Abstract
Teucrium leucocladum is among the most used traditional medicinal plants in Palestine, which is used for the treatment of hyperglycemia and colon spasms from ancient times. Therefore, the current investigation aimed for the first time to determine the hypoglycemic, hypolipidemic, and oxidative stress inhibitory effects of the aerial parts (stem and leaves) of T. leucocladum hydrophilic (water) extract in streptozotocin- (STZ-) induced diabetic rats (65 mg/kg), given intraperitoneally at a dose of 100 mg/kg for 21 days. The rats were divided into four groups as control (C), control + T. leucocladum extract (C + TL), diabetes (D), and diabetes + T. leucocladum extract (D + TL). The antioxidant activity was analyzed using in vitro 2,2-diphenyl-1-picrylhydrazyl and in vivo methods by measuring the plasma and tissue malondialdehyde (MDA) levels using a colorimetric assay. On the other hand, glutathione peroxidase (GSH-Px), erythrocyte superoxide dismutase (SOD) enzyme levels, serum paraoxonase (PON), and arylesterase (ARE) enzyme activities were assessed by utilizing standard biochemical kits. Besides, the blood glucose and serum insulin levels were assessed by a glucometer and Rat ELISA Kit, respectively. However, the autoanalyzer was used to evaluate the lipid profile. The diabetic rat group that administered T. leucocladum extract showed the best reduction in the tissue and plasma MDA levels and an increase of insulin-releasing potentials. Besides, the serum PON and ARE activities and erythrocyte superoxide dismutase and whole blood glutathione peroxidase enzyme levels were significantly increased in all animals treated with T. leucocladum extract. The current investigation demonstrated that T. leucocladum manifests antihyperglycemic and antihyperlipidemic effects and also increased the antioxidative defense system and reduced the lipid peroxidation process in experimental diabetic rats.
1. Introduction
For thousands of years, people tried to cure their diseases by utilizing various available natural materials and among these were medicinal plants [1, 2]. Recently, it is well recognized that diabetes mellitus is considered one of the most common diseases worldwide, which is characterized as a type of metabolic disorder known by hyperglycemia, hypoinsulinemia, hyperlipidemia, and increased oxidative stress [3]. However, hyperglycemia diminishes pro-oxidant/antioxidant balance by reducing antioxidant levels and raising the standards of free radical [4]. Oxidative stress can cause various pathological complications in diabetic patients, including cardiovascular diseases, cancer, neurological disorders, acute respiratory distress syndrome, and many other diseases [5, 6]. The human body utilizes various antioxidant mechanisms of actions to decrease the damages caused by oxidative stress including enzymatic and nonenzymatic systems [7]. These mechanisms counterbalance the effects of toxic reactive oxygen species (ROS) in human cells and tissues. The enzymatic and nonenzymatic antioxidant compounds include vitamins A, C, and E, catalase (CAT), glutathione reductase (GRx), glutathione (GSH), glutathione peroxidase (GPx), and superoxide dismutase (SOD) [8].
Hypoglycemic oral drugs and insulin injections have many side effects such as syncope, dizziness, nervousness, anxiety, depression, diarrhea, nausea, and vomiting [9]. Therefore, there are increases of interest by diabetic patients and healthcare global systems to search for traditional and natural herbal remedies with antihyperglycemic effect due to their fewer side effects [10].
However, few studies on medicinal plants have investigated their antihyperglycemic, antihyperlipidemic activities, and antioxidative effects in vivo. These plants mainly consist of bioactive secondary metabolic molecules such as polyphenols, vitamins, glycosides, and steroids which have antioxidant, antihyperlipidemic, and antidiabetic effects at the same time [11].
Many investigations have shown that Teucrium plant species contain various bioactive compounds such as terpenoids, flavonoids, and iridoids [12]. Teucrium leucocladum Boiss. is a very rare aromatic shrubby plant (20–50 cm) with white appressed woolly stem [13]. Previous investigations showed that Teucrium plant species contain different types of sesquiterpene and flavonoid contents including cirsimaritin, apigenin 7-glucoside, vicenin-2, apigenin 5-galloyl-glucoside, and luteolin 7-glucoside [14, 15].
To the best of our knowledge, this is the first study investigating the effects of T. leucocladum plant antihyperglycemic, antihyperlipidemic, and antioxidant potential in STZ-induced diabetic rats. For that, the current experimental work aims to measure the erythrocyte superoxide dismutase (SOD) and whole blood glutathione peroxidase (GSH-Px) levels and serum paraoxonase (PON) and arylesterase (ARE) activities. In addition, it aims to determine the plasma and tissue (Musculus gastrocnemius, heart, liver, and kidney) malondialdehyde (MDA) levels to investigate the oxidative status of the rats and also aims to assess the blood lipid profile, including total cholesterol (TC), triglyceride (TG), and high-density lipoprotein-cholesterol (HDL-C) levels.
2. Materials and Methods
2.1. Plant Materials and Extraction Methods
The aerial parts of T. leucocladum (stems and leaves) were collected from the Nablus region of Palestine in May 2017. The plant was identified by pharmacognosist Dr. Nidal Jaradat. The voucher specimen was deposited in the Herbal Products Laboratory at An-Najah National University with a specific code: Pharm-PCT-2411. The collected plant materials were washed several times with distilled water. Afterward, the aerial parts were dried in the shade at a stable normal humidity level and ordinary temperature.
The aqueous extract of T. leucocladum was exhaustively extracted by boiling 100 g of plant powder in 1 L distilled water for 2 h at 100°C. The produced extract was then filtered using Whatman filter paper No.1 and dried using a freeze dryer (Mill-Rock Technology, 85bt, Nanjing, China). The obtained dried aqueous extracts were weighed and stored at 4°C until being used.
2.2. Animal Models
The current study was conducted with 40 Wistar Albino male rats which were brought from Uludağ University Animal House; their weight average was 200–250 g. The rats were kept in individual cages and were housed in groups of 4 per cage. All the utilized rats were kept in a temperature-controlled environment (25 ± 2°C) on a 12 : 12 h light: dark photoperiod. They were given free access to tap water and basic laboratory food which consists of 25% proteins, 3% vitamins, 35% carbohydrates, and 7% lipids for one week before starting the trial. Experimental protocols, ethical procedures, and policies were authorized according to the Animal Care and Use Committee of Uludağ University (2018-04/13).
2.3. Induction of Diabetes
The rats were inducted with diabetes by using single intraperitoneal injections of STZ (65 mg/kg) (BioShop, Ontario, Canada) which was infused with a freshly prepared solution of sodium citrate buffer with pH 4.5, while control rats (C) were received an injection of citrate buffer solution only. The blood glucose levels were measured after 48 h of STZ inductions.
The rats which had blood glucose levels more than 200 mg/dl were considered diabetic and were included in the conducted study. Blood glucose concentration was measured with a Glucostix strip test in a glucometer (Abbott Glucometer Med. Prod., California, United States). Since streptozotocin injection may result in fatal hypoglycemia related to massive insulin release, the diabetic rats were kept on a 5% glucose solution diet for 24 h after STZ injection to prevent hypoglycemia.
2.4. Experimental Design and Sample Collection
The Wistar rats were selected randomly and allotted into 4 groups of 10 rats in each one: Group I consists of normal control rats (C), Group II control rats were given intraperitoneally T. leucocladum extract (C + TL), Group III consists of STZ-induced diabetic rats (D), and Group IV diabetic rats were given intraperitoneally T. leucocladum extract 100 mg/kg (D + TL).
2.5. T. leucocladum Treatment
One week after injection of STZ, the aqueous extract of T. leucocladum was given intraperitoneally for 21 days at a dose of 100 mg/kg for groups II and IV. The daily food and fluid intake, weekly body weight, and blood glucose levels for all groups were recorded through the experiment [16].
At the end of the experimental stage and after 10–12 h of fasting, the blood samples were obtained from all the studied rat groups under light anesthesia by cardiac puncture. Immediately after the collection of blood samples, the skeletal muscles (Musculus gastrocnemius), heart, kidney, and liver tissues were removed, rinsed with a standard normal saline cold solution, dried with gauze, and kept in the refrigerator at −20°C for further use. All the blood samples were drawn in individual additive test tubes which contained EDTA and coated with heparin. However, the whole blood samples and erythrocytes in blood were used for the determination of the activities of antioxidant enzymes. Blood samples were stored at −20°C until they were analyzed.
2.6. Determination of Biochemical Parameters
Glucose levels in blood samples obtained from each experimental group by cutting the tail of rats every week of the experiment were estimated using blood Glucostix strips (Abbott-Glucometer, USA). The levels of HDL-C, TG, and TC in serum samples were assessed using standard biochemical apparatus as an autoanalyzer (Abbott, USA). However, the serum insulin level in the serum was evaluated using Rat ELISA kit (Lab science, E-EL-R2466, USA). Moreover, the plasma SOD and GSH-Px levels were estimated by utilizing available commercial kit (YL Biotech, Shanghai), and the PON1 and ARE enzyme activities in serum were estimated using a commercial kit (Rel Assay Diagnostics, Mega Tıp, Gaziantep, Turkey).
The kidney, heart, liver, and muscle tissue MDA levels were determined by the thiobarbituric acid (TBA) method using a spectrophotometer at a wavelength of 532 nm (Beckman Coulter Du 730 UV/Vis, USA) [17]. The plasma MDA concentrations were determined using thiobarbituric acid (TBA) assay using a spectrophotometer at a wavelength of 535 nm [18].
2.7. Antioxidant Activity of T. leucocladum Extract
Stock solutions at a concentration of 1 mg/ml in methanol were prepared from T. leucocladum extract and Trolox (Sigma-Aldrich, Germany). Each one of these stock solutions was diluted with methanol to prepare 12 of the working solutions with the following concentrations: 1, 2, 3, 5, 7, 10, 20, 30, 40, 50, 80, and 100 μg/ml. A freshly prepared DPPH solution (Sigma-Aldrich, Germany) (0.002% w/v) was mixed with methanol and with each of the abovementioned working solutions at a 1 : 1 : 1 ratio. Besides, a negative control solution was prepared by mixing the mentioned DPPH solution with methanol in a 1 : 1 ratio, while Trolox which is a vitamin E analog and considered strong antioxidant reagent was used as a positive control. All of these solutions were incubated at room temperature in a dark cabinet for 30 min. At the end of the incubation period, the optical density of these solutions was determined spectrophotometrically at a wavelength of 517 nm. The antioxidant activity of Trolox and T. leucocladum extract was estimated using the following formula:
| (1) |
where A and B represent the absorbance of the blank and the extract, respectively.
The antioxidant half-maximal inhibitory concentration (IC50) for T. leucocladum extract and Trolox and their standard deviations were calculated by using BioData Fit edition 1.02 (data fit for biologist) [19]. The antioxidant activity of T. leucocladum at the different concentrations mentioned above was expressed in terms of the antioxidant activity of the Trolox standard. This was determined by using the following equation:
| (2) |
2.8. Statistical Analysis
As the data were normally distributed and variables were expressed as mean ± SEM. One-way ANOVA tests were used to investigate statistically significant differences. A level of p value <0.05 was accepted as statistically significant. In addition, the determination of in vitro antioxidant activity was carried out in triplicate. The obtained results were presented as means ± standard deviation (SD). Statistical analyses were conducted using SPSS version 13.0 for Windows.
3. Results
The result of the current study showed that the food and water consumption, blood glucose, and serum TC and TG levels were significantly increased in the D group, while the bodyweight of this group significantly decreased compared with the C group rats. However, there were no differences between the C + TL and C groups, while the food and water intake decreased in the D + TL group in comparison with the D group; otherwise, food and water intake decreased in the C + TL group compared with the D + TL group as presented in Table 1. Moreover, the serum TG levels were significantly decreased in the C + TL group and also food and water consumption, blood glucose, and TC and TG levels were significantly decreased in the D + TL group compared with the D group. Also, in the D group, the blood glucose and serum TG and TC levels were elevated, while the serum insulin and HDL-C levels were significantly decreased in comparison with the C group. Furthermore, high levels of insulin and low levels of blood glucose, TG, and TC were significantly detected in the D + TL group in comparison with the D group. The levels of serum HDL-C did not change between the D and D + TL groups as shown in Table 1.
Table 1.
Body weight, food and water consumption, and metabolic parameters of the control and experimental groups of rats.
| Group | C | C + TL | D | D + TL |
|---|---|---|---|---|
| Food intake (g/24 h) | 17.4 ± 0.46 | 18.8 ± 0.56d | 36.6 ± 0.82a | 31.9 ± 1b |
| Water intake (mL/24 h) | 34.5 ± 1.5 | 36.1 ± 1.1d | 182.7 ± 3.3a | 61.2 ± 1b |
| Final body weight (g) | 255.4 ± 3.2 | 226.4 ± 8 | 202.7 ± 4a | 226.8 ± 12 |
| Glucose (mg/dl) | 126.2 ± 3.4 | 125.8 ± 1.1 | 534.2 ± 10.6a | 507.3 ± 10.8b |
| Insulin (ng/ml) | 1.7 ± 0.23 | 1.6 ± 0.24 | 0.5 ± 0.08a | 0.99 ± 0.08b |
| TC (mg/dl) | 58 ± 1.3 | 54.8 ± 0.8 | 89.8 ± 3.7 a | 54.4 ± 2.2b |
| TG (mg/dl) | 78 ± 2.3 | 47.8 ± 2.8c | 328.5 ± 38a | 67.8 ± 3.1b |
| HDL-C (mg/dl) | 53.6 ± 1.4 | 55.7 ± 3.2 | 47.1 ± 2.1a | 55.4 ± 1.7 |
Values are expressed as mean ± SEM for ten rats in each group. Data analyzed using two-way ANOVA. ap < 0.05 between the normal control (C) and diabetic control (D). bp < 0.05 between the diabetic control (D) and diabetic TL treatment (D + TL). cp < 0.05 between the normal control (C) and the normal TL treatment (C + TL). dp < 0.05 between the normal TL treatment (C + TL) and diabetic TL treatment (D + TL).
In addition, Table 2 shows that the plasma GSH-Px level was significantly higher in the D + TL group in comparison with the D group while plasma GSH-Px and SOD levels decreased in the C + TL group compared with the D + TL group. Furthermore, the serum PON and ARE activities were increased dramatically in the C + TL group in comparison with the control one. In diabetic rats, the activities of PON1 and ARE were decreased significantly compared with the C group
Table 2.
The GSH-Px, SOD, PON1, and ARE activities in the control and experimental groups of rats.
| Group | C | C + TL | D | D + TL |
|---|---|---|---|---|
| Plasma GSH-Px (ng/mL) | 8.5 ± 0.24 | 9.2 ± 0.31d | 9.9 ± 0.42a | 11.7 ± 0.59b |
| Plasma SOD (ng/mL) | 0.89 ± 0.1 | 0.97 ± 0.1d | 1.37 ± 0.04a | 1.39 ± 0.08 |
| PON (U/L) | 135.3 ± 8.8 | 187.9 ± 11.3c | 61.5 ± 2.1a | 137.8 ± 20b |
| ARE (U/L) | 141.8 ± 1.6 | 175.3 ± 5.2c | 60.7 ± 2.8 a | 158.5 ± 9.5b |
Values are expressed as mean ± SEM for ten rats in each group. Data analyzed using two-way ANOVA. ap < 0.05 between the normal control (C) and diabetic control (D). bp < 0.05 between the diabetic control (D) and diabetic TL treatment (D + TL). cp < 0.05 between the normal control (C) and the normal TL treatment (C + TL). dp < 0.05 between the normal TL treatment (C + TL) and diabetic TL treatment (D + TL).
On the other hand, both the PON1 and ARE activities in the D + TL group were increased significantly in comparison with the D group. Moreover, Figure 1 depicts that the MDA levels did not change in the C and C + TL groups in the kidney and heart tissues, while a significant increase was observed in the MDA levels in the diabetic group. In addition, MDA levels in the heart and kidney tissues were decreased in the C + TL group compared with the D + TL group.
Figure 1.
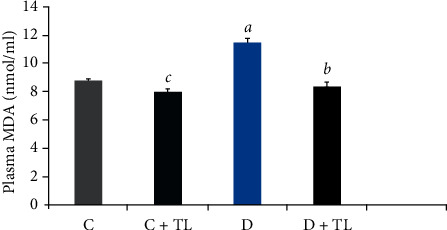
Malondialdehyde (MDA) levels in plasma (nmol/ml) of the control and experimental rats. Data analyzed using one-way ANOVA. Values are expressed as mean ± SEM. ap < 0.05 between the normal control (C) and diabetic control (D). bp < 0.05 between the diabetic control (D) and diabetic TL treatment (D + TL). cp < 0.05 between the normal control (C) and the normal TL treatment (C + TL). dp < 0.05 between the normal TL treatment (C + TL) and diabetic TL treatment (D + TL).
On the other hand, MDA levels in the blood plasma, heart, skeletal muscle, liver, and kidney tissues were decreased in the D + TL group compared with the D group rats as shown in Figures 1–5.
Figure 2.
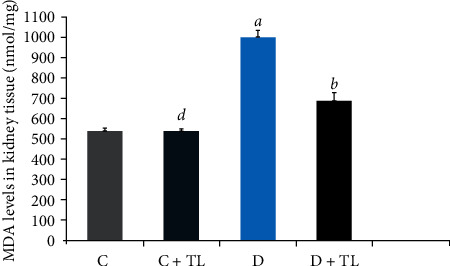
Malondialdehyde (MDA) levels in the kidney tissues (nmol/mg) of the control and experimental rats. Data analyzed using one-way ANOVA. Values are expressed as mean ± SEM. ap < 0.05 between the normal control (C) and diabetic control (D). bp < 0.05 between the diabetic control (D) and diabetic TL treatment (D + TL). cp < 0.05 between the normal control (C) and the normal TL treatment (C + TL). dp < 0.05 between the normal TL treatment (C + TL) and diabetic TL treatment (D + TL).
Figure 3.
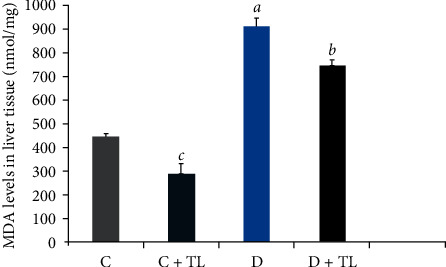
Malondialdehyde (MDA) levels in the liver tissues (nmol/mg) of the control and experimental rats. Data analyzed using one-way ANOVA. Values are expressed as mean ± SEM. ap < 0.05 between the normal control (C) and diabetic control (D). bp < 0.05 between the diabetic control (D)and diabetic TL treatment (D + TL). cp < 0.05 between the normal control (C) and the normal TL treatment (C + TL). dp < 0.05 between the normal TL treatment (C + TL) and diabetic TL treatment (D + TL).
Figure 4.
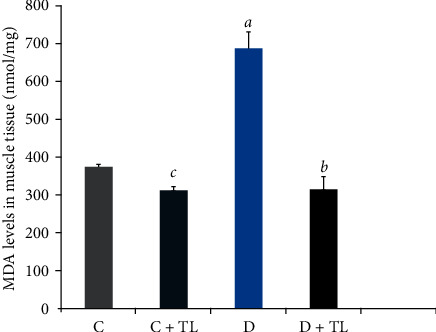
Malondialdehyde (MDA) levels in the muscle tissues (nmol/mg) of the control and experimental rats. Data analyzed using one-way ANOVA. Values are expressed as mean ± SEM. ap < 0.05 between the normal control (C) and diabetic control (D). bp < 0.05 between the diabetic control (D) and diabetic TL treatment (D + TL). cp < 0.05 between the normal control (C) and the normal TL treatment (C + TL). dp < 0.05 between the normal TL treatment (C + TL) and diabetic TL treatment (D + TL).
Figure 5.
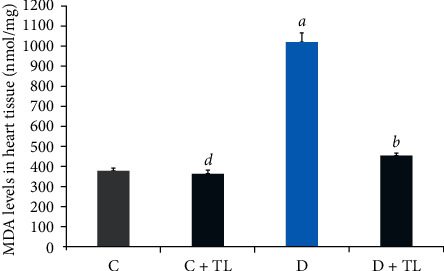
Malondialdehyde (MDA) levels in the heart tissues (nmol/mg) of the control and experimental rats. Data analyzed using one-way ANOVA. Values are expressed as mean ± SEM. ap < 0.05 between the normal control (C) and diabetic control (D). bp < 0.05 between the diabetic control (D) and diabetic TL treatment (D + TL). cp < 0.05 between the normal control (C) and the normal TL treatment (C + TL). dp < 0.05 between the normal TL treatment (C + TL) and diabetic TL treatment (D + TL).
The antioxidant activity of T. leucocladum extract was tested by DPPH assay using Trolox as a reference compound. The used concentrations ranged from 1 to 100 μg/ml for the T. leucocladum extract as well as for standard Trolox as shown in Figure 6.
Figure 6.
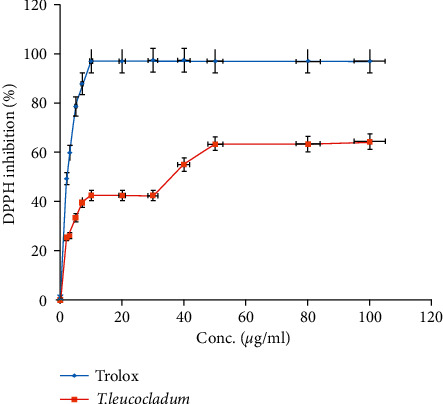
DPPH inhibitory activity by T. leucocladum aqueous extract and Trolox. This test was conducted in triplicate and the results were expressed as mean ± SD.
The result revealed that the free radical scavenging property was exhibited by T. leucocladum extract which has an IC50 value of 25.7 μg/ml, while the free radical scavenging property of Trolox was 2.09 μg/ml.
4. Discussion
Most of the recently used medicines are initially evaluated on animal models for various purposes. Firstly, it may not be necessary to examine new medicines on humans if preliminary evaluations on animals show that they are not clinically useful. Secondly, studies on animals provide a degree of genetic and environmental manipulation rarely possible in humans. Thirdly, animal studies provide unique insights into the etiology and pathophysiology of the disease and can reveal new targets for the tested medicines. Finally, regulatory authorities concerned with public protection require extensive animal testing to evaluate new medicines for toxicity and to establish safety [20, 21].
Recently, global interests are focusing on the search for organic nutrients, nonnutrient, and traditional medicinal herbs which have antidiabetic and antioxidant potentials. They mainly contain biologically active phytochemical classes such as stilbenes, flavonoids, polyphenols, and carotenoids for reducing the negative impacts of free radicals and oxidative stress damages especially in patients suffering from diabetes mellitus [22, 23].
Hyperglycemia and hyperlipidemia as well as polyuria, polyphagia, and polydipsia are the most prominent symptoms in diabetic patients observed in experimental animals (rats and mice) administration by STZ or alloxan with diabetes mellitus [24]. In this regard, the results of the current study showed that in the diabetic rat group, the food consumption, water intake, blood glucose, and TC and TG levels were significantly increased. At the same time, bodyweight, HDL-C, and serum insulin secretions were decreased in comparison with the C group. The current study showed that T. leucocladum extract decreased blood glucose levels and elevated serum insulin levels in the D + TL group. However, one of the active substances of T. leucocladum was cirsimaritin; an investigation conducted by Lee et al. showed that it is a biologically active compound that prevented apoptosis in pancreatic beta cells caused by STZ. In this study, the increase of the insulin levels in all groups treated with the plant extract may be caused by the regeneration of the pancreas by cirsimaritin compound [25].
Another one of the biologically active compounds found in T. leucocladum is apigenin flavonoid. A study conducted by Park found that the glucose uptake in U937 cells decreased when the cells were processed by apigenin [26].
In our study, the decrease in the blood glucose level in the D + TL group may be caused by the effect of apigenin and other contents presented in the plant. The increase of the insulin levels in parallel with the decrease in blood glucose levels in the D + TL group suggested that this plant has a hypoglycemic therapeutic effect.
Actually, hyperlipidemia is a group of metabolic disorders characterized by hypertriglyceridemia and hypercholesterolemia which is considered the cause of many diseases such as macro-microangiopathy, cardiovascular complications, and metabolic syndrome. Epidemiological studies have shown that the consumption of flavonoid-rich diets, diabetic dyslipidemia (hypercholesterolemia, hypertriglyceridemia, hyperphospho-lipidemia), and coronary heart disease risk can be reduced. In fact, flavonoids act directly by activating enzymes regulating lipid and carbohydrate metabolism in the liver and intestines, by stimulating the fat absorption or by increasing the fat excretion [27].
Agreeing with these facts, the current studied plant species contains as observed in previous investigations luteolin and apigenin flavonoids, which may be the main cause of these observed results. In this study, the decrease in serum TC and serum TG levels in both the C + TL and D + TL groups may have been affected by one or all of the abovementioned biological properties of flavonoids. For that, T. leucocladum improves hyperlipidemia in diabetic condition and it is important to show a protective effect in healthy rats.
In the D group, the antioxidant potential has increased because of the deficiency of the antioxidant defense system as well as the increase in the blood glucose and lipid levels. In fact, free radicals may produce damaging effects on the cells, causing lipid peroxidation in the cell membranes such as MDA, which is the last product of lipid peroxidation and one of the oxidative stress biomarkers [28].
The current study has shown a significant increase in MDA levels in blood plasma and tissues (heart, muscle, liver, and kidney) in the D group in comparison with the C group. There was a significant decrease in plasma and tissue MDA levels in the C + TL group (only muscle and liver tissues) and a decrease in the MDA levels in the heart, kidney, liver, muscle, and plasma in the diabetic group given T. leucocladum (D + TL) extract compared with the C and D groups. On the other hand, the decrease in MDA levels of the blood plasma, heart, skeletal muscle, and kidney tissues was not observed only in the diabetic group but also in the control group. This is an important point in terms of the protective effect of T. leucocladum in healthy individuals.
For human health, it is very important to consume vegetable and fruits or any food supplements containing phenolic molecules which play an essential role in protecting the human organisms against cancer, aging, and inflammations, through the management of oxidative stress [29].
Moreover, in vitro, the antioxidant activity results showed that T. leucocladum has a high antioxidant potential compared with Trolox as a reference antioxidant compound. In agreement with our results, many previously conducted studies of Teucrium species showed a potential antioxidant effect, for example, extracts derived from T. polium, T. chamaedrys, and T. montanum revealed a significant inhibitory effect with IC50 values ranging from 10 to 70 mg/mL [30, 31].
There are enzymatic and nonenzymatic antioxidant defense systems in the human body to prevent the damage caused by reactive oxygen species. SOD and GSH-Px are antioxidant enzymes [32, 33].
Regarding oxidative stress inhibitory activity, it was noticed that the GSH-Px was significantly increased in the D + TL group. In addition, it significantly increased the GSH-Px and SOD activities, while decreased the PON and ARE activities in the D group. At the same time, the PON1 enzyme is involved in the PON enzyme family which is a component of HDL-C. The primary role of PON1 is to protect lipoproteins from the oxidative process. For such a reason, the antioxidant effect of PON1 is usually related with the ability to neutralize hydrogen peroxide and other free radicals. Also, ARE enzyme level is an essential indicator for the PON1 activity. Many conditions can increase oxidative stress such as diabetes, hyperlipidemia, and coronary heart diseases [34]. As shown in this study, the decrease in serum PON1 and ARE activities in diabetic rats may be associated with hyperglycemia, hyperlipidemia, and/or oxidative stress [35].
While the revealed results in the current study showed that the activities of PON1 and ARE enzymes were increased significantly in the C + TL and D + TL groups compared with the C and D groups, respectively. It is important that the PON and ARE activities increased in the C + TL and D + TL groups because this suggests that this plant has a strong antioxidant effect in increasing the synthesis of enzymes in both groups. As previously documented, the decrease in ARE activity in the diabetic condition may be due to oxidative modification of the nucleic material and/or transcription factors or due to the glycation process [36]. Polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism [37].
To the best of the authors' knowledge, the current study provides the first evidence of the hypoglycemic, hypolipidemic, and antioxidant potential of the current studied traditional medicinal plant.
5. Conclusion
The findings of the current study concluded that T. leucocladum extract can be used as an antihyperglycemic, antihyperlipidemic, and powerful antioxidant agent as a treatment of diabetes or as a supportive therapy. A literature survey on T. leucocladum extract has shown that no biomedical analysis has previously been established on the serum insulin, TC, TG, HDL-C, SOD, GSH-Px, PON, ARE, and MDA potentials. Further, toxicological and clinical experiments are required to evaluate this plant species and its possible applications in the pharmaceutical fields.
Acknowledgments
This study was supported by a Grant from Uludağ University Research Foundation (number (KUAP (F)- 2017/2)).
Contributor Information
Najlaa Bassalat, Email: nbassalat@gmail.com.
Sibel Taş, Email: smeral@uludag.edu.tr.
Nidal Jaradat, Email: nidaljaradat@najah.edu.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
This study covers the master thesis of the first author.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
Najlaa Bassalat, Sibel Taş, and Nidal Jaradat designed and performed the experiment, analyzed the data, and wrote and reviewed the paper. All authors have read and agreed to the published version of the manuscript and contributed equally to this work.
References
- 1.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):p. 559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amer J., Jaradat N., Hattab S., Al-hihi S., Juma’a R. E. Traditional palestinian medicinal plant cercis siliquastrum (Judas tree) inhibits the DNA cell cycle of breast cancer-antimicrobial and antioxidant characteristics. European Journal of Integrative Medicine. 2019 doi: 10.1016/j.eujim.2019.03.005. [DOI] [Google Scholar]
- 3.Le Lay S., Simard G., Martinez M. C., Andriantsitohaina R. Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxidative Medicine and Cellular Longevity. 2014;2014:18. doi: 10.1155/2014/908539.908539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoarau E., Chandra V., Rustin P., Scharfmann R., Duvillie B. Pro-oxidant/antioxidant balance controls pancreatic β-cell differentiation through the ERK1/2 pathway. Cell Death and Disease. 2014;5(10):p. e1487. doi: 10.1038/cddis.2014.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elahi M. M., Kong Y. X., Matata B. M. Oxidative stress as a mediator of cardiovascular disease. Oxidative Medicine and Cellular Longevity. 2009;2(5):259–69. doi: 10.4161/oxim.2.5.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birben E., Sahiner U. M., Sackesen C., Erzurum S., Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organization Journal. 2012;5(1):9–14. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaradat N., Shawarb N., Hussein F., et al. Antibacterial and antioxidant screening of semi-synthetic naringin based hydrazone and oxime derivatives. Jundishapur Journal of Microbiology. 2018;11(6):1–9. [Google Scholar]
- 8.Matough F. A., Budin S. B., Hamid Z. A., Alwahaibi N., Mohamed J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos University Medical Journal. 2012;12(1):p. 5. doi: 10.12816/0003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avilés-Santa L., Sinding J., Raskin P. Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Annals of Internal Medicine. 1999;131(3):182–188. doi: 10.7326/0003-4819-131-3-199908030-00004. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhury A., Duvoor C., Dendi R., et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Frontiers in Endocrinology. 2017;8:p. 6. doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiménez-Aguilar D. M., Grusak M. A. Minerals, vitamin C, phenolics, flavonoids and antioxidant activity of amaranthus leafy vegetables. Journal of Food Composition and Analysis. 2017;58:33–39. [Google Scholar]
- 12.Piozzi F., Bruno M., Rosselli S. Advances on the chemistry of furano-diterpenoids from teucrium genus. Heterocycles. 2015;65(5):1221–1234. [Google Scholar]
- 13.Boulos L. Flora of Egypt: Verbenaceae-Compositae. Cairo, Egypt: Al Hadara Publishing; 2002. [Google Scholar]
- 14.Bruno M., Maria C., Rodríguez B., Omar A. A. Guaiane sesquiterpenes from Teucrium leucocladum. Phytochemistry. 1993;34(1):245–247. [Google Scholar]
- 15.Kawashty S., El-Din E. G., Saleh N. The flavonoid chemosystematics of two teucrium species from Southern Sinai, Egypt. Biochemical Systematics Ecology. 2020;27(6):657–660. [Google Scholar]
- 16.Barati S., JoB Momtaz H. J. A., Sciences P. The effect of hydro-alcoholic extract of morus nigra leaf on lipids and sugar in serum of diabetic rats. Asian Journal of Biomedical and Pharmaceutical Sciences. 2017;2(15):p. 38. [Google Scholar]
- 17.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analythical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Young I., Trimble E. Measurement of malondialdehyde in plasma by high performance liquid chromatography with fluorimetric detection. Annals of Clinical Biochemistry. 1991;28(5):504–508. doi: 10.1177/000456329102800514. [DOI] [PubMed] [Google Scholar]
- 19.Jaradat N. A., Shawahna R., Hussein F., Al-Lahham S. Analysis of the antioxidant potential in aerial parts of Trigonella arabica and Trigonella berythea grown widely in palestine: A comparative study. European Journal of Integrative Medicine. 2016;8(5):623–630. [Google Scholar]
- 20.Pecoraro V., Moja L., Dall’Olmo L., Cappellini G., Garattini S. J. E. J. o. C. I. Most appropriate animal models to study the efficacy of statins: a systematic review. European Journal of Clinical Investigation. 2014;44(9):848–871. doi: 10.1111/eci.12304. [DOI] [PubMed] [Google Scholar]
- 21.Hackam D. G. Translating animal research into clinical benefit. British Medical Journal. 2007;334:7586–7591. doi: 10.1136/bmj.39104.362951.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apak R., Ozyurek M., Guclu K., Capanoglu E. Antioxidant activity/capacity measurement. 3. Reactive oxygen and nitrogen species (ROS/RNS) scavenging assays, oxidative stress biomarkers, and chromatographic/chemometric assays. Journal of Agricultural and Food Chemistry. 2016;64(5):1046–1070. doi: 10.1021/acs.jafc.5b04744. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y. J., Gan R. Y., Li S., et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tas S., Sarandol E., Ziyanok S., Aslan K., Dirican M. Effects of green tea on serum paraoxonase/arylesterase activities in streptozotocin-induced diabetic rats. Nutrition research. 2005;25(12):1061–1074. [Google Scholar]
- 25.Lee D., Kim K. H., Lee J., et al. Protective effect of cirsimaritin against streptozotocin‐induced apoptosis in pancreatic beta cells. Journal of Pharmacy and Pharmacology. 2017;69(7):875–883. doi: 10.1111/jphp.12719. [DOI] [PubMed] [Google Scholar]
- 26.Park J. B. Flavonoids are potential inhibitors of glucose uptake in U937 cells. Biochemical Biophysical Research Communication. 1999;260(2):568–574. doi: 10.1006/bbrc.1999.0890. [DOI] [PubMed] [Google Scholar]
- 27.Hertog M. G., Kromhout D., Aravanis C., et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Archieve of Internal Medicine. 1995;155(4):381–386. [PubMed] [Google Scholar]
- 28.Khoubnasabjafari M., Ansarin K., Jouyban A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. BioImpacts. 2015;5(3):123–129. doi: 10.15171/bi.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin D., Xiao M., Zhao J., et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules. 2016;21(10):p. 1374. doi: 10.3390/molecules21101374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macedonia R. In vitro antioxidant activity of some teucrium species (Lamiaceae) Acta Pharmaceutica. 2005;55:207–214. [PubMed] [Google Scholar]
- 31.Jaradat N.A. Review of the taxonomy, ethnobotany, phytochemistry, phytotherapy and phytotoxicity of germander plant (Teucrium polium L.) Asian Journal of Pharmaceutical Clinical Research. 2015;8(2):13–19. [Google Scholar]
- 32.Mirończuk-Chodakowska I., Witkowska A. M., Zujko M. E. Endogenous non-enzymatic antioxidants in the human body. Advances in Medical Science. 2017;63(1):68–78. doi: 10.1016/j.advms.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Sies H. Oxidative stress: oxidants and antioxidants. Experimental Physiology. 1997;82(2):291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 34.Zhu R., Wang Y., Zhang L., Guo Q. Oxidative stress and liver disease. Hepatology Research. 2014;42(8):741–749. doi: 10.1111/j.1872-034X.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 35.Costa L. G., Giordano G., Furlong C. E. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: the hunt goes on. Biochemical Pharmacology. 2011;81(3):337–344. doi: 10.1016/j.bcp.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valko M., Leibfritz D., Moncol J., Cronin M. T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The International Journal of Biochemistry and Cell Biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Gouédard C., Barouki R., Morel Y. Dietary polyphenols increase paraoxonase 1 gene expression by an aryl hydrocarbon receptor-dependent mechanism. Molecular and Cellular Biology. 2004;24(12):5209–5222. doi: 10.1128/MCB.24.12.5209-5222.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


