Structured Abstract
Background
Aim of this study was to assess if pre-procedural computed tomography CT could identify high-risk operative features and predict increased procedural complexity.
Methods
Consecutive patients who had CTs performed ≤ 90 days of their planned lead extraction (LE) were included. CTs were reviewed blinded to outcome according to a preset checklist. The outcome was a combined endpoint of procedural complication and major complications.
Results
Between January 1, 2015- July 1, 2018 n=143 patients underwent CT and LE. Median age 68 (IQR 54.4-76.5), 35 % female. Median age of extracted leads were 111 months, and 126 (43%) were > 10 years.
CT detected lead perforation > 5 mm (n=13), < 5 mm (n= 55), severe lead adhesions (n=65), leads touching vessel wall > 1 cm (n=102), lead fracture (n= 8), and severe ipsilateral venous stenosis/occlusion (n= 36).
The procedure was complex in 63 cases. There were 2 deaths, and 6 major complications.
Patients with severe lead adhesions had more complex procedures, n=36 vs 29, p=0.04, whereas none of the other findings on CT were significantly associated with worse outcome. In patients with leads that had an indwelling time below 10 years, n=72, severe lead adhesions on CT was associated with worse outcome in multivariable analysis, OR 6.4 (95 % CI 1.4-30.2), p = 0.02.
Conclusions
Pre-procedural CT can be used to locate severe lead adhesions in patients planned for lead extraction. In patients with indwelling leads < 10 years pre-procedural CT aid in identifying patients prone to complex extractions.
Keywords: Cardiac implantable electrical devices, percutaneous extraction, preprocedural imaging, computed tomography
Condensed abstract
Unpredictable lead adhesions can make lead extraction more complicated. Adhesions are known to affect leads with longer indwelling time but can unpredictably affect newer leads. In this retrospective study of 143 patients who had a pre-procedural computed tomography (CT) within 90 days of their lead extraction, lead adhesions identified on CT were associated with a more difficult extraction procedure, and major complications. Patients with leads that had an indwelling time less than 10 years, lead adhesions identified on CT was significantly associated with a more difficult extraction, or major complications, OR 6.4 (95 % CI 1.4-30.2), p = 0.02.
Summary
Lead adhesions are feared in extraction cases, especially if they are unexpected. In this retrospective study CT could aid in identifying lead adhesions. CT verified severe adhesions were associated with a more difficult extraction procedure, and major complications.
#cvImaging #cvEP #epPPM
Introduction
An increasing prevalent population of cardiac implanted device (CIED) have led to a commensurate increase in the need for removal of device systems. Lead complications such as infection, malfunction, thromboembolism originating from leads, or redundant leads after device upgrades might necessitate lead extraction (1,2).
Percutaneous lead extraction is not without risk, as lead perforation, fracture and adhesions can make extraction more difficult (3). Known risks for lead extraction are commonly based on the following factors: 1) lead (age, type of lead, possible perforation, presence of vegetations) 2) the cause for extraction (infection/other) 3) clinical risk factors (female gender, body mass index < 25, prior stroke, heart failure, end stage renal failure requiring dialysis, diabetes, oral anticoagulant therapy, and anemia) (1).
However, the inherent potential of the lead to form adhesions to the vascular wall, the myocardium or other leads can cause avulsion of venous or myocardial tissues resulting in potentially life-threatening complications (3). Leads with longer indwelling times form adhesions, but adhesions may also unpredictably appear in patients with newer leads (4). Computed tomography (CT) provides a way to visualize the leads as they course through the veins and into the cardiac chambers and is currently recommended in the Heart Rhythm Society’s expert consensus document to identify lead perforation (1). In addition, areas of adhesions, patency of vasculature and CIED leads can be assessed using CT. The potential of CT to preoperatively identify areas of calcification, and sites where the leads have attached to vessel walls, might enable identification of patients who are at risk of a complicated lead extraction procedure, or at risk of a complication (5).
The aim of this study was to assess if preoperative CT could be used to identify high-risk features that are associated with the difficulty of the extraction procedure.
Method
Information about all patients scheduled for CIED laser lead extraction (LLE) at the University of California, San Diego have been registered into a specific registry since August 17th, 2010. Patient data including medical history, age, height, weight, BMI and procedural data including type of CIED, number of leads removed, equipment used for removal, fluoroscopy times and complications have been collected.
In this retrospective single-center study, consecutive patients included in the LLE registry between January 1, 2015 and July 1, 2018 and who had a CT within 90 days of their procedure were identified and included in the study. Patients with CT performed > 90 days, or patients with outcome data missing from the registry were excluded, Figure 1.
Figure 1. Laser lead CIED extraction procedures from January 1 2015- July 1 2018.
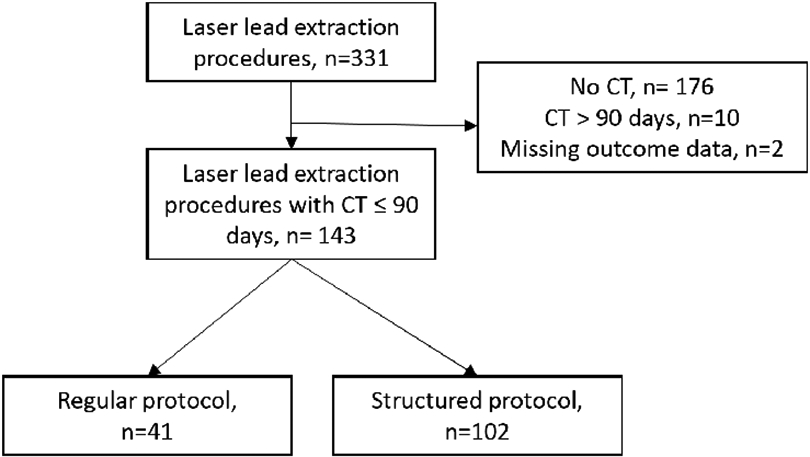
Flow chart of all CIED laser lead extraction procedures performed at a single-center January 1 2015- July 1 2018
Computed tomography
Between 2015-2017, CTs were mainly performed in patients with CIED leads aged ≥ 10 years, whereas from 2018 high quality CTs were ordered for all patients admitted for LLE. All CT examinations were performed on Toshiba 320-slice, General Electric 64-slice, or General Electric 256-slice scanners with 0.5 - 0.625 mm longitudinal resolution. Initially, CT of the thorax was obtained with or without intravenous contrast using variable contrast volume and injection rates depending on patient BMI and whether concurrent body parts were being scanned. In 2016-2017, CT protocol was gradually revised to a more specific lead extraction protocol utilizing contrast-enhancement, ECG-gating, and 3D reconstruction. This structured protocol consisted of 120 mL iodinated contrast (Omnipaque™ 350mg iodine/mL) diluted in 120 mL of saline injected at 2.5 mL/sec, z-axis coverage through the entire chest covering 2 x 16 cm stations, patient positioned supine with arms-up, and dose-modulated prospective or retrospective cardiac gating with image acquisition in end-diastole on prospectively gated examinations (centered at 75% of the R-R interval). 3D reconstructions were created using TeraRecon 3D software (version 4.4.12). Coronal and sagittal multiplanar reformats of axial images were used in addition to the source axial images for analysis. Off-axis review of imaging data was performed using TeraRecon. For retrospective cardiac-gated exams, review of different phases of the cardiac cycle in 10% intervals was also performed with TeraRecon software.
A specific checklist was filled out for all CTs including lead termination, lead perforation, presence of lead fracture, lead calcifications, lead thrombus, lead adhesions and presence of venous occlusion/severe stenosis. Lead perforation was defined as lead termination beyond the epicardial margin, measured as the distance between the epicardial margin and lead tip utilizing multiplanar reformats in addition to off-axis manipulation of source axial images using TeraRecon 3D software. If multiple cardiac phases were available, the phase with least cardiac motion was used for assessment of lead termination and perforation. Severe lead adhesion was determined present if any portion of the lead was embedded within the wall, with no visible surrounding intravenous contrast or blood. CTs were reviewed by a radiologist blinded to outcome of the LLE procedure. In order to categorize findings, we used a model suggested by Ehieli et al (6) where findings were categorized according to major findings which might have impact on the procedure or outcome, and moderate findings which might impact procedural difficulty, Table 1.
Table 1.
Classification of findings on pre-procedural computed tomography, modified from Ehieli et al (6)
| Major findings | |
|---|---|
| Lead | |
| Lead perforations ≥ 5 mm | |
| Moderate findings | |
| Lead | Lead perforations < 5 mm Lead fracture Lead thrombus |
| Adhesions | Moderate lead adhesion: Lead touching the wall of a central vein ≥ 1 cm Severe lead adhesion: Lead embedded in vessel wall |
| Vein patency | Ipsilateral occlusion or Ipsilateral severe stenosis |
Procedure & outcome
The primary outcome was a combined endpoint of major complications and the complexity of the procedure. Major complications were defined as per 2017 HRS expert consensus statement (1).
The procedure was classified as complex if it required any of the following: upgrade in laser sheath size, a combination of laser and mechanical tools, required a femoral snare or if it required a prolonged fluoroscopy time, determined as exceeding the 90th percentile.
Statistics
Data analysis was carried out using the SPSS software version 25 (IBM Corp. Armonk, NY). All tests were two-sided with statistical significance at p< 0.05. Continuous variables were described as median (IQR) or mean (SD) and were compared with student’s t-test or Wilcoxon Rank-sum test depending on distribution. For comparison of categorical variables, the χ2 test was used. Binary logistic regression was used to identify factors associated with a worse outcome or procedural difficulty. For multivariable analysis the patient’s age, BMI < 25 and severe lead adhesions were included in the final model.
Ethics
The study was approved by the UCSD Institutional Review Board and granted a waiver of consent.
Results
Demographics & Leads
In total, 331 LLE procedures were performed between January 1, 2015 and July 1, 2018 at the University of California, San Diego. A CT was obtained within 90 days before n=145 of LLE. Two patients in the CT cohort had missing data, and were excluded, leaving n=143 patients in our cohort, Figure 1.
Median age in our cohort was 68 years, and clinical risk factors for lead extraction such as female gender n=51 (35%), BMI below 25 n=41 (29%), history of cerebrovascular disease n=23 (16%), heart failure (52.9 %), diabetes n=41 (29%) and end-stage renal disease were common, Table 1.
Extractions of CIEDs of ICD/CRT type was more common than pacemakers. In 53 patients (37.1 %) the reason for extraction was infection, Table 2. In general, the leads extracted were old, with a median dwelling time of over 9 years, and close to half of the procedures involved at least one lead with a dwelling time of > 10 years. Of the n=294 leads removed, the majority had active fixation, and were pacing leads, Supplementary table 1.
Table 2.
Baseline demographics for patients undergoing laser lead extraction
| Demographics | CT ≤ 90 days, n=143 |
|---|---|
| Age, median (IQR) | 68.0 (54.4-76.5) |
| Gender, female | 50 (35%) |
| Weight in kg, median (IQR) | 80.5 (68.3-97.2) |
| Height in cm, mean (SD) | 171.7 (10.2) |
| BMI, median (IQR) | 27.5 (23.5-32.0) |
| BMI < 25, n (%) | 41 (28.7) |
| Current smoker, n (%) | 6 (5.2) |
| Comorbidities | |
| Heart failure, n (%) | 74 (52.9) |
| Prior MI, n (%) | 22 (15.6) |
| History of VT | 33 (23.4) |
| Atrial fibrillation, n (%) | 59 (41.8) |
| Hypertension, n (%) | 82 (58.2) |
| On dialysis, n (%) | 6 (4.3) |
| Diabetes mellitus, n (%) | 41 (29.1) |
| COPD, n (%) | 18 (12.8) |
| Cerebrovascular disease, n (%) | 23 (16.3) |
| Chronic renal insuffiency, n (%) | 21 (14.9) |
| Prior open heart surgery, n (%) | 32 (22.9) |
| Left ventricular assist device, n (%) | 2 (1.4) |
| CIED prior to explant | |
| Pacemaker dependent, n (%) | 52 (36.4) |
| Pacemaker, n (%) | 63 (45.3) |
| ICD, n (%) | 44 (31.7) |
| CRT-P, n (%) | 5 (3.6) |
| CRT-D, n (%) | 26 (18.7) |
| ECHO findings (n=108) | |
| EF, mean (SD) | 46.3 (16.4) |
| Vegetation on lead per ECHO, n (%) | 24 (21.8) |
| Indication for extraction | |
| Infection, n (%) | 53 (37.1) |
| Malfunction, n (%) | 72 (50.3) |
| System upgrade, n (%) | 8 (5.6) |
| Other, n (%) | 10 (7.0) |
| Leads | |
| Age of leads, median months | 111 (65-142) |
| Number of procedures with at least one lead with dwelling time > 10 years, n (%) | 71 (49.6) |
| Median number of leads extracted | 2 (1-2) |
| Number of procedures with 3 or more leads extracted, n (%) | 30 (21) |
BMI= Body Mass Index CIED= Cardiovascular Implantable Electronic device COPD= Chronic Obstructive Pulmonary Disease CRT-P= Cardiac Resynchronization Therapy Pacemaker CRT-D= Cardiac Resynchronization Therapy Defibrillator CT= Computed Tomography ECHO= Echocardiogram EF=Ejection Fraction ICD= Implantable Cardioverter Defibrillator IQR=Interquartile range MI=Myocardial infarction SD= Standard Deviation VT= Ventricular tachycardia
CT findings
Images were acquired using contrast in n=128 (89.5%), ECG-gated in n=81 (56.6 %) and 3-D reconstructed in n=31 (25.4%).
In n=13 (9 %) a perforation ≥ 5 mm was detected, categorized as a major finding. Moderate findings affecting at least one lead were as follows: lead perforation < 5 mm (n= 55, 39 %), lead fracture (n= 8, 6 %), moderate lead adhesion with lead touching vessel wall of central vein> 1 cm (n=102, 71%), severe lead adhesion with lead embedded in vessel wall (n=65, 45%), and severe ipsilateral venous stenosis/occlusion (n= 36, 25%). No lead thrombus was detected. Of the 143 procedures, 141 had at least one major or moderate finding. An example of severe lead adhesion is shown in Figure 2.
Figure 2. Severe lead adhesion in patient with abandoned lead before successful extraction.
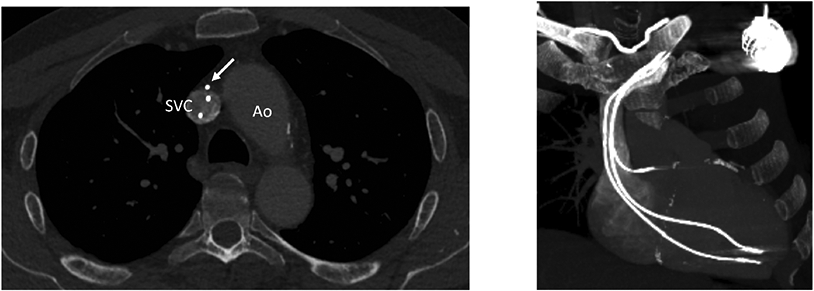
A) Axial CT image at the level of the aorta arch (Ao) and superior vena cava (SVC) demonstrates an abandoned lead embedded within the wall of the upper SVC (white arrow). Note lack of contrast opacification circumferentially about this lead. B) 3D maximum intensity projection (MIP) reconstruction shows course and termination of the three transvenous leads.
In a sensitivity analysis, CT image acquisition was compared before and after the cardiac-gated, contrast enhanced, named “structured”, CT protocol was initiated in April 2016. In n=102 (71 %) patients the CT was performed after the structured protocol was initiated. Significantly more contrast was given to patients in the structured CT group compared to the regular group (number of patients receiving contrast n= 97 (95%) vs n=31 (76%)), ECG gating was performed in 74 % vs 15% and 3-D reconstruction in 32% vs 7 %, p < 0.05 for all comparisons.
There was no significant difference with regards to detection of a major finding in the structured vs the non-structured group (8 % vs 12 %). Of the moderate findings only moderate lead adhesion >1 cm was significantly more commonly detected using the structured CT protocol (77% vs 59 %), p = 0.025.
Procedural complexity and outcome
The procedure was complex in 63 cases requiring at least one of the following: upgrade in laser sheath size, n=18, laser and/or mechanical tool needed n=24, three or more tools needed in one lead n=15, femoral snare n=2, or fluoroscopy-time exceeding 90th percentile (> 14.2 minutes) n=20. In total there were 2 deaths, both due to tears in the superior vena cava (SVC). There were 6 other major complications; 3 patients with right ventricular tears, and 3 with SVC tears, all requiring sternotomy.
Of the patients with major findings on CT, none had a major complication nor a significantly more complicated procedure. Of the moderate findings detected on CT only patients with severe lead adhesions (leads embedded in vessel wall) had a more complex procedure compared to patients without severe lead adhesions, n=36 (54.5%) vs 29 (37.7%), p=0.04. None of the other moderate findings of CT were significantly associated with a more complex procedure nor complication. Major complications, n=8, only occurred in patients with at least one lead aged > 10 years, n=71, which was significant compared to no major complications in the remaining patients with leads with indwelling time less than 10 years, n=72, p=0.003. None of the CT findings were associated with the endpoint of major complication alone. Patients with a major complication and/or complex procedure were significantly younger, more commonly had a BMI< 25, and their leads were older, Table 3.
Table 3.
Outcome with regards to known risk factors for lead extraction and CT findings
| Combined endpoint*, n=66 |
Not reaching combined endpoint, n= 77 |
p-value combined endpoint |
|
|---|---|---|---|
| Demographics | |||
| Age, median (IQR) | 66.0 (21.6) | 72.0 (19.4) | 0.042 |
| Women, n (%) | 26 (39.4%) | 24 (31.2%) | NS |
| BMI < 25 | 25 (37.9) | 16 (20.8) | 0.033 |
| Diabetes, n (%) | 17 (26.2) | 24 (31.6%) | NS |
| Heart failure | 31 (48.4) | 43 (56.6) | NS |
| History of cerebrovascular disease, n (%) | 8 (12.3) | 15 (19.7) | NS |
| ESRD | 2 (3.1) | 4 (5.3) | NS† |
| Prior open-heart surgery, n (%) | 19 (25) | 13 (20.3) | NS† |
| Age of lead, median (IQR) | 135.0 (75.1) | 74.0 (73.8) | < 0.001 |
| Number of leads extracted, median (IQR) | 2 (1) | 2 (1) | NS |
| Infection | 21 (32.3) | 32 (43.2) | NS |
| ICD leads, yes/no | 36 (54.5) | 31 (40.3) | NS |
| Major finding CT | |||
| Lead perforation ≥ 5 mm | 6 (9.1) | 7 (9.1) | NS |
| Moderate finding CT | |||
| Lead perforation < 5 mm | 25 (37.9) | 30 (39) | NS |
| Lead fracture | 6 (9.1) | 2 (2.6) | NS† |
| Moderate adhesion | 45 (69.2) | 57 (74) | NS |
| Severe adhesion | 36 (54.5) | 29 (37.7) | 0.043 |
| Ipsilateral venous occlusion/severe stenosis | 19 (28.8) | 17 (22.1) | NS |
Combined endpoint = complicated procedure and/or major complication
Fisher’s exact test
BMI= Body Mass Index CT= Computed Tomography ESDR= End stage renal disease IQR=Interquartile range SD= Standard Deviation
Using univariate logistic regression age (OR 0.98, 95 % CI 0.96-1.0), BMI < 25 (OR 2.34, 95 % CI 1.07-5.16), mean duration of leads (OR 1.02, 95 % CI 1.01-1.03), number of leads extracted (OR 1.53, 95 % CI 1.06-2.16), severe lead adhesions on CT (OR 1.99, 95 % CI 1.02-3.88) were all significantly associated with the combined outcome. In a multivariable analysis using significant variables from the univariate analysis only age (OR 0.96, 95 % CI 0.93-0.99), p=0.01, and mean duration of leads (OR 1.02, 95% CI 1.01-1.03), p = 0.001, remained significantly associated with the outcome.
Longer indwelling time is known risk factor for lead extraction, as leads adhere over time, and surgical precautions are common in the management of these patients(4). However, in patients with leads with an indwelling time of <10 years, lead adhesions are not always foreseen or anticipated. We wanted to assess if CT could enhance risk stratification in patients with leads < 10 years. Hence, a stratified analysis comparing extractions in which no leads were aged > 10 years, n=72 to those with at least one lead > 10 years, n=71, was performed. In the univariate analysis severe lead adhesions on CT in patients with leads aged <10 years, had a significantly increased risk of the combined endpoint with an OR of 3.8. This remained significant in the multivariable analysis where presence of severe lead adhesions showed the strongest risk of a complex procedure or major complications, Table 4.
Table 4.
Stratified analysis for extraction procedures with at least one lead older than 10 years compared to extraction procedures with no leads over 10 years
| Lead/s < 10 years, n=72 | Lead/s > 10 years, n=71 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) or n (%) |
OR (95 % CI) * |
p- value |
OR (95 % CI) † |
p- value |
Median (IQR) or n (%) |
OR * |
p- value |
OR† | p- value |
|
| Age, year | 68 (23) | 0.98 (0.9-1.01) | 0.192 | 0.94 (0.88-0.995) | 0.03 | 67 (22) | 0.97 (0.93-1.00) | 0.056 | 0.95 (0.90-0.999) | 0.04 |
| BMI < 25 | 18 (32%) | 1.94 (0.56-6.77) | 0.300 | 3.94 (0.72-21.4) | 0.11 | 23 (32%) | 2.61 (0.71-9.64) | 0.149 | 2.11 (0.53-8.34) | 0.29 |
| Severe lead adhesion on CT (Lead embedded in wall) | 27 (38%) | 3.82 (1.20-12.21) | 0.024 | 6.37 (1.34-30.3) | 0.02 | 38 (54%) | 0.81 (0.29-2.27) | 0.692 | 1.22 (0.34-4.41) | 0.76 |
Univariate analysis
Multivariable analysis
BMI= Body Mass Index CT= Computed Tomography OR= Odds ratio
Discussion
In this single-center study, age of the indwelling lead was significantly associated with major complications and/or a more complex procedure. This is an expected finding, however, in patients with leads with an indwelling time < 10 years, CTs were able to identify severe lead adhesions that in this group showed the strongest associated with a more complex procedure and major complications. This association was more pronounced if a structured protocol for CT image acquisition was used. Hence, the use of a structured CT protocol showed the most clinical value in the group with younger leads, where severe adhesions are less expected.
Lead age is a known risk factor for lead extraction and surgical precaution, such as the use of a hybrid operative room and prophylactic placement of endovascular occlusion balloon in vena cava, may already be in place in patients with indwelling lead age > 10 years. However, for patients with leads < 10 years, CT might add important information with regards to lead adhesions and indicate that a more complex procedure, and surgical precaution might be warranted. In one smaller study of 30 patients, lead adhesions detected on CT were associated with longer laser times, and larger laser sheaths needed (5). In our study, severe lead adhesions were the only CT finding significantly associated with a more complex procedure, especially in patients with indwelling leads < 10 years. Young patient age and very old leads were in our study associated with lead adhesions, which has been noted previously (4).
Our study used a classification system for CT suggested by Ehieli et al, where a major finding was determined as a lead perforating over ≥ 5 mm. (6) In their study 100 patients with a mean age of 63 underwent preprocedural CT, and the results were used to guide lead removal strategy. They detected 6 patients (6 %) with lead perforations > 5 mm and changed the removal strategy in 4 of these patients (3 abandoned leads, 1 open surgical extraction), whereas two patients underwent percutaneous extraction (complications not reported). In a German study of 30 patients, preprocedural CT revealed myocardial perforations in 5 (16.7%) of their patients. In this study the definition for perforation was different as it only specified that the lead tip should extrude from the myocardium and did not use any length criterion. (7) We showed a detection rate of lead perforations > 5 mm at 9 %, which is similar to Ehieli’s study with similar criteria for detection. (6) (7) Surprisingly, in our study lead perforation > 5 mm was not significantly associated with our combined outcome. There could be several reasons for this; we might not have been powered to show such a difference; a perforated lead tip through the relatively thicker myocardium might be a lesser risk than leads adhesing to the relatively thinner veins; or the difficulty in determining lead perforations due to metallic artefacts from lead tips might have over-or underestimated the presence of perforations. However, perforations extending > 5 mm past the epicardial border are less likely to be artifact.
Compared to other studies there was a pronounced difference in the most severe type of adhesion (with leads embedded in the vessel wall) in our study with a detection rate of 45.5 % compared to only 11% in Ehieli’s study. Ipsilateral stenosis/occlusion was less common in our study with 25% detection rate compared to 43% (6). These differences might be due to different CT protocols, but also patients selected for CT. In Ehieli’s study consecutive patients were included, whereas in our study initially only patients perceived as higher risk had a CT. This might have introduced a selection bias, and more significant findings would have been detected on CT compared to a consecutive inclusion. In addition, the introduction of a structured protocol for image acquisition mattered as we detected a difference in adhesions after introduction of a structured CT protocol. The average age of the indwelling leads in our study was high, which might affect lead adhesion as a fibrous capsule might form over time (4).
In a German study of 30 patients there were adhesions in 57% of patients, although the classification differed from our study, making comparisons difficult (7).
A preoperative CT increases radiation to the patient, but this risk might be justified as surgical precautions might improve. A standardized protocol for CT acquisition and larger studies might shed additional light on risk assessment prior to lead extraction.
Limitations
The major limitation of this study is that it is a small non-randomized retrospective study performed in a single center. This might affect generalizability and introduce bias.
In the initial phase of the study only patients with older leads had a preprocedural CT. This might have introduced a selection bias. Patients with older leads are more likely to form lead adhesions, and if lead adhesions are very common a difference will be more difficult to detect. Indeed, lead adhesions were not significant in a multivariable analysis in the entire group, whereas when stratification per lead age was done, we could show that lead adhesions detected in patients with newer leads were significantly associated with a worse outcome.
In addition, the method for CT image acquisition changed, with 3-D reconstruction and ECG-gating added in more exams. This might introduce a detection bias, causing an increased detection in patients with the new protocol. We used stratification according to date of CT imaging to try to address this, showing that lead adhesions were identified easier using the new protocol.
We only had one radiologist reviewing the CTs, hence we cannot account for interobserver differences.
Conclusions
Pre-procedural computed tomography can be used to detect severe lead adhesions in patients planned for lead extraction. In patients with indwelling leads < 10 years CT may identify patients prone to more complex extraction procedures and could be of use in preoperative surgical planning.
Supplementary Material
Central illustration. Clinical application of computed tomography prior to lead extraction.
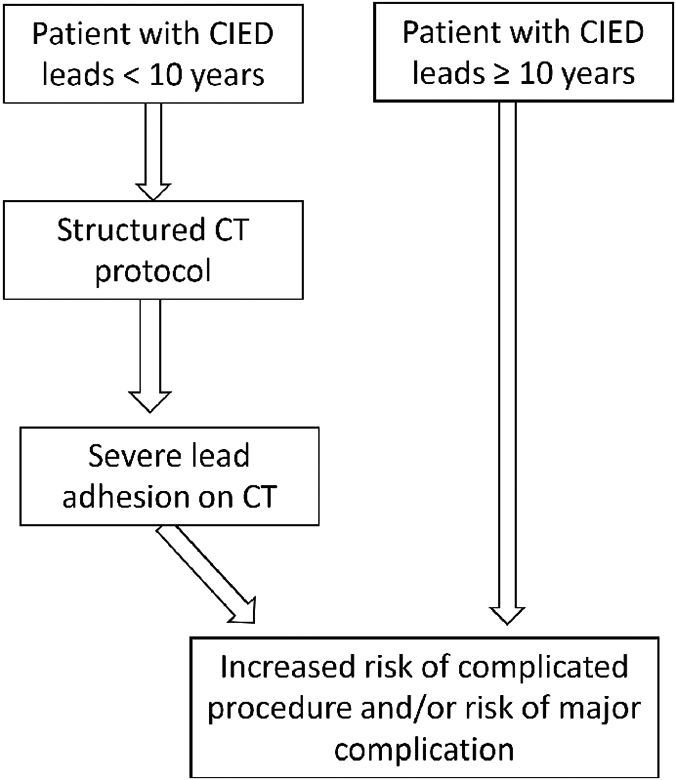
Suggested use of computed tomography in patients with Cardiac implantable electrical devices (CIEDs) for risk management
Clinical perspectives.
In patients with cardiac implantable devices and leads with an indwelling time less than 10 years computed tomography using a structured protocol can aid in preprocedural risk assessment and surgical planning prior to lead extraction.
Acknowledgement
We would like to acknowledge Ms Niki Aramburo, Cardiac Electrophysiology Data Manager, for her excellent knowledge and upkeeping of the laser lead registry and Ms Gini Roberts for all administrative support.
Abbreviations list
- BMI
Body Mass Index
- CIED
Cardiac implantable electrical devices
- CT
Computed tomography
- LLE
Laser lead extraction
- OR
Odds ratio
References
- 1.Kusumoto FM, Schoenfeld MH, Wilkoff BL et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart rhythm : the official journal of the Heart Rhythm Society 2017;14:e503–e551. [DOI] [PubMed] [Google Scholar]
- 2.Rusanov A, Spotnitz HM. A 15-year experience with permanent pacemaker and defibrillator lead and patch extractions. The Annals of thoracic surgery 2010;89:44–50. [DOI] [PubMed] [Google Scholar]
- 3.Rickard J, Wilkoff BL. Extraction of implantable cardiac electronic devices. Current cardiology reports 2011;13:407–14. [DOI] [PubMed] [Google Scholar]
- 4.Smith MC, Love CJ. Extraction of transvenous pacing and ICD leads. Pacing and clinical electrophysiology : PACE 2008;31:736–52. [DOI] [PubMed] [Google Scholar]
- 5.Lewis RK, Pokorney SD, Greenfield RA et al. Preprocedural ECG-gated computed tomography for prevention of complications during lead extraction. Pacing and clinical electrophysiology : PACE 2014;37:1297–305. [DOI] [PubMed] [Google Scholar]
- 6.Ehieli WL, Boll DT, Marin D, Lewis R, Piccini JP, Hurwitz LM. Use of Preprocedural MDCT for Cardiac Implantable Electric Device Lead Extraction: Frequency of Findings That Change Management. AJR American journal of roentgenology 2017;208:770–776. [DOI] [PubMed] [Google Scholar]
- 7.Vogler J, Pecha S, Azarrafiy R et al. Navigation of lead extraction-is it possible? Impact of preprocedural electrocardiogram-triggered computed tomography on navigation of lead extraction. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery 2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


