Abstract
Genitourinary syndrome of menopause (GSM) is a chronic and progressive condition with a series of vulvovaginal, sexual, and lower urinary tract discomforts, mainly due to hypoestrogenism. Menopausal hormone therapy (MHT) has generally been considered as the most effective treatment for GSM. In addition, vaginal microbiota is of particular significance to gynecological and reproductive illnesses and potentially has some intimate connections with GSM. Consequently, we sought to evaluate how MHT impacts the composition and structure of vaginal microbiota while alleviating GSM in Chinese menopausal women aged 45–65 years, which has not been investigated previously. 16S rRNA gene sequencing was performed to analyze microbial diversity and composition using vaginal swabs obtained from 100 menopausal women, classified as MHT women who have been taking tibolone regularly (n = 50) and non-treated women who never received any treatment (n = 50). Vaginal Health Index Score (VHIS) and GSM symptoms inquiry were also performed. We found that the vaginal microbial diversity decreased and that the abundance of Lactobacillus increased to be the dominant proportion significantly in the MHT group, in considerable contrast to vaginal microbiota of the non-treated group, which significantly comprised several anaerobic bacteria, namely, Gardnerella, Prevotella, Escherichia-Shigella, Streptococcus, Atopobium, Aerococcus, Anaerotruncus, and Anaerococcus. In this study, women without any MHT had significantly more severe GSM symptoms than those receiving tibolone, especially with regard to vulvovaginal dryness and burning, as well as decreased libido (P < 0.01). However, there was no significant difference in the severity of urological symptoms between the groups (P > 0.05). Furthermore, Lactobacillus was demonstrated to be associated with VHIS positively (r = 0.626, P < 0.001) and with GSM negatively (r = −0.347, P < 0.001). We also identified Chlamydia (r = 0.277, P < 0.01) and Streptococcus (r = 0.270, P < 0.01) as having a prominent association with more serious GSM symptoms. Our study provided an elucidation that MHT could notably alleviate GSM and conspicuously reshape the composition of the vaginal microbiota, which is of extreme importance to clinical practice for the management of GSM.
Keywords: menopause, vaginal microbiota, genitourinary syndrome of menopause, menopausal hormone therapy, 16S rRNA gene sequencing, tibolone
Introduction
Genitourinary syndrome of menopause (GSM), previously known as vulvovaginal atrophy or atrophic vaginitis, is reported in approximately half of perimenopausal and postmenopausal women worldwide (Portman and Gass, 2014; Geng et al., 2018) and characterized by bothersome disorders interfering with quality of life. It is a collection of symptoms and signs associated with low circulating estrogen levels, commonly including vaginal dryness; dyspareunia; frequent, urgent, and painful urination; recurrent urinary tract infection; incontinence; etc. (Portman and Gass, 2014). However, GSM has not received enough attention from health care providers and patients, causing a delay in management. It is reported that only 25% of patients in Asia were willing to seek medical assistance, and only 24% of them had access to treatment (Chua et al., 2017). Menopausal hormone therapy (MHT) previously has been shown to be the most effective management for GSM (Gandhi et al., 2016; Faubion et al., 2017). As estrogen receptors distribute in diverse tissues and organs of our body, women in the menopausal stage tend to manifest various types of symptoms requiring treatment due to estrogen deficiency. We found that systemic hormone replacement therapy is suitable for a great many cases in our practical clinical work. The human microbiota inhabiting the vagina alter dynamically across different reproductive stages (Gajer et al., 2012). After menopause, endocrine level and vaginal environment change gradually accompanied by changes in vaginal microbial community structure. In general, vaginal dominant colonization with Lactobacillus is infrequent compared to premenopausal women (Muhleisen and Herbst-Kralovetz, 2016; Kaur et al., 2020). However, the correlation between menopausal related genitourinary symptoms and vaginal flora is still full of ambiguity. Some studies reported that women with examination findings of atrophy had lower proportion of Lactobacillus (Shen et al., 2016; Brotman et al., 2018), whereas other studies did not show this significant association (Mitchell et al., 2017, 2018). While there is evidence of associations between different types of hormone therapy and increased detection of Lactobacillus in vaginal environment (Devillard et al., 2004; Mitchell et al., 2018; Gliniewicz et al., 2019), relatively little is known about effects of MHT on the overall vaginal microbial composition and symptoms and signs of GSM, especially in Chinese women.
In this cross-sectional study, we sought to explore vaginal microbial structure and severity of GSM in women who received regular and uniform MHT in comparison with those who did not by using 16S rRNA gene amplicon sequencing to identify microbial taxa. Moreover, we evaluated the severity of common symptoms in GSM, confirmed a transformation in abundance and diversity of vaginal bacteria after MHT, and elucidated particular associations among vaginal microbiota, MHT, and clinical characteristics.
Materials and Methods
Subjects’ Data and Sample Collection
Menopausal women were recruited and selected from patients attending the menopause clinic in the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital in China, from February 2017 to December 2018. These participants were divided into two groups of 50 subjects each, according to whether they have received regular MHT (tibolone, a synthetic steroid, 2.5 mg orally per day, was selected as the uniform treatment in this study) at least in the past year. Women were included in the study if they were 45–65 years old and did not have menstruation in the past 60 days. In addition, women were excluded if they had any contraindications of MHT; irregular menstrual bleeding due to disease; induced menopause caused by drug, operation, or chemoradiotherapy; a history of diabetes or severe chronic diseases; or they were pregnant or lactating. Moreover, women receiving intravaginal operation such as gynecological examination within the past 24 h, systemic or vaginal topical antibiotic treatment in the past 30 days, or those with no sexual partner were also excluded from this study. All study subjects signed an informed consent form before participation.
Participants responded to one-on-one questionnaires conducted by trained investigators. Demographic characteristics were first assessed by a questionnaire including age, height, weight, reproductive history, menstruation, employment status, economic level, and years of education. Height and weight were measured to calculate body mass index (BMI). In addition, serum estradiol (E2) and follicular stimulating hormone (FSH) levels were abstracted from medical records archived at the Shanghai Jiao Tong University Affiliated Sixth People’s Hospital with participants’ permission. Common menopausal genitourinary symptoms, such as (1) vulvovaginal symptoms including dryness, itching, and burning; (2) urological symptoms including frequent, urgent, and painful urination; recurrent urinary tract infection; and incontinence; and (3) sexual symptom including only decreased libido, were also evaluated by asking women if they had bothersome symptoms described above on a five-point scale ranging from 0 to 4, corresponding to “asymptomatic,” “mild,” “moderate,” “severe,” and “extremely severe,” respectively. Objectively, the severity of GSM was represented by the sum of scores for all seven symptoms.
Then, women underwent a gynecological examination by an experienced gynecologist, during which the Vaginal Health Index Score (VHIS) was measured. The VHIS evaluates the condition of vaginal mucosa through elasticity, vaginal discharge, pH, mucosal integrity, and moisture. A lower VHIS means a worse vaginal physical condition, and if the score is less than 15, it indicates the status of vaginal atrophy (Arêas et al., 2019). Also during this visit, two sterile swabs were used to obtain vaginal secretions samples from the posterior vaginal fornix while avoiding touching the cervix and vulva to avert contamination. One swab was used to detect vaginal pH by a pH test strip (Merck, Darmstadt, Germany) from 4.0 to 7.0 (Huang et al., 2010), and the other was immediately placed in a cryopreservation tube and stored in a −80°C freezer until processed.
DNA Extraction, Polymerase Chain Reaction Amplification, and Sequencing
Before the DNA extraction, frozen swabs containing vaginal secretions were thawed on the ice in a decontaminated environment. The bacterial genomic DNA was isolated from each specimen using the FastDNA® SPIN Kit for Soil (MP Biomedicals, Irvine, CA, United States), according to the standard manufacturer’s protocol, and stored at −20°C prior to subsequent detection. DNA concentrations and purities were quantified by the NanoDrop® ND-2000 UV-Vis spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, United States), and DNA quality was verified by 1% agarose gel electrophoresis.
The V3–V4 hypervariable region of the bacterial 16S ribosomal RNA gene was amplified using primers (Ling et al., 2017) 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GACTACHVGGGTWTCTAAT-3′) under the following thermal-cycling parameters: an initial temperature of 95°C for 3 min, followed by 28 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s, and a final extension at 72°C for 10 min. The polymerase chain reaction (PCR) reactions were performed in a mixture in volume of 20 μL, containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of forward/reverse primer (5 μM), 0.4 μL of FastPfu Polymerase, 10 ng of template DNA, and 10 μL of ddH2O. The PCR products were checked on a 2% agarose gel, extracted from the gel, and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States) and then quantified using QuantiFluorTM-ST (Promega, Madison, WI, United States). Subsequently, purified amplicons were pooled in equal amounts and were sent for paired-end sequencing (2 ∗ 300) on an Illumina MiSeq platform.
Bioinformatics and Statistical Analysis
Bioinformatics analysis was conducted in QIIME (v 1.9.0) or using R project (v 3.6.3) basically. Raw 16S rRNA gene sequences were analyzed using the Quantitative Insights into Microbial Ecology (QIIME) pipeline (v 1.9.0) as previously described (Caporaso et al., 2010). Briefly, raw sequences were assigned to respective samples based on the sample specific barcode sequences and trimmed through removal of both barcode and primer sequences for further quality filtering. Criteria used to filter the low-quality sequence reads were mainly as follows: sequences with a length less than 150 bp, sequences with an average quality score less than 20, sequences with mononucleotide repeats greater than 8, and sequences containing one or more ambiguous base (Huang et al., 2018). Retained high-quality sequences were clustered into distinct operational taxonomic units (OTUs) at a 97% similarity threshold using QIIME’s UCLUST program (Edgar, 2010), and chimeric sequences were detected and removed with ChimeraSlayer implemented in QIIME (Haas et al., 2011). Finally, representative sequences were aligned against the SILVA database (Quast et al., 2013)1 using the Ribosomal Database Project (RDP)2 Bayesian classifier that was retrained to assign taxonomy to each OTU at a 70% confidence threshold. Vaginal microbial taxa were confirmed at the phylum, class, order, family, and genus levels in this portion of the study analysis. In order to eliminate the difference of sequencing depth among samples and to minimize the sequencing errors caused by different library sizes, an average and uniform OTUs table was generated by subsampling under the minimum sequencing depth for downstream analysis.
Based on the OTU information, alpha and beta diversities were utilized to examine vaginal microflora structure. Alpha diversity (de la Cuesta-Zuluaga et al., 2019) (considered as “within” sample diversity) indices were calculated using the vegan package in R (v 3.6.3), including Shannon diversity index, Ace richness index, Chao estimator, Simpson diversity index, and Good’s coverage. Beta diversity (Chu et al., 2017) (known as “between” sample diversity) was analyzed using the Bray–Curtis and weighted UniFrac distance matrices. Then the principal coordinate analysis (PCoA) was plotted using R package ggplot with significant clustering in the intergroup Adonis test. The hierarchically clustered heatmap was generated to visualize microbial taxonomy composition and abundance by the R package pheatmap. Briefly, some relevant tables reflecting community structures were generated to reinforce argument explicitly. In addition, the linear discriminant analysis (LDA) effect size (LEfSe) (Segata et al., 2011) modeling was applied for the identification of significantly differential genera between groups based on relative abundance performed in the online Galaxy tools3. To define multivariate correlations of clinical characteristics and microbial community structure, Pearson correlation coefficient between them was calculated, and canonical correlation analysis (CCA) (Guo et al., 2016) was implemented in the R package vegan.
Further statistical analysis was performed using SPSS software (v 22.0). For continuous variables, data with a normal distribution were expressed as mean ± standard deviation; otherwise, data were shown as median (lower quartile, upper quartile). For categorical variables, the percentage was used to describe information. To judge differences between groups, independent-samples t-test (normal distribution and homogeneity of variance), Mann-Whitney U-test (non-normal distribution), χ2-test, or Fisher exact test (comparison of rate) was applied. Differences were considered statistically significant with P < 0.05.
Results
Study Cohorts and the Severity of GSM Symptoms
A total of 100 vaginal samples from 100 menopausal women were available for analysis in this study and classified into two groups: non-treated women without any MHT (n = 50) and women on MHT over the past year (n = 50, tibolone was selected as the unified medication regimen for MHT in our study). Table 1 describes the main demographic characteristics of each group, and no significant difference was observed between groups except for years since menopause that was longer in women with MHT than in non-treated women.
TABLE 1.
Demographic characteristics of participants.
| Demographics | Non-treated group | MHT group | P-values | |
| (n = 50) | (n = 50) | |||
| Age (y, mean ± SD) | 53.34 ± 3.33 | 54.76 ± 4.27 | 0.067a | |
| BMI (kg/m2, mean ± SD) | 22.50 ± 3.54 | 21.85 ± 2.50 | 0.291a | |
| E2 [pg/mL, median (Q1–Q3)] | 26.5 (17.00–33.25) | 28.5 (17.75–37.75) | 0.909b | |
| FSH [mIU/mL, median (Q1–Q3)] | 68.16 (51.29–80.08) | 59.14 (47.52–68.29) | 0.107b | |
| No. of parity (n, %) | ≤ 1 | 43 (86.0%) | 43 (86.0%) | 1.000c |
| ≥ 2 | 7 (14.0%) | 7 (14.0%) | ||
| No. of abortion (n, %) | ≤ 1 | 29 (58.0%) | 31 (62.0%) | 0.683c |
| ≥ 2 | 21 (42.0%) | 19 (38.0%) | ||
| Occupational status (n, %) | In work | 23 (46.0%) | 21 (42.0%) | 0.687c |
| Retired/unemployed | 27 (54.0%) | 29 (58.0%) | ||
| Educational length (y, n, %) | ≤ 6 | 2 (4.0%) | 1 (2.0%) | 0.638d |
| 6–9 | 9 (18.0%) | 12 (24.0%) | ||
| 9–12 | 19 (38.0%) | 22 (44.0%) | ||
| > 12 | 20 (40.0%) | 15 (30.0%) | ||
| Economic state (monthly income, yuan, n, %) | ≤ 3,000 | 17 (34.0%) | 21 (42.0%) | 0.533c |
| 3,000–5,000 | 15 (30.0%) | 16 (32.0%) | ||
| > 5,000 | 18 (36.0%) | 13 (26.0%) | ||
BMI, body mass index; E2, estradiol; FSH, follicular stimulating hormone. aIndependent-samples t-test. bMann-Whitney U-test. cPearson χ2-test. dFisher exact test.
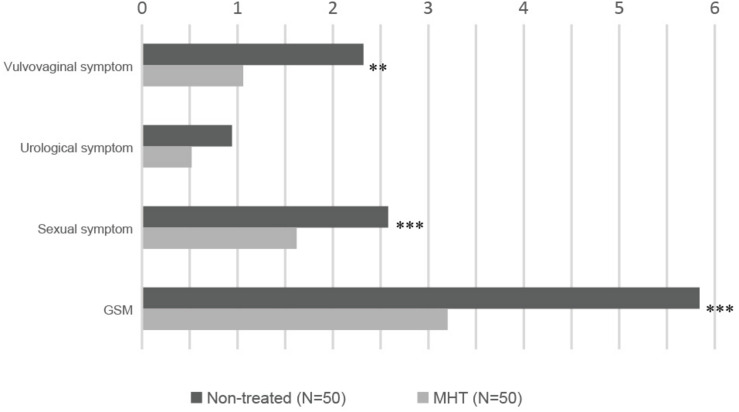
Table 2 shows common symptoms of GSM by severity, from asymptomatic to extremely severe, and Figure 1 demonstrates intuitively the mean score for main symptoms by group. Scores of vaginal symptoms and sexual symptom, as well as GSM, were higher in the non-treated group, indicating that a majority of GSM symptoms were significantly relieved in women treated with MHT (P < 0.01). However, there was no significant drop in the severity scores of urological symptoms between the groups (P > 0.05).
TABLE 2.
Common symptoms of GSM.
| Common symptoms | Non-treated group (n = 50) |
MHT group (n = 50) |
P-values | ||||||||||
| Asymptomatic | Mild | Moderate | Severe | Extremely severe | Mean ± SD (Mean) | Asymptomatic | Mild | Moderate | Severe | Extremely severe | Mean ± SD (Mean) | ||
| Vulvovaginal symptom | 2.32 ± 0.36 (2) | 1.06 ± 0.18 (1) | < 0.01 | ||||||||||
| Dryness | 30.00% | 36.00% | 20.00% | 10.00% | 4.00% | 1.22 ± 0.16 (1) | 58.00% | 26.00% | 16.00% | 0.00% | 0.00% | 0.58 ± 0.11 (0) | <0.005 |
| Itching | 62.00% | 20.00% | 6.00% | 12.00% | 0.00% | 0.68 ± 0.15 (0) | 68.00% | 22.00% | 10.00% | 0.00% | 0.00% | 0.42 ± 0.10 (0) | 0.366 |
| Burning | 78.00% | 10.00% | 4.00% | 8.00% | 0.00% | 0.42 ± 0.13 (0) | 96.00% | 2.00% | 2.00% | 0.00% | 0.00% | 0.06 ± 0.04 (0) | < 0.01 |
| Urological symptom | 0.94 ± 0.19 (0) | 0.52 ± 0.16 (0) | 0.051 | ||||||||||
| Frequent, urgent, and painful urination | 70.00% | 18.00% | 10.00% | 2.00% | 0.00% | 0.44 ± 0.11 (0) | 84.00% | 14.00% | 2.00% | 0.00% | 0.00% | 0.18 ± 0.06 (0) | 0.072 |
| Recurrent urinary tract infection | 90.00% | 2.00% | 8.00% | 0.00% | 0.00% | 0.18 ± 0.08 (0) | 96.00% | 0.00% | 4.00% | 0.00% | 0.00% | 0.08 ± 0.06 (0) | 0.436 |
| Incontinence | 74.00% | 20.00% | 6.00% | 0.00% | 0.00% | 0.32 ± 0.08 (0) | 84.00% | 10.00% | 2.00% | 4.00% | 0.00% | 0.26 ± 0.10 (0) | 0.305 |
| Sexual symptom | 2.58 ± 0.17 (3) | 1.62 ± 0.20 (1) | < 0.005 | ||||||||||
| Decreased libido | 8.00% | 8.00% | 32.00% | 22.00% | 30.00% | 2.58 ± 0.17 (3) | 77.80% | 24.00% | 20.00% | 14.00% | 14.00% | 1.62 ± 0.20 (1) | <0.005 |
| GSM | 5.84 ± 0.49 (5) | 3.20 ± 0.41 (2.5) | < 0.005 | ||||||||||
P-value in bold indicates statistical difference is significant.
FIGURE 1.
Severity of GSM main symptoms in each group: non-treated vs. MHT. Higher score means more severe symptom; statistical differences are shown in this figure: ∗∗P < 0.01, ∗∗∗P < 0.005.
Sequencing Data, Microbial Distribution, and Abundance
In this work, a total of 3,965,265 sequence reads (range 30,214–72,367 reads per sample) were acquired after quality control, with an average length of 444 bp per sample. Then high-quality sequences were classified into 27 phyla, 52 classes, 103 orders, 176 families, and 356 genera through bioinformatics processing.
The alpha diversity indices differed significantly between the non-treated group and MHT group as shown in Table 3. Shannon diversity index, Ace richness index, and Chao estimator were greater in the non-treated samples, whereas Simpson diversity index was lower in the non-treated samples relative to the MHT samples (P < 0.005). These results reflected a higher microbial diversity of vaginal environment in the non-treated women than in the MHT-treated women. However, there was no significant difference in Good’s coverage value between two groups, and the value for each group was 1.00, suggesting that the sequencing depth was sufficient to saturate the taxa.
TABLE 3.
Alpha diversity indices for vaginal microbiota.
| Alpha diversity indices | Non-treated group (n = 50) |
MHT group (n = 50) |
P-values | ||||
| Median | Q1 | Q3 | Median | Q1 | Q3 | ||
| Shannon | 0.99 | 0.59 | 1.46 | 0.02 | 0.01 | 0.52 | < 0.005 |
| Simpson | 0.48 | 0.30 | 0.70 | 1.00 | 0.70 | 1.00 | < 0.005 |
| Ace | 51.53 | 24.43 | 111.52 | 30.58 | 12.54 | 63.07 | < 0.005 |
| Chao | 35.13 | 21.75 | 107.00 | 22.10 | 10.75 | 30.44 | < 0.005 |
| Coverage | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.109 |
P-values were obtained from Mann-Whitney U-test. Bold value means there exists significant statistical difference.
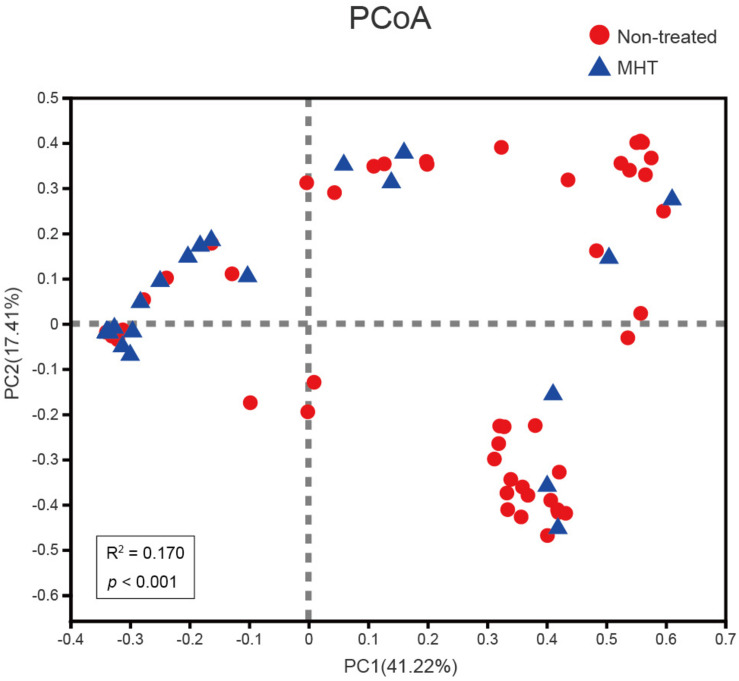
To examine how the vaginal microbiota varied between these two groups, beta diversity analysis was performed by PCoA on the Bray–Curtis dissimilarity. As illustrated in Figure 2, the community structure of MHT-treated women was mostly separated from non-treated women (Adonis P < 0.001), with an R2-value (0.170) suggestive of this significant differentiation.
FIGURE 2.
Comparing samples distributions belonging to the non-treated (red) or MHT (blue) group by using principal coordinate analysis (PCoA) on the Bray–Curtis dissimilarity. Each sample is shown as a dot.
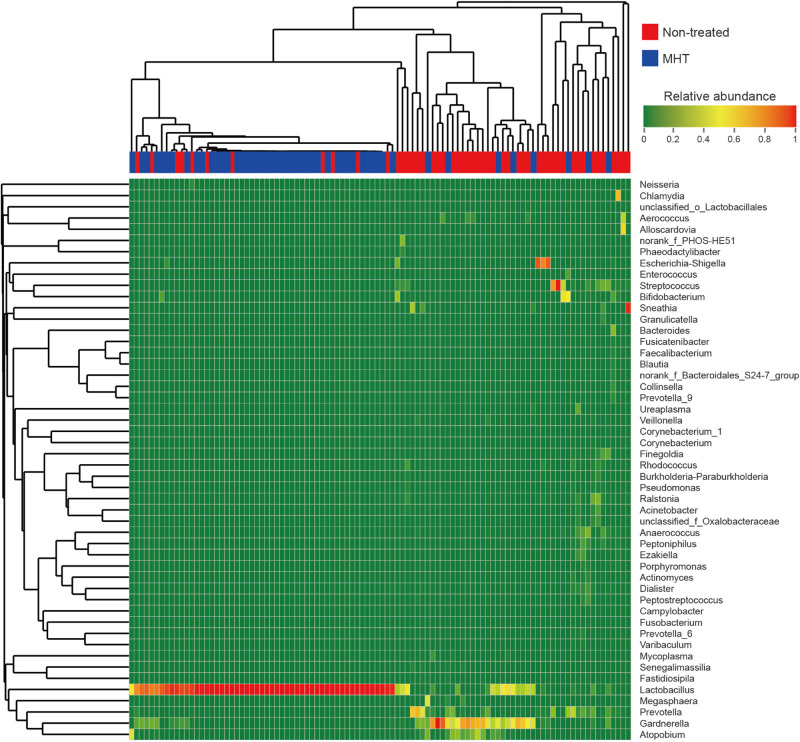
For more details on microbial community at the genus level, we analyzed the relative abundance of the top 50 classified genera in all samples, which was demonstrated as a hierarchically clustered heatmap (Figure 3). Table 4 lists the most abundant genera in two groups and included only the genera with abundance proportions greater than 0.005 in each sample; those failing to meet the condition were expressed as “others.” According to Figure 3, samples within the same group were more interrelated. Furthermore, microbial community profiles from the two groups were markedly different (Figure 3 and Table 4), which was also verified by PCoA results. Lactobacillus was the most representative bacterial genus in both groups, but was dominant only in the MHT group (Lactobacillus proportion of 29.1% in the non-treated samples vs. 83.1% in the MHT samples). In the non-treated group, Gardnerella, Prevotella, Streptococcus, Escherichia-Shigella, Atopobium, Sneathia, Bifidobacterium, etc., made great contributions, which was demonstrated by both data and graphics.
FIGURE 3.
Heatmap analysis of relative abundance of microbial taxa found in all vaginal samples at the genus level (top 50). Each sample is shown as vertical bar, and different color of each cell represents different relative abundance: the low abundance and high abundance are highlighted by green and red, respectively. The horizontal bar on the top indicates the non-treated (red) or MHT (blue) group; dendrograms reflect hierarchical clustering on Bray–Curtis dissimilarity (columns) or Euclidean distance (rows) using average linkage.
TABLE 4.
Proportion of most abundant genera in vaginal microbiota of different groups.
| Genus | Abundance proportion in non-treated group |
Abundance proportion in MHT group |
||||
| Mean | Max | Min | Mean | Max | Min | |
| Lactobacillus | 29.1% | 100.0% | 0.0% | 83.1% | 100.0% | 0.0% |
| Gardnerella | 22.7% | 99.9% | 0.0% | 6.4% | 55.5% | 0.0% |
| Prevotella | 7.4% | 63.5% | 0.0% | 1.7% | 31.4% | 0.0% |
| Streptococcus | 5.8% | 97.4% | 0.0% | 0.7% | 21.4% | 0.0% |
| Escherichia-Shigella | 5.1% | 81.4% | 0.0% | 0.2% | 9.2% | 0.0% |
| Atopobium | 4.4% | 37.0% | 0.0% | 2.1% | 46.0% | 0.0% |
| Sneathia | 3.1% | 92.5% | 0.0% | 0.1% | 6.6% | 0.0% |
| Bifidobacterium | 2.1% | 54.1% | 0.0% | 1.4% | 52.0% | 0.0% |
| Aerococcus | 1.8% | 38.4% | 0.0% | 0.1% | 1.9% | 0.0% |
| Ralstonia | 1.5% | 22.2% | 0.0% | 0.1% | 1.3% | 0.0% |
| Anaerococcus | 1.3% | 15.1% | 0.0% | 0.6% | 23.6% | 0.0% |
| Chlamydia | 1.2% | 58.6% | 0.0% | 0.0% | 0.0% | 0.0% |
| Alloscardovia | 1.1% | 56.1% | 0.0% | 0.0% | 0.2% | 0.0% |
| Rhodococcus | 0.8% | 11.3% | 0.0% | 0.0% | 0.8% | 0.0% |
| Peptoniphilus | 0.7% | 13.2% | 0.0% | 0.2% | 7.5% | 0.0% |
| Finegoldia | 0.6% | 15.1% | 0.0% | 0.4% | 17.6% | 0.0% |
| Acinetobacter | 0.6% | 14.1% | 0.0% | 0.0% | 0.1% | 0.0% |
| Ezakiella | 0.6% | 12.3% | 0.0% | 0.0% | 2.1% | 0.0% |
| Dialister | 0.5% | 5.4% | 0.0% | 0.3% | 11.6% | 0.0% |
| Ureaplasma | 0.5% | 18.0% | 0.0% | 0.2% | 4.5% | 0.0% |
| Megasphaera | 0.2% | 9.8% | 0.0% | 1.1% | 46.4% | 0.0% |
| Others | 8.7% | 72.9% | 0.0% | 1.4% | 30.3% | 0.0% |
Others represent those genera with abundance proportion less than 0.005 in each sample. Mean, the average proportion of all samples in one group; Min, minimal proportion of a sample in one group; Max, maximal proportion of a sample in one group.
Significant Differences in Microbial Communities
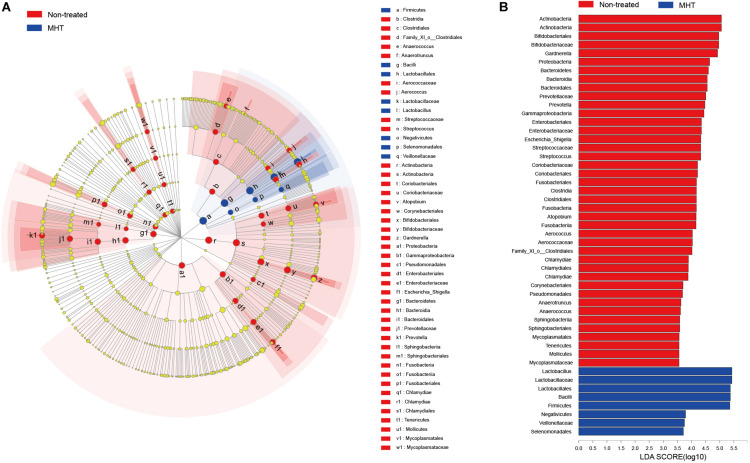
Subsequently, biomarker analysis was conducted to identify the special microbial taxa with significant abundance differences between non-treated and MHT samples using the LEfSe modeling. As seen in Figure 4, there were 49 bacterial clades presenting significant intergroup differences with a LDA threshold of 3.5, and most bacteria were enriched in non-treated samples. Focusing on the genus level, we found that there were as many as eight significantly different genera in the non-treated group, namely, Gardnerella, Prevotella, Escherichia-Shigella, Streptococcus, Atopobium, Aerococcus, Anaerotruncus, and Anaerococcus (all P < 0.05). In contrast, only Lactobacillus manifested the abundance advantage in the MHT group (P < 0.05).
FIGURE 4.
Linear discriminant analysis (LDA) effect size (LEfSe) analysis for comparing microbial variations at the genus level between the non-treated (red) and MHT (blue) group. (A) LEfSE cladogram of microbial taxa in different group (P < 0.05). (B) LDA score identifying effect size of difference between groups with a threshold value of 3.5, a higher score represents a greater contribution to the observed intergroup difference, indicating this taxon is a more important biomarker.
Multivariate Analysis
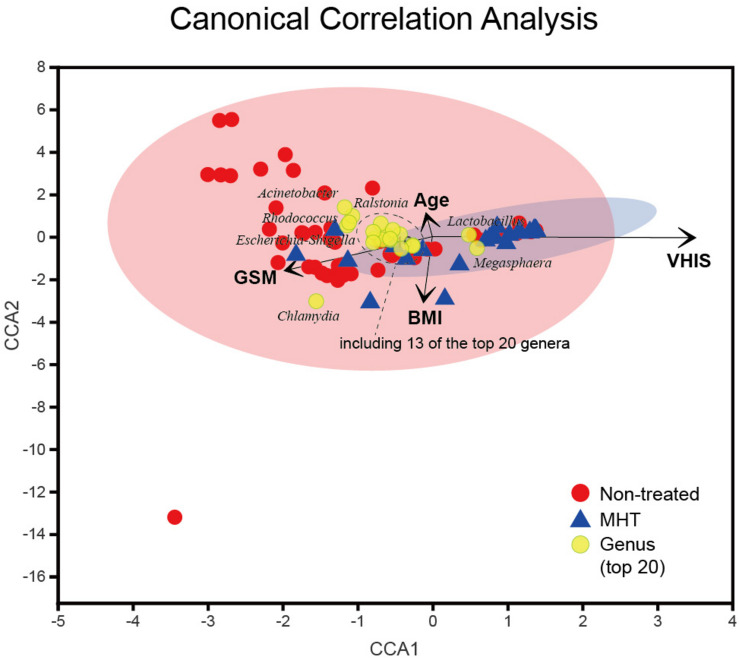
Finally, the CCA was applied to reveal the potentially multivariate correlation of clinical characteristics and primary microbial community (Figure 5). In the CCA, the length of the vector is used to interpret this correlation (a longer vector means a greater correlation with bacterial distribution), and the angle between vectors could reflect associations of clinical variables (a positive association is plotted as an acute angle and a negative association as an obtuse angle). Additionally, a greater distance between samples indicates a stronger dissimilarity in microbial structure. According to Figure 5, we found that the dissimilarity between samples in the non-treated group was larger than that in the MHT group, this phenomenon was consistent with PCoA results. Moreover, the distribution of non-treated samples was generally in accordance with the GSM vector, whereas MHT samples were mostly distributed in the direction of the VHIS vector, which was similar to the comparison of the severity of GSM between these two groups. The first axis had a significantly positive correlation with VHIS (P < 0.001), as well as a negative correlation with GSM (P < 0.005); it was obvious that these two variables had an obtuse relationship. Lactobacillus was also associated with the first axis; similarly, it corrected with VHIS positively (r = 0.626, P < 0.001) and with GSM negatively (r = −0.347, P < 0.001). As for GSM, other vaginal genera had some connections with it as well, including Chlamydia (r = 0.277, P < 0.01), Streptococcus (r = 0.270, P < 0.01), Enterococcus (r = 0.236, P < 0.05), Corynebacterium (r = 0.234, P < 0.05), Porphyromonas (r = 0.223, P < 0.05), and Escherichia-Shigella (r = 0.199, P < 0.05), all of which increased with the GSM severity score. Additionally, the second axis only presented a significantly inverse relationship with BMI (P < 0.001), but age could not affect the microbial community distribution in our study (P > 0.05).
FIGURE 5.
Canonical correlation analysis (CCA) of the above vaginal microbiota and clinical characteristics. Red and blue points represent non-treated and MHT samples, respectively, the 95% confidence intervals of samples’ distribution are visualized by ellipses, and the top 20 microbial genera are colored with yellow. Clinical characteristic variables (age, BMI, VHIS, GSM) are indicated by vectors in black.
Discussion
In the current study, we performed a cross-sectional survey to determine a vaginal microbial restoration and GSM-related symptom improvement with MHT application based on 16S rRNA gene sequencing and to demonstrate the salient relevance among them. Taking into account the possible impact of different MHT options on the outcome, we chose tibolone as the only unified treatment. The foremost reason is that the vast majority of women treated with tibolone had no menstrual period; in this way, effects of menstruation on vaginal flora could be eliminated. What needs to be explained was that in our study cohort, patients received tibolone treatment due to multiple systematic symptoms rather than genitourinary symptoms alone, in strict accordance with the medicine guidelines (Pinkerton, 2020). Tibolone, a synthetic steroid with estrogenic, progestogenic, and androgenic properties, has long been used worldwide for menopausal symptoms (Formoso et al., 2016). Obviously, we proved that women receiving tibolone had significantly milder GSM symptoms than non-treated women, especially for vulvovaginal dryness and burning, as well as decreased libido, which was similar to previously reported studies (Kenemans and Speroff, 2005). However, there was no significant distinction in vulvovaginal itching and all urological symptoms between the two populations. The underlying basis for these findings is presumably the medicinal property. Tibolone is rapidly metabolized into three metabolites that play diverse roles in target tissues in the body through binding with estrogen, progesterone, or androgen receptors and modulating enzymatic activity (Kloosterboer, 2004), which explains why the levels of serum E2 and FSH were steady in all study participants. Tibolone itself and its Δ4 isomer have slight androgenic effects contributing to the increases in free testosterone and consequently to improving sexual desire (Laan et al., 2001). The female reproductive and lower urinary tracts share the same embryonic origin—urogenital sinus; furthermore, estrogen, and progesterone receptors have been identified in both tissues (Tincello et al., 2009). In theory, it could be anticipated that hormone therapy would help to ameliorate both genital and urinary symptoms, whereas the present researches still remain controversial about the effect of systemic estrogen on urinary symptoms (Robinson and Cardozo, 2011). We supposed that the urinary tract symptoms were mainly mild in the cohort, which to some extent may explain the lack of significant remission of symptoms after tibolone. Hence further clinical surveys and basic experiments should be carried out to clarify the management of postmenopausal lower urinary tract symptoms.
Herein, we demonstrated the vaginal microflora of women with MHT differed from that of non-treated women. First, the differences in all alpha diversity indices between groups implied a higher microbial diversity in samples of non-treated women, which suggested that there might be no definitely preponderant bacteria in these samples. Then, the analysis results revealed distinct vaginal microbial signatures in each group. Lactobacillus accounted for the highest proportion in both of them, but it was only dominant in the vaginal community of women with MHT. We also found it correlated positively with a healthier vaginal condition as well as negatively with more severe GSM, corresponding to the findings of previous studies (Muhleisen and Herbst-Kralovetz, 2016; Mitchell et al., 2018; Gliniewicz et al., 2019). However, it is pertinent to note that the non-treated vaginal microflora significantly included several anaerobic bacteria, namely, Gardnerella, Prevotella, Escherichia-Shigella, Streptococcus, Atopobium, Aerococcus, Anaerotruncus, and Anaerococcus. Interestingly, Prevotella has been shown to promote the growth of Gardnerella by producing polyamines during metabolism, in the same way, the existence of biofilm in Gardnerella could stimulate the growth of Prevotella (Randis and Ratner, 2019). Additionally, not only Gardnerella and Prevotella but also Streptococcus and Atopobium were strongly associated with bacterial vaginosis (Onderdonk et al., 2016). Numerous studies have affirmed that Escherichia coli, as the primary pathogen of urinary tract infections, could adhere to or even invade into vaginal epithelial cells prior to inducing an ascending infection (Brannon et al., 2020). Likewise, a few particular species of Aerococcus were also believed to involve in urinary tract infections (Rasmussen, 2016). One previous research detected that Anaerotruncus in the female reproductive tract might contribute to the development of endometrial cancer (Walther-António et al., 2016). Moreover, Anaerococcus was reported to be correlated with cervical intraepithelial neoplasia disease progression (Mitra et al., 2015). Taken together, these findings hint that ecological interactions are meaningful for vaginal environment and reproductive health; however, the potential interactions are far from definitive.
Our work specifically addressed relationships between the severity of GSM and vaginal microbiota, and except for a strikingly negative correlation between Lactobacillus and GSM, we demonstrated that other taxa correlated with GSM positively, including Chlamydia and Streptococcus, with high correlation coefficients. It has been suggested that a distinct bacterial community state characterized by Streptococcus was associated with vulvovaginal atrophy (Brotman et al., 2018), and our results were consistent with this finding. Moreover, it is well known that Chlamydia infection could result in acute and chronic reproductive disorders. Nevertheless, a few cross-sectional studies found that women in menopause were not susceptible to Chlamydia (Jackson et al., 2015; Stemmer et al., 2018). Notably, there was probably a complex mutual mechanism underlying the definite association between Chlamydia and more severe symptoms of GSM, as shown in our findings. Infection with Chlamydia in the female genital tract could drive increased expression of TLR2 and TLR4 accompanied by upregulation of cytokines, such as tumor necrosis factor α, interleukin 1α (IL-1α), and IL-6; these cytokines together trigger the inflammatory response during which tissue damage occurs (Agrawal et al., 2009). Unfortunately, our study did not involve immune-relevant research, so there was no way to directly provide an explicit conclusion of the interaction mechanism between vaginal bacteria and the local immune response. This aspect was one of limitations in the present study. However, based on the available evidence, we may speculate that the composition and stability of the vaginal microbiota play an essential part in determining the mucosal immune response and susceptibility to infection. Other limitations included that sample sizes were relatively small and participants included in this study were only from outpatients in one clinical center, which might lead to the issue that our results only partly reflected the situation of menopausal women in Shanghai, China. In this regard, additional studies with larger sample sizes and multicenter are required to further investigate the latent microbes associated with GSM and the microbiological mechanism in the occurrence and development of GSM.
In conclusion, GSM emerges with a decline in circulating estrogen levels, which may worsen gradually without intervention. Understanding the microbiological effect of MHT on GSM could help to improve novel strategies for GSM treatment and prevent discomfort. Our study demonstrated that Lactobacillus was distinctly abundant in the vagina and that symptoms of GSM were notably alleviated after regular hormone therapy. Furthermore, several special anaerobic bacteria were found to be significantly enriched in menopausal women without any treatment, and these bacteria were bound up with gynecological and reproductive diseases. Women infected with Chlamydia or Streptococcus in the vagina may be prone to suffer from GSM, which is necessary to be taken into account by medical staff. These findings are of great importance to clinical practice for the management of GSM, although the microbiological mechanism in the progression of GSM remains to be explored and perfected further.
Data Availability Statement
The datasets presented in this study have been deposited in an online repository. The name of the repository and accession number can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA669119.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai Sixth People’s Hospital. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article.
Author Contributions
MT conceived, directed and coordinated all aspects of this study. LG, WH, SJ, YanwZ, and PL participated in sample collection, the data collation, and result analysis. YiZ, YangZ, and JH contributed to DNA extraction and PCR amplification from samples. LG and MT prepared and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge participants in this study and sincerely thank colleagues at the Obstetrics and Gynecology Department of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital for the questionnaire survey, vaginal samples collection, and data arrangement.
Funding. This work was supported by the Shanghai Health and Family Planning Commission (20184Y0362) and the Science and Technology Commission of Shanghai Municipality (15411950202).
References
- Agrawal T., Vats V., Salhan S., Mittal A. (2009). The mucosal immune response to Chlamydia trachomatis infection of the reproductive tract in women. J. Reprod. Immunol. 83 173–178. 10.1016/j.jri.2009.07.013 [DOI] [PubMed] [Google Scholar]
- Arêas F., Valadares A. L. R., Conde D. M., Costa-Paiva L. (2019). The effect of vaginal erbium laser treatment on sexual function and vaginal health in women with a history of breast cancer and symptoms of the genitourinary syndrome of menopause: a prospective study. Menopause 26 1052–1058. 10.1097/GME.0000000000001353 [DOI] [PubMed] [Google Scholar]
- Brannon J. R., Dunigan T. L., Beebout C. J., Ross T., Wiebe M. A., Reynolds W. S., et al. (2020). Invasion of vaginal epithelial cells by uropathogenic Escherichia coli. Nat. Commun. 11:2803. 10.1038/s41467-020-16627-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman R. M., Shardell M. D., Gajer P., Fadrosh D., Chang K., Silver M. I., et al. (2018). Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 25 1321–1330. 10.1097/GME.0000000000001236 [DOI] [PubMed] [Google Scholar]
- Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D. M., Ma J., Prince A. L., Antony K. M., Seferovic M. D., Aagaard K. M. (2017). Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 23 314–326. 10.1038/nm.4272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua Y., Limpaphayom K. K., Cheng B., Ho C. M., Sumapradja K., Altomare C., et al. (2017). Genitourinary syndrome of menopause in five Asian countries: results from the Pan-Asian revive survey. Climacteric 20 367–373. 10.1080/13697137.2017.1315091 [DOI] [PubMed] [Google Scholar]
- de la Cuesta-Zuluaga J., Kelley S. T., Chen Y., Escobar J. S., Mueller N. T., Ley R. E. (2019). Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems 4:e00261-19. 10.1128/mSystems.00261-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillard E., Burton J. P., Hammond J. A., Lam D., Reid G. (2004). Novel insight into the vaginal microflora in postmenopausal women under hormone replacement therapy as analyzed by PCR-denaturing gradient gel electrophoresis. Eur. J. Obstet. Gynecol. Reprod. Biol. 117 76–81. 10.1016/j.ejogrb.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- Faubion S. S., Sood R., Kapoor E. (2017). Genitourinary syndrome of menopause: management strategies for the clinician. Mayo Clin. Proc. 92 1842–1849. 10.1016/j.mayocp.2017.08.019 [DOI] [PubMed] [Google Scholar]
- Formoso G., Perrone E., Maltoni S., Balduzzi S., Wilkinson J., Basevi V., et al. (2016). Short-term and long-term effects of tibolone in postmenopausal women. Cochrane Database. Syst. Rev. 10:CD008536. 10.1002/14651858.CD008536.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajer P., Brotman R. M., Bai G., Sakamoto J., Schütte U. M., Zhong X., et al. (2012). Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4:132ra52. 10.1126/scitranslmed.3003605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi J., Chen A., Dagur G., Suh Y., Smith N., Cali B., et al. (2016). Genitourinary syndrome of menopause: an overview of clinical manifestations, pathophysiology, etiology, evaluation, and management. Am. J. Obstet. Gynecol. 215 704–711. 10.1016/j.ajog.2016.07.045 [DOI] [PubMed] [Google Scholar]
- Geng L., Zheng Y., Zhou Y., Li C., Tao M. (2018). The prevalence and determinants of genitourinary syndrome of menopause in Chinese mid-life women: a single-center study. Climacteric 21 478–482. 10.1080/13697137.2018.1458832 [DOI] [PubMed] [Google Scholar]
- Gliniewicz K., Schneider G. M., Ridenhour B. J., Williams C. J., Song Y., Farage M. A., et al. (2019). Comparison of the vaginal microbiomes of premenopausal and postmenopausal women. Front. Microbiol. 10:193. 10.3389/fmicb.2019.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Ding X., Liu C., Xue J. H. (2016). Sufficient canonical correlation analysis. IEEE Trans. Image Process 25 2610–2619. 10.1109/TIP.2016.2551374 [DOI] [PubMed] [Google Scholar]
- Haas B. J., Gevers D., Earl A. M., Feldgarden M., Ward D. V., Giannoukos G., et al. (2011). Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21 494–504. 10.1101/gr.112730.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. J., Moore E. E., Boyko E. J., Scholes D., Lin F., Vittinghoff E., et al. (2010). Vaginal symptoms in postmenopausal women: self-reported severity, natural history, and risk factors. Menopause 17 121–126. 10.1097/gme.0b013e3181acb9ed [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Li C., Li F., Zhao J., Wan X., Wang K. (2018). Cervicovaginal microbiota composition correlates with the acquisition of high-risk human papillomavirus types. Int. J. Cancer 143 621–634. 10.1002/ijc.31342 [DOI] [PubMed] [Google Scholar]
- Jackson J. A., McNair T. S., Coleman J. S. (2015). Over-screening for chlamydia and gonorrhea among urban women age ≥25 years. Am. J. Obstet. Gynecol. 212 40.e1–40.e6. 10.1016/j.ajog.2014.06.051 [DOI] [PubMed] [Google Scholar]
- Kaur H., Merchant M., Haque M. M., Mande S. S. (2020). Crosstalk between female gonadal hormones and vaginal microbiota across various phases of women’s gynecological Lifecycle. Front. Microbiol. 11:551. 10.3389/fmicb.2020.00551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenemans P., Speroff L. (2005). Tibolone: clinical recommendations and practical guidelines. A report of the international tibolone consensus group. Maturitas 51 21–28. 10.1016/j.maturitas.2005.02.011 [DOI] [PubMed] [Google Scholar]
- Kloosterboer H. J. (2004). Tissue-selectivity: the mechanism of action of tibolone. Maturitas 48(Suppl. 1), S30–S40. 10.1016/j.maturitas.2004.02.012 [DOI] [PubMed] [Google Scholar]
- Laan E., van Lunsen R. H., Everaerd W. (2001). The effects of tibolone on vaginal blood flow, sexual desire and arousability in postmenopausal women. Climacteric 4 28–41. 10.1080/713605033 [DOI] [PubMed] [Google Scholar]
- Ling Z., Liu F., Shao L., Cheng Y., Li L. (2017). Dysbiosis of the urinary microbiota associated with urine levels of proinflammatory chemokine interleukin-8 in female Type 2 diabetic patients. Front. Immunol. 8:1032. 10.3389/fimmu.2017.01032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. M., Srinivasan S., Plantinga A., Wu M. C., Reed S. D., Guthrie K. A., et al. (2018). Associations between improvement in genitourinary symptoms of menopause and changes in the vaginal ecosystem. Menopause 25 500–507. 10.1097/GME.0000000000001037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C. M., Srinivasan S., Zhan X., Wu M. C., Reed S. D., Guthrie K. A., et al. (2017). Vaginal microbiota and genitourinary menopausal symptoms: a cross-sectional analysis. Menopause 24 1160–1166. 10.1097/GME.0000000000000904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra A., MacIntyre D. A., Lee Y. S., Smith A., Marchesi J. R., Lehne B., et al. (2015). Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci. Rep. 5:16865. 10.1038/srep16865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhleisen A. L., Herbst-Kralovetz M. M. (2016). Menopause and the vaginal microbiome. Maturitas 91 42–50. 10.1016/j.maturitas.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Delaney M. L., Fichorova R. N. (2016). The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 29 223–238. 10.1128/CMR.00075-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton J. V. (2020). Hormone therapy for postmenopausal women. N. Engl. J. Med. 382 446–455. 10.1056/NEJMcp1714787 [DOI] [PubMed] [Google Scholar]
- Portman D. J., Gass M. L. (2014). Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the international society for the study of women’s sexual health and the North American menopause society. Climacteric 17 557–563. 10.3109/13697137.2014.946279 [DOI] [PubMed] [Google Scholar]
- Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., et al. (2013). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41 D590–D596. 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randis T. M., Ratner A. J. (2019). Gardnerella and prevotella: co-conspirators in the pathogenesis of bacterial vaginosis. J. Infect. Dis. 220 1085–1088. 10.1093/infdis/jiy705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M. (2016). Aerococcus: an increasingly acknowledged human pathogen. Clin. Microbiol. Infect. 22 22–27. 10.1016/j.cmi.2015.09.026 [DOI] [PubMed] [Google Scholar]
- Robinson D., Cardozo L. (2011). Estrogens and the lower urinary tract. Neurourol. Urodyn. 30 754–757. 10.1002/nau.21106 [DOI] [PubMed] [Google Scholar]
- Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12:R60. 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J., Song N., Williams C. J., Brown C. J., Yan Z., Xu C., et al. (2016). Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci. Rep. 6:24380. 10.1038/srep24380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemmer S. M., Mordechai E., Adelson M. E., Gygax S. E., Hilbert D. W. (2018). Trichomonas vaginalis is most frequently detected in women at the age of peri-/premenopause: an unusual pattern for a sexually transmitted pathogen. Am. J. Obstet. Gynecol. 218 328.e1–328.e13. 10.1016/j.ajog.2017.12.006 [DOI] [PubMed] [Google Scholar]
- Tincello D. G., Taylor A. H., Spurling S. M., Bell S. C. (2009). Receptor isoforms that mediate estrogen and progestagen action in the female lower urinary tract. J. Urol. 181 1474–1482. 10.1016/j.juro.2008.10.104 [DOI] [PubMed] [Google Scholar]
- Walther-António M. R., Chen J., Multinu F., Hokenstad A., Distad T. J., Cheek E. H., et al. (2016). Potential contribution of the uterine microbiome in the development of endometrial cancer. Genome Med. 8:122. 10.1186/s13073-016-0368-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study have been deposited in an online repository. The name of the repository and accession number can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA669119.