glycol electrolyte solution for colonoscopy preparation. Endoscopy. Endoscopy 2010;42:633–8. [DOI] [PubMed] [Google Scholar]
- [20].Melicharkova A, Flemming J, Vanner S, et al. A low-residue breakfast improves patient tolerance without impacting quality of low-volume colon cleansing prior to colonoscopy: a randomized trial. Am J Gastroenterol 2013;108:1551–5. [DOI] [PubMed] [Google Scholar]
- [21].Stolpman DR, Solem CA, Eastlick D, et al. A randomized controlled trial comparing a low-residue diet versus clear liquids for colonoscopy preparation: impact on tolerance, procedure time, and adenoma detection rate. J Clin Gastroenterol 2014;48:851–5. [DOI] [PubMed] [Google Scholar]
- [22].Butt J, Bunn C, Paul E, et al. The White Diet is preferred, better tolerated, and non-inferior to a clear-fluid diet for bowel preparation: a randomized controlled trial. J Gastroenterol Hepatol 2016;31:355–63. [DOI] [PubMed] [Google Scholar]
- [23].Walter J, Francis G, Matro R, et al. The impact of diet liberalization on bowel preparation for colonoscopy. Endosc Int Open 2017;5:E253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Park DI, Park SH, Lee SK, et al. Efficacy of prepackaged, low residual test meals with 4L polyethylene glycol versus a clear liquid diet with 4L polyethylene glycol bowel preparation: a randomized trial. J Gastroenterol Hepatol 2009;24:988–91. [DOI] [PubMed] [Google Scholar]
- [25].Sipe BW, Fischer M, Baluyut AR, et al. A low-residue diet improved patient satisfaction with split-dose oral sulfate solution without impairing colonic preparation. Gastrointest Endosc 2013;77:932–6. [DOI] [PubMed] [Google Scholar]
- [26].Scott SR, Raymond PL, Thompson WO, et al. Efficacy and tolerance of sodium phosphates oral solution after diet liberalization. Gastroenterol Nurs 2005;28:133–9. [DOI] [PubMed] [Google Scholar]
- [27].Clark BT, Protiva P, Nagar A, et al. Quantification of adequate bowel preparation for screening or surveillance colonoscopy in men. Gastroenterology 2016;150:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US multi-society task force on colorectal cancer. Gastroenterology 2014;147:903–24. [DOI] [PubMed] [Google Scholar]
- [29].Hassan C, East J, Radaelli F, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy 2019;51:775–94. [DOI] [PubMed] [Google Scholar]
- [30].McNutt LA, Wu C, Xue X, et al. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol 2003;157:940–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


