Abstract
To assess whether MR diffusion imaging may be applied for non-invasive detection of renal changes correlating with clinical diagnosis of acute kidney injury (AKI) in patients after lung transplantation (lutx).
Fifty-four patients (mean age 49.6, range 26–64 years) after lutx were enrolled in a prospective clinical study and underwent functional MR imaging of the kidneys in the early postoperative period. Baseline s-creatinine ranged from 39 to 112 μmol/L. For comparison, 14 healthy volunteers (mean age 42.1, range 24–59 years) underwent magnetic resonance imaging (MRI) using the same protocol. Renal tissue injury was evaluated using quantification of diffusion and diffusion anisotropy with diffusion-weighted (DWI) and diffusion-tensor imaging (DTI). Renal function was monitored and AKI was defined according to Acute-Kidney-Injury-Network criteria. Statistical analysis comprised one-way ANOVA and Pearson correlation.
67% of lutx patients (36/54) developed AKI, 47% (17/36) had AKI stage 1, 42% (15/36) AKI stage 2, and 8% (3/36) severe AKI stage 3. Renal apparent diffusion coefficients (ADCs) were reduced in patients with AKI, but preserved in transplant patients without AKI and healthy volunteers (2.07 ± 0.02 vs 2.18 ± 0.05 vs 2.21 ± 0.03 × 10–3 mm2/s, P < .05). Diffusion anisotropy was reduced in all lutx recipients compared with healthy volunteers (AKI: 0.27 ± 0.01 vs no AKI: 0.28 ± 0.01 vs healthy: 0.33 ± 0.02; P < .01). Reduction of renal ADC correlated significantly with acute loss of renal function after lutx (decrease of renal function in the postoperative period and glomerular filtration rate on the day of MRI).
MR diffusion imaging enables non-invasive assessment of renal changes correlating with AKI early after lutx. Reduction of diffusion anisotropy was present in all patients after lutx, whereas marked reduction of renal ADC was observed only in the group of lutx recipients with AKI and correlated with renal function impairment.
Keywords: acute kidney injury, diffusion imaging, functional magnetic resonance imaging, lung transplantation
1. Introduction
Loss of renal function is a frequent complication after cardiothoracic surgery.[1] Thirty to 60% of patients after lung transplantation (lutx) develop acute kidney injury (AKI).[2–6] Hemodynamic instability during lutx can lead to impairment of renal perfusion, which is aggravated by renal vasoconstriction due to immunosuppression with calcineurin inhibitors.[7,8] Pre-existing renal insufficiency is an additional risk factor for AKI.[2,9] Perioperative AKI is associated with an increased risk for progression to chronic kidney disease (CKD) and poor prognosis.[6,10–13] Understanding the mechanisms for the development of AKI, identification of risk factors and development of strategies for early diagnosis of the presence and severity of AKI in patients after lutx would address an unmet clinical need to improve the management of patients.
Functional magnetic resonance imaging (MRI) allows for non-invasive quantification of local and global changes of renal perfusion, tissue integrity and microstructure without administration of potentially nephrotoxic contrast agents.[14,15] Diffusion weighted imaging (DWI) measures the Brownian motion of molecules within a tissue and is quantified by the apparent diffusion coefficient (ADC). The ADC reflects diffusion and microperfusion (pseudodiffusion), which can be extracted and analyzed separately by using the intravoxel incoherent motion (IVIM) model.[16] In experimental studies, DWI allowed for accurate and early detection of AKI[17]: diffusion was associated with the severity of kidney injury and the amount of inflammatory cell infiltration at histology.[17] In patients, diffusion within renal tissue has been found to be reduced in different renal disorders such as CKD[18,19] or renal allograft dysfunction.[20,21]
With diffusion tensor imaging (DTI), three-dimensional diffusion properties and the degree of fractional anisotropy (FA) are quantified.[22] The normal renal medulla is highly structured with tubules, collecting ducts and vessels oriented radially to the renal pelvis resulting in a high degree of diffusion anisotropy.[23,24] Tubular injury and alterations of renal microstructure contribute to reduced diffusion anisotropy as it has been shown in patients with renal allograft dysfunction[25,26] and in experimental studies.[27]
We hypothesized that diffusion imaging is able to detect renal changes correlating with clinical diagnosis of AKI in lung transplant patients, and may help to further characterize the severity of kidney injury in the context of renal function impairment.
2. Methods
2.1. Patients and study design
This prospective study was approved by the institutional review board of Hannover Medical School (ethical number: 6379). Written informed consent was obtained from all participants. Between July 2013 and December 2014, 54 patients (38 men, 16 women, mean age 49.6, range 26–64 years) underwent MRI at 14 ± 2 days after surgery and were included in the present analysis. For comparison, 14 healthy volunteers without the history of renal disease or nephrotoxic medication (9 men, 5 women, mean age 42.1, range 24–59 years) were enrolled as a control group.
2.2. Clinical parameters
Renal function was monitored before transplantation, daily or every other day at least until day 16 after surgery. Serum creatinine (s-creatinine) and estimated glomerular filtration rate (eGFR) according to the modification-of-diet-in-renal-disease (MDRD) formula[28] as well as stages of CKD[29] were determined. Perioperative AKI was defined and graded according to Acute-Kidney–Injury-Network (AKIN) criteria within 48 hours after surgery.[30] Patient characteristics, medical history, and details of the surgical procedure (duration of surgery, ischemia times) were recorded.
2.3. MRI protocol and analysis
MRI was conducted on a 1.5 Tesla magnet (Avanto, Siemens Healthcare, Erlangen, Germany) using a 6-channel body coil. To assess renal morphology a respiratory-triggered, fat-saturated T2-weighted turbo spin echo sequence was applied in the coronal plane with the following parameters: TR/TE 2000/100 ms, FOV 420 × 420 mm2, matrix 384 × 269, slice thickness 5 mm. For DWI and DTI, echo planar, respiratory-triggered, fat-saturated sequences were acquired in coronal planes that corresponded to the morphological T2-weighted sequence. DWI parameters were: TR/TE 2000/78 ms, FOV 420 × 420 mm2, matrix 192 × 192, slice thickness 5 mm, 10 b-values 0, 50, 100, 150, 200, 300, 400, 600, 800, 1000 s/mm2. DTI parameters were: TR/TE 4000/92 ms, FOV 420 × 420 mm2, matrix 192 × 192, slice thickness 5 mm, 20 diffusion direction, 2 b-values 0, 600 s/mm2.
In order to correct for motion DWI and DTI images were coregistered with T2-weighed images using non-rigid registration and the software Elastix (open source: http://elastix.isi.uu.nl/). From DWI images, ADC values were calculated using a monoexponential model. Additional bi-exponential analysis was conducted with a multistep approach and maps of pure diffusion (ADCd), pseudodiffusion/microperfusion (ADCp), and perfusion fraction (Fp) were calculated as described previously.[31] For DTI analysis, diffusion measurements along 20 axes were fitted to a 3 × 3 matrix, representing the diffusion tensor. The degree of diffusion anisotropy (fractional anisotropy = FA) was calculated, and depicted in parameter maps.[31]
On all parameter maps 3 regions of interest were drawn manually into renal cortex and medulla in the upper, middle, and lower third of the kidney by 1 investigator who was blinded to patient details (4 years experience with renal MRI). The average size of an individual region of interest was 125 mm2 (range 45–295 mm2) for the renal cortex and 72 mm2 (range 21–181 mm2) for the renal medulla (Supplement Figure 1). Mean diffusion values of both kidneys were calculated for the renal cortex and renal medulla.
2.4. Statistical analysis
Statistical analysis was performed with SPSS (version 24.0, IBM Corporation, USA) and GraphPad Prism (version 6.0, GraphPad Software, Inc., USA). Data were normally distributed according to the Kolmogorov–Smirnov test (P-values between .2 and .74). Therefore, parametric tests were used for analysis. Clinical parameters and patients’ characteristics were compared between patients with and without AKI by using unpaired t test for continuous and Fisher exact test for categorical variables. Differences of MR parameters in patients with and without AKI and healthy volunteers as well as in groups of different AKI stages were determined using one-way ANOVA. Furthermore, the correlations of MR diffusion parameters with eGFR at different time points were determined using Pearson coefficient of correlation. Values are given as mean ± SEM. P-values <.05 are considered statistically significant.
3. Results
3.1. Patient characteristics and surgical details
Patient characteristics and clinical parameters of lutx recipients and donors in this study were not significantly different between patients that developed perioperative AKI and patients who did not develop AKI. There was no significant difference in the frequency of underlying lung diseases that led to lutx (P = .33–1.0; Table 1). All patients received double lutx. Total ischemia time, representing the time between explantation at the donor site and reperfusion at the recipient site, for the right lung was 404 ± 20 minutes in AKI and 364 ± 32 minutes in non AKI patients (P = .27). There was a trend towards longer ischemia times for the left lung in the AKI group (529 ± 25 minutes vs 449 ± 37; P = .08). The left lung is transplanted after the right lung and therefore has a longer ischemia time. Duration of surgery was 5.6 ± 0.5 hours in AKI and 5.0 ± 0.2 hours in non AKI patients (P = .47). The duration of postoperative mechanical ventilation (12.3 ± 1.8 vs 11.9 ± 1.7 hours, P = .88) and the frequency of postoperative extracorporeal membrane oxygenation (ECMO) treatment (19% vs. 22%, P = 1.0) were similar in patients with and without AKI (Table 1). Immunosuppressive regimen was identical in all patients. They received triple immunosuppressive therapy with tacrolimus, mycophenolate mofetil, and prednisolone.
Table 1.
Clinical parameters of patients with AKI and without AKI.
| Parameter | AKI n = 36 | no AKI n = 18 | P-value |
| Reason for lung transplantation | |||
| Emphysema, number/percentage Alpha-1-Antitrypsin deficiency, number/percentage | 12/36 (33%) 1/36 (3%) | 7/18 (39%) 1/18 (6%) | ns, .77 ns, 1.0 |
| Pulmonary fibrosis, number/percentage | 16/36 (44%) | 7/18 (39%) | ns, .78 |
| Cystic fibrosis, number/percentage | 3/36 (8%) | 1/18 (6%) | ns, 1.0 |
| CLAD/re lutx, number/percentage | 2/36 (6%) | 1/18 (6%) | ns, 1.0 |
| Sarcoidosis, number/percentage | 1/36 (3%) | 1/18 (6%) | ns, 1.0 |
| Bronchiectasis, number/percentage | 1/36 (3%) | 0/18 (0%) | ns, 1.0 |
| GvHD, number/percentage | 0/36 (0%) | 1/18 (6%) | ns, .33 |
| Lymphangioleiomyomatosis, number/percentage | 1/36 (3%) | 0/18 (0%) | ns, 1.0 |
| Clinical parameters lung transplant recipients | |||
| Male, number/percentage | 25/36 (69%) | 13/18 (72%) | ns, 1.0 |
| Height, cm | 174 ± 2 | 175 ± 2 | ns, .77 |
| Weight, kg | 70 ± 2 | 68 ± 3 | ns, .68 |
| Age, y | 48 ± 2 | 41 ± 3 | ns, .35 |
| History of smoking, number/percentage | 16/36 (44%) | 9/18 (50%) | ns, .78 |
| Secondary pulmonary hypertension, number/percentage | 15/36 (42%) | 6/18 (33%) | ns, .77 |
| Diabetes mellitus, number/percentage | 4/36 (11%) | 1/18 (6%) | ns, .65 |
| ECMO preoperative, number/percentage | 2/36 (6%) | 0/18 (0%) | ns, .55 |
| Clinical parameters of lung donors | |||
| Male, number/percentage | 24/36 (67%) | 13/18 (72%) | ns, .76 |
| Height, cm | 175 ± 3 | 177 ± 2 | ns, .54 |
| Weight, kg | 83.8 ± 3.1 | 92.0 ± 10.7 | ns, .35 |
| Age, y | 47.3 ± 2.9 | 52.1 ± 3.6 | ns, .32 |
| Duration of ventilation, d | 5.0 ± 0.6 | 4.1 ± 1.0 | ns, .39 |
| Transplantation/ post-transplantation details | |||
| Double lutx, number/percentage | 36/36 (100%) | 18/18 (100%) | ns, 1.0 |
| Minimal invasive lutx, number/percentage | 34/36 (94%) | 16/18 (89%) | ns, .59 |
| Clamshell technique, number/percentage | 2/36 (6%) | 2/18 (11%) | ns, .59 |
| Ischemia time right lung, min | 404 ± 20 | 364 ± 32 | ns, .27 |
| Ischemia time left lung, min | 529 ± 25 | 449 ± 37 | ns, .08 |
| Duration of surgery, h | 5.6 ± 0.5 | 5.0 ± 0.2 | ns, .47 |
| ECMO postoperative | 7/36 (19%) | 4/18 (22%) | ns, 1.0 |
| Duration of ventilation after lutx, h | 12.3 ± 1.8 | 11.9 ± 1.7 | ns, .88 |
| Triple immunosuppressive therapy with tacrolimus, mycophenolate mofetil and prednisolone, number/percentage | 36/36 (100%) | 18/18 (100%) | ns, 1.0 |
| Tacrolimus levels at the day of MRI, μg/L | 10.5 ± 0.7 | 10.1 ± 0.7 | ns, .76 |
All values are given as numbers and percentage or mean ± SEM.
AKI = acute kidney injury, CLAD = chronic lung allograft dysfunction, EC = erythrocyte concentrate, ECMO = extracorporeal membrane oxygenation, FFP = fresh frozen plasma, GvHD = graft-versus-host-disease, lutx = lung transplantation, ns = not significant, re lutx = re lung transplantation, TC = thrombocyte concentrate.
3.2. Acute kidney injury and renal function in patients after lutx
Perioperative AKI was detected in 36/54 (67%) patients, and classified as mild (AKIN stage 1) in 17 patients, as moderate (AKIN stage 2) in 16, and as severe (AKIN stage 3) in 3 patients. Baseline renal function parameters before lutx were not significantly different between lutx patients with (s-creatinine ranged from 39 to 112 μmol/L) and without perioperative AKI (s-creatinine ranged from 48 to 95 μmol/L, Table 2). Within 48 hours after transplantation, maximum s-creatinine increased significantly to 138 ± 9 μmol/L in AKI patients compared with patients without AKI (86 ± 7 μmol/L, P < .01). Renal function partially recovered until the day of MRI with s-creatinine levels of 89 ± 10 μmol/L in patients with perioperative AKI and 64 ± 4 μmol/L in patients without AKI (P < .05; Table 2).
Table 2.
Renal function parameters of patients with AKI and without AKI.
| Parameter | AKI n = 36 | no AKI n = 18 | P-value |
| Preoperative renal function | |||
| s-Creatinine, μmol/L | 66 ± 3 | 68 ± 4 | ns, .72 |
| eGFR, mL/min/1.73 m2 | 106 ± 3 | 99 ± 4 | ns, .16 |
| Postoperative renal function (most severe renal impairment within 48 hours after surgery) | |||
| s-Creatinine (max.), μmol/L | 138 ± 9 | 86 ± 7 | <.01 |
| s-Creatinine increase (max.), factor | 2.1 ± 0.1 | 1.3 ± 0.1 | <.001 |
| eGFR (min.), mL/min/1.73 m2 | 64 ± 5 | 92 ± 6 | <.01 |
| Renal function at the day of MRI (14 ± 2 days after lutx) | |||
| s-Creatinine, μmol/L | 89 ± 10 | 64 ± 4 | ns, .09 |
| eGFR, mL/min/1.73 m2 | 91 ± 4 | 106 ± 4 | <.05 |
Significant differences between groups of patients with and without AKI are given in the last column.
AKI = acute kidney injury, CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, lutx = lung transplantation, MRI = magnetic resonance imaging, ns = not significant.
3.3. Renal diffusion was reduced in patients with perioperative AKI after lutx and correlates with renal function impairment
A significant reduction of diffusion in renal tissue of patients with perioperative AKI was observed 2 weeks after lutx compared with patients without AKI and to healthy volunteers (P < .05, Fig. 1). ADC of renal medulla was 2.08 ± 0.02 × 10–3 mm2/s in patients with AKI, 2.18 ± 0.05 × 10–3 mm2/s in lutx recipients without AKI (P < .05) and 2.20 ± 0.03 × 10–3 mm2/s in healthy volunteers (P < .05). Significant differences of renal diffusion between different stages of AKI were not observed (Table 3). Comparable but less pronounced differences between groups were detected for the IVIM parameters of pure diffusion (renal medulla: healthy volunteers 1.57 ± 0.05 10–3 mm2/s, no AKI 1.50 ± 0.04 10–3 mm2/s, not significant, AKI 1.49 ± 0.02 10−3 mm2/s, not significant) and microperfusion (renal cortex: healthy volunteers 10.03 ± 0.44 10−3 mm2/s, no AKI 9.89 ± 0.52 10−3 mm2/s, AKI 8.58 ± 0.26 10−3 mm2/s, P < .05, Table 3, Supplement Fig. 2).
Figure 1.
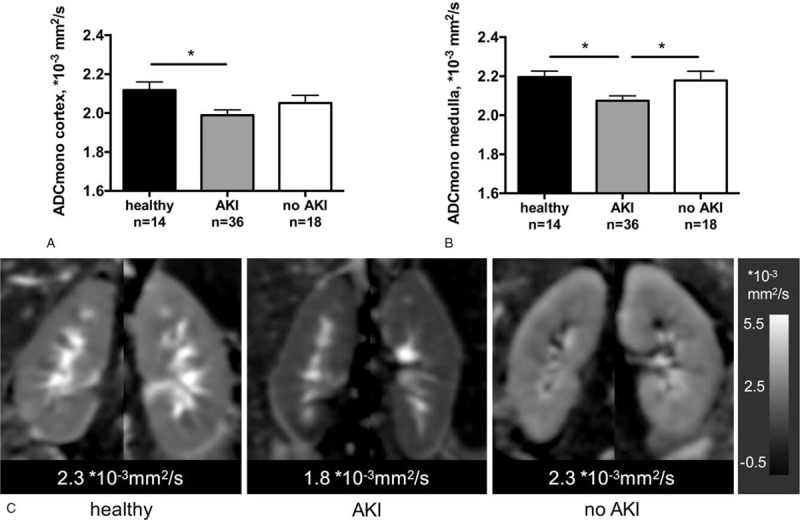
MR diffusion is reduced in patients with perioperative AKI after lutx. Mean ± SEM values of MR diffusion, quantified by ADC, in renal cortex (A) and medulla (B). Representative parameter maps show reduced diffusion in a patient after lutx with perioperative AKI compared with a patient without perioperative AKI and a healthy volunteer (C). ∗P < .05. ADC = apparent diffusion coefficient; AKI = acute kidney injury; lutx = lung transplantation.
Table 3.
Diffusion parameters in groups of AKI stages.
| Parameter | Healthy n = 14 | No AKI n = 18 | AKI n = 36 | AKIN 1 n = 17 | AKIN 2 n = 15 | AKIN 3 n = 3 | |
| ADC ×10−3 mm2/s | Cortex | 2.12 ± 0.04 | 2.05 ± 0.04 | 1.99 ± 0.03∗ | 1.97 ± 0.04∗ | 2.01 ± 0.05 | 1.99 ± 0.12 |
| Medulla | 2.20 ± 0.03 | 2.18 ± 0.05 | 2.07 ± 0.02∗# | 2.06 ± 0.04∗# | 2.09 ± 0.04 | 2.06 ± 0.12 | |
| FA | Cortex | 0.15 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.004 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.12 ± 0.01 |
| Medulla | 0.33 ± 0.02 | 0.28 ± 0.01∗∗ | 0.27 ± 0.01∗∗∗ | 0.27 ± 0.01∗∗ | 0.26 ± 0.01∗∗∗ | 0.27 ± 0.05∗ | |
| ADCd | Cortex | 1.47 ± 0.04 | 1.44 ± 0.04 | 1.43 ± 0.02 | 1.41 ± 0.03 | 1.46 ± 0.03 | 1.40 ± 0.11 |
| ×10−3 mm2/s | Medulla | 1.57 ± 0.05 | 1.50 ± 0.04 | 1.49 ± 0.02 | 1.49 ± 0.03 | 1.52 ± 0.03 | 1.38 ± 0.13 |
| ADCp | Cortex | 10.03 ± 0.44 | 9.89 ± 0.52 | 8.58 ± 0.26∗# | 8.25 ± 0.35∗# | 9.13 ± 0.43 | 8.09 ± 0.99 |
| ×10−3 mm2/s | Medulla | 9.89 ± 0.36 | 10.22 ± 0.47 | 9.31 ± 0.25 | 8.92 ± 0.25# | 9.78 ± 0.47 | 9.13 ± 1.26 |
| Fp | Cortex | 0.36 ± 0.02 | 0.34 ± 0.02 | 0.31 ± 0.01∗ | 0.30 ± 0.01∗ | 0.32 ± 0.01 | 0.32 ± 0.06 |
| Medulla | 0.35 ± 0.02 | 0.36 ± 0.02 | 0.33 ± 0.01 | 0.33 ± 0.01 | 0.32 ± 0.01 | 0.36 ± 0.01 |
Significant differences compared to healthy volunteers (∗P < .05, ∗∗P < .01, ∗∗∗P < .001) and patients without AKI (#P < .05) are indicated. No significant differences were found between groups of different AKI stages.
ADC = apparent diffusion coefficient, quantitative value of diffusion, ADCd = ADC diffusion, quantitative value of pure diffusion, ADCp = ADC perfusion, quantitative value of pseudodiffusion, AKI = acute kidney injury, AKIN 1-3 = AKI stage 1–3 according to the Acute Kidney Injury Network criteria corresponding to mild, moderate and severe AKI, FA = fractional anisotropy, quantitative value of diffusion anisotropy, Fp = perfusion fraction.
Renal diffusion correlated with eGFR at the day of MRI (cortex r = 0.50, P < .001; medulla r = 0.30, P < .05) and acute decrease of eGFR within 48 hours after lutx (cortex r = –0.31, P < .05; medulla r = –0.34, P < .05; Table 4, Fig. 2).
Table 4.
Correlation of MR diffusion parameters with renal function and clinical parameters.
| Parameter | ADC (cortex) | ADC (medulla) | FA (medulla) |
| Renal function | |||
| Minimum postoperative eGFR | r = 0.373∗∗ | r = 0.234 | r = 0.134 |
| eGFR decrease | r = –0.310∗ | r = –0.344∗ | r = –0.094 |
| eGFR at the day of MRI | r = 0.499∗∗∗ | r = 0.302∗ | r = 0.235 |
ADC = apparent diffusion coefficient, eGFR = estimated glomerular filtration rate, FA = fractional anisotropy, quantitative value of diffusion anisotropy, LuTx = lung transplantation, quantitative value of diffusion.
P < .05.
P < .01.
P < .001.
Figure 2.
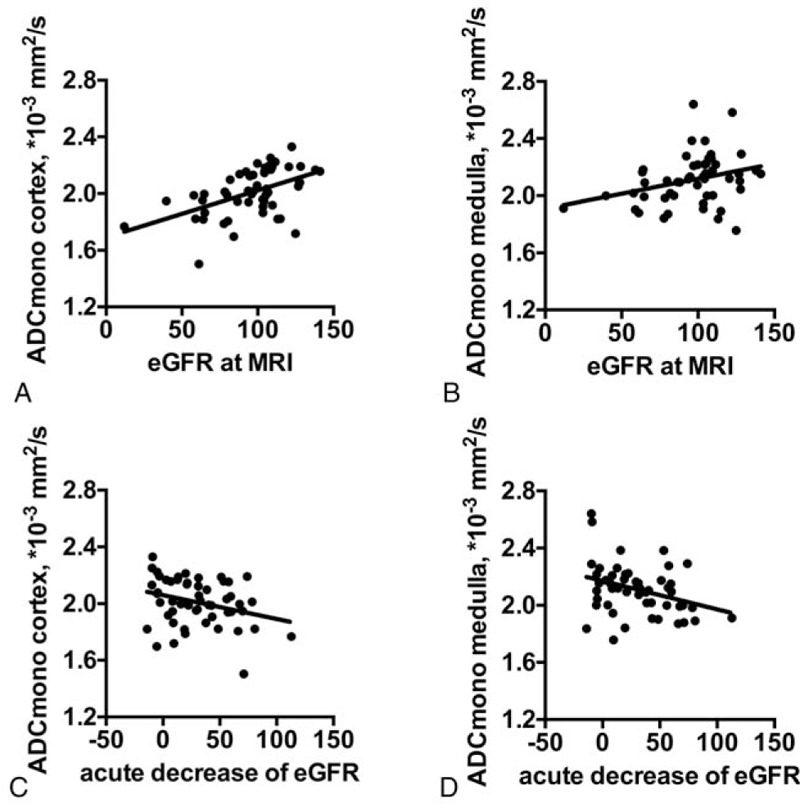
Correlations of renal diffusion and renal function parameters. Significant correlations of ADCmono in renal cortex and medulla with eGFR at the day of MRI (A, P < .001; B, P < .05) and with acute decrease of eGFR within 48 hours after lutx (C, P < .05; D, P < .05) are shown. ADCmono = ADC calculated by mono-exponential analysis, eGFR = estimated glomerular filtration, MRI = magnetic resonance imaging.
3.4. Diffusion anisotropy is reduced in all patients after lutx and is not related to severity of acute renal function impairment
Diffusion anisotropy of renal medulla quantified by FA values was significantly reduced compared with healthy volunteers in patients with and without AKI with similar magnitude (healthy volunteer 0.33 ± 0.02, AKI 0.27 ± 0.01, no AKI 0.28 ± 0.01, P < .01; Fig. 3). No differences of diffusion anisotropy were observed in the renal cortex. Diffusion anisotropy in patients after lutx did not correlate with eGFR at the day of MRI or acute decrease of eGFR within 48 hours after lutx (Table 4). Note that in 1 patient with and another without AKI FA values were not available due to technical issues.
Figure 3.
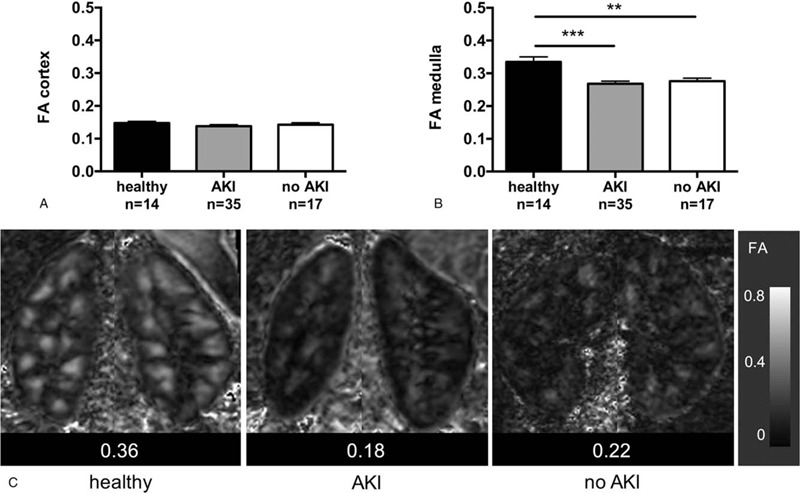
Diffusion anisotropy is reduced in all patients after lutx. Mean ± SEM values of MR diffusion anisotropy (FA) in renal cortex (A) and medulla (B). Representative parameter maps show reduced diffusion anisotropy within the renal medulla in a patient after lutx with and another patient without perioperative AKI, and normal diffusion properties in a healthy volunteer (C). ∗∗P < .01, ∗∗∗P < .001. AKI = acute kidney injury; lutx = lung transplantation.
4. Discussion
We have shown that renal diffusion 2 weeks after lutx was significantly reduced in patients with perioperative AKI compared with patients without AKI and to healthy volunteers. Diffusion anisotropy was reduced in all lung transplant patients compared with volunteers irrespective of whether AKI was diagnosed. Reduced diffusion was significantly correlated with acute perioperative decline of renal function and eGFR at the day of MRI.
The incidence of perioperative AKI after double lutx was 67% in our prospective study and was comparable to that reported in larger retrospective analyses.[2] In studies including unilateral lutx incidence rates of AKI are lower: in a study cohort of approximately 50% single lutx, one-third developed AKI.[3] However, in our study, established risk factors for perioperative AKI in lutx patients such as pulmonary disease other than COPD, lower baseline eGFR, and duration of mechanical ventilation for >1 day[2] were not significantly different between patients with and without AKI. This may be explained by the fact that only patients, who had a mild clinical course without prolonged stay at the intensive care unit, were included into the study. This caused a selection bias towards patients with better initial postoperative course after lutx. Furthermore, the number of patients included is relatively low compared with the large retrospective analyses that found an association with the aforementioned risk factors.
Perioperative AKI after non-renal organ transplantation has been identified to increase the risk for CKD, and the onset of CKD to increase the risk of death (relative risk increased by factor 2.13 and 4.55).[11] In another retrospective analysis of 657 patients 1-year patient survival was lower in AKI versus no AKI patients and over a period of 5 years CKD reached 22% (no AKI), 44% (AKI with recovery), and 45% (AKI with no recovery).[13] Beside the surgery associated AKI, several other factors contribute to decline of renal function over time such as nephrotoxic medication (e.g., immunosuppressive calcineurin inhibitors and aminoglycoside antibiotics) or comorbidities (e.g., hypertension or diabetes).[11] Early identification of patients at risk for CKD is important since different treatment strategies might lead to better renal outcome and improved renal function. In a very recent clinical trial where stable lung transplant recipients at 3 to 6 months after surgery were randomized into the standard triple therapy versus a quadruple therapy with low calcineurin inhibitor regimen in combination with an mTOR inhibitor demonstrated superiority of the quadruple low calcineurin inhibitor regimen in preservation of renal function at 12 months after surgery. eGFR was 64.5 mL/min in the quadruple therapy group versus 54.6 mL/min for the standard triple group (P < .001).[32] That stresses the importance of early patient identification at risk of CKD in order to consider alternative therapy regimens with better renal outcome and less morbidity for the patients.
With quantitative functional MRI local and global changes of renal tissue can be examined specifically and non-invasively in the early phase after transplantation.[17,26] Experimental studies evidenced a predictive value of functional MR parameters early after renal ischemia reperfusion injury in mice for subsequent CKD and kidney volume loss.[17,33] Functional MRI is safely applicable in patients with impaired renal function as no contrast-agent is needed and might help to elucidate severity of AKI and monitor renal injury in patients undergoing transplantation.
In the present study, MR diffusion techniques such as DWI and DTI were used to quantify early renal injury due to perioperative AKI. Renal ADC as determined by DWI 2 weeks after lutx was significantly reduced in patients who experienced AKI, and was preserved in patients without AKI and healthy volunteers. Furthermore, ADC correlated with renal function at the day of MRI and acute decline of renal function after lutx. These findings suggest that renal ADC values are a non-invasive imaging-based correlate for clinical relevant renal injury in the early postoperative period. Reduced ADC of renal tissue may be explained by several factors that lead to narrowing of the interstitial space such as infiltration of inflammatory cells, tissue edema, cell swelling, and tubular injury due to perioperative ischemia reperfusion injury as it has been shown in experimental studies[17,33] and patients early after kidney transplantation.[31] IVIM analysis in our study revealed that reduction of microperfusion and pure diffusion both contributed to reduced ADC values in AKI patients.
Diffusion anisotropy, measured by DTI, was significantly reduced in all lutx patients, independent of the presence of AKI and did not correlate with renal function. This points towards possible subclinical changes, which might be present in all patients after lutx. Diffusion anisotropy in renal medulla requires normal renal function, resulting in tubular flow and perfusion oriented radially to the renal pelvis, as well as normal renal microstructure, with structures oriented towards the renal pelvis. It has been shown for renal allografts that impairment of renal function and alterations of renal microstructure such as tubular injury are associated with reduced diffusion anisotropy.[25,26,31] Our data highlight that diffusion parameters applied in the present study provide complementary information of AKI in the context of major surgery as DTI is sensitive to detect subclinical renal injury, while ADC reduction is associated with manifest AKI and the severity of renal function impairment measured by eGFR.
Some limitations of this study should be acknowledged. First, a relatively small number of patients with lutx and healthy volunteers was included. Therefore, dedicated subgroup or multilinear regression analyses were not possible. However, we aimed to investigate the value of MR diffusion imaging for assessment of AKI in a well-defined patient cohort of lutx recipients. Second, only patients with mild clinical course without prolonged ICU stay were included in the study. This caused a selection bias towards patients with a better overall outcome after surgery. However, this was the first study to evaluate different MR diffusion techniques, that is, DWI and DTI, to provide non-invasive and quantitative imaging-based biomarkers for renal injury due to perioperative AKI in the early phase after lutx. Third, MRI scans were performed in patients with and without perioperative increase of s-creatinine and MRI parameters were correlated to clinical parameters. Therefore, in our study, information from renal diffusion parameters was not used to detect AKI and did not change patients’ treatment. Fourth, we did not characterize changes in renal diffusion over time, as this study was designed to characterize postoperative renal changes by MR diffusion imaging in patients after lutx and to correlate MR findings with clinical diagnosis of AKI and established laboratory parameters. Future research projects on lutx patients to assess AKI and CKD in a longitudinal study design will be needed.
In conclusion, MR diffusion imaging with DWI and DTI enables detection of renal changes correlating with clinical diagnosis of AKI shortly after lutx. Marked ADC reduction was observed only in the group of lutx recipients with AKI and correlated with renal function impairment. Thus, renal diffusion, measured by functional MRI, can provide additional information in the early postoperative period after lutx. In addition, it might help to improve patient management by adapting therapy plans early in order to prevent further renal injury.
Acknowledgments
Frank Wacker, Marcel Gutberlet and Katja Derlin have research collaborations with Siemens healthcare outside the submitted work.
Author contributions
Conceptualization: Katja Derlin, Susanne Hellms, Marcel Gutberlet, Dagmar Hartung, Faikah Gueler.
Data curation: Katja Derlin, Susanne Hellms, Marcel Gutberlet, Peperhove Matti Joonas, Mi-Sun Jang, Christine Fegbeutel, Igor Tudorache, Bjoern Juettner, Faikah Gueler.
Formal analysis: Katja Derlin, Susanne Hellms, Marcel Gutberlet, Peperhove Matti Joonas, Thorsten Derlin, Igor Tudorache, Birgitt Wiese, Ralf Lichtinghagen, Faikah Gueler.
Funding acquisition: Katja Derlin, Susanne Hellms, Faikah Gueler.
Investigation: Katja Derlin, Susanne Hellms, Peperhove Matti Joonas, Mi-Sun Jang, Dagmar Hartung, Christine Fegbeutel, Bjoern Juettner.
Methodology: Katja Derlin, Susanne Hellms, Dagmar Hartung, Thorsten Derlin, Faikah Gueler.
Project administration: Katja Derlin, Susanne Hellms, Dagmar Hartung.
Resources: Susanne Hellms, Axel Haverich, Frank Wacker, Gregor Warnecke, Faikah Gueler.
Software: Marcel Gutberlet, Birgitt Wiese.
Supervision: Katja Derlin, Dagmar Hartung, Hermann Haller, Axel Haverich, Frank Wacker, Gregor Warnecke, Faikah Gueler.
Validation: Susanne Hellms, Marcel Gutberlet.
Visualization: Katja Derlin, Susanne Hellms.
Writing – original draft: Katja Derlin, Susanne Hellms, Peperhove Matti Joonas, Thorsten Derlin.
Writing – review & editing: Katja Derlin, Susanne Hellms, Marcel Gutberlet, Peperhove Matti Joonas, Mi-Sun Jang, Dagmar Hartung, Thorsten Derlin, Christine Fegbeutel, Igor Tudorache, Bjoern Juettner, Birgitt Wiese, Ralf Lichtinghagen, Hermann Haller, Axel Haverich, Frank Wacker, Gregor Warnecke, Faikah Gueler.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ADC = apparent diffusion coefficient, ADCd = IVIM parameter, pure diffusion calculated by bi-exponential analysis, ADCmono = ADC calculated by mono-exponential analysis, ADCp = IVIM parameter, pseudodiffusion calculated by bi-exponential analysis, AKI = acute kidney injury, AKIN = acute-kidney-injury-network, CKD = chronic kidney disease, DTI = diffusion tensor imaging, DWI = diffusion weighted imaging, eGFR = estimated glomerular filtration, FA = fractional anisotropy, Fp = IVIM parameter, perfusion fraction of diffusion, IVIM = intravoxel incoherent motion, lutx = lung transplantation, MRI = magnetic resonance imaging.
How to cite this article: Derlin K, Hellms S, Gutberlet M, Peperhove M, Jang MS, Greite R, Hartung D, Derlin T, Fegbeutel C, Tudorache I, Jüttner B, Wiese B, Lichtinghagen R, Haller H, Haverich A, Wacker F, Warnecke G, Gueler F. Application of MR diffusion imaging for non-invasive assessment of acute kidney injury after lung transplantation. Medicine. 2020;99:49(e22445).
KD and SH authors have contributed equally to this work.
FG deceased.
AH, FW, and GW are Member of the German Centre for Lung Research, BREATH site.
SH and KD received funding from the Young faculty program of Hannover Medical School.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Ho J, Lucy M, Krokhin O, et al. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: a nested case-control study. Am J Kidney Dis 2009;53:584–95. [DOI] [PubMed] [Google Scholar]
- [2].Rocha PN, Rocha AT, Palmer SM, et al. Acute renal failure after lung transplantation: incidence, predictors and impact on perioperative morbidity and mortality. Am J Transplant 2005;5:1469–76. [DOI] [PubMed] [Google Scholar]
- [3].Bennett D, Fossi A, Marchetti L, et al. Postoperative acute kidney injury in lung transplant recipients. Interact Cardiovasc Thorac Surg 2019;28:929–35. [DOI] [PubMed] [Google Scholar]
- [4].Peperhove M, Vo Chieu VD, Jang MS, et al. Assessment of acute kidney injury with T1 mapping MRI following solid organ transplantation. Eur Radiol 2018;28:44–50. [DOI] [PubMed] [Google Scholar]
- [5].Balci MK, Vayvada M, Salturk C, et al. Incidence of early acute kidney injury in lung transplant patients: a single-center experience. Transplant Proc 2017;49:593–8. [DOI] [PubMed] [Google Scholar]
- [6].Wehbe E, Duncan AE, Dar G, et al. Recovery from AKI and short- and long-term outcomes after lung transplantation. Clin J Am Soc Nephrol 2013;8:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zaltzman JS, Pei Y, Maurer J, et al. Cyclosporine nephrotoxicity in lung transplant recipients. Transplantation 1992;54:875–8. [DOI] [PubMed] [Google Scholar]
- [8].Puschett JB, Greenberg A, Holley J, et al. The spectrum of ciclosporin nephrotoxicity. Am J Nephrol 1990;10:296–309. [DOI] [PubMed] [Google Scholar]
- [9].Navis G, Broekroelofs J, Mannes GP, et al. Renal hemodynamics after lung transplantation. A prospective study. Transplantation 1996;61:1600–5. [DOI] [PubMed] [Google Scholar]
- [10].Broekroelofs J, Navis GJ, Stegeman CA, et al. Long-term renal outcome after lung transplantation is predicted by the 1-month postoperative renal function loss. Transplantation 2000;69:1624–8. [DOI] [PubMed] [Google Scholar]
- [11].Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003;349:931–40. [DOI] [PubMed] [Google Scholar]
- [12].Hadem J, Gottlieb J, Seifert D, et al. Prolonged mechanical ventilation after lung transplantation-a single-center study. Am J Transplant 2016;16:1579–87. [DOI] [PubMed] [Google Scholar]
- [13].Wehbe E, Brock R, Budev M, et al. Short-term and long-term outcomes of acute kidney injury after lung transplantation. J Heart Lung Transplant 2012;31:244–51. [DOI] [PubMed] [Google Scholar]
- [14].Zhang JL, Rusinek H, Chandarana H, et al. Functional MRI of the kidneys. J Magn Reson Imaging 2013;37:282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang JL, Morrell G, Rusinek H, et al. New magnetic resonance imaging methods in nephrology. Kidney Int 2014;85:768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Le Bihan D, Breton E, Lallemand D, et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 1988;168:497–505. [DOI] [PubMed] [Google Scholar]
- [17].Hueper K, Rong S, Gutberlet M, et al. T2 relaxation time and apparent diffusion coefficient for noninvasive assessment of renal pathology after acute kidney injury in mice: comparison with histopathology. Invest Radiol 2013;48:834–42. [DOI] [PubMed] [Google Scholar]
- [18].Inoue T, Kozawa E, Okada H, et al. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol 2011;22:1429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Thoeny HC, De Keyzer F, Oyen RH, et al. Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology 2005;235:911–7. [DOI] [PubMed] [Google Scholar]
- [20].Thoeny HC, Zumstein D, Simon-Zoula S, et al. Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: initial experience. Radiology 2006;241:812–21. [DOI] [PubMed] [Google Scholar]
- [21].Blondin D, Lanzman RS, Mathys C, et al. [Functional MRI of transplanted kidneys using diffusion-weighted imaging]. Rofo 2009;181:1162–7. [DOI] [PubMed] [Google Scholar]
- [22].Hagmann P, Jonasson L, Maeder P, et al. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics 2006;26: suppl: S205–23. [DOI] [PubMed] [Google Scholar]
- [23].Notohamiprodjo M, Glaser C, Herrmann KA, et al. Diffusion tensor imaging of the kidney with parallel imaging: initial clinical experience. Invest Radiol 2008;43:677–85. [DOI] [PubMed] [Google Scholar]
- [24].Ries M, Jones RA, Basseau F, et al. Diffusion tensor MRI of the human kidney. J Magn Reson Imaging 2001;14:42–9. [DOI] [PubMed] [Google Scholar]
- [25].Hueper K, Gutberlet M, Rodt T, et al. Diffusion tensor imaging and tractography for assessment of renal allograft dysfunction-initial results. Eur Radiol 2011;21:2427–33. [DOI] [PubMed] [Google Scholar]
- [26].Lanzman RS, Ljimani A, Pentang G, et al. Kidney transplant: functional assessment with diffusion-tensor MR imaging at 3T. Radiology 2013;266:218–25. [DOI] [PubMed] [Google Scholar]
- [27].Cheung JS, Fan SJ, Chow AM, et al. Diffusion tensor imaging of renal ischemia reperfusion injury in an experimental model. NMR Biomed 2010;23:496–502. [DOI] [PubMed] [Google Scholar]
- [28].Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006;145:247–54. [DOI] [PubMed] [Google Scholar]
- [29].Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet 2016. [DOI] [PubMed] [Google Scholar]
- [30].Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017;13:241–57. [DOI] [PubMed] [Google Scholar]
- [31].Hueper K, Khalifa AA, Brasen JH, et al. Diffusion-Weighted imaging and diffusion tensor imaging detect delayed graft function and correlate with allograft fibrosis in patients early after kidney transplantation. J Magn Reson Imaging 2016;44:112–21. [DOI] [PubMed] [Google Scholar]
- [32].Gottlieb J, Neurohr C, Muller-Quernheim J, et al. A randomized trial of everolimus-based quadruple therapy vs standard triple therapy early after lung transplantation. Am J Transplant 2019;19:1759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hueper K, Peperhove M, Rong S, et al. T1-mapping for assessment of ischemia-induced acute kidney injury and prediction of chronic kidney disease in mice. Eur Radiol 2014;24:2252–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


