Abstract
Objectives
The coronavirus disease 2019 (COVID-19) pandemic is seriously threatening public health and setting off huge economic crises across the world. In the absence of specific drugs for COVID-19, there is an urgent need to look for alternative approaches. Therefore, the aim of this paper was to review the roles of micronutrients and bioactive substances as potential alternative approaches in combating COVID-19.
Methods
This review was based on the literature identified using electronic searches in different databases.
Results
Vitamins (A, B, C, D, and E), minerals (selenium and zinc), and bioactive substances from curcumin, echinacea, propolis, garlic, soybean, green tea, and other polyphenols were identified as having potential roles in interfering with spike glycoproteins, angiotensin converting enzyme 2, and transmembrane protease serine 2 at the entry site, and inhibiting activities of papain-like protease, 3 chymotrypsin-like protease, and RNA-dependent RNA polymerase in the replication cycle of severe acute respiratory syndrome coronavirus 2. Having immunomodulating, antiinflammatory, antioxidant, and antiviral properties, such micronutrients and bioactive substances are consequently promising alterative nutritional approaches to combat COVID-19.
Conclusions
The roles of micronutrients and bioactive substances in the fight against COVID-19 are exciting areas of research. This review may suggest directions for further study.
Keywords: COVID-19, SARS-CoV-2, Cytokine storms, Lung injury, Micronutrients, Bioactive substances
Introduction
Outbreaks of coronavirus disease 2019 (COVID-19) infections began in late 2019 in Wuhan, China [1]. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is an enveloped, positive-sense, single-stranded RNA virus that belongs to the genus β-coronavirus [2,3]. SARS-CoV-2 follows the steps of attachment, endocytosis, biosynthesis, maturation, and exocytosis in its replication cycle [4].
A genome-wide phylogenetic analysis indicated that SARS-CoV-2 shares a 79.5% sequence identity with SARS-CoV and 50% with the Middle-East respiratory syndrome coronavirus (MERS-CoV) [3,5,6]. A structural and functional analysis showed that the spike (S) glycoprotein for SARS-CoV-2 bound to angiotensin (Ang) converting enzyme (ACE) 2 receptors [4] with 10- to 20-folds higher affinity than SARS-CoV [7]. SARS-CoV-2 has a basic reproduction number (RO) of 2.2 [1] that doubles the RO of SARS-CoV and MERS-CoV (RO < 1) [8], and suggests that SARS-CoV-2 is extremely contagious. Given the high RO, mutation, and recombination, SARS-CoV-2 infections spread very rapidly and pose a serious threat to public health [6], causing huge economic crises across the world [9].
Many countries have implemented public health measures, including social distancing and lockdowns, to mitigate further spreading of the virus [4]. Nonetheless, thousands of patients with severe cases have been dying every day worldwide due to a lack of specific antiviral drugs and the pressure of clinical treatment [6]. The direct cause of death is generally due to ensuing severe atypical pneumonia [10] as a result of cytokine storms. The early death cases of the COVID-19 outbreak occurred primarily in elderly people, possibly due to their weak immune system that permits a faster progression of the viral infection [1,11].
In the absence of specific drugs for SARS-CoV-2, there is an urgent need to find alternative approaches to prevent and control the spread of the virus. Public health measures that can mitigate the risk of infection and death are desperately required. A recent increase in the popularity of alternative medicine and natural products has renewed interest in micronutrients and bioactive substances as potential alternative approaches. Exploring the repurposing of already studied nutritional interventions for SARS-CoV, MERS-CoV, and other viral infections can provide alternative approaches to combat COVID-19. Therefore, the aim of this paper was to review the potential roles of micronutrients and bioactive substances in combating COVID-19. Our narrative review focuses on micronutrients and bioactive substances with potential effects on replication cycles and complexes, which have potential antiviral properties for SARS-CoV-2 and are capable of boosting host immune systems. In this review, the following questions were addressed in detail: 1) What kind of roles can micronutrients and bioactive substances play at the entry site and in the replication processes of SARS-CoV-2; 2) can micronutrients and bioactive substances play roles in lessening the replication complex of SARS-CoV-2; 3) what are their potential impacts on immune responses to infection with SARS-CoV-2; 4) do they likely mitigate the clinical features of COVID-19; and 5) do they have antiviral properties?
Methods
Literature searches were performed in the Pubmed, Scopus, Embase, CENTRAL, and Google Scholar databases, as well as citation tracking on original research articles between March 26, 2020 and June 25, 2020. The online search was done using a combination of the following keywords: “micronutrients and coronaviruses”; “micronutrients and SARS”; “micronutrients and MERS”; “micronutrients and RNA viruses”; “bioactive substances and coronaviruses”; “bioactive substances and SARS”; “bioactive substances and MERS”; and “bioactive substances and RNA viruses”. In this review, articles were screened based on titles and abstracts, and subsequently included when they met the inclusion criteria. The inclusion criteria included articles defining outcome measures; reporting on antiinflammatory, antioxidant, and antiviral effects, as well as immunologic responses of micronutrients and bioactive substances on SARS CoV-2, SARS, MERS, and other viral infections; and published in English. Unpublished articles were excluded, as well as duplicates, noninterventional studies (e.g., case-control, cross-sectional, cohort, and case report studies, as well as commentaries and letters to editors). Based on the inclusion and exclusion criteria, 351 articles were retrieved. Of these, 58 articles were selected and included in the review processes.
Results and discussion
Pathobiology of SARS-CoV-2
SARS-CoV-2 entry into the host cell
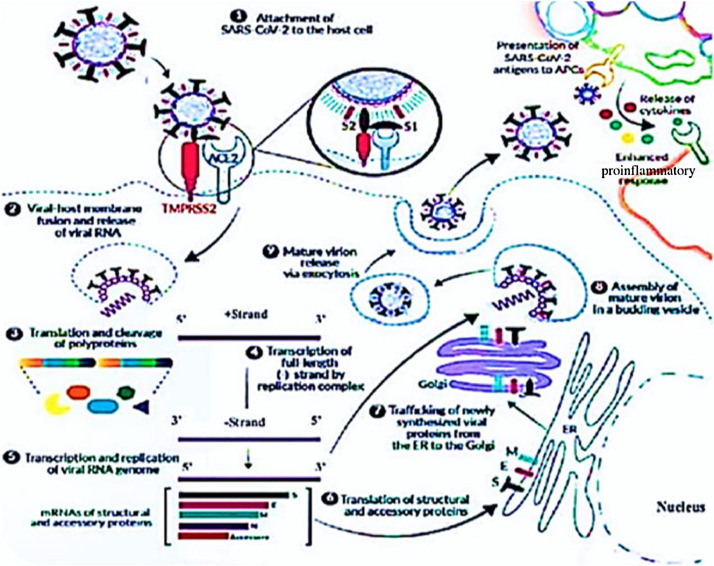
The receptor-binding domain of the virus S attaches to the ACE2 receptor of lung epithelial cells [12,13]. S glycoprotein comprises two functional subunits, S1 and S2, which are responsible for attachment and fusion, respectively [14]. The two subunits are subjected to protease cleavage to be cleaved and primed by transmembrane protease serine 2 (TMPRSS2) and cathepsin L [15,16]. Once the viruses attach to ACE2 receptors, they enter the host cells either through endocytosis or membrane fusion (Fig. 1 ) [4,17].
Fig. 1.
SARS-CoV-2 enters the host cell through the binding of the viral S protein to the host ACE2 receptor. The S protein is cleaved into S1 and S2 by a cell-derived protease. S1 binds to ACE2 and S2 is activated by the host serine protease TMPRSS2 and results in membrane fusion. Once inside, SARS-CoV-2 hijacks the host machinery to transcribe, replicate, and translate its RNA genome and structural proteins before being reassembled, encapsulated, and exocytosed from the cell. SARS-CoV-2 antigens are presented to host APCs, which produce a range of cytokines. The release of cytokines causes an enhanced, unbalanced, and devastating proinflammatory response in the host. Adapted from Review Invivogen (www.invivogen.com). APCs, antigen-presenting cells; ER, Endoplasmic Reticulum; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SARS-CoV-2 replication cycle
Once the virus is endocytosed, its RNA genome is released into the host cell cytoplasm. During the process of replication, the host translational machinery is hijacked for the translation of polyproteins and essential viral proteases [18,19]. The 5’ two-thirds of the genome encodes two polyproteins, pp1a and pp1ab, collectively termed the replicase. These polyproteins are cleaved by 3 chymotrypsin-like protease (3CLpro) and papain-like protease into 16 nonstructural proteins, including RNA-dependent RNA polymerase (RdRp) [3,20]. In the 3’, one-third of the SARS-CoV-2 genome, like other β-coronavirus, encodes four essential structural proteins (S, envelope, matrix/membrane, and nucleocapsid), along with a set of accessory proteins [3,19,20].
The newly synthesized structural and accessory proteins are then trafficked from the endoplasmic reticulum through the Golgi apparatus, after which new virions assemble in budding Golgi vesicles [19]. At the end, the mature SARS-CoV-2 virions are exocytosed and released from the host cell into the surrounding environment to repeat the infection cycle (Fig. 1) [21].
SARS-CoV-2 replication complex
Infection triggers inflammatory responses. Studies have shown that patients with severe disease have increased plasma concentrations of proinflammatory cytokines, such as interleukin (IL) 6, IL-8, IL-10, IL-17, monocyte chemoattractant protein 1, macrophages inflammatory protein 1 alpha, granulocyte-macrophage colony-stimulating factor, interferon (IFN)- γ, and tumor necrosis factor-alpha [22], [23], [24], [25]. Infiltration of many inflammatory cells were observed in patients with a case of severe COVID-19 infection [22,26]. High concentrations of proinflammatory cytokines may lead to cytokine storms. Accumulated evidence suggests that patients with severe COVID-19 infections had cytokine storm syndrome [27].
Cytokine storms induce lung injuries that results in acute respiratory distress syndrome (ARDS), a life-threatening lung disorder [28]. Overactivation of T cells, manifested by increased Th17 levels, and high cytotoxicity of cluster of differentiation (CD) 8 T cells account for, in part, the severe immune injury in patients infected with COVID-19 [22]. Lipopolysaccharide (LPS), a potent proinflammatory molecule, triggers strong inflammatory responses. At the tissue level, LPS causes acute lung injury (ALI)/ARDS by directly or indirectly damaging pulmonary microvascular endothelial cells, resulting in increased alveolar capillary membrane permeability and subsequent pulmonary oedema, refractory hypoxemia, pulmonary hypertension, and intense cellular infiltration, particularly neutrophilic infiltration [29], [30], [31].
Immune responses
Coronaviruses display tropism for epithelial cells of the respiratory or gastrointestinal tract [32]. Respiratory epithelial cells contribute to respiratory health beyond the barrier function and the initiation of immune responses [33]. Epithelial cells, alveolar macrophages, and dendritic cells are the main components for innate immunity [24] and combat viruses in the first line of defense until adaptive immunity gets involved [4].
Studies have demonstrated that cytokines, such as IFN-α/β, are secreted to inhibit viral replication [34], [35], [36]. Neutralizing antibody directed against capsid proteins blocks the initiation of coronavirus infection [32]. The immunity conferred by the infection, apparently immunoglobulin A, is short lived. Combined, the antigenic variability of coronaviruses may contribute to frequent reinfections [37]. Although immunity after infection appears to be brief and reinfection can occur [38], there is no question on having robust immune responses to combat coronavirus infections.
Clinical features
After the incubation period of 2 to 14 d, patients with a COVID-19 infection manifest mild-to-severe respiratory illness, with symptoms including fever, cough, and dyspnea [8,23]. In fact, the signs and symptoms usually vary at the time of the onset of the illness and some patients may experience malaise, headache, sore throat, runny nose, and tachypnea [39], as well as fatigue, anorexia, myalgia, and sputum production over the course of the disease [23]. All patients had pneumonia with abnormal findings on chest computed tomography (CT) scans [23] and several patients had lymphocytopenia at the time of admission [23,40].
Micronutrients
Vitamins
Vitamin A
Vitamin A plays a substantial role in maintaining the integrity of respiratory epithelial cells [33,41] (Table 1 ). The active form of vitamin A, retinoic acid, has protective effects in several respiratory pathologies [42]. Diets low in vitamin A pronounce disease severity, as observed with the infectious bronchitis virus [43], and compromise the effectiveness of vaccines, such as the bovine coronavirus vaccine [43,44]. Chronic vitamin A deficiency has been associated with histopathological changes in the pulmonary epithelial lining [42]. Multiple genes respond to the signals of retinoic acid through transcriptional and nontranscriptional mechanisms [42].
Table 1.
Micronutrients and bioactive substances with immunomodulating, antiinflammatory, antioxidant, and antiviral properties
| Micronutrients and bioactive substances | Targets | Specific effects | Reference |
|---|---|---|---|
| Vitamin A | Immune response | Maintains integrity of respiratory epithelium | [33,41] |
| Has protective effects in many respiratory pathologies | [42] | ||
| Regulates the expression of retinoic acid-inducible gene I and interferon-regulatory factor 1 | [46] | ||
| Riboflavin | Antiviral property | Together with ultraviolet light effectively reduced Middle-East respiratory syndrome coronavirus titer | [55] |
| Nicotinamide | Immune response | Inhibits neutrophil infiltration | [56] |
| Pyridoxine | Immune response | Improves immune responses | [57] |
| Folate | Immune response | Plays crucial role in DNA and protein synthesis in cells | [58] |
| Vitamin B12 | Immune response | Improves high CD4/CD8 ratio and suppresses natural killer cells | [59] |
| Vitamin C | Antioxidant | Protects against reactive oxygen species | [65] |
| Inhibits the production of cytokines storm | [28] | ||
| Immune response | Highly concentrated in phagocytes and lymphocytes, suggesting a physiological role in immune cells | [60] | |
| Supports epithelial barrier function against pathogens | [62] | ||
| Improves immune system activities | [65] | ||
| Increases production of interferon-α/β | [68] | ||
| Antiviral property | Dehydroascorbic acid showed strong antiviral activity | [69] | |
| Vitamin D | Antiinflammatory | Downregulates proinflammatory cytokines (cytokine storm) | [23,78] |
| Increases expression of antiinflammatory cytokines | [83] | ||
| Stimulates T reg cells development | [84] | ||
| Enhances expression of glutathione reductase and glutamate–cysteine ligase | [79,80] | ||
| Immune response | Stimulates maturation of immune cells | [9] | |
| Attenuates lipopolysaccharide-induced acute lung injury by, at least partially, inducing ACE2/Ang 1-7 axis activity and inhibiting renin and the ACE/Ang II/Ang II type 1 receptor cascade | [31] | ||
| Antiviral property | Explained by inducing the release of cathelicidin LL-37 and human b defensin 2 | [76] | |
| Vitamin E | Antioxidant | Reduces oxidative stress through binding to free radicals | [93] |
| Zinc | Replication cycle | Interferes with 3 chymotrypsin-like protease | [106], [107], [108], [109] |
| Antiinflammatory | Inhibits the expression of proinflammatory cytokines, chemokines, acute phase proteins (C-reactive protein and fibrogen) through inhibiting nuclear factor κB signaling | [113,116,117] | |
| Modulation of regulatory T – cell functions that may limit the cytokine storm in coronavirus disease 2019 | [118] | ||
| Immune response | Involves in maintenance and development of innate and adaptive immune system | [119] | |
| Considered as second messenger of immune cells | [113] | ||
| Antiviral property | In combination with pyrithione at low concentration inhibits the replication of severe acute respiratory syndrome coronavirus | [109] | |
| Selenium | Antioxidant | Antioxidant properties of amino acid selenocysteine | [100] |
| Inhibits inflammatory process (cell injury) through Se-dependent glutathione peroxidase | [99] | ||
| Immune response | Induces immune response | [101] | |
| Antiviral property | Diminishes viral mutation and improves the immunocompetence of patients with selenium deficiency | [100] | |
| Anthraquinone emodin | Entry site | Interferes with attachment and fusion of spike protein and ACE2 receptor | [126] |
| Curcumin | Entry site | Interferes with attachment and fusion of spike protein and ACE2 receptor | [125] |
| Antiviral property | Reduces infectivity of virus in dose–time dependent manner | [125] | |
| Echinacea purpurea | Antiinflammatory | Suppresses proinflammatory responses | [150] |
| Antiviral property | Has effect on virus during initial infection and at time of transmission | [150] | |
| Garlic | Replication cycle | Interferes with RNA-dependent RNA polymerase | [148] |
| Antiviral property | Has antiviral activities | [146,147] | |
| Used in management of common cold | [122] | ||
| Ginseng | Immune response | Has immunomodulatory effects | [122,158,159] |
| Green tea (containing EGCG, ECG, and EGC) | Entry site | Inhibits transmembrane protease serine 2 | [134] |
| Antiviral property | Possesses broad range of antiviral spectrum | [135,136] | |
| Has antiviral effect on influenza virus by altering physical properties of viral membrane | [137] | ||
| Nicotianamine | Entry site | Interferes with attachment and fusion of S-protein and ACE2 receptor | [127] |
| Propolis | Antiinflammatory | Antiinflammatory activity | [151,154,155] |
| Antiviral property | Has antiviral activities | [154] | |
| Quercetin | Entry site | Reduces endocytosis | [142] |
| Replication cycle | Interferes with 3 chymotrypsin-like protease | [9,[143], [144], [145] | |
| Antiviral property | Has antiviral activities | [140] | |
| Resveratrol | Antiviral property | Has antiviral activities | [157] |
| Sulforaphane | Entry site | Inhibits transmembrane protease serine 2 | [33,128,129] |
| Antioxidants | Decreases oxidative stress and inflammation | [132] |
ACE, angiotensin converting enzyme; Ang, angiotensin; CD, cluster of differentiation.
The active retinol metabolite, all trans-retinoic acid (ATRA), is responsible for mediating many of the important functions of retinoids, which are the synthetic derivatives and metabolites of vitamin A. ATRA is the natural ligand for retinoic acid receptors (RARs), which form heterodimers with retinoid X receptors within the nucleus [45,46]. RAR-retinoid X receptor heterodimers bind to retinoid acid response elements on the promoters of target genes to activate transcription of these genes when bound by ligand [47]. As indicated by Soye et al. [46], retinoids are implicated in the regulation of the expression of many IFN-stimulated genes, including the retinoic acid-inducible gene I (RIG-I) and IFN-regulatory factor 1 (IRF-1).
RIG-I functions as a cytosolic pathogen recognition receptor [48] and drives immune signaling after binding to pathogen-associated molecular pattern motifs within viral RNA that accumulate during acute infection of many RNA viruses [49]. RIG-I initiates signaling events, resulting in the production of cytokines, such as type I IFNs [50,51]. IFNs have antiviral, antiproliferative, and immunomodulatory activities, and thus play crucial roles in host defenses [52].
IFN has been reported to induce RIG-I expression by causing the IRF-1 transcription factor to bind to the RIG-I promoter [53]. RIG-I has been shown to recognize a variety of RNA viruses, including the measles virus [54]. Soye et al. [46] concluded that ATRA inhibits measles virus replication through the RARα-dependent regulation of RIG-I and IRF-1 and via an IFN feedback loop. Table 2 indicates the dietary sources of vitamin A and other micronutrients and bioactive substances.
Table 2.
Dietary sources for micronutrients and bioactive substances with potential effects on coronavirus disease 2019
| Micronutrients and bioactive substances | Food source | Reference |
|---|---|---|
| Vitamin A | Animal-derived foods (liver, beef, poultry, fish oils); green leafy vegetables (kale, spinach, broccoli); orange and yellow vegetables (carrots, sweet potatoes, pumpkin, squash, tomatoes, red bell pepper); fruits (mango, papaya and melon), and red palm oil | [162], [163], [164], [165] |
| Riboflavin | Milk and dairy products, organ meats, lean meats, eggs, fish, green vegetables, cereals and grain products | [165], [166], [167], [168] |
| Nicotinamide | Meat and meat products, milk and dairy products, fish, legumes, cereals and grains | [165,168] |
| Pyridoxine | Meat and meat products, milk and dairy products, fish, potatoes and other vegetables, cereals and grain, peanuts and soybeans | [164,165,168] |
| Folate | Vegetables, fruits, legumes, beef, liver and eggs, seafoods | [164,165,169] |
| Vitamin B12 | Dairy products (milk, butter, cheese yogurt), eggs, meat, poultry, meat, liver, fish, fermented vegetables | [163,170] |
| Vitamin C | Fresh fruits (oranges, kiwi, lemon, grapefruit, strawberries) and vegetables (broccoli, Brussels sprouts, cabbage, cauliflower, tomatoes, peppers, white potatoes) | [72,163,164] |
| Vitamin D | Sun exposure, cod liver oil, oily fish, sun-exposed mushroom | [163,164,171] |
| Vitamin E | Plant-based oils, sunflower seeds, almonds, peanuts, pumpkin, asparagus, mango, avocado, red bell pepper, wheat germ | [163,164,172] |
| Zinc | Refined diets low in cereal fiber and phytic acid, with adequate protein primarily from meats, fish and dairy products | [162,164] |
| Selenium | Seafood, fish, organ meats, poultry, eggs, dairy products, nuts, beans, lentils, whole-wheat bread | [163], [164], [165] |
| Anthraquinone emodin | Genus Rheum and polygonum | [126] |
| Curcumin extract | Turmeric | [125] |
| Echinacea | Echinacea purpurea (purple coneflower) | [150,151] |
| Garlic extract | Garlic | [173] |
| Ginseng extract | Ginseng | [122] |
| EGCG, ECG, and EGC | Green tea (Camellia sinensis) | [137] |
| Nicotianamine | Soybean | [127] |
| Propolis | Bee products, bee glue | [151,174] |
| Quercetin | Apples, honey, raspberries, onions, red grapes, cherries, tea (Camellia sinensis), citrus fruits, and green leafy vegetables | [175] |
| Resveratrol | Grapes, red wine, peanuts, cocoa, and some berries | [176], [177], [178], [179] |
| Sulforaphane | Broccoli, Brussels sprouts, and cabbage | [33] |
ECG, epicatechin gallate; EGC, epigallocatechin; EGCG, epigallocatechin-3-gallate.
Vitamin B complexes
Vitamin B complexes have an important role in immune system regulation. Riboflavin and ultraviolet light effectively reduced MERS-CoV titer [55]. A study on mice revealed that nicotinamide significantly inhibited neutrophil infiltration into the lungs, but paradoxically led to the development of hypoxemia [56]. Appropriate supplementation of pyridoxine has improved immune responses [57]. Folate plays a crucial role in DNA and protein synthesis, which suggests its role in cellular proliferation [58]. The provision of methyl–vitamin B12 treatment for vitamin B12-deficient patients improved the CD4/CD8 ratio and suppressed natural killer cells [59].
Vitamin C
Vitamin C is believed to inhibit the production of cytokine storms due to COVID-19 infection [28]. A high dose of vitamin C is used to treat ARDS. For instance, Fowler et al. [29] reported that infusing high-dose, intravenous vitamin C into patients with virus-induced ARDS was associated with a rapid resolution of lung injuries with no evidence of post-ARDS fibroproliferative sequelae. Another study indicated that the timely administration of high-dose, intravenous vitamin C improves the outcome of COVID-19 infection [28].
Vitamin C is highly concentrated in phagocytes and lymphocytes, which suggests its physiological role in immune cells [60]. Mucoid surface film of lung alveolae, which contain high vitamin C concentrations, may behave as a defensive barrier [61]. Vitamin C supports epithelial barrier function against pathogens [62]. Ingestion of high-dose ascorbic acid effectively prevents or ameliorates the common cold [63,64].
Vitamin C supplementation improves resistance to infection by improving the immune system [65]. For instance, a study in 1997 indicated that the incidence of pneumonia was significantly decreased as the result of vitamin C supplementation [66], possibly through immune system improvements. Walsh et al. [67] reported that vitamin C supplementation increased chicks’ resistance to infection with the avian coronavirus. Vitamin C is essential in antiviral immune response at an early time of infection, especially against the influenza virus, through an increased production of IFN-α/β [68]. Dehydroascorbic acid, which is an oxidized form of ascorbic acid, showed strong antiviral activity [69]. Vitamin C is widely administered to shorten the duration of illness from the common cold [70,71]. The use of vitamin C reduces runny nose and relieves pain in limbs and muscles [72].
Vitamin D
Lung epithelial cells express high basal levels of CYP27 B1 and low levels of CYP24 A1, favoring the conversion of vitamin D to its active form. Vitamin D stimulates the maturation of immune cells [9] and plays a major role in mediating immune systems in response to infection [73]. Vitamin D upregulates the production of human cathelicidin LL-37 [74] and defensins [75], which have antimicrobial and antiendotoxin activities [74]. The antiviral effects of vitamin D could be explained by cathelicidin LL-37, human β defensin 2, and perhaps through the release of reactive oxygen species [76]. Cathelicidin LL-37 tends to disrupt viral lipid envelopes [76] and appears to be effective in combating septicemia [77].
Sundaram and Coleman [78] indicated that 1α, 25 dihydroxy (OH)2D downregulates proinflammatory cytokines, such as IL-6, IL-8, and tumor necrosis factor-alpha, in different cells in vitro [78]. Vitamin D enhances the expression of genes related to antioxidation, such as glutathione reductase and the glutamate-cysteine ligase modifier subunit [79,80]. The increased production of glutathione spares the use of vitamin C [80], [81], [82]. Vitamin D increases the expression of antiinflammatory cytokines [83] and stimulates the development of T reg cells [84]. The antiinflammatory effect of vitamin D has been carried out in part through nuclear factor κB inhibition [85]. Vitamin D induces IκBα, which is an inhibitor of nuclear factor κB, and results in the reduction of the viral induction of inflammatory genes [86]. The vitamin D receptor, which is the mediator of 1, 25 (OH)2 D activities, is highly expressed in the lungs and involves the protection against sepsis-induced lung injury [31,87]. Shi et al. [88] demonstrated that vitamin D receptor knockout mice experienced a higher severity of LPS-induced ALI. Recently, Biesalski [89] indicated in his review that a low level of vitamin D may contribute to increased activity of the renin-Ang system and subsequent higher blood pressure.
The renin-Ang system, which includes ACE and ACE2, is a complex network that plays a major role in various biological functions, including blood pressure regulation and water balance. ACE cleaves Ang I into Ang II, while ACE2, a homologue of ACE, functions as an endogenous counter-regulator of ACE by hydrolyzing Ang II into Ang 1-7 [31,90]. Upon binding to the Ang II type 1 receptor, Ang II causes vasoconstriction, inflammation, and apoptosis, whereas Ang 1-7 opposes the effects of Ang II by interacting with its own receptor, Mas [31,91. Vitamin D may attenuate LPS-induced ALI by inhibiting nuclear factor κB and the renin-Ang system homolog family member A/Rho kinase signaling pathways [92]. To alleviate injury of the lung, vitamin D at least partially induces ACE2/Ang 1-7 axis activity and inhibits renin and the ACE/Ang II/Ang II type 1 receptor cascade [31].
Vitamin E
Vitamin E plays an important role in reducing oxidative stress [93]. Studies have shown that vitamin E deficiency increases the severity of diseases and causes injury. Beck et al. [94] reported that vitamin E deficiency intensified the myocardial injury of coxsackievirus B3 infection in mice. The virulence of coxsackievirus B3 was increased in mice due to vitamin E and selenium deficiency [95]. Low levels of vitamins E and D exacerbated bovine coronavirus infection in calves [9,96], which suggests that there is an inverse association between the status of vitamin E and viral infection. Thus, improving the status of vitamin E is believed to minimize the deleterious effect of oxidative stress in patients with COVID-19 infection.
Minerals
Selenium
Selenium is an essential trace element for redox biology [97]. Selenoproteins contain selenocysteine, and are involved in antioxidant defense systems [98]. A deficiency of selenium may account for lung injury. Studies have shown that mice fed with a selenium-deficient diet developed much more severe lung injuries than selenium-adequate mice in post-influenza virus infection [99]. This injury may be attributed to the increased expression of proinflammatory cytokines and chemokines. Beck et al. [99] indicated that selenium-dependent glutathione peroxidase may play an important role during influenza-induced inflammatory processes [99]. In concert with vitamin E, selenium prevents the formation of free radicals and oxidative damage to cells and tissues [100].
Selenium has an impact on immune responses. A study on influenza-infected mice revealed that immune responses in the lungs of selenium-deficient mice skewed toward Th2 rather than Th1 responses [99]. Ma et al. [101] reported that selenium in combination with ginseng stem leaf saponins has a synergistic effect on the induction of immune responses to vaccines against the infectious bronchitis virus [101]. A lack of selenium may be related to the mutation of viral genomes [98]. Benign forms of coxsackievirus B3 and influenza A viruses rapidly mutated to virulence forms in hosts with a selenium-deficient status [102,103]. Pandemics of SARS and the influenza A virus originated in biogeochemical selenium-poor regions of China [100,104,105]. On the other hand, selenium supplementation was shown to diminish viral mutation and improve immunocompetence of patients with a selenium deficiency [100].
Zinc
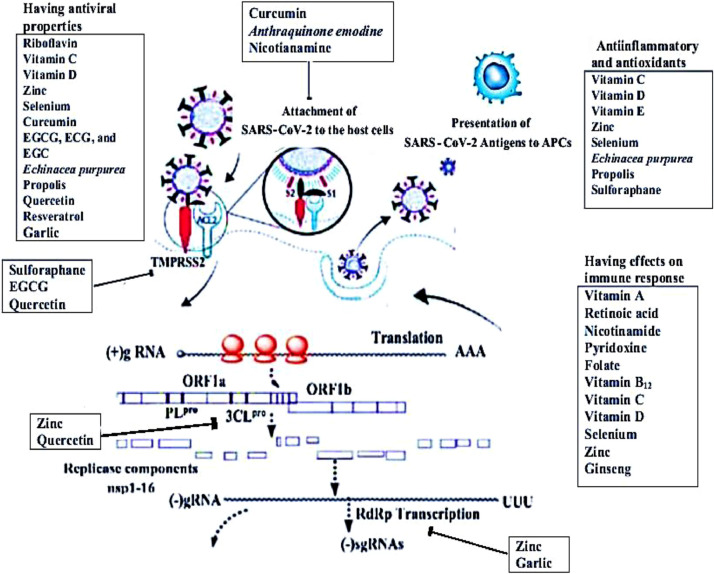
Some studies have revealed that zinc has an impact on the viral replication cycle. For instance, studies on the rhinovirus and poliovirus 3CLpro showed that zinc ions interfered with protease activity [106,107]. Krenn et al. [108] observed the inhibition of polyprotein processing by zinc ions in cells infected with the human rhinovirus and coxsackievirus B3. Zinc ions impaired the replication of RNA viruses by interfering with the proteolytic processing of viral polyproteins (Fig. 2 ) [109]. Uchide et al. [110] suggested the inhibitory effect of zinc ions on RdRp. Zinc ions were noted to inhibit RdRps from the rhinovirus and hepatitis C virus [111,112]. In combination with zinc ionophore pyrithione, zinc ions were shown to inhibit SARS-CoV RdRp activity [109].
Fig. 2.
Potential effects of micronutrients and bioactive substances on COVID-19. Micronutrients and bioactive substances interfere with the attachments of S glycoproteins and ACE2 receptors, 3CLpro, and RdRp transcription. They have antiviral, antiinflammatory, and antioxidant properties and can bolster the immune responses. APCs, antigen-presenting cells; COVID-19, coronavirus disease 2019; ECG, epicatechin gallate; EGC, epigallocatechin; EGCG, epigallocatechin gallate; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Zinc possesses antioxidant and antiinflammatory effects [113]. Prasad et al suggested that zinc reduces oxidative stresses caused by the common cold [114]. When taken early and appropriately, zinc was found to be effective in reducing the duration and severity of the common cold [115]. In addition, zinc can inhibit the expression of proinflammatory cytokines, chemokines, acute phase proteins (C-reactive protein and fibrinogen) and other factors involved in inflammatory responses through inhibiting nuclear factor κB signaling [116,117] and the modulation of regulatory T-cell functions, which may limit cytokine storms in COVID-19 infections [118].
Zinc is considered the second messenger of immune cells [113] due to its importance in developing and maintaining innate and adaptive immune systems [119]. Several randomized trials revealed that zinc has a beneficial effect on treating the common cold, particularly when used during the first 24 h of symptom onset [120], [121], [122]. Zinc supplement given to zinc-deficient children could reduce measles-related morbidity and mortality [123]. In vitro studies revealed that zinc salts were found to inhibit rhinovirus replication, possibly by interfering with rhinovirus cleavage [65]. Zinc in combination with pyrithione at a low concentration inhibits SARS-CoV replication [109]. The administration of zinc gluconate lozenges every 2 h was effective in decreasing the severity and duration of the common cold [124]. Zinc acetate lozenges decreased the total severity scores for all symptoms, and effectively shortened the overall duration of the common cold [113].
Bioactive substances
Bioactive substances from curcumin, echinacea, propolis, garlic, soybean, green tea, and other polyphenols were identified as playing potential roles in combating COVID-19 infection. Curcumin, a component of turmeric, has been used as a food additive and herbal supplement. A study has shown that curcumin interfered with the binding of enveloped viruses to cell surface [125]. Derivatives of curcumin exhibited antiviral activity against enveloped viruses. Direct treatment of a virus with curcumin reduced the infectivity of the virus in a dose–time-dependent manner for enveloped viruses, as well as the vesicular stomatitis virus [125]. Curcumin also exhibited antiviral properties against dengue virus and hepatitis C virus [125].
Anthraquinone emodin, derived from the genus Rheum and polygonum, has the potential to block the interaction of S glycoprotein and ACE2 in a dose-dependent manner [126]. Takahashi et al. [127] identified nicotianamine in soybean and demonstrated its role as a novel ACE2 inhibitor (Fig. 2) [127]. Sulforaphane (SFN), a phytochemical, is commonly found in cruciferous vegetables, such as broccoli, cabbage, and Brussels sprouts (Table 2). Studies have shown that SFN modifies respiratory protease/antiprotease balances that determine susceptibility to viral infection [33,128,129]. The use of SFN increases the secretion of antiprotease-like, secretory, leukocyte, protease inhibitors [130] and decreases TMPRSS2 activity [33,131]. SFN is involved in decreasing oxidative stress and inflammation [132]. Studies have revealed that SFN-containing broccoli sprouts significantly decreased proinflammatory cytokines, such as IL-6, in nasal lavage fluid from subjects inoculated with a live attenuated influenza virus vaccine [133].
Green tea possesses a broad range of antiviral spectrum on both enveloped and nonenveloped viruses [134,135]. Polyphenolic compound catechins, including epigallocatechin gallate (EGCG), epicatechin gallate, and epigallocatechin from green tea, were observed to have an antiviral effect on the influenza virus by altering the physical properties of the viral membrane [136]. EGCG has been shown to induce antiprotease-like, secretory, leukocyte, protease inhibitor secretion, and inhibit TMPRSS2 secretion to protect against viral infection [137]. A study on mice infected with the influenza virus revealed that the oral administration of EGCG had a nearly 50% decrease in viral titers and 50% increase in survival rates [33,138]. The distinct antiviral activities of EGCG were also observed on the Epstein–Barr virus by inhibiting the expression of viral proteins [136,139].
Several studies indicated the antiviral properties of quercetin [140]. Quercetin had effect on influenza A virus infection [141]. Pretreatment of quercetin efficiently reduced influenza A virus endocytosis [142]. Flavonoids, including quercetin 3-β-d-glucose, helichrysetin, herbacetin, rhoifolin, pectolinarin, bioflavonoids, and isobavachalcone, were found to block 3CLpro in patients infected with the coronavirus [9,[143], [144], [145]. Garlic also has antiviral properties [146,147]. Viral RNA polymerase is likely affected by garlic. Allicin from garlic was able to inhibit viral RNA polymerase [148]. Garlic in combination with other herbs was used in the management of the common cold [122,149].
Extracts of Echinacea purpurea, purple coneflower, suppresses proinflammatory responses [150]. Echinacea is generally considered an immune stimulant, and typically used to prevent and treat upper respiratory tract infections [151]. Echinacea has strong antiviral effects against certain viruses [152] depending on the time of application. The administration of echinacea extracts for acute upper respiratory tract infections may be beneficial at early treatment of an existing illness [151]. Echinacea purpurea has an effect on viruses during initial infection and at the time of transmission [150]. Echinacea has been used for several decades to prevent the common cold and the flu [153]. A multiherbal formula (Immumax) containing echinacea extract, garlic powder, Nigella sativa oil, panax ginseng extract, vitamin C, and elemental zinc is helpful to reduce the duration and severity of the common cold [122].
Propolis has been shown to have antiinflammatory [151,154,155] and antiviral activities [154]. A study revealed that propolis had an effect on the influenza virus [151,15]6 and herpes simplex virus type 1 [154,155]. Likewise, resveratrol has antiviral properties. Lin et al. [157] reported that resveratrol influenced the MERS virus. Extracts from ginseng (Panax quinquefolium) were shown to have immunomodulatory effects [122,158,159]. Ginseng extracts effectively prevented acute respiratory illness due to influenza and respiratory syncytial viruses [160]. Herba houttuyniae has been extensively used in symptomatic therapy for pneumonia, fever, sore throat, and cough [161], suggesting its potential use for patients with COVID-19 infection.
Conclusions
Vitamins (A, B, C, D, and E), minerals (selenium and zinc), and bioactive substances from curcumin, echinacea, propolis, garlic, soybean, green tea, and other polyphenols have shown promising effects in interrupting transmission, reducing susceptibility, and ameliorating the severity of SARS-CoV, MERS-CoV, and other viral infections. These micronutrients and bioactive substances play significant roles in interfering with S glycoproteins, ACE2 receptors, and TMPRSS2 at the site of entry, as well as inhibiting activities of papain-like protease, 3CLpro, and RdRp during the replication processes. The effects of these micronutrients and bioactive substances on SARS-CoV propose the same effects on SARS-CoV-2 due to their similarities in the phylogenetic and replication cycle. Having immunomodulating, antiinflammatory, antioxidant, and antiviral properties, all identified micronutrients and bioactive substances can be considered as alternative nutritional approaches in combating COVID-19 infection. The proper use of such nutrients in daily diets can support not only currently existing therapies but also upcoming vaccines and drugs by enhancing their efficacy. The roles of micronutrients and bioactive substances in COVID-19 are exciting areas of research and further studies are needed to substantiate their benefits in combating COVID-19 infection.
References
- 1.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12:372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wrapp D, Wang N, Corbett KS, Corbett KS, Goldsmith JA, Hsieh C. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: A modelling study. Lancet. 2020;395:689–697. doi: 10.1016/S0140-6736(20)30260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song Z, Xu Y, Bao L, Zhang L, Yu P, Qu Y. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11:59. doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS. J Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;180 doi: 10.1016/j.cell.2020.02.052. 271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosch BJ, van der Zee R, de Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein: Structural and functional characterization of the fusion core complex. J Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Central Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehr AR, Perlman S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - An update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshikawa T, Hill T, Li K, Peters CJ, Tseng CT. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian S, Hu W, Niu L, Liu H, Xu H, SY Xiao. Pulmonary pathology of early phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boretti A, Banik BK. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 2020;12 doi: 10.1016/j.phanu.2020.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fowler AA, III, Kim C, Lepler L, Malhotra R, Debesa O, Natarajan R. Intravenous vitamin C as adjunctive therapy for enterovirus/ rhinovirus induced acute respiratory distress syndrome. World J Crit Care Med. 2017;6:85–90. doi: 10.5492/wjccm.v6.i1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su X, Wang L, Song Y, Bai C. Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide. Intensive Care Med. 2004;30:133–140. doi: 10.1007/s00134-003-2001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Yang J, Chen J, Luo Q, Zhang Q, Zhang H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol Med Rep. 2017;16:7432–7438. doi: 10.3892/mmr.2017.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butel JS. Coronaviruses in section of virology. In: Brooks F, Carroll KC, Butel JS, Morse SA, Mietzner TA, editors. Jawetz, Melnick and Adelberg's medical microbiology. 26th Edition. McGraw-Hill; New York, NY: 2013. pp. 613–617. A Lange medical book. [Google Scholar]
- 33.Meyer M, Jaspers I. Respiratory protease/antiprotease balance determines susceptibility to viral infection and can be modified by nutritional antioxidants. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1189–L1201. doi: 10.1152/ajplung.00028.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner RB, Felton A, Kosak K, Kelsey DK, Meschievitz CK. Prevention of experimental coronavirus colds with intranasal alpha-2b interferon. J Infect Dis. 1986;154:443–447. doi: 10.1093/infdis/154.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pei J, Sekellick MJ, Marcus PI, Choi IS, Collisson EW. Chicken interferon type I inhibits infectious bronchitis virus replication and associated respiratory illness. J Interferon Cytokine Res. 2001;21:1071–1077. doi: 10.1089/107999001317205204. [DOI] [PubMed] [Google Scholar]
- 36.Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J., Jr Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326:905–908. doi: 10.1016/j.bbrc.2004.11.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bienz KA. Coronaviruses in section of virology. In: Kayser FH, Bienz KA, Eckert J, Zinkernagel RM, editors. Medical microbiology. Thieme; New York, NY: 2005. pp. 446–448. [Google Scholar]
- 38.Levinson W. Review of Medical Microbiology and Immunology. 14th Edition. McGraw; New York NY: 2016. Coronaviruses in chapter of RNA enveloped viruses; pp. 323–324. A Lange medical book. [Google Scholar]
- 39.Tabata S, Imai K, Kawano S, Ikeda M, Kodama T, Miyoshi K. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: A retrospective analysis. Lancet Infect Dis. 2020;20:1043–1050. doi: 10.1016/S1473-3099(20)30482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan W, Ni Z, Hu Y, Liang W, Ou C, He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biesalski HK, Nohr D. Importance of vitamin – A for lung function and development. Mol Aspects Med. 2003;24:431–440. doi: 10.1016/s0098-2997(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 42.Timoneda J, Rodríguez-Fernández L, Zaragozá R, Marín MP, Cabezuelo MT, Torres L. Vitamin A deficiency and the lung. Nutrients. 2018;10:1132. doi: 10.3390/nu10091132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West CE, Sijtsma SR, Kouwenhoven B, Rombout JH, van der Zijpp AJ. Epithelia-damaging virus infections affect vitamin A status in chickens. J Nutr. 1992;122:333–339. doi: 10.1093/jn/122.2.333. [DOI] [PubMed] [Google Scholar]
- 44.Jee J, Hoet AE, Azevedo MP, Vlasova AN, Loerch SC, Pickworth CL. Effects of dietary vitamin A content on antibody responses of feedlot calves inoculated intramuscularly with an inactivated bovine coronavirus vaccine. Am J Vet Res. 2013;74:1353–1362. doi: 10.2460/ajvr.74.10.1353. [DOI] [PubMed] [Google Scholar]
- 45.Germain P, Chambon P, Eichele G, Evans RM, Lazar MA, Leid M. Retinoic acid receptors. Pharmacol Rev. 2006;58:712–725. doi: 10.1124/pr.58.4.4. [DOI] [PubMed] [Google Scholar]
- 46.Soye KJ, Trottier C, Richardson CD, Ward BJ, Jr, Miller WH. RIG-I is required for the inhibition of measles virus by retinoids. PLoS One. 2011;6:e22323. doi: 10.1371/journal.pone.0022323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–630. doi: 10.1002/neu.20242. [DOI] [PubMed] [Google Scholar]
- 48.Loo YM, Gale MJR. Immune signaling by RIG-I-like receptors. Immunity. 2011;34:680–692. doi: 10.1016/j.immuni.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kell A, Stoddard M, Li H, Marcotrigiano J, Shaw GM, Gale MJR. Pathogen-associated molecular pattern recognition of hepatitis C virus transmitted/founder variants by RIG-I is dependent on U-core length. J Virol. 2015;89:11056–11068. doi: 10.1128/JVI.01964-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 51.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 52.Meurs EF, Breiman A. The interferon inducing pathways and the hepatitis C virus. World J Gastroenterol. 2007;13:2446–2454. doi: 10.3748/wjg.v13.i17.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su ZZ, Sarkar D, Emdad L, Barral PM, Fisher PB. Central role of interferon regulatory factor-1 (IRF-1) in controlling retinoic acid inducible gene- I (RIG-I) expression. J Cell Physiol. 2007;213:502–510. doi: 10.1002/jcp.21128. [DOI] [PubMed] [Google Scholar]
- 54.Plumet S, Herschke F, Bourhis JM, Valentin H, Longhi S, Gerlier D. Cytosolic 59-triphosphate ended viral leader transcript of measles virus as activator of the RIG I-mediated interferon response. PLoS One. 2007;2:e279. doi: 10.1371/journal.pone.0000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keil SD, Bowen R, Marschner S. Inactivation of Middle East respiratory syndrome coronavirus (MERS-CoV) in plasma products using a riboflavin-based and ultraviolet light-based photochemical treatment. Transfusion. 2016;56:2948–2952. doi: 10.1111/trf.13860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones HD, Yoo J, Crother TR, Kyme P, Ben-Shlomo A, Khalafi R. Nicotinamide exacerbates hypoxemia in ventilator-induced lung injury independent of neutrophil infiltration. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qian B, Shen S, Zhang J, Jing P. Effects of vitamin B6 deficiency on the composition and functional potential of T cell populations. J Immunol Res. 2017;2017 doi: 10.1155/2017/2197975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dhur A, Galan P, Hercberg S. Folate status and the immune system. Prog Food Nutr Sci. 1991;15:43–60. [PubMed] [Google Scholar]
- 59.Tamura J, Kubota K, Murakami H, Sawamura M, Matsushima T., Tamura T. Immunomodulation by vitamin B12: Augmentation of CD8þ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol. 1999;116:28–32. doi: 10.1046/j.1365-2249.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Washko PW, Wang Y, Levine M. Ascorbic acid recycling in human neutrophils. J Biol Chem. 1993;268:5531–5535. [PubMed] [Google Scholar]
- 61.Atherton JG, Kratzing CC, Fisher A. The effect of ascorbic acid on infection chick-embryo ciliated tracheal organ cultures by coronavirus. Arch Virol. 1978;56:195–199. doi: 10.1007/BF01317848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pauling L. Freeman; San Francisco, CA: 1970. Vitamin C and common cold. [Google Scholar]
- 64.Dunitz JD. Pauling LC. Biographical memoirs of fellows of the Royal Society. 28 February 1901–19 August 1994. 1996;42:316–8. [DOI] [PubMed]
- 65.Wintergerst ES, Maggini S, Hornig DH. Immune enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50:85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 66.Hemila H. Vitamin C intake and susceptibility to pneumonia. Pediatr Infect Dis J. 1997;16:836–837. doi: 10.1097/00006454-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Walsh TJ, Goodman JL, Pappas P, Bekersky I, Buell DN, Roden M. Safety, tolerance and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: Maximum tolerated dose study. Antimicrob Agents Chemother. 2001;45:3487–3496. doi: 10.1128/AAC.45.12.3487-3496.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim Y, Kim H, Bae S, Choi J, Lim SY, Lee N. Vitamin C is an essential factor on the anti-viral immune responses through the production of interferon-α/β at the initial stage of influenza A virus (H3N2) infection. Immune Network. 2013;13:70–74. doi: 10.4110/in.2013.13.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furuya A, Uozaki M, Yamasaki H, Arakawa T, Arita M, Koyama AH. Antiviral effects of ascorbic and dehydroascorbic acids in vitro. Int J Mol Med. 2008;22:541–545. [PubMed] [Google Scholar]
- 70.Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heimer KA, Hart AM, Martin LG, Rubio-Wallace S. Examining the evidence for the use of vitamin C in the prophylaxis and treatment of the common cold. J Am Assoc Nurse Prac. 2009;21:295–300. doi: 10.1111/j.1745-7599.2009.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ran L, Zhao W, Wang J, Wang H, Zhao Y, Tseng Y. Extra dose of vitamin C based on a daily supplementation shortens the common cold: A meta-analysis of 9 randomized controlled trials. BioMed Res Int. 2018;2018 doi: 10.1155/2018/1837634. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Bikle DD. Vitamin D and immune function: understanding common pathways. Curr Osteoporos Rep. 2009;7:58–63. doi: 10.1007/s11914-009-0011-6. [DOI] [PubMed] [Google Scholar]
- 74.Grant WB, Giovannucci E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918–1919 influenza pandemic in the United States. Dermatoendocrinol. 2009;1:215–219. doi: 10.4161/derm.1.4.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laaksi I. Vitamin D and respiratory infection in adults. Proc Nutr Soc. 2012;71:90–97. doi: 10.1017/S0029665111003351. [DOI] [PubMed] [Google Scholar]
- 76.Beard JA, Allison B, Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50:194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mookherjee N, Rehaume LM, Hancock RE. Cathelicidins and functional analogues as antisepsis molecules. Expert Opin Ther Targets. 2007;11:993–1004. doi: 10.1517/14728222.11.8.993. [DOI] [PubMed] [Google Scholar]
- 78.Sundaram ME, Coleman LA. Vitamin D and influenza. Adv Nutr. 2012;3:517–525. doi: 10.3945/an.112.002162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lei GS, Zhang C, Cheng BH, Lee CH. Mechanisms of action of vitamin D as supplemental therapy for pneumocystis pneumonia. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01226-17. e01226–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mousavi S, Bereswill S, Heimesaat MM. Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbiol Immunol. 2019;9:73–79. doi: 10.1556/1886.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Colunga Biancatelli RML, Berrill M, Marik PE. The antiviral properties of vitamin C. Expert Rev Anti Infect Ther. 2020;18:99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- 83.Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system-Working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M. 1,25-dihydroxyvitamin D3 and IL2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gruber-Bzura BM. Vitamin D and influenza—Prevention or therapy? Int J Mol Sci. 2018;19:2419. doi: 10.3390/ijms19082419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunnighakw GW. Vitamin D decreases respiratory syncytial virus induction of NF-kB chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kong J, Zhu X, Shi Y, Liu T, Chen Y, Bhan I. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol Endocrinol. 2013;27:2116–2125. doi: 10.1210/me.2013-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi YY, Liu TJ, Fu JH, Xu W, Wu LL, Hou AN. Vitamin D/VDR signaling attenuates lipopolysaccharideinduced acute lung injury by maintaining the integrity of the pulmonary epithelial barrier. Mol Med Rep. 2016;13:1186–1194. doi: 10.3892/mmr.2015.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Biesalski HK. Vitamin D deficiency and co-morbidities in COVID-19 patients – A fatal relationship? NFS J. 2020;20:10–21. [Google Scholar]
- 90.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 91.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin 1-7 and Mas: New players of the renin-angiotensin system. J Endocrinol. 2013;216:R1–17. doi: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 92.Zhang M, Dong M, Liu W, Wang L, Luo Y, Li Z. 1α,25dihydroxyvitamin D3 ameliorates seawater aspiration-induced acute lung injury via NFκB and RhoA/Rho kinase pathways. PLoS One. 2014;9 doi: 10.1371/journal.pone.0104507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galmes S, Serra F, Palou A. Vitamin E metabolic effects and genetic variants: A challenge for precision nutrition in obesity and associated disturbances. Nutrients. 2018;10:1919. doi: 10.3390/nu10121919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beck MA, Kolbeck PC, Rohr LH, Shi Q, Morris VC, Levander OA. Vitamin E deficiency intensifies the myocardial injury of coxsackievirusB3 infection of mice. J Nutr. 1994;124:345–358. doi: 10.1093/jn/124.3.345. [DOI] [PubMed] [Google Scholar]
- 95.Beck MA. Increased virulence of coxsackievirus B3 in mice due to vitamin E or selenium deficiency. J Nutr. 1997;127:966S–970S. doi: 10.1093/jn/127.5.966S. [DOI] [PubMed] [Google Scholar]
- 96.Nonnecke BJ, McGill JL, Ridpath JF, Sacco RE, Lippolis JD, Reinhardt TA. Acute phase response elicited by experimental bovine diarrhea virus (BVDV) infection is associated with decreased vitamin D and E status of vitamin-replete preruminant calves. J Dairy Sci. 2014;97:5566–5579. doi: 10.3168/jds.2014-8293. [DOI] [PubMed] [Google Scholar]
- 97.Rayman MP. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 98.Guillin OM, Vindry C, Ohlmann T, Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11:2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beck MA, Nelson HK, Shi Q, Dael PV, Schiffrin EJ, Blum S. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001;15:1481–1483. [PubMed] [Google Scholar]
- 100.Harthill M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol Trace Elem Res. 2011;143:1325–1336. doi: 10.1007/s12011-011-8977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma X, Bi S, Wang Y, Chi X, Hu S. Combined adjuvant effect of ginseng stem leaf saponins and selenium on immune responses to a live bivalent vaccine of Newcastle disease virus and infectious bronchitis virus in chickens. Poult Sci. 2019;98:3548–3556. doi: 10.3382/ps/pez207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beck MA, Shi Q, Morris VC, Levander OA. Rapid genomic evolution of a non-virulent Coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med. 1995;1:433–436. doi: 10.1038/nm0595-433. [DOI] [PubMed] [Google Scholar]
- 103.Nelson HK, Shi Q, van Dael P, Schiffrin EJ, Blum S, Barclay D. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J. 2001;15:1846–1848. [PubMed] [Google Scholar]
- 104.Alfthan G, Xu GL, Tan WH, Aro A, Wu J, Yang YX. Selenium supplementation of children in a selenium-deficient area in China: Blood selenium levels and glutathione peroxidase activities. Biol Trace Elem Res. 2000;73:113–125. doi: 10.1385/BTER:73:2:113. [DOI] [PubMed] [Google Scholar]
- 105.Xia Y, Hill KE, Byrne DW, Xu J, Burk RF. Effectiveness of selenium supplements in a low-selenium area of China. Am J Clin Nutr. 2005;81:829–834. doi: 10.1093/ajcn/81.4.829. [DOI] [PubMed] [Google Scholar]
- 106.Baum EZ, Bebernitz GA, Palant O, Mueller T, Plotch SJ. Purification, properties, and mutagenesis of poliovirus 3C protease. Virol. 1991;165:140–150. doi: 10.1016/0042-6822(91)90762-z. [DOI] [PubMed] [Google Scholar]
- 107.Cordingley MG, Register RB, Callahan PL, Garsky VM, Colonno RJ. Cleavage of small peptides in vitro by human rhinovirus 14 3C protease expressed in Escherichia coli. J Virol. 1989;63:5037–5045. doi: 10.1128/jvi.63.12.5037-5045.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krenn BM, Gaudernak E, Holzer B, Lanke K, Van Kuppeveld FJM, Seipelt J. Antiviral activity of the zinc ionophores pyrithione and hinokitiol against picornavirus infections. J Virol. 2009;83:58–64. doi: 10.1128/JVI.01543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.te Velthuis AJW, van den Worm SHE, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uchide N, Ohyama K, Bessho T, Yuan B, Yamakawa T. Effect of antioxidants on apoptosis induced by influenza virus infection: Inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res. 2002;56:207–217. doi: 10.1016/s0166-3542(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 111.Hung M, Gibbs CS, Tsiang M. Biochemical characterization of rhinovirus RNA-dependent RNA polymerase. Antiviral Res. 2002;56:99–114. doi: 10.1016/s0166-3542(02)00101-8. [DOI] [PubMed] [Google Scholar]
- 112.Ferrari E, Wright-Minogue J, Fang JWS, Baroudy BM, Lau JY, Hong Z. Characterization of soluble hepatitis C virus RNA-dependent RNA polymerase expressed in Escherichia coli. J Virol. 1999;73:1649–1654. doi: 10.1128/jvi.73.2.1649-1654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prasad AS. Discovery of human zinc deficiency: Its impact on human health and disease. Adv Nutr. 2013;4:176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Prasad AS, Bao B, Beck FWJ, Kucuk O, Sarkar FH. Antioxidant effect of zinc in humans. Free Radic Biol Med. 2004;37:1182–1190. doi: 10.1016/j.freeradbiomed.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 115.Singh M, Das R. Zinc for the common cold. Cochrane Database Syst Rev. 2011;2:1–58. doi: 10.1002/14651858.CD001364.pub3. [DOI] [PubMed] [Google Scholar]
- 116.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1 doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hayden MS, Ghosh S. Regulation of NF-kappaB by TNF family cytokines. Semin Immunol. 2014;26:253–266. doi: 10.1016/j.smim.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Skalny AV, Rink L, Ajsuvakova OP, Aschner M, Gritsenko VA, Alekseenko SI. Zinc and respiratory tract infections: Perspectives for COVID‑19 (review) Int J Mol Med. 2020;46:17–26. doi: 10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maares M, Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys. 2016;611:58–65. doi: 10.1016/j.abb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 120.Petrus EJ, Lawson KA, Bucci LR, Blum K. Randomized, doublemasked, placebo-controlled clinical study of the effectiveness of zinc acetate capsules on common cold symptoms in allergy-tested subjects. Curr Ther Res. 1998;59:595–607. doi: 10.1016/S0011-393X(98)85058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Godfrey JC, Conant Sloane B, Smith DS, Turco JH, Mercer N, Godfrey NJ. Zinc gluconate and the common cold: A controlled clinical study. J Int Med Res. 1992;20:234–246. doi: 10.1177/030006059202000305. [DOI] [PubMed] [Google Scholar]
- 122.Yakoot M, Salem A. Efficacy and safety of a multiherbal formula with vitamin C and zinc (Immumax) in the management of the common cold. Int J Gen Med. 2011;4:45–51. doi: 10.2147/IJGM.S16266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Awotiwon AA, Oduwole O, Sinha A, Okwundu CI. Zinc supplementation for the treatment of measles in children. Cochrane Database Syst Rev. 2017;6 doi: 10.1002/14651858.CD011177.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Eby GA, Davis DR, Halcomb WW. Reduction in duration of common cold by zinc gluconate lozenges in a double-blind study. Antimicrob Agents Chemother. 1984;25:20–24. doi: 10.1128/aac.25.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mounce BC, Cesaro T, Carrau L, Vallet T, Vignuzzi M. Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding. Antivir Res. 2017;142:148e157. doi: 10.1016/j.antiviral.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 126.Ho TY, Wu SL, Chen JC, Li CC, Hsiang CY. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Takahashi S, Yoshiya T, Yoshizawa-Kumagaye K, Sugiyama T. Nicotianamine is a novel angiotensin-converting enzyme 2 inhibitor in soybean. Biomed Res. 2015;36:219–224. doi: 10.2220/biomedres.36.219. [DOI] [PubMed] [Google Scholar]
- 128.Fahey JW, Talalay P. Antioxidant functions of sulforaphane: A potent inducer of phase II detoxication enzymes. Food Chem Toxicol. 1999;37:973–979. doi: 10.1016/s0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 129.Kim JK, Park SU. Current potential health benefits of sulforaphane. EXCLI J. 2016;15:571–577. doi: 10.17179/excli2016-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meyer M, Kesic MJ, Clarke J, Ho E, Simmen RC, Diaz-Sanchez D. Sulforaphane induces SLPI secretion in the nasal mucosa. Respir Med. 2012;107:472–475. doi: 10.1016/j.rmed.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gibbs A, Schwartzman J, Deng V, Alumkal J. Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. Proc Natl Acad Sci U S A. 2009;106:16663–16668. doi: 10.1073/pnas.0908908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev. 2012;32:687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Noah TL, Zhang H, Zhou H, Glista-Baker E, Muller L, Bauer RN. Effect of broccoli sprouts on nasal response to live attenuated influenza virus in smokers: A randomized, double-blind study. PLoS One. 2014;9:e98671. doi: 10.1371/journal.pone.0098671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eterpi M, McDonnell G, Thomas V. Disinfection efficacy against parvoviruses compared with reference viruses. J Hosp Infect. 2009;73:64–70. doi: 10.1016/j.jhin.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 135.Lee YH, Jang YH, Kim Y, Kim J, Seong BL. Evaluation of green tea extract as a safe personal hygiene against viral infections. J Biol Eng. 2018;12:1. doi: 10.1186/s13036-017-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Song J, Lee K, Seong B. Antiviral effect of catechins in green tea on influenza virus. Antivir Res. 2005;68:66–74. doi: 10.1016/j.antiviral.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 137.Kesic MJ, Meyer M, Bauer R, Jaspers I. Exposure to ozone modulates human airway protease/antiprotease balance contributing to increased influenza A infection. PLoS One. 2012;7:e35108. doi: 10.1371/journal.pone.0035108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ling JX, Wei F, Li N, Li JL, Chen LJ, Liu YY. Amelioration of influenza virus-induced reactive oxygen species formation by epigallocatechin gallate derived from green tea. Acta Pharmacol Sin. 2012;33:1533–1541. doi: 10.1038/aps.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang LK, Wei TT, Chiu YF, Tung CP, Chuang JY, Hung SK. Inhibition of Epstein–Barr virus lytic cycle by (−)-epigallocatechin gallate. Biochem Biophys Res Commun. 2003;301:1062–1068. doi: 10.1016/s0006-291x(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 140.Choi HJ, Song JH, Park KS, Kwon DH. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur J Pharm Sci. 2009;37:329–333. doi: 10.1016/j.ejps.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 141.Uchide N, Toyoda H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Mol. 2011;16:2032–2052. doi: 10.3390/molecules23100000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vaidya B, Cho SY, Oh KS, Kim SH, Kim YO, Jeong EH. Effectiveness of periodic treatment of quercetin against influenza A virus H1N1 through modulation of protein expression. J Agric Food Chem. 2016;64:4416–4425. doi: 10.1021/acs.jafc.6b00148. [DOI] [PubMed] [Google Scholar]
- 143.Jo S, Kim S, Shin DH, Kim MS. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jo S, Kim H, Kim S, Shin DH, Kim MS. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem Biol Drug Des. 2019;94:2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ryu YB, Jeong HJ, Kim JH, Kim YM, Park JY, Kim D. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL (pro) inhibition. Bioorg Med Chem. 2010;18:7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weber ND, Andersen DO, North JA, Murray BK, Lawson LD, Hughes BG. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992;58:417–423. doi: 10.1055/s-2006-961504. [DOI] [PubMed] [Google Scholar]
- 147.Bayan L, Koulivand PH, Gorji A. Garlic: A review of potential therapeutic effects. Avicenna J Phytomed. 2014;4:1–14. [PMC free article] [PubMed] [Google Scholar]
- 148.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–129. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 149.Josling P. Preventing the common cold with a garlic supplement: A double-blind, placebo-controlled survey. Adv Ther. 2001;18:189–193. doi: 10.1007/BF02850113. [DOI] [PubMed] [Google Scholar]
- 150.Hudson JB. Applications of the phytomedicine echinacea purpurea (purple coneflower) in infectious diseases. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/769896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cohen HA, Varsano I, Kahan E, Sarrell EM, Uziel Y. Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin C in preventing respiratory tract infections in children. A randomized, double-blind, placebo-controlled, multicenter study. Arch Pediatr Adolesc Med. 2004;158:217–221. doi: 10.1001/archpedi.158.3.217. [DOI] [PubMed] [Google Scholar]
- 152.Jawad M, Schoop R, Suter A, Klein P, Eccles R. Safety and efficacy profile of echinacea purpurea to prevent common cold episodes: A randomized, double-blind, placebo-controlled trial. Evid Based Complementary Altern Med. 2012;2012 doi: 10.1155/2012/841315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Foster S. Healing Arts Press; Rochester, VT: 1991. Echinacea nature's immune enhancer. [Google Scholar]
- 154.Amoros M, Simeos CM, Girre L, Sauager F, Cormier M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture: Comparison with the antiviral activity of propolis. J Nat Prod. 1992;55:1732–1740. doi: 10.1021/np50090a003. [DOI] [PubMed] [Google Scholar]
- 155.Dumitrescu M, Crisan I, Esanu V. Mechanism of the anti-herpetic activity of aqueous extract of propolis, II: Activity of lectins from the aqueous extract of propolis. Rom J Virol. 1993;441:49–54. [PubMed] [Google Scholar]
- 156.Serkedja J, Manolova N, Bankova V. Anti-influenza virus effect of some propolis constituents and their analogue esters of substitute cinnamic acids. J Nat Prod. 1992;55:294–302. doi: 10.1021/np50081a003. [DOI] [PubMed] [Google Scholar]
- 157.Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17:144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang M, Guilbert LJ, Ling L, Li J, Wu Y, Xu S. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium) J Pharm Pharmacol. 2001;53:1515–1523. doi: 10.1211/0022357011777882. [DOI] [PubMed] [Google Scholar]
- 159.Lee YS, Chung IS, Lee IR, Kim KH, Hong WS, Yun YS. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginseng isolated from Panax ginseng. Anticancer Res. 1997;17:323–331. [PubMed] [Google Scholar]
- 160.McElhaney JE, Gravenstein S, Cole S, Davidson E, O'neill D, Petitjean S. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J Am Geriatr Soc. 2004;52:13–19. doi: 10.1111/j.1532-5415.2004.52004.x. [DOI] [PubMed] [Google Scholar]
- 161.Li S, Wang R, Zhang Y, Zhang X, Layon AJ, Li Y. Symptom combinations associated with outcome and therapeutic effects in a cohort of cases with SARS. Am J Chinese Med. 2006;34:937–947. doi: 10.1142/S0192415X06004417. [DOI] [PubMed] [Google Scholar]
- 162.Dietary Reference Intakes . National Academies Press; Washington, DC: 2001. Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc: A report of the Panel on Micronutrients, Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. [Google Scholar]
- 163.Food and Agriculture Organization of the United Nations Human vitamin and mineral requirements. Report of a joint FAO/WHO expert consultation Bangkok; Thailand; 2001. Available at: Accessed July 25, 2020. [Google Scholar]
- 164.National Health Service. Vitamins and minerals. Available at: https://www.nhs.uk/conditions/vitamins-and-minerals/vitamin-b/. Accessed July 25, 2020.
- 165.National Institutes of Health. Office of Dietary supplement. Available at: https://ods.od.nih.gov/factsheets/Riboflavin-Consumer/. Accessed July 25, 2020.
- 166.Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;77:1352–1360. doi: 10.1093/ajcn/77.6.1352. [DOI] [PubMed] [Google Scholar]
- 167.Pinto JT, Zempleni J. Riboflavin. Adv Nutr. 2016;7:973–975. doi: 10.3945/an.116.012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mielgo-Ayuso J, Aparicio-Ugarriza R, Olza J, Aranceta-Bartrina J, Gil Á, Ortega RM. Dietary intake and food sources of niacin, riboflavin, thiamin and vitamin B6 in a representative sample of the Spanish population. The ANIBES study. Nutrients. 2018;10:846. doi: 10.3390/nu10070846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dietary Reference Intakes . National Academies Press; Washington, DC: 1998. Thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. [PubMed] [Google Scholar]
- 170.Vogiatzoglou A, Smith AD, Nurk E, Berstad P, Drevon CA, Ueland PM. Dietary sources of vitamin B-12 and their association with plasma vitamin B-12 concentrations in the general population: The Hordaland homocysteine study. Am J Clin Nutr. 2009;89:1078–1087. doi: 10.3945/ajcn.2008.26598. [DOI] [PubMed] [Google Scholar]
- 171.Keflie TS, Nölle N, Lambert C, Nohr D, Biesalski HK. Impact of the natural resource of UVB on the content of vitamin D2inoyster mushroom (Pleurotus ostreatus) under subtropical settings. Saudi J Biol Sci. 2019;26:1724–1730. doi: 10.1016/j.sjbs.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bellizzi MC, Franklin MF, Duthie GG, James WPT. Vitamin E and coronary heart disease: The European paradox. Eur J Clin Nutr. 1994;48:822–831. [PubMed] [Google Scholar]
- 173.Agarwal KC. Therapeutic actions of garlic constituents. Med Res Rev. 1996;16:111–124. doi: 10.1002/(SICI)1098-1128(199601)16:1<111::AID-MED4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 174.Castaldo S, Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73:S1–S6. doi: 10.1016/s0367-326x(02)00185-5. [DOI] [PubMed] [Google Scholar]
- 175.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 176.Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 177.Rimando AM, Kalt W, Magee JB, Dewey J, Ballington JR. Resveratrol, pterostilbene, and piceatannol in vaccinium berries. J Agric Food Chem. 2004;52:4713–4719. doi: 10.1021/jf040095e. [DOI] [PubMed] [Google Scholar]
- 178.Sanders TH, McMichael RW, Jr, Hendrix KW. Occurrence of resveratrol in edible peanuts. J Agric Food Chem. 2000;48:1243–1246. doi: 10.1021/jf990737b. [DOI] [PubMed] [Google Scholar]
- 179.Hurst WJ, Glinski JA, Miller KB, Apgar J, Davey MH, Stuart DA. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J Agric Food Chem. 2008;56:8374–8378. doi: 10.1021/jf801297w. [DOI] [PubMed] [Google Scholar]