Abstract
Cannabinoid (CB) receptor agonists are of growing interest as targets for anti-seizure therapies. Here we examined the effect of systemic administration of the CB receptor agonist WIN 55,212-2 (WIN) against audiogenic seizures (AGSs) in the Genetically Epilepsy Prone Rat (GEPR)-3 strain, and against seizures evoked focally from the Area Tempestas (AT). We compared these results to the effect of focal administration of the CB1/2 receptor agonist CP 55940 into the deep layers of the superior colliculus (DLSC), a brain site expressing CB1 receptors. While systemic administration of WIN dose-dependently decreased AGS in GEPR-3s, it was without effect in the AT model. By contrast, intra-DLSC infusion of CP 55940 decreased seizures in both models. To determine if the effects of systemic WIN were dependent upon activation of CB1 receptors in the DSLC, we next microinjected the CB1 receptor antagonist SR141716, before WIN systemic treatment, and tested animals for AGS susceptibility. The pretreatment of the DLSC with SR141716 was without effect on its own and did not alter the anti-convulsant action of WIN systemic administration. Thus, while CB receptors in the DLSC are a potential site of anticonvulsant action, they are not necessary for the effects of systemically administered CB agonists.
Keywords: cannabinoid, superior colliculus (SC), area tempestas, piriform cortex, genetically epilepsy prone rat (GEPR)
Introduction
The epilepsies, as a group, are one of the most common neurological disorders and are associated with substantial morbidity, mortality, and economic burden. Identifying therapies to treat epilepsies can be a challenge due to the diversity of the condition. Epileptic seizures can arise in a variety of brain networks, resulting in differing semiology, pharmacosensitivity, and degree of impairment—a drug that works for one type of seizure may be ineffective or exacerbate another. Studies suggested that the deep layers of the superior colliculus (DLSC) are part of the network that generates audiogenic seizures (AGSs), a form of reflex seizures triggered by intense sounds (Faingold and Randall, 1999b). While AGSs are uncommon in humans, they can serve as a model for brainstem seizure activity. In rats and mice, bilateral lesions of the superior colliculus, microinjection of GABA antagonist (Depaulis et al., 1990), N-methyl-D-aspartate (NMDA) antagonist (Faingold and Casebeer, 1999a; Raisinghani and Faingold, 2003) or alpha1-noradrenergic agonist (Faingold and Casebeer, 1999a) markedly attenuated AGS severity (Merrill et al., 2003). In a different approach, optogenetic activation of DLSC also attenuates seizures in Genetically Epilepsy-Prone Rat (GEPR) 3 substrain (Soper et al., 2016; Wicker et al., 2019). Activation of the DLSC also potently suppresses seizures in other models of epilepsy, including the maximal electroshock model (Redgrave et al., 1992; Gale et al., 1993), evoked and genetic absence seizure models (Nail-Boucherie et al., 2002, 2005; Soper et al., 2016); the Area Tempestas (AT) model of forebrain seizures (Soper et al., 2016), and the pentylenetetrazole model of generalized seizures (Weng and Rosenberg, 1992; Soper et al., 2016). Further elucidating the neurotransmitter systems in the networks for brainstem and forebrain seizures may offer novel therapeutic targets for the treatment of generalized seizures.
The endocannabinoid system has shown promise as a target for seizure control, but there are conflicting reports about the efficacy of cannabinoid (CB) receptor targeting compounds in suppressing seizures (Rosenberg et al., 2017). One of the most promising candidates are CB1 receptor agonists, which exert anticonvulsant effects against generalized tonic seizures in the maximal electroshock test (Luszczki et al., 2006; Florek-Luszczki et al., 2014b; Tutka et al., 2018), but produce mixed effects against generalized seizures evoked by pentylenetetrazole (Bahremand et al., 2008a; Andres-Mach et al., 2012; Vilela et al., 2013; Aghaei et al., 2015; Huizenga et al., 2017) and limbic seizures in the kainic acid model (Bojnik et al., 2012; Shubina et al., 2015). These models are characterized by different types of seizures: the former by tonic seizures that critically rely on brainstem networks (Merrill et al., 2003, 2005), and the later by limbic seizures that critically rely on forebrain networks (Browning et al., 1993).
The CB1 receptor is the major CB receptor found in the central nervous system, and is coupled to Gi signaling cascades; its activation is typically associated with decreased transmitter release. Endocannabinoid signaling mediated through presynaptic CB1 receptors reduces both glutamate and GABA release (Kathmann et al., 1999; Sullivan, 1999; Hájos et al., 2000), and therefore is a potent regulator of neuronal excitability. Somewhat paradoxically, in the context of seizure suppression, the CB1 receptor co-localizes with GABA neurons in many brain regions it has been suggested that the primary cell type that expresses CB1 receptor is inhibitory (Katona et al., 1999, 2001). This is of particular interest in the context of the DLSC, as disinhibition of the DLSC by silencing GABAergic inputs from the substantia nigra pars reticulata (SNpr) is also potently anticonvulsant (Wicker et al., 2019). Both the DLSC and the SNpr express CB1 receptors, with the SNpr displaying some of the highest receptor density in the brain (Herkenham et al., 1991). Thus, here, we sought to determine the effect of CB1 receptor agonists on brainstem seizures in GEPR-3 rats and forebrain seizures in the Area Tempestas model while comparing the effects of systemic and intra-DLSC delivery of CB1 agonist.
Materials and Methods
Animals
Experiments were performed on male Sprague–Dawley (SD) purchased from Envigo (Frederick, MD, USA) rats and GEPR-3s obtained from a colony maintained at Georgetown University. Animals weighed 270–285 g at the time of surgery and were housed two per cage in the Georgetown University Division of Comparative Medicine under environmentally controlled conditions (12 h light/dark cycle, lights on between 6:00 AM and 6:00 PM; ambient temperature 23°C ± 1°C; controlled humidity) with food (Lab Diet, #5001) and water provided ad libitum. All the procedures and experiments were conducted following the Association for Assessment and Accreditation of Laboratory Animal Care standards, the Guide for the Care and Use of Laboratory Animals (Rowan, 1979), and were approved by the Georgetown University Care and Use Committee.
Surgery
Surgeries were performed as previously described (Santos et al., 2018). Briefly, rats were anesthetized with equithesin (2.8 mg/kg, i.p.) and placed into a stereotaxic frame (David Kopf, Tujunga, CA, USA). Surgery was conducted with the incisor bar at +5.0 mm above the interaural line according to the atlas of Pellegrino and Cushman (1967). For all microinjection experiments, a 22 g guide cannula (Plastics One, Roanoke, VA, USA) was paired with a 28 g internal cannula with the internal cannula extended 2 mm deeper than the guide. A screw cap was placed on the guide cannula to keep cannula tracks clean. Cannulae were implanted unilaterally into the AT (Piredda and Gale, 1985; 4.0 mm anterior to Bregma, 3.5 mm lateral to the midline, 5.5 mm ventral to the dura) and/or into the DLSC (5.0 mm posterior to Bregma, 2.5 mm lateral to the midline, and 4.5 mm ventral to the dura; Pellegrino and Cushman, 1967). Atlas panels shown in the Figures are reproduced in modified form from the Brain Maps 4.0 atlas (Swanson, 2018). Cannulae were fixed in place with jeweler’s screws and dental acrylic. Following surgery, all animals were given at least 1 week to recover before behavioral testing.
Systemic Drug Administration
The CB1 receptor agonist, WIN [WIN 55212-2 mesylate (R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl) methyl]pyrrolol[1,2, 3 de]-1,4-benzoxazinyl]-(1-naphthalenyl) methanone)], was obtained from Tocris Biosciences (Bristol, UK) and dissolved in 50% of DMSO and 0.9% saline. Solutions were prepared at a concentration of 2 mg/ml and drug or vehicle was administered by intraperitoneal (i.p.) injection at a volume of 1 ml/kg. The drug was administered 20 min before seizure testing.
CB Drug Microinfusion
The CB1/2 receptor agonist CP 55940 (CP) was dissolved in 50% DMSO-50% saline, and 26.5 nmol (in 0.5 μl) was microinfused bilaterally into the DLSC. We selected CP 55940 over WIN for microinjection, because it has a higher affinity for the CB1 receptor than does WIN. By contrast, we selected WIN for systemic administration because its anticonvulsant profile has been better characterized than that of systemic CP. The CB1 receptor antagonist SR 141716A (SR, rimonabant) was dissolved in 50% DMSO-50% saline, and 1 μg (4.3 nmol) in 0.5 μl was infused into the DLSC. These drug doses were chosen based on published reports (Sañudo-Peña et al., 2000; Citraro et al., 2013; Florek-Luszczki et al., 2014a). Drug microinfusions were performed essentially as we have previously described (Wicker and Forcelli, 2016; Wicker et al., 2019). In brief, rats were gently restrained and a 28-gauge internal cannula was inserted. Drug infusion was controlled by a syringe pump driving a Hamilton syringe programmed to deliver the drug at a rate of 0.2 μl/min. After the completion of the infusion, the internal cannula was left in place for at least 1 min to reduce drug reflux up the cannula track and then removed. Drug microinfusion was performed 5 min before seizure testing.
Piriform Cortex (Area Tempestas) Seizures
Twelve SD rats were used for these experiments. A stainless-steel guide cannula was stereotaxically implanted above the left AT as described above. Bicuculline methiodide (BMI; Sigma–Aldrich) was dissolved in 0.9% saline at a concentration of 1 mM, and a dose of 100–280 pmols (0.2 μl/min) was used to induce seizures. Bicuculline was injected immediately following intra-DLSC injections or 20 min following systemic WIN. Initially, a 100 pmol dose of bicuculine was microinjected into the AT; in the absence of seizure activity, the amount of bicuculline injected was increased (increments of 20 pmol) on a subsequent day until a seizure with the score of 3–5 on the modified Racine scale (see below) was reached. After AT injections were completed, SD rats were monitored for 60 min in a transparent plastic box, and behaviors were documented by an observer in real-time. In some SD rats, electroencephalogram (EEG) was used to monitor electrical activity correlated with behavioral seizures. During stereotaxic surgery, six holes were made in the skull so that six EEG screw wire electrodes could be screwed in for dura-mater contact. Placement of these screws was bilateral as follows: frontal lobe, parietal lobe, and cerebellum. These EEG wire electrodes were routed into an EEG pedestal (Plastics One, Roanoke, VA, USA) that was secured to the skull with dental acrylic. EEG recordings were performed in awake animals with the EEG pedestal coupled to an EEG preamplifier and amplifier (Pinnacle Technologies, Lawrence, KS, USA). The raw signal was routed to a PowerLab analog-to-digital converted and data were recorded with LabChart software (AD Instruments, Colorado Springs, CO, USA) with a 60 Hz low pass filter. After each experimental session, rats were returned to their home cage and given at least 48 h in between sessions. We used a modified Racine scale for seizure severity described as follows: 0.5 = jaw clonus, 1 = myoclonic jerks, 2 = single-arm forelimb clonus, 3 = bilateral forelimb clonus and facial clonus, 4 = bilateral forelimb and facial clonus with rearing, 5 = loss of balance after rearing with forelimb and facial clonus (Piredda and Gale, 1985; Cassidy and Gale, 1998).
Audiogenic Seizure Testing
GEPR-3 rats were first tested for response to AGSs by challenging them with 105–110 dB pure tones (Med Associates, St. Albans, VT, USA) for 60 s. In the cases of no seizure activity, the animals were given a 120 dB mixed sounds produced by an electrical bell. Following the establishment of baseline AGS score, the animals were given 60 min to recover and then tested again under a given experimental condition. The animals were then tested a final time for AGSs after an additional 20 min. Only GEPR-3s that exhibited AGSs with a score of 2 or greater were used in the pharmacological experiments. AGS seizure severity was classified into four stages as follows: 0 = no seizures, 1 = one episode of wild running seizures (WRS), 2 = two or more episodes of WRS, 3 = one episode of WRS followed by tonic-clonic seizures. Behavioral outcomes assessed included maximum seizure severity score reached, latency to seizure onset, and duration of the seizure. Seizure severity was scored by two observers in real-time and recorded with a video monitoring system (Med Associates, St. Albans, VT, USA).
Statistics and Data Analysis
Statistical analyses were performed in GraphPad Prism version 6 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS version 25 (IBM). Non-parametric data and data that failed tests of normality (e.g., seizure severity, seizure frequency, and seizure count) were analyzed using the Wilcoxon Matched Pairs test for paired data or Friedman’s test with Dunn’s multiple comparison test.
Results
We first evaluated the ability of systemic administration of WIN to attenuate AGSs in GEPR-3s; the testing strategy is shown in Figure 1A. GEPR-3s were first tested for a baseline AGS susceptibility. Thirty minutes later, GEPR-3s were injected with either vehicle or WIN (1, 1.5, or 2 mg/kg) and re-tested for AGS 30 min after injection. As shown in Figure 1B, the 1 mg/kg dose of WIN did not alter AGS responses (Friedman’s test, Q = 0.625, p = 0.94). In all cases (Baseline 1, Vehicle, Baseline 2, and 1 mg/kg of WIN) the median seizure score was 3. We found a similar lack of effect following the 1.5 mg/kg dose of WIN (Figure 1C); the median seizure score for each group was 3, and thus did not differ as a function of treatment (Friedman’s test, Q = 5.0, p = 0.24). Unlike the lower doses, 2 mg/kg of WIN produced a robust suppression of seizure activity (median seizure = 0), concurrent with mild sedation. This significant reduction in seizure severity (Q = 24.97, p = 0.000016, Friedman’s test) was driven by the difference between the second baseline session and the 2 mg/kg treated post-test (p = 0.0021, Dunn’s multiple comparison test). The 2 mg/kg dose also differed significantly from the vehicle (p = 0.0073). Importantly, the two baseline sessions did not differ from one another (p > 0.999), nor did the vehicle treatment differ from the baseline (p > 0.999).
Figure 1.
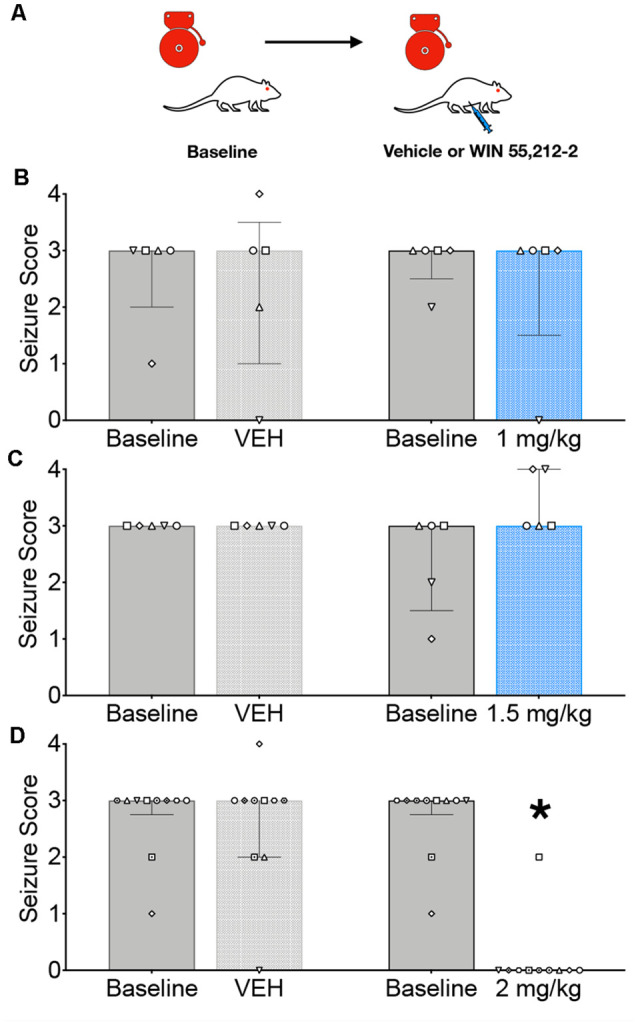
Systemic administration of the CB1/2 receptor agonist, WIN 55,212-2 (WIN) attenuates audiogenic seizures in genetically epilepsy-prone rats (GEPRs). (A) Schematic of the testing scheme; animals were tested with a baseline test session before a vehicle-treated session. On a separate day, animals were tested on a baseline session and then with one of three doses of WIN. The testing order was counterbalanced across subjects. (B) Against audiogenic seizures (AGS) scores showing baseline sessions and comparing vehicle (left) and 1 mg/kg WIN (right). (C) AGS scores showing baseline sessions and comparing vehicle (left) and 1.5 mg/kg WIN (right). (D) AGS scores showing baseline sessions and comparing vehicle (left) and 2 mg/kg (right). Bars indicate median and interquartile range, symbols show individual subjects. *p < 0.05, according to Friedman test followed by Dunn’s post hoc test.
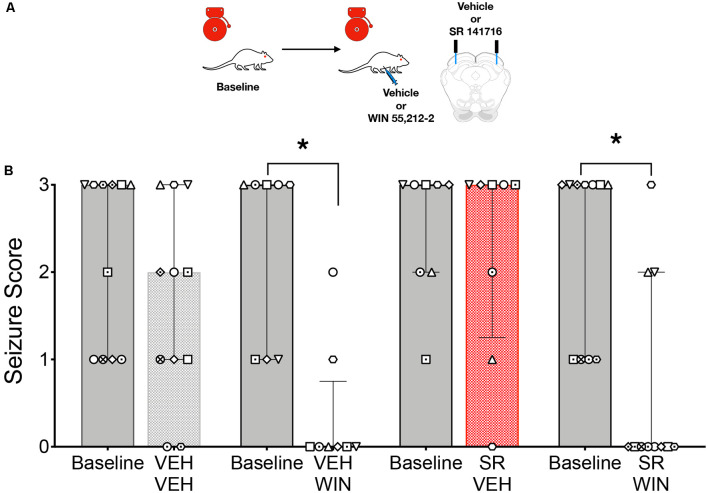
We next sought to determine if focal activation of CB1 receptors in the DLSC would impair the effect of systemic drug administration; the testing scheme for these experiments is shown in Figure 2A. GEPR-3s were tested for baseline AGS susceptibility and then injected with vehicle or CP in a counterbalanced manner. During the un-injected (baseline) audiogenic susceptibility test and the vehicle injection test, the median seizure score was a 3. However, CP injection produced a significant suppression of seizure responses (median = 0; Q = 15.08, p = 0.0002; Friedman’s test, Figure 2B). Baseline and vehicle-injected conditions did not differ from one another (p > 0.999, Dunn’s multiple comparison test). CP injection, by contrast, significantly reduced AGS as compared both to the baseline session (p = 0.0065) and the vehicle-injected session (p = 0.029).
Figure 2.
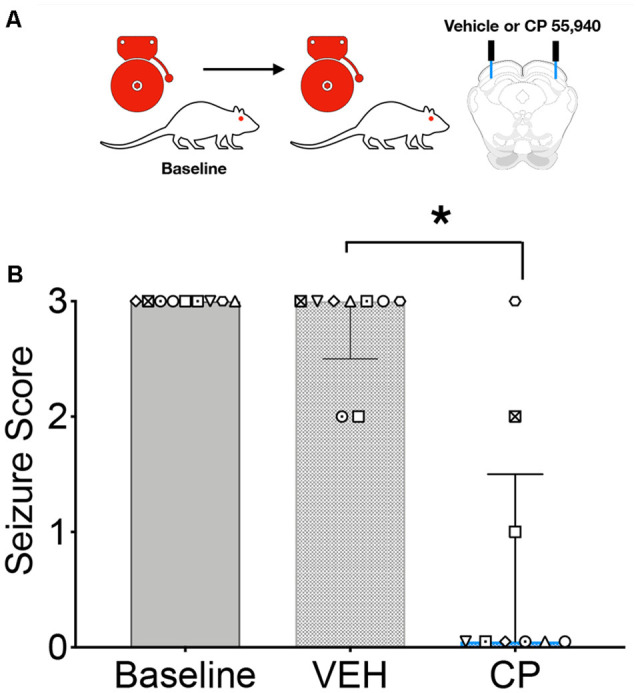
Intracollicular injection of the CB1/2 receptor agonist, CP 55940 (CP) attenuates audiogenic seizures in GEPRs. (A) Schematic of the testing scheme; animals were tested with a baseline test session before a vehicle-treated session and a CP treated session. A baseline is shown only once, as in this experiment all animals displayed baseline scores of three across both tests. (B) Microinfusion of CP significantly decreased audiogenic seizure score as compared to vehicle (VEH) infusion into the deep layers of the superior colliculus (DLSC). Bars indicate median and interquartile range, symbols show individual subjects. *p < 0.05, according to Friedman test followed by Dunn’s post hoc test.
Given that focal delivery of CB1/2 agonist to the DLSC resulted in a suppression of seizures that was reminiscent of that seen after systemic administration of agonist, we next sought to determine if activation of CB1/2 receptors in the DLSC was necessary for the seizure-suppressive effects of systemic drug treatment. The experimental scheme is shown in Figure 3A. GEPR-3s were tested on a baseline AGS test and then retested following systemic administration of vehicle or WIN paired with an intra-DLSC injection of vehicle or the CB1 receptor antagonist SR141716. Due to premature implant loss, not all animals received all treatments; we, therefore, analyzed these sessions independently. When the systemic vehicle was paired with the intra-DLSC vehicle, seizure responses did not differ from the baseline session (W = −11, p = 0.25, Figure 3B). Similar to what we previously observed (Figure 1), systemic administration of WIN when paired with an intra-DLSC vehicle infusion produced a significant reduction in seizure severity when compared to the baseline session (W = −36, p = 0.0078). Intra-DLSC administration of the CB antagonist SR141716, when paired with systemic vehicle injection was without effect on seizure severity as compared to the baseline session (W = −2, p = 0.75). By contrast, we observed robust seizure suppression following systemic administration of WIN, paired with intra-DLSC administration of SR141716 (W = −55, p = 0.002). Thus, blockade of CB receptors in the DLSC did not alter the anticonvulsant action of WIN.
Figure 3.
Activation of collicular CB1/2 receptors is sufficient, but not necessary to account for the anticonvulsant effect of systemic WIN 55,212-2 (WIN) in GEPRs. (A) Schematic of the testing scheme; animals were tested with a baseline test session before each of the four test conditions. Animals received either vehicle (VEH) or SR 141716 (SR) infusion into the DLSC followed by VEH or WIN (2 mg/kg, i.p.). (B) AGS score as a function of drug treatments. WIN administration significantly reduced AGS severity as compared to the pre-administration baseline. SR administration was without effect on audiogenic seizures (AGSs) but failed to reverse the anticonvulsant action of WIN. Bars show median and interquartile range, symbols show individual subjects. *p < 0.05, according to Friedman test followed by Dunn’s post hoc test.
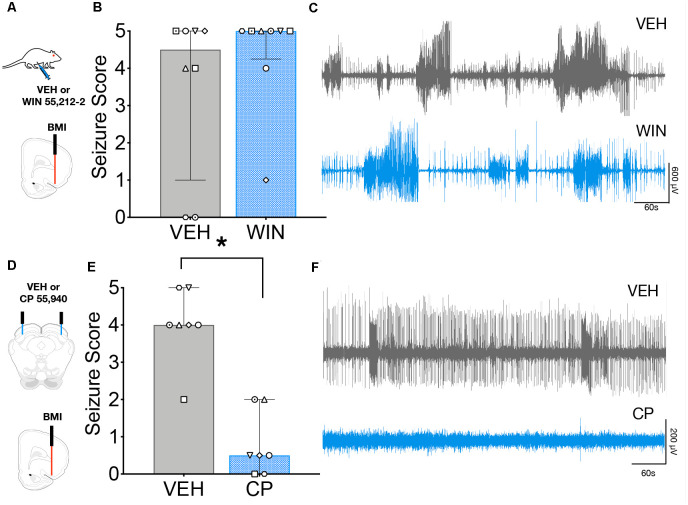
To determine if this anticonvulsant profile held for a seizure model reliant on forebrain networks, we examined the effect of systemic (Figure 4A) and intra-DLSC (Figure 4B) administration of CB1/2 agonist against seizures evoked focally from the AT. We used a high dose of WIN (2 mg/kg) for these experiments. We found that while this dose produced mild sedation, it did not suppress behavioral (Figure 4B) or electrographic (Figure 4C) seizures. Systemic injection of the vehicle was associated with a median seizure severity of 4.5, whereas WIN administration at the dose of 2 mg/kg was associated with a median seizure score of 5 (W = 13, p = 0.41). In contrast to the lack of effect following systemic administration of WIN, intra-DLSC injection of CP was associated with robust suppression of AT-evoked seizures. Following vehicle injection into the DLSC (Figure 4D), the median AT evoked seizure response was 4 (Figure 4E). By contrast, after the intra-DLSC injection of CP, the median response was 0.5, an effect that reached the level of statistical significance (W = −28, p = 0.016). Thus, unlike the case of AGSs, systemic CB agonists did not protect against AT-evoked seizures, but intra-DLSC activation of CB receptors potently suppressed behavioral (Figure 4E) and electrographic (Figure 4F) seizures.
Figure 4.
Intracollicular, but not systemic cannabinoid (CB) receptor activation suppresses seizures evoked from Area Tempestas (AT). (A) Seizures were evoked by injection of the GABA-A receptor antagonist, bicuculline methiodide (BMI) into the AT after systemic administration of VEH or WIN 55,212-2. (B) Behavioral seizure severity under the vehicle (VEH) and WIN treated conditions did not differ. (C) Representative electrographic seizures from the same subject under VEH and WIN treated conditions, showing multiple high amplitude events and isolated interictal spiking. (D) Seizures were evoked by injection of BMI into the AT following either VEH or CP infusion into the DLSC. (E) Seizure severity was significantly reduced by CP infusion into the DLSC. (F) Representative electrographic activity following treatment of the DLSC with VEH (top) and CP (bottom) from the same subject, showing that behavioral seizure suppression co-occurred with suppression of electrographic seizure activity. Bars show median and interquartile range, symbols show individual subjects. *p < 0.05.
Discussion
Here we report a surprising dissociation between the effects of CB agonists when administered systemically and intra-DLSC across two models of experimental seizures. While systemic administration of the CB1/2 receptor agonist WIN exerted a dose-dependent suppression of AGSs in GEPR-3s, systemic administration was without effect against seizures evoked from AT. By contrast, intra-DLSC administration of the CB1 receptor agonist CP was effective in both models. Interestingly, while activation of the CB1 receptor in the DLSC was sufficient to suppress seizure activity, these CB1 receptors are not necessary—for the anticonvulsant action of WIN.
CB receptor agonists have been widely studied for anti-seizure effects across an array of models of seizures; this has been reviewed extensively elsewhere (Wallace et al., 2001; Skaper and Di Marzo, 2012; Cristino et al., 2020). Our results are generally consistent with previous reports examining the effect of WIN on seizure susceptibility in two other AGS models. For example, a single dose of WIN failed to suppress acute AGS in Krushinsky-Molodkina rats, it delays the development of audiogenic kindling suggestive of an antiepileptogenic effect (Vinogradova and van Rijn, 2015). Furthermore, chronic treatment with CB1 antagonist SR141716A worsens AGS (Vinogradova et al., 2011). While the GEPR-3 and the Krushinsky–Molodkina rat share phenotypic similarities, we cannot exclude a contribution of strain to the differences between published reports and our present studies. Our results are likewise consistent with the findings of Citraro et al. (2016) who reported that WIN reduced AGS severity in DBA/2 mice. Similarly, activation of CB1 receptors protected against acute clonic and generalized tonic-clonic seizures in pentylenetetrazole model (Bahremand et al., 2008b) and maximal electroshock test (Wallace et al., 2001; Luszczki et al., 2011; Florek-Luszczki et al., 2014b; Florek-Luszczki et al., 2015). Thus, activation of CB1 receptors is effective in suppressing seizures that require hindbrain circuitry.
In the present study, we found that systemic administration of WIN did not alter the occurrence of AT-evoked seizure. By contrast, systemic administration of WIN reduced the incidence of spontaneous seizures in the post-status epilepticus pilocarpine model (Wallace, 2003; Di Maio et al., 2015), and reduced the seizure severity of acute pilocarpine-induced status epilepticus (Colangeli et al., 2019; Di Maio et al., 2019). Moreover, WIN protects against acute temporal-lobe like seizures in the maximal dentate activation model (Carletti et al., 2016). The AT model differs from pilocarpine in both its focal nature (vs. systemic pilocarpine) and its pharmacology (GABA antagonist vs. muscarinic agonist). It also differs in the locus of seizure origination (piriform cortex) as compared to the maximal dentate activation model (dentate gyrus). Of particular note is the dense expression of CB1 receptors on interneurons of the anterior piriform cortex (Terral et al., 2019), notably this area of dense receptor expression overlaps with the region functionally defined as AT. This raises the possibility that systemic administration of WIN may have further disinhibited the piriform cortex, negating anticonvulsant actions occurring at other sites in the brain.
We had hypothesized that the DLSC might be a critical site of action for CB-mediated anti-convulsant effects. This was based on: (1) the well-established role the DLSC plays in audiogenic seizures, and in particular, in the wild-running phase which is typical of the GEPR-3 strain (Merrill et al., 2005); (2) volumetric changes in the SC in GEPR-3 rats (Lee et al., 2018); (3) the interconnections between the substantia nigra pars reticulata and the DLSC and the density of CB1 receptors in the SNpr (Herkenham et al., 1991; Matsuda et al., 1992; Glass et al., 1997; Tsou et al., 1998); (4) the presence of CB1 immunopositive axons in the DLSC (Tsou et al., 1998; Sañudo-Peña et al., 2000); (5) the anticonvulsant effects of activation of the DLSC (Soper et al., 2016); and (6) the anticonvulsant effects of disinhibition of the SC through inhibition of nigrotectal projections (Wicker et al., 2019). Consistent with our hypothesis, we found that the focal application of CP to the DLSC suppressed both AGSs and AT-evoked seizures. These findings are, however, generally consistent with a disinhibitory action of CP in the DLSC. While we found that focal microinjection of CB1/2 agonist into the DLSC attenuated both AGSs and AT-evoked seizures, we found that the focal blockade of CB1 receptors in the DLSC was without effect on the anticonvulsant action of systemic agonist, suggesting an extra-DLSC site of the action of the agonist. A potential site of interest is the inferior colliculus that is critical in AGS initiation (see Faingold et al., 2014 for a review of audiogenic seizure networks), however, while there is an expression of CB1 receptors in the IC, the expression is less than that observed in the SC (Herkenham et al., 1991).
While the CB1 receptor is the predominant CB receptor in the brain, the CB2 receptor is also expressed in the brainstem (Van Sickle et al., 2005), cerebellum (Ashton et al., 2006; Suárez et al., 2008), and other brain regions (Gong et al., 2006). While selective CB1 receptor agonists and mixed CB1/CB2 receptor agonists have well-documented anticonvulsant effects, selective CB2 agonists are both less studied and less robust (for review see: Rosenberg et al., 2017). While WIN and CP are mixed CB1/2 agonists, the profile of anticonvulsant effects seen with WIN is similar to those of the highly selective CB1 agonist, ACEA (Rosenberg et al., 2017), suggesting that anticonvulsant effects are CB1, rather than CB2 mediated. Moreover, CB2 receptor expression in the SC (Gong et al., 2006; Duff et al., 2013) has been predominantly detected in the visual (superficial) as compared to the deep and intermediate layers, which are the sites associated with anticonvulsant action (Gale et al., 1993; Redgrave et al., 1993). Thus, it is unlikely that the anticonvulsant effects of intracollicular CP are mediated by CB2 receptors.
In sum, our data suggest that focal modulation of CB receptors within the DLSC controls both brainstems- and forebrain-evoked seizures, but also show that this site of action is not necessary for the anticonvulsant effect of systemic CB1/2 receptor agonists. While in the brainstem model, both systemic and intra-DLSC drug treatment reduced seizures, in the forebrain model, intra-DLSC drug infusion but not systemic drug treatment reduced seizures. These data add to our understanding of potential sites of action of CBs in the context of anti-seizure therapy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Georgetown University Animal Care and Use Committee.
Author Contributions
VS and RH contributed equally. VS, RH, PN’G, and PF designed research. VS, RH, and EW performed experiments. VS, RH, and PF analyzed data. VS, RH, and PF drafted the manuscript, which all authors edited. PN’G and PF obtained funding and supervised the study. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by R01NS097762 from the National Institute of Neurological Disorders and Stroke to PF and R01 AA027660 from the National Institute on Alcohol Abuse and Alcoholism to PN’G. PF was supported in part by KL2TR001432 from the National Center for Advancing Translational Sciences.
References
- Aghaei I., Rostampour M., Shabani M., Naderi N., Motamedi F., Babaei P., et al. (2015). Palmitoylethanolamide attenuates PTZ-induced seizures through CB1 and CB2 receptors. Epilepsy Res. 117, 23–28. 10.1016/j.eplepsyres.2015.08.010 [DOI] [PubMed] [Google Scholar]
- Andres-Mach M., Zolkowska D., Barcicka-Klosowska B., Haratym-Maj A., Florek-Luszczki M., Luszczki J. J. (2012). Effect of ACEA-a selective cannabinoid CB1 receptor agonist on the protective action of different antiepileptic drugs in the mouse pentylenetetrazole-induced seizure model. Prog. Neuropsychopharmacol. Biol. Psychiatry 39, 301–309. 10.1016/j.pnpbp.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Ashton J. C., Friberg D., Darlington C. L., Smith P. F. (2006). Expression of the cannabinoid CB2 receptor in the rat cerebellum: an immunohistochemical study. Neurosci. Lett. 396, 113–116. 10.1016/j.neulet.2005.11.038 [DOI] [PubMed] [Google Scholar]
- Bahremand A., Shafaroodi H., Ghasemi M., Nasrabady S. E., Gholizadeh S., Dehpour A. R. (2008a). The cannabinoid anticonvulsant effect on pentylenetetrazole-induced seizure is potentiated by ultra-low dose naltrexone in mice. Epilepsy Res. 81, 44–51. 10.1016/j.eplepsyres.2008.04.010 [DOI] [PubMed] [Google Scholar]
- Bahremand A., Shafaroodi H., Ghasemi M., Nasrabady S. E., Gholizadeh S., Dehpour A. R. (2008b). The cannabinoid anticonvulsant effect on pentylenetetrazole-induced seizure is potentiated by ultra-low dose naltrexone in mice. Epilepsy Res. 81, 44–51. 10.1016/j.eplepsyres.2008.04.010 [DOI] [PubMed] [Google Scholar]
- Bojnik E., Turunç E., Armağan G., Kanıt L., Benyhe S., Yalçın A., et al. (2012). Changes in the cannabinoid (CB1) receptor expression level and G-protein activation in kainic acid induced seizures. Epilepsy Res. 99, 64–68. 10.1016/j.eplepsyres.2011.10.020 [DOI] [PubMed] [Google Scholar]
- Browning R., Maggio R., Sahibzada N., Gale K. (1993). Role of brainstem structures in seizures initiated from the deep prepiriform cortex of rats. Epilepsia 34, 393–407. 10.1111/j.1528-1157.1993.tb02579.x [DOI] [PubMed] [Google Scholar]
- Carletti F., Gambino G., Rizzo V., Ferraro G., Sardo P. (2016). Involvement of TRPV1 channels in the activity of the cannabinoid WIN 55, 212-2 in an acute rat model of temporal lobe epilepsy. Epilepsy Res. 122, 56–65. 10.1016/j.eplepsyres.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Cassidy R. M., Gale K. (1998). Mediodorsal thalamus plays a critical role in the development of limbic motor seizures. J. Neurosci. 18, 9002–9009. 10.1523/JNEUROSCI.18-21-09002.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citraro R., Russo E., Ngomba R. T., Nicoletti F., Scicchitano F., Whalley B. J., et al. (2013). CB1 agonists, locally applied to the cortico-thalamic circuit of rats with genetic absence epilepsy, reduce epileptic manifestations. Epilepsy Res. 106, 74–82. 10.1016/j.eplepsyres.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Citraro R., Russo E., Leo A., Russo R., Avagliano C., Navarra M., et al. (2016). Pharmacokinetic-pharmacodynamic influence of N-palmitoylethanolamine, arachidonyl-2′-chloroethylamide and WIN 55, 212-2 on the anticonvulsant activity of antiepileptic drugs against audiogenic seizures in DBA/2 mice. Eur. J. Pharmacol. 791, 523–534. 10.1016/j.ejphar.2016.09.029 [DOI] [PubMed] [Google Scholar]
- Colangeli R., Di Maio R., Pierucci M., Deidda G., Casarrubea M., Di Giovanni G. (2019). Synergistic action of CB1 and 5-HT 2B receptors in preventing pilocarpine-induced status epilepticus in rats. Neurobiol. Dis. 125, 135–145. 10.1016/j.nbd.2019.01.026 [DOI] [PubMed] [Google Scholar]
- Cristino L., Bisogno T., Di Marzo V. (2020). Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 16, 9–29. 10.1038/s41582-019-0284-z [DOI] [PubMed] [Google Scholar]
- Depaulis A., Liu Z., Vergnes M., Marescaux C., Micheletti G., Warter J. M. (1990). Suppression of spontaneous generalized non-convulsive seizures in the rat by microinjection of GABA antagonists into the superior colliculus. Epilepsy Res. 5, 192–198. 10.1016/0920-1211(90)90038-w [DOI] [PubMed] [Google Scholar]
- Di Maio R., Cannon J. R., Timothy Greenamyre J. (2015). Post-status epilepticus treatment with the cannabinoid agonist WIN 55, 212-2 prevents chronic epileptic hippocampal damage in rats. Neurobiol. Dis. 73, 356–365. 10.1016/j.nbd.2014.10.018 [DOI] [PubMed] [Google Scholar]
- Di Maio R., Colangeli R., Di Giovanni G. (2019). WIN 55, 212-2 reverted pilocarpine-induced status epilepticus early changes of the interaction among 5-HT2C/NMDA/CB1 receptors in the rat hippocampus. ACS Chem. Neurosci. 10, 3296–3306. 10.1021/acschemneuro.9b00080 [DOI] [PubMed] [Google Scholar]
- Duff G., Argaw A., Cecyre B., Cherif H., Tea N., Zabouri N. (2013). Cannabinoid receptor CB2 modulates axon guidance. PLoS One 8:e70849. 10.1371/journal.pone.0070849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold C., Casebeer D. (1999a). Modulation of the audiogenic seizure network by noradrenergic and glutamatergic receptors of the deep layers of superior colliculus. Brain Res. 821, 392–399. 10.1016/s0006-8993(99)01101-4 [DOI] [PubMed] [Google Scholar]
- Faingold C. L., Randall M. E. (1999b). Neurons in the deep layers of superior colliculus play a critical role in the neuronal network for audiogenic seizures: mechanisms for production of wild running behavior. Brain Res. 815, 250–258. 10.1016/s0006-8993(98)01136-6 [DOI] [PubMed] [Google Scholar]
- Faingold C. L., Raisinghani M., N’Gouemo P. (2014). “Neuronal networks in epilepsy: comparative audiogenic seizure networks,” in Neuronal Networks in Brain Function, CNS Disorders and Therapeutics, eds Faingold C. L., Blumenfeld H. (Sandiego, CA: Elsevier/Academic Press; ), 349–373. [Google Scholar]
- Florek-Luszczki M., Wlaz A., Kondrat-Wrobel M. W., Tutka P., Luszczki J. J. (2014a). Effects of WIN 55, 212-2 (a non-selective cannabinoid CB1 and CB2 receptor agonist) on the protective action of various classical antiepileptic drugs in the mouse 6 Hz psychomotor seizure model. J. Neural Transm. 121, 707–715. 10.1007/s00702-014-1173-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florek-Luszczki M., Zagaja M., Luszczki J. J. (2014b). Influence of WIN 55, 212-2 on the anticonvulsant and acute neurotoxic potential of clobazam and lacosamide in the maximal electroshock-induced seizure model and chimney test in mice. Epilepsy Res. 108, 1728–1733. 10.1016/j.eplepsyres.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Florek-Luszczki M., Zagaja M., Luszczki J. J. (2015). Influence of arachidonyl-2′-chloroethylamide, a selective cannabinoid CB1 receptor agonist, on the anticonvulsant and acute side-effect potentials of clobazam, lacosamide and pregabalin in the maximal electroshock-induced seizure model and chimney test in mice. Fundam. Clin. Pharmacol. 29, 382–393. 10.1111/fcp.12123 [DOI] [PubMed] [Google Scholar]
- Gale K., Pazos A., Maggio R., Japikse K., Pritchard P. (1993). Blockade of GABA receptors in superior colliculus protects against focally evoked limbic motor seizures. Brain Res. 603, 279–283. 10.1016/0006-8993(93)91248-q [DOI] [PubMed] [Google Scholar]
- Glass M., Dragunow M., Faull R. L. M. (1997). Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77, 299–318. 10.1016/s0306-4522(96)00428-9 [DOI] [PubMed] [Google Scholar]
- Gong J.-P., Onaivi E. S., Ishiguro H., Liu Q.-R., Tagliaferro P. A., Brusco A., et al. (2006). Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 1071, 10–23. 10.1016/j.brainres.2005.11.035 [DOI] [PubMed] [Google Scholar]
- Hájos N., Katona I., Naiem S. S., MacKie K., Ledent C., Mody I., et al. (2000). Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur. J. Neurosci. 12, 3239–3249. 10.1046/j.1460-9568.2000.00217.x [DOI] [PubMed] [Google Scholar]
- Herkenham M., Lynn A. B., de Costa B. R., Richfield E. K. (1991). Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 547, 267–274. 10.1016/0006-8993(91)90970-7 [DOI] [PubMed] [Google Scholar]
- Huizenga M. N., Wicker E., Beck V. C., Forcelli P. A. (2017). Anticonvulsant effect of cannabinoid receptor agonists in models of seizures in developing rats. Epilepsia 58, 1593–1602. 10.1111/epi.13842 [DOI] [PubMed] [Google Scholar]
- Kathmann M., Bauer U., Schlicker E., Göthert M. (1999). Cannabinoid CB1 receptor-mediated inhibition of NMDA- and kainate-stimulated noradrenaline and dopamine release in the brain. Naunyn Schmiedebergs Arch. Pharmacol. 359, 466–470. 10.1007/pl00005377 [DOI] [PubMed] [Google Scholar]
- Katona I., Sperlágh B., Sík A., Käfalvi A., Vizi E. S., Mackie K., et al. (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 19, 4544–4558. 10.1523/JNEUROSCI.19-11-04544.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I., Rancz E. A., Acsady L., Ledent C., Mackie K., Hajos N., et al. (2001). Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J. Neurosci. 21, 9506–9518. 10.1523/JNEUROSCI.21-23-09506.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Rodriguez O. C., Albanese C., Santos V. R., de Oliveira J. A. C., Donatti A. L. F., et al. (2018). Divergent brain changes in two audiogenic rat strains: a voxel-based morphometry and diffusion tensor imaging comparison of the genetically epilepsy prone rat (GEPR-3) and the wistar audiogenic rat (WAR). Neurobiol. Dis. 111, 80–90. 10.1016/j.nbd.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino L. J., Cushman A. J. (1967). A Stereotaxic Altasl of The Rat Brain. New York, NY: Appleton-Century-Crofts. [Google Scholar]
- Luszczki J. J., Czuczwar P., Cioczek-Czuczwar A., Czuczwar S. J. (2006). Arachidonyl-2′-chloroethylamide, a highly selective cannabinoid CB1 receptor agonist, enhances the anticonvulsant action of valproate in the mouse maximal electroshock-induced seizure model. Eur. J. Pharmacol. 547, 65–74. 10.1016/j.ejphar.2006.07.037 [DOI] [PubMed] [Google Scholar]
- Luszczki J. J., Andres-Mach M., Barcicka-Klosowska B., Florek-Luszczki M., Haratym-Maj A., Czuczwar S. J. (2011). Effects of WIN 55, 212-2 mesylate (a synthetic cannabinoid) on the protective action of clonazepam, ethosuximide, phenobarbital and valproate against pentylenetetrazole-induced clonic seizures in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1870–1876. 10.1016/j.pnpbp.2011.07.001 [DOI] [PubMed] [Google Scholar]
- Matsuda L. A., Bonner T. I., Lolait S. J. (1992). Cannabinoid receptors: which cells, where, how and why? NIDA Res. Monogr. 126, 48–56. [PubMed] [Google Scholar]
- Merrill M. A., Clough R. W., Jobe P. C., Browning R. A. (2003). Role of the superior colliculus and the intercollicular nucleus in the brainstem seizure circuitry of the genetically epilepsy-prone rat. Epilepsia 44, 305–314. 10.1046/j.1528-1157.2003.31802.x [DOI] [PubMed] [Google Scholar]
- Merrill M. A., Clough R. W., Jobe P. C., Browning R. A. (2005). Brainstem seizure severity regulates forebrain seizure expression in the audiogenic kindling model. Epilepsia 46, 1380–1388. 10.1111/j.1528-1167.2005.39404.x [DOI] [PubMed] [Google Scholar]
- Nail-Boucherie K., Lê-Pham B. T., Marescaux C., Depaulis A. (2002). Suppression of absence seizures by electrical and pharmacological activation of the caudal superior colliculus in a genetic model of absence epilepsy in the rat. Exp. Neurol. 177, 503–514. 10.1006/exnr.2002.7997 [DOI] [PubMed] [Google Scholar]
- Nail-Boucherie K., Lê-Pham B. T., Marescaux C., Depaulis A. (2005). Evidence for a role of the parafascicular nucleus of the thalamus in the control of epileptic seizures by the superior colliculus. Epilepsia 46, 141–145. 10.1111/j.0013-9580.2005.30304.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piredda S., Gale K. (1985). A crucial epileptogenic site in the deep prepiriform cortex. Nature 317, 623–625. 10.1038/317623a0 [DOI] [PubMed] [Google Scholar]
- Raisinghani M., Faingold C. L. (2003). Identification of the requisite brain sites in the neuronal network subserving generalized clonic audiogenic seizures. Brain Res. 967, 113–122. 10.1016/s0006-8993(02)04232-4 [DOI] [PubMed] [Google Scholar]
- Redgrave P., Simkins M., Overton P., Dean P. (1992). Anticonvulsant role of nigrotectal projection in the maximal electroshock model of epilepsy-I. Mapping of dorsal midbrain with bicuculline. Neuroscience 46, 379–390. 10.1016/0306-4522(92)90059-b [DOI] [PubMed] [Google Scholar]
- Redgrave P., Westby G. W. M., Dean P. (1993). Functional architecture of rodent superior colliculus: relevance of multiple output channels. Prog. Brain Res. 95, 69–77. 10.1016/s0079-6123(08)60358-1 [DOI] [PubMed] [Google Scholar]
- Rosenberg E. C., Patra P. H., Whalley B. J. (2017). Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis and epilepsy-related neuroprotection. Epilepsy Behav. 70, 319–327. 10.1016/j.yebeh.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A. N. (1979). Guide for the care and use of laboratory animals. J. Med. Primatol. 8:128 10.1159/000460187 [DOI] [Google Scholar]
- Santos V. R., Kobayashi I., Hammack R., Danko G., Forcelli P. A. (2018). Impact of strain, sex and estrous cycle on gamma butyrolactone-evoked absence seizures in rats. Epilepsy Res. 147, 62–70. 10.1016/j.eplepsyres.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sañudo-Peña M. C., Tsou K., Romero J., Mackie K., Walker J. M. (2000). Role of the superior colliculus in the motor effects of cannabinoids and dopamine. Brain Res. 853, 207–214. 10.1016/s0006-8993(99)02291-x [DOI] [PubMed] [Google Scholar]
- Shubina L., Aliev R., Kitchigina V. (2015). Attenuation of kainic acid-induced status epilepticus by inhibition of endocannabinoid transport and degradation in guinea pigs. Epilepsy Res. 111, 33–44. 10.1016/j.eplepsyres.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Skaper S. D., Di Marzo V. (2012). Endocannabinoids in nervous system health and disease: the big picture in a nutshell. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367, 3193–3200. 10.1098/rstb.2012.0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soper C., Wicker E., Kulick C. V., N’Gouemo P., Forcelli P. A. (2016). Optogenetic activation of superior colliculus neurons suppresses seizures originating in diverse brain networks. Neurobiol. Dis. 87, 102–115. 10.1016/j.nbd.2015.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez J., Bermúdez-Silva F. J., Mackie K., Ledent C., Zimmer A., Cravatt B. F. (2008). Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J. Comp. Neurol. 509, 400–421. 10.1002/cne.21774 [DOI] [PubMed] [Google Scholar]
- Sullivan J. M. (1999). Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J. Neurophysiol. 82, 1286–1294. 10.1152/jn.1999.82.3.1286 [DOI] [PubMed] [Google Scholar]
- Swanson L. W. (2018). Brain maps 4.0-Structure of the rat brain: an open access atlas with global nervous system nomenclature ontology and flatmaps. J. Comp. Neurol. 526, 935–943. 10.1002/cne.24381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terral G., Busquets-Garcia A., Varilh M., Achicallende S., Cannich A., Bellocchio L., et al. (2019). CB1 receptors in the anterior piriform cortex control odor preference memory. Curr. Biol. 29, 2455.e5–2464.e5. 10.1016/j.cub.2019.06.041 [DOI] [PubMed] [Google Scholar]
- Tsou K., Brown S., Sañudo-Peña M. C., Mackie K., Walker J. M. (1998). Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, 393–411. 10.1016/s0306-4522(97)00436-3 [DOI] [PubMed] [Google Scholar]
- Tutka P., Wlaz A., Florek-Łuszczki M., KoŁodziejczyk P., Bartusik-Aebisher D., Łuszczki J. J. (2018). Arvanil, olvanil, AM 1172 and LY 2183240 (various cannabinoid CB1 receptor agonists) increase the threshold for maximal electroshock-induced seizures in mice. Pharmacol. Rep. 70, 106–109. 10.1016/j.pharep.2017.08.006 [DOI] [PubMed] [Google Scholar]
- Van Sickle M. D., Duncan M., Kingsley P. J., Mouihate A., Urbani P., Mackie K., et al. (2005). Neuroscience: identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332. 10.1126/science.1115740 [DOI] [PubMed] [Google Scholar]
- Vilela L. R., Medeiros D. C., Rezende G. H. S., de Oliveira A. C. P., Moraes M. F. D., Moreira F. A. (2013). Effects of cannabinoids and endocannabinoid hydrolysis inhibition on pentylenetetrazole-induced seizure and electroencephalographic activity in rats. Epilepsy Res. 104, 195–202. 10.1016/j.eplepsyres.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Vinogradova L. V., van Rijn C. M. (2015). Long-term disease-modifying effect of the endocannabinoid agonist WIN55, 212-2 in a rat model of audiogenic epilepsy. Pharmacol. Rep. 67, 501–503. 10.1016/j.pharep.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Vinogradova L. V., Shatskova A. B., van Rijn C. M. (2011). Pro-epileptic effects of the cannabinoid receptor antagonist SR141716 in a model of audiogenic epilepsy. Epilepsy Res. 96, 250–256. 10.1016/j.eplepsyres.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Wallace M. J. (2003). The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J. Pharmacol. Exp. Ther. 307, 129–137. 10.1124/jpet.103.051920 [DOI] [PubMed] [Google Scholar]
- Wallace M. J., Wiley J. L., Martin B. R., DeLorenzo R. J. (2001). Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur. J. Pharmacol. 428, 51–57. 10.1016/s0014-2999(01)01243-2 [DOI] [PubMed] [Google Scholar]
- Weng X., Rosenberg H. C. (1992). Infusion of bicuculline methiodide into the tectum: model specificity of pro- and anticonvulsant actions. Epilepsy Res. 12, 1–8. 10.1016/0920-1211(92)90085-8 [DOI] [PubMed] [Google Scholar]
- Wicker E., Forcelli P. A. (2016). Chemogenetic silencing of the midline and intralaminar thalamus blocks amygdala-kindled seizures. Exp. Neurol. 283, 404–412. 10.1016/j.expneurol.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker E., Beck V. C., Kulick-Soper C., Kulick-Soper C. V., Hyder S. K., Campos-Rodriguez C., et al. (2019). Descending projections from the substantia nigra pars reticulata differentially control seizures. Proc. Natl. Acad. Sci. U S A 116, 27084–27094. 10.1073/pnas.1908176117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.