Abstract
Depression is a common neurological disease that seriously affects human health. There are many hypotheses about the pathogenesis of depression, and the most widely recognized and applied is the monoamine hypothesis. However, no hypothesis can fully explain the pathogenesis of depression. At present, the brain-derived neurotrophic factor (BDNF) and neurogenesis hypotheses have highlighted the important role of plasticity in depression. The plasticity of neurons and glial cells plays a vital role in the transmission and integration of signals in the central nervous system. Plasticity is the adaptive change in the nervous system in response to changes in external signals. The hippocampus is an important anatomical area associated with depression. Studies have shown that some antidepressants can treat depression by changing the plasticity of the hippocampus. Furthermore, caloric restriction has also been shown to affect antidepressant and hippocampal plasticity changes. In this review, we summarize the latest research, focusing on changes in the plasticity of hippocampal neurons and glial cells in depression and the role of BDNF in the changes in hippocampal plasticity in depression, as well as caloric restriction and mitochondrial plasticity. This review may contribute to the development of antidepressant drugs and elucidating the mechanism of depression.
1. Introduction
According to the latest epidemiological survey, the incidence of depression worldwide is 4.7%, and the incidence in China is 4%; depression is a serious disease, and only 10% of patients respond effectively to treatment [1]. Depression severely affects human health and quality of life, including but not limited to excessive negative emotions, anhedonia, and cognitive impairment. The hippocampus is a key anatomical brain region associated with depression. Numerous studies have confirmed that changes in hippocampal plasticity (hippocampal volume, number of synapses, synaptic plasticity, changes in glutamate receptors, neurogenesis, and glial cell plasticity) occur in patients with depression [2]. It has been reported that the volume of the hippocampus in patients with major depression disorder (MDD) is significantly reduced [3]. After using antidepressant drugs, patient hippocampal tail volume increases in proportion to symptom relief [4]. The change in volume could be due to changes in neurons and glial cells.
Studies have shown that stress (especially chronic stress and early life stress) is closely related to the development of depression [5]. Chronic stress can cause apoptosis in hippocampal subregions and affect the integrity of hippocampal cells [6]. Long-term potentiation (LTP) and long-term depression (LTD) are the two main forms of synaptic plasticity changes. Stress causes changes in synaptic efficacy and stimulates the hypothalamic-pituitary-adrenal (HPA) axis to increase the levels of glucocorticoid (cortisol in human, corticosterone in rodents), resulting in a decrease in hippocampal LTP [7] that contributes to LTD [8]. Cell-level studies have found that stress leads to decreases in dendritic complexity and dendritic spine density [9]. On the other hand, excitatory synaptic neurotransmission is also affected in chronically stressed animals [10]. Central nervous system neurotransmitter dysfunction is one of the aspects of the pathogenesis of depression. Glutamate is widely distributed in the central nervous system and is an important excitatory neurotransmitter. Glutamate works by binding to the corresponding glutamate receptors, and pharmacological studies have shown that glutamate receptor modulators have antidepressant effects.
There are many hypotheses about the pathogenesis of depression, but the biological mechanisms remain unclear. The monoamine hypothesis is the most widely accepted hypothesis, and most current treatments for depression are based on the monoamine hypothesis. However, there are many limitations to the monoamine hypothesis. For example, the latency of antidepressant drugs cannot be explained, not all drugs that enhance monoamine functions have antidepressant activity, and a reduction in monoamines does not induce depression in healthy humans [11]. The neuroendocrine hypothesis proposes that abnormal function of the HPA axis leads to depression. Patients with depression often exhibit excessive secretion of glucocorticoids [12]. In a rodent model of chronic stress (classic depression model), plasma corticosterone levels are significantly increased [13]. Long-term administration of exogenous corticosterone can cause depression-like behavior in rodents [14]. With the development of immunological research, clinical studies have shown that chronic inflammation and some viral infectious diseases can significantly increase the incidence of depression, and the neuroinflammatory response hypothesis has thus been proposed. Studies have shown that the levels of proinflammatory cytokines in patients with depression are significantly increased [15]. Proinflammatory cytokines can affect the metabolism of monoamine transmitters and can also act on the HPA axis, impairing the negative feedback regulation of the HPA axis [16].
In recent years, people have gradually realized that information processing in the brain is not just the transfer of chemicals between neurons, but the result of the complex effects of neural networks. This finding puts forward the concept of neuroplasticity, that is, the ability of the nervous system to adapt and respond to the environment, including neurogenesis, neuronal remodeling, and synapse formation. The theory proposes that neuroplasticity disorders are involved in the pathological process of depression and that neurotrophic factors are important indicators for evaluating neuroplasticity. Brain-derived neurotrophic factor (BDNF) is a kind of neurotrophic factor. BDNF plays a key role in regulating synapse, hippocampal LTP, and neurogenesis [17].
Unfortunately, there is no single hypothesis that can explain the pathogenesis of depression. Moreover, the current clinical antidepressant treatment method is simplistic, the cure rate of antidepressant drugs is low, their side effects are high, and they are costly. The role of calorie restriction (CR) in depression has recently been revealed [18]. Previous research confirmed that CR is positively correlated with lifespan and negatively correlated with neurological diseases [19]. Studies have shown that CR can increase hippocampal neurogenesis and BDNF expression [20]. Neurogenesis is closely related to depression. Mitochondria are the sites at which cells metabolize energy and control programmed cell death [21]. The mitochondrial network plays a key role in CR [22]. Therefore, in addition to exploring the effect of CR on hippocampal plasticity, we also discuss the role of mitochondrial plasticity of the hippocampus.
At present, there have been many studies on antidepressants, and there are also different views on the pathogenesis of depression. However, the shortcomings of antidepressants are obvious to everyone. Because CR is simple to implement and has almost no side effects, it may be used to treat depression or to assist with existing antidepressant treatments to improve their efficacy. In this review, we introduce the various changes in hippocampal plasticity in depression and discuss the role of BDNF and CR in the treatment of depression. This review of hippocampal neuroplasticity may contribute to the development of new drugs and treatments.
2. Changes in Hippocampal Plasticity in Depression
The hippocampus is part of the limbic system and plays a vital role in depression. Numerous changes in hippocampal plasticity can be seen in both human depression patients and rodent depression models. There is a significant reduction in hippocampal volume. Depression can cause changes in various subregions, glutamate receptors, and glial cells in the hippocampus. Stress exists at the core of the many influencing factors. Some antidepressants also act through plasticity regulation. The different structures and functions of different hippocampal subregions may help to better understand hippocampal plasticity.
2.1. Hippocampal Volume
Clinical studies and neuroimaging results have shown that the hippocampal volume of patients with depression is reduced [23]. Similar results were observed in rodent models [24]. Both unipolar and bipolar depression (BD) patients have decreased hippocampal volume [25]. Telomere length is positively correlated with hippocampal volume, and reduced telomere length increases the risk of BD [26]. Chronic stress reduces hippocampal volume and inhibits neurogenesis in rats [27]. Early life stress increases the risk of MDD, which is associated with reduced left hippocampal volume [28]. The relationship between hippocampal volume and depression seems very complicated. Electroconvulsive therapy (ECT) increases the volume of the left hippocampus in patients, but there is no positive correlation with the treatment effect on depression [29]. The reduction in the hippocampus is not only caused by depression but is also a cause of depression [30]. Moreover, changes in hippocampal volume in depression are regulated by gene polymorphisms and gene expressions such as oxytocin receptor genes [31], monoamine-related genes [32], and neuroinflammatory genes [33].
2.2. Hippocampal Synaptic Neuroplasticity in the Different Subregions
In depression, the changes in hippocampal synaptic plasticity are also reflected in the hippocampal subregions, especially the cornu ammonis 3 (CA3) and the dentate gyrus (DG). In this section, we summarize the relationship between different hippocampal subregions and LTP and LTD and introduce some antidepressant drugs or methods to improve depression (based on the plasticity of the different subregions).
2.2.1. Cornu Ammonis (CA1-CA3)
CA1 neurons accept 2 different glutamatergic inputs: temporoammonic- (TA-) CA1 and the CA3 region and the Schaffer collateral- (SC-) CA1. The JAK-STAT signaling pathway plays a role in the LTD induced by both pathways [34]. Chronic unpredictable stress (CUS) and corticosterone administration can reduce the excitatory signaling of TA-CA1 in rats [35]. Long-term plasticity in CA1 is induced by GABAergic interneurons (parvalbumin-expressing (PV+), nitric oxide synthase-expressing (NOS+)) [36]. Acute stress activates μ-opioid receptors on GABAergic neurons to facilitate low-frequency stimulation-induced LTD at SC-CA1 glutamatergic synapses in mice [37]. Electroacupuncture (EA) can improve depression by restoring CA1 synaptic plasticity, which may be mediated by regulating 5-HT receptor levels [38]. The perineuronal net (PNN) restricts LTD in the CA1 area in mice [39]. Interlaminar CA1-CA1 networks exhibit NMDA receptor-dependent LTP rather than LTD [40].
CA2 is a region that tends to be overlooked. The volume of CA2 is negatively correlated with depressive symptoms [41]. It is difficult to induce LTP and LTD in the CA2 region, which showed unique synaptic stability compared with that of other regions. A study showed that neurons in CA2 require more current to generate action potentials [42]. The function of CA2 in regulating synaptic plasticity may be different from other CA regions. The inhibitory LTD of PV+ interneurons in the CA2 region does not exist in young mice and may require the maturation of PNN and ErbB4 [43]. The plasticity of PV+ neurons is mediated by δ-opioid receptors [44].
CA3 pyramidal cells receive three types of excitatory synaptic afferent neurons: mossy fibers (MFs), perforant paths (PPs), and associational/commissural (AC) fibers. The MF-CA3 synapse has been reported to play a role in antidepressant drugs [45]. Novel spatial learning contributes to hippocampal plasticity and is termed learning-facilitated plasticity (LFP). When rats explore different environments, the MF-CA3 and AC-CA3 synapses show different responses to LTD. However, when exploring a new empty environment, both synapses promote LTP [46]. LFP-induced LTD and LTP in the MF-CA3 synapse require the activation of β-adrenergic receptors [47]. In addition, ECT treatment can increase dopamine regulation in MF [48]. The antidepressant drugs can enhance D1 receptor-dependent synaptic potentiation in the MF-CA3 [49]. Vagus nerve stimulation (a treatment for depression) can cause an increase in PP-CA3 synaptic transmission [50]. In an animal model of depression induced by chronic unpredictable mild stress (CUMS), LTP, basal synaptic transmission, and dendrite spine density were decreased in the CA3-CA1 synapse [51]. The CA3-CA1 synapse is also thought to be involved in the acquisition of associative learning [52]. CA3-CA3 synapses are widely distributed [53] and have strong plasticity [54]. Studies have shown that the spike timing-dependent plasticity of the CA3-CA3 synapses contributes to information storage and retrieval [55].
2.2.2. Dentate Gyrus
Apoptosis in the CA1 and CA4 regions and the DG can be observed in patients with MDD [56]. Patients with ECT treatment that had larger right CA4/DG volumes were associated with symptom remission [57]. DG plasticity changes are also involved in the mechanism of some antidepressant drugs. Tianeptine is a special tricyclic antidepressant. It mainly acts on the 5-HT system, which can increase the activity of hippocampal pyramidal cells and the reuptake of 5-HT by hippocampal neurons (as opposed to traditional antidepressants) [58]. Tianeptine was reported to reduce DG apoptosis in a tree shrew model of depression [59]. Maternal separation (MS) can impair learning and memory and cause depression-like behavior. Studies have shown that MS can induce the apoptosis in the DG and reduce cell proliferation in rats [60]. The expression of glutamate receptor 1 and protein kinase B phosphorylation in the DG were also decreased [60]. These factors may be involved in depression caused by MS. Fluoxetine, a selective 5-HT reuptake inhibitor widely used in clinical practice, can selectively inhibit 5-HT transporter and block the reuptake of 5-HT by the presynaptic membrane, thereby producing antidepressant effects. A study has shown that after 2 weeks of MS in rats, 7 days of fluoxetine treatment can reverse cell apoptosis, increase cell proliferation (reduce the number of terminal deoxynucleotidyl transferase-mediated dUTP gap terminal marker-positive cells), and has an antidepressant effect [61]. Chronic stress can damage LTP in the DG of rats, but this effect is reversible [62].
The effect of sirtuin (SIRT) 2 knockdown in the DG is similar to that of chronic stress, and both downregulate plasticity-related genes [63]. In addition, chronic stress reduces SIRT1 activity in mice, leading to depression-like behavior [64]. Physical activity can modulate LTP and LTD in DG of rodents, but the effects are complex and depend on the studies and the conditions (exercise regime, duration, and intensity) [65]. Both acute stress and dexamethasone injection increased the release of somatostatin (SST) in DG hilar cells [66]. Several studies have shown that SST promotes LTP in the DG [67]. However, the opposite effect has also been reported [68]. The reason for this phenomenon may be that SST has different effects on different hippocampal synapses.
There are other factors, such as dopamine [69], 5-HT [70], sex hormones (estradiol, testosterone) [71], paracrine signaling factor (Wnt [72], Notch1 [73]), proinflammatory cytokines [74], and epigenetic changes [75], that are also involved in regulating DG plasticity, which we will not elaborate here.
2.3. Glutamate Receptor Involvement in Synaptic Plasticity
Glutamate receptors are mainly divided into two categories: one is ionic receptors including the N-methyl-d-aspartate receptor (NMDAR), the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR), and the kainite receptor (KAR), and the metabolic receptor, mGluR. These receptors are closely related to hippocampal plasticity to varying degrees.
2.3.1. N-Methyl-d-aspartate Receptor (NMDAR)
NMDAR has multiple subtypes, including NR1 and NR2A-D. The function of NMDAR is determined by the different functions, structure and distribution of various subunits, and different combinations of subunits [76]. Generally, NR1 is considered to be an important component of NMDAR functionality [77]. NMDAR ligand-gated ion channels are regulated by glycine [78], and glycine has a binding site on NR1 [79]. Previous studies have shown that NMDAR is mainly distributed in the postsynaptic density (PSD) of the postsynaptic membrane. Studies in recent years have confirmed that NMDAR is also distributed in the presynaptic membrane and non-PSD areas [80]. PSD95 participates in NMDA receptor regulation [81]. PSD95 expression promotes the maturation of excitatory (glutamatergic) synapses, increasing the number and size of dendritic spines [82]. PSD95 stabilizes NMDAR by binding to GluN2B or degrading STEP61 [83]. The activity-dependent regulation of STEP61 and its substrates GluN2B and GluA2 (a subunit of AMPAR) may contribute to the homeostasis of excitatory synapses [84]. In the absence of stimulation, the NMDA ion channel is not opened due to the blocking of Mg2+. Under various stimuli, the postsynaptic membrane depolarizes. The blocking effect of Mg2+ disappears, and Ca2+ and Na+ enter the cell, increasing free Ca2+ in the cell and producing various biochemical reactions. NMDAR-dependent LTP is widely reported in the hippocampus [85]. Ketamine is an NMDAR antagonist, and its rapid antidepressant mechanism has been confirmed in human and animal models. Ketamine can reverse the decrease of hippocampal CA3 and DG dendritic spine density in depressed mice, activate AMPAR, and increase BDNF expression and release and activation of the mammalian target of rapamycin (mTOR) [86]. Depressed rats treated with ketamine exhibit increased LTP and levels of EPSCs mediated by NMDA receptors in the hippocampus [87]. In addition to PSD95, NMDAR also binds with multiple molecules including CaMKII [88] and microtubule-associated protein 2 [89], to form a multiprotein complex, which plays a role in plasticity, learning, and memory.
2.3.2. α-Amino-3-hydroxy-5-methyl-4-isoxazole Propionic Acid Receptor (AMPAR)
AMPAR regulates synaptic plasticity through changes in the number of postsynaptic membranes (insertion or removal of AMPAR). AMPAR regulates synaptic transport during LTP through two ways: lateral movement and exocytosis [90]. Lateral diffusion exchanges AMPAR at the dendritic spines, depending on the spinal morphology [91]. AMPAR surface diffusion is the key to hippocampal LTP and learning [92]. Acute stress can increase the AMPAR phosphorylation and surface expression in the hippocampal CA1 region of mice and alleviate LTP impairment [93]. Another way to recruit AMPAR to the synapse is through exocytosis [94]. Cyclin Y inhibits AMPAR exocytosis of dendritic spines, thereby inhibiting LTP [95]. This exocytosis in LTP passes through the RAS/ERK signaling pathway [96]. In addition, the silent synapses (the synapse only expresses NMDAR before inducing LTP) express AMPAR after inducing LTP and then, they become a functional synapse [97]. Several studies have suggested that hippocampal LTD is associated with AMPAR endocytosis. Calcyon can regulate endocytosis, and knocking out the gene that encodes calcyon leads to the LTD disappearance in the hippocampal CA1 region [98]. Inhibition of AMPAR endocytosis can increase the AMPAR level and decrease LTD [99]. However, one study suggested that AMPAR-induced LTD was the result of inhibiting exocytosis rather than enhancing endocytosis [100].
2.3.3. Kainate Receptor (KAR)
There has been less focus on KAR than on the other two ionic glutamate receptors. However, the function of KAR associated with plasticities such as the modulation of excitability [101], transmitter release [102], neuronal development [103], and neurogenesis [104]. The neto protein is an important auxiliary protein that regulates the KAR in many interneurons [105]. The distribution of KAR also shows some differences, and it is expressed in the presynaptic regions, in addition to the postsynaptic regions [106]. Furthermore, the bidirectional regulation of KAR is also considered to play an important role in plasticity [107]. Several studies have found a link between KAR subtypes and depression. GluR7 is a susceptibility gene associated with recurrent MDD [108]. GluK4 (a KAR subunit) deficiency is associated with increased cognitive ability [109]. Rodent models have also confirmed that GRIK4 (GluK4 gene) knockout causes an antidepressant phenotype [110].
Chronic stress and corticosteroids increase KAR subunit mRNA expression in rats [111]. KAR is reported to be involved in glutamate release in the CA1 and CA3 regions [112]. The short-term and long-term potentiation of KAR participation in the hippocampal MF synapse has been generally confirmed, in which presynaptic KAR plays a central role [113]. Similarly, KAR is also involved in the induction and expression of hippocampal LTD [114]. KAR regulates not only the glutamatergic system but also the GABAergic system. KAR inhibits GABA release and the synaptic transmission to CA1 [115]. However, another study came to the opposite conclusion. KAR increased the efficacy of GABAergic synapses [116]. This effect could be related to the agonist concentration, but the specific mechanism is still unclear.
2.3.4. Metabolic Receptor (mGluR)
mGluR is a type of G-protein-coupled receptor and is divided into three categories (I, II, and III) with 8 subtypes (mGluR1-8). Type I mGluRs include two subtypes: mGluR1 and mGluR5. Chronic stress can increase type I mGluR-mediated LTD in the hippocampal CA1 region [117], while acute stress has only a promoting effect [118]. This phenomenon indicates that type I mGluR-mediated plasticity changes require repeated stress stimulation. Besides, DHPG (a type I glutamate receptor agonist) induced synaptic plasticity of the Schaffer collateral NMDAR (induced LTD) [119]. Studies on type I mGluRs mainly focus on the feedback circuit of the hippocampal stratum oriens, in which type I mGluRs induce LTP [120]. The type I mGluRs induced anti-Hebbian LTP in interneurons of rat hippocampal stratum oriens [121]. Type I mGluRs are also involved in TEA-induced LTP in rat MF-CA3 synapses [122]. Studies have shown that type I mGluR-mediated synaptic plasticity occurs via the β-arrestin signaling pathway [123]. The TRPC1 channel plays a critical role in the process of mGluR5-regulated plasticity [124].
Although type II and III mGluRs are rarely reported in terms of hippocampal plasticity, their important role cannot be ignored. Fluoxetine combined with a low-dose ly379268 (a mGluR2/3 agonist) can increase cell proliferation and neurogenesis of cultured cerebellar granule neurotransmitters and shorten the incubation period required for the downregulation of hippocampal β-adrenergic receptors [125]. The study of type III mGluRs showed that MS significantly reduced the expression of mGluR4 in the hippocampus of rats, while fluoxetine reversed the changes induced by MS [126]. Behavioral pharmacology studies have shown that LSP4-2022 (a selective agonist of mGluR4) has a strong effect in promoting depression in mice. This effect does not exist in mGluR4-knockout mice, but whether an mGluR4 antagonist can induce an antidepressant effect needs further verification [127]. In mGluR4-knockout mice, improved spatial learning is associated with hippocampal LTP [128]. AMN082 (an mGluR7 allosteric agonist) produces antidepressant-like effects by regulating glutamatergic signaling [129]. mGluR7-knockout mice show antidepressant phenotype, causing HPA axis dysregulation and increasing hippocampal BDNF protein levels [130].
2.4. Hippocampal Apoptosis and Neurogenesis
The neurogenesis hypothesis is to some extent an extension of the BDNF hypothesis. Apoptosis of hippocampal neurons has been observed in MDD patients and rodent depression models [131, 132]. Apoptosis of hippocampal neurons may be one of the causes of depression. Venlafaxine is a dual inhibitor of 5-HT and norepinephrine reuptake, which can inhibit the reuptake of NA and 5-HT and slightly inhibit the reuptake of dopamine. Venlafaxine also inhibits hippocampal neuron apoptosis in depression by upregulating the expression of BDNF in the hippocampus of rats [133].
Hippocampal neurogenesis is the process by which adult neural stem cells become progenitor cells and then functional DG cells, providing functional and structural plasticity [134]. Although the number of newborn neurons is much smaller than that in the hippocampal granular cell layer, these cells have important implications in hippocampal function. Increased adult hippocampal neurogenesis can reduce depression-like behavior in mice [135]. Chronic corticosterone injection reduced the neurogenesis of DG in rats [136] and the dendritic complexity of mature granule cells [137]. Adult neurogenesis altered the excitability of the DG [138] and enforced chronic stress adaptability by inhibiting mature granulosa cells in the ventral DG [139]. Ketamine increased DG cell proliferation in depressed rats [140]. Stress and corticosterone also inhibit progenitor cell proliferation, possibly by increasing nitric oxide levels [141].
2.5. Glial Cell Plasticity
Glial cells are another prominent cell type in nerve tissue and are widely distributed in the central and peripheral nervous systems. The glial cells in the central nervous system mainly include astrocyte, oligodendrocyte, and microglia. There is evidence that astrocytes affect adult neurogenesis in hippocampal DG [142]. The activity of p38α mitogen-activated protein kinase (MAPK) in astrocytes is required for hippocampal LTD [143]. Chronic mild stress-induced depression may be related to a decrease in the number of astrocytes and the activation of microglia [144]. The pruning of synapses by microglia is necessary for brain development [145]. Microglia reshape synapses through presynaptic phagocytosis and spine head filopodia induction. Microglial inhibitors can eliminate the decrease in hippocampal LTP and LTD caused by peripheral inflammation [146]. In CUS-induced depression model rats, the number of oligodendrocytes in the hippocampal CA3 and DG regions is decreased [147]. Oligodendrocyte depolarization can induce short-term and long-term plasticity in hippocampal white matter [148]. Oligodendrocytes can regulate axonal excitability and nerve conduction [149]. The inhibition of oligodendrocyte formation impairs memory consolidation in mice [150]. Myelin basic protein is a marker of mature oligodendrocytes. Chronic social frustration stress can cause changes in the plasticity of ventral hippocampal myelin, but this effect depends on genetic background [151].
3. Role of Brain-Derived Neurotrophic Factor in Depression
The neurotrophic factor and neurogenesis hypotheses connect BDNF with plasticity and depression. Clinical studies have shown that plasma BDNF levels are reduced in patients with depression [152], and antidepressant treatment can increase BDNF levels [153]. BDNF is an indispensable factor in the antidepressant effects of ketamine [154]. BDNF plays an important role in regulating hippocampal plasticity. BDNF regulates LTP in the hippocampus by enhancing synaptic responses [155]. In addition, BDNF also affects the expression of monoamine genes in the hippocampus [156]. Current studies have mainly focused on BDNF and its precursor (proBDNF) and mature forms (mBDNF) [157]. CUMS caused a decrease in the BDNF/proBDNF ratio in the rodent's hippocampus: BDNF rescued CUMS-induced behaviors and spine loss, while proBDNF resulted in a decrease in spine density [158]. The high-affinity receptor of BDNF is tropomyosin receptor kinase B (TrkB), which has low affinity for p75 [159]. Furthermore, BDNF activates multiple signaling pathways (Figure 1). Therefore, in this section, we focus on these signaling pathways and discuss the role of the BDNF signaling pathway in hippocampal plasticity in depression and antidepressant drugs.
Figure 1.
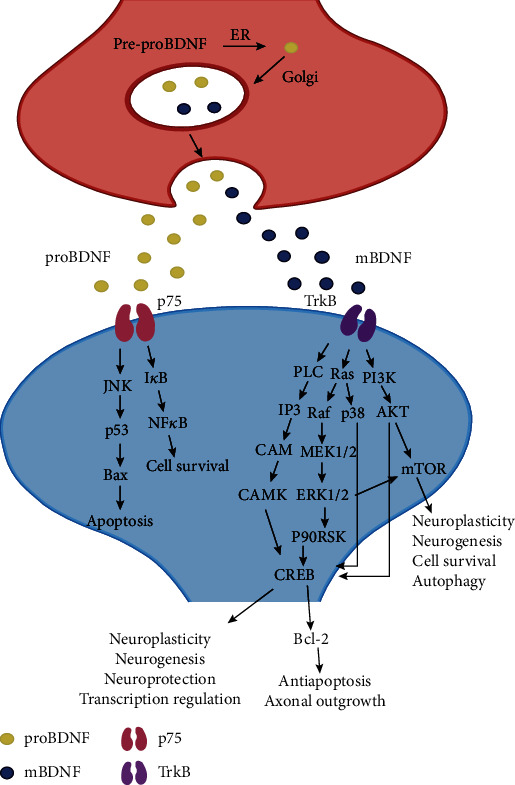
Diagrams of the BDNF-relevant signaling pathways. BDNF: brain-derived neurotrophic factor; ER: endoplasmic reticulum; TrkB: tropomyosin receptor kinase B; PLC: phospholipase C; IP3: inositol triphosphate; CAM: calmodulin; CAMK: CAM-dependent protein kinase; p90RSK: 90 kDa ribosomal S6 kinase; ERK1/2: extracellular-regulated kinase 1/2; PI3K: phosphoinositide 3-kinase; AKT: protein kinase B; mTOR: mammalian target of rapamycin; CREB: cAMP-response element binding protein; JNK: c-Jun N-terminal kinase; Bax: Bcl-2-associated X protein; NF-κB: nuclear factor-κB; IκB: an inhibitor of NF-κB.
3.1. Mitogen-Activated Protein Kinase (MAPK) Pathway
After binding to TrkB, BDNF phosphorylates Shc and alters the conformation of the TrkB-Shc complex [160]. Phosphorylated Shc then acts on the downstream growth factor receptor-bound protein 2 (GRB2) and the son of sevenless (SOS), which in turn activate the MAPK signaling pathway, leading to ERK1/2 phosphorylation [161]. BDNF may increase nuclear CREB activity through 2 main pathways: p38 phosphorylation and 90 kDa ribosomal S6 kinase (p90RSK) phosphorylation [162, 163]. CREB is an important hub in neuroplasticity and neuroprotection [164]. Many examples of antidepressants work through the BDNF/MAPK pathway. For example, the plant Centella asiatica supposed to have antidepressant properties improves the memory of chronic electrical stress rats (increased hippocampal BDNF levels) via the BDNF/TrkB/ERK pathway [165]. The plant Angelica sinensis plays a neuroprotective effect through the BDNF/CREB/p90RSK signaling pathway [162]. Imipramine, a selective inhibitor of monoamine reuptake, can exert neuroprotective effects via the BDNF/MAPK/Bcl-2 pathway [166].
3.2. Phospholipase Cγ (PLCγ)
Activated PLC hydrolyzes the membrane component phosphatidylinositol 4,5-bisphosphate (PIP2) to produce a second messenger inositol triphosphate (IP3). IP3 promotes the release of Ca2+ from the cellular calcium reservoir and increases the concentration of Ca2+ in the cytoplasm. Ca2+ then binds to calmodulin (CAM) to transmit the signal. The signal transduction molecule downstream of the Ca2+/M complex is a protein kinase that can be activated by the Ca2+/CAM complex called CAM-dependent protein kinase [167]. One study showed that the TrkB/PLC pathway mediated mouse hippocampal LTP and regulated CREB activity [168].
3.3. Phosphoinositide 3-Kinase (PI3K) Pathway
Activated PI3K can catalyze the production of phosphatidylinositol 3,4,5-triphosphate (PIP3). PIP3 activates AKT by binding to its pleckstrin homology (PH) domain and can also activate AKT by activating 3-phosphoinositide-dependent protein kinase 1 (PDK1) [169]. BDNF has been reported to inhibit autophagy through the PI3K-Akt signaling pathway [170].
Most studies on downstream molecules of the BDNF-mediated PI3K-Akt signaling pathway have focused on mammalian target of rapamycin (mTOR). The BDNF/mTOR signaling pathway is involved in the rapid antidepressant mechanism of the antidepressant drug ketamine [154], and ketamine enhances the structural plasticity of dopaminergic neurons through the AMPA receptor-driven BDNF/mTOR signaling pathway [171]. S 47445 (positive allosteric modulator of AMPAR) reduced the motor activity of olfactory bulb-excised mice, showing an antidepressant effect, and reversed the changes in the expression of BDNF/mTOR [172]. Hypidone hydrochloride (YL-0919) showed a faster antidepressant effect than fluoxetine and also reversed the activity of BDNF/mTOR signaling and some key synaptic proteins [173]. Sulforaphane can play an antidepressant effect in chronic mild stress mice and can block the elevation of corticosterone, corticotropin, IL-6, and TNF-α in serum of mice [174]. The antidepressant effect of sulforaphane may be through inhibition of the HPA axis and inflammatory response. Furthermore, sulforaphane can reverse the decrease of BDNF and dendritic spine density in depressed mice [175]. Knockdown of hyperpolarization-activated cyclic nucleotide-gated channel 1 (HCN1) increased the excitability of cells in the mouse CA1 region, resulting in showing an antidepressant phenotype [176]. Inhibition of HCN1 can reduce depression and improve learning ability in rats [177]. These effects are all related to the upregulation of the BDNF/mTOR signaling pathway and synaptic transmission. Studies on the binding of mTOR with different proteins to form functional polymer complexes suggested that BDNF/mTOR1 can be used as a research focus for a new generation of antidepressant drugs [178]. The BDNF/mTOR1 signaling pathway may be the target of the antidepressant effect of traditional prescriptions (lily bulb and Rehmannia decoction) [179].
4. Caloric Restriction as a Putative Treatment in Depression
The antidepressant effect of CR has been confirmed in rodent depression models (Figure 2). During aging, neuroinflammation and oxidative stress increase in the hippocampus, and synaptic plasticity and neurogenesis decrease, but these adverse reactions can be alleviated by CR [180]. CR reduces cell death in the hippocampal CA3 area after kainate administration [181]. At the genetic level, CR reduces basic DNA loss [182] and affects the expression of genes associated with synaptic plasticity in the hippocampal CA1 and CA3 region [183]. CR can stabilize the gene expression of presynaptic proteins [184] and prevent the reduction in key synaptic proteins in the CA3 [185]. In terms of neuroendocrine, CR increased the expression of adiponectin in rodent adipose tissue and blood adiponectin levels [186]. Patients with BD often have decreased plasma adiponectin levels [187]. Adiponectin and antidepressant drugs can improve the symptoms of depression [188]. Adiponectin has a beneficial effect in fighting neuroinflammation [189] and also plays an important role in the remodeling and neurogenesis of dendrites and dendritic spines of mouse hippocampal neurons [190]. All of these factors have beneficial effects on depression. Furthermore, CR can also lead to reduced leptin levels [191]. There have been different research results on the relationship between leptin and depression. Studies have shown that patients with depression have reduced leptin levels [192], and the same conclusions have been reached in animal models of depression [193]. However, in another study, it was found that patients with MDD had increased leptin levels [194]. Leptin is considered to be a proinflammatory factor, and the reduced level caused by CR may have a positive effect on depression [195]. CR also increases hippocampal synaptic plasticity by changing the morphology and function of astrocytes [196]. A study has confirmed that CR can preserve LTP that is lost in the hippocampus of aging rats [197]. cAMP and its response element-binding protein CREB are involved in the CR-mediated regulation of plasticity [198]. However, the regulation of plasticity by CR is bidirectional, as long-term CR decreased hippocampal neurogenesis and granulosa cell density [199] and leads to reduced levels of some lipids in the DG, impairing spatial memory [200], whereas short-term CR has beneficial effects on hippocampal plasticity. Proteomics analysis showed that CR could improve glutamate disorders, impaired protein synthesis, and mitochondrial dysfunction [201].
Figure 2.
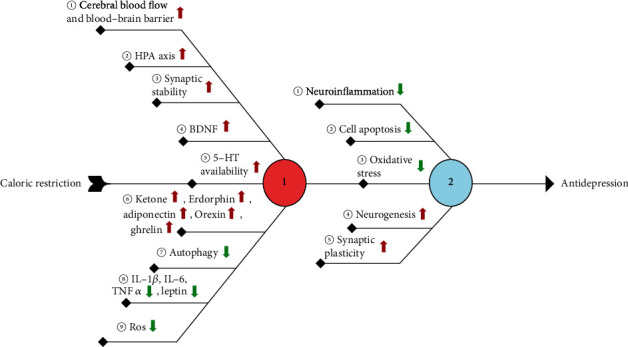
The molecular mechanism involved in the antidepressant effect of caloric restriction.
Depression may be related to impaired hippocampal mitochondrial plasticity, which is restored by antidepressant treatment [202]. Mitochondria contribute to increasing dendritic spines, synapse regeneration, and synaptic plasticity [203]. When mitochondria increase synaptic plasticity, BDNF levels increase [204]. Chronic stress leads to mitochondrial dysfunction and mitochondrial protein imbalance [205]. Importantly, LTP requires a rapid burst and fission of dendritic mitochondria [206]. In terms of treatment, physical exercise can improve posttraumatic stress disorder through an increase in hippocampal mitochondrial function [207]. Mitochondria are associated with BDNF-mediated synapses and vascular plasticity in ECT [208].
5. Conclusion
Different degrees of hippocampal plasticity changes have been observed in clinical depression patients and rodent depression models (Figure 3). BDNF plays a central role in hippocampal neuroplasticity, and a variety of changes involve the activation or inhibition of the BDNF signaling pathways. The downstream mTOR signaling pathway has received widespread attention since it was discovered to have a role in the antidepressant mechanism of ketamine. BDNF also promotes neurogenesis and remodeling of synaptic morphology and structure through the activation of the mTOR signaling pathway. However, due to the many side effects of ketamine and the fact that the direct activation or inhibition of the mTOR signaling pathway may have adverse effects on humans, the research on mTOR has been restrained to a certain extent. However, this does not affect its potential as a new generation, fast-acting antidepressant treatment target. The positive effects of CR on a variety of neurological diseases (including depression) have been confirmed. Because CR has almost no side effects and is simple to perform, it does not burden patients further with discomfort or treatment costs. CR may be used as a physical form of clinical antidepressant treatment in the future and support other antidepressant treatments to further reduce the suffering of patients with depression. However, the deeper molecular and cellular mechanisms of CR need to be further explored, and its possible defects should be clarified.
Figure 3.
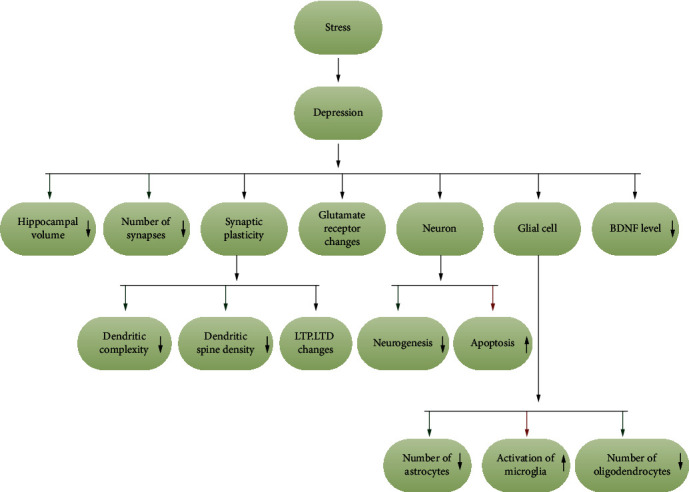
Changes in hippocampal plasticity in depression.
Acknowledgments
This work was supported by the National Key R&D Program of China (Grant No. 2018YFC1311600), the Jilin Province Medical and Health Talents (2017F012, 2019SCZT007, and 2019SCZT013), the Jilin Science and Technology Agency (20180414050GH, 20190701078GH, 20200201465JC, and 20200301005RQ), and Scientific Research Foundation of the Education Department of Jilin Province (Grant Nos. JJKH20201038KJ and JJKH20201032KJ).
Contributor Information
Songbai Xu, Email: xusongbai@jlu.edu.cn.
Bingjin Li, Email: libingjin@jlu.edu.cn.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
WX and RC contributed to the drafting of the article. WX, XY, FZ, HZ, ZC, WY, RC, SX, and BL provided comments. All authors have revised the manuscript critically for important intellectual content and approved the final version to be published.
References
- 1.Huang Y., Wang Y., Wang H., et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi: 10.1016/S2215-0366(18)30511-X. [DOI] [PubMed] [Google Scholar]
- 2.Sheline Y. I., Liston C., McEwen B. S. Parsing the hippocampus in depression: chronic stress, hippocampal volume, and major depressive disorder. Biological Psychiatry. 2019;85(6):436–438. doi: 10.1016/j.biopsych.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Cao B., Passos I. C., Mwangi B., et al. Hippocampal subfield volumes in mood disorders. Molecular Psychiatry. 2017;22(9):1352–1358. doi: 10.1038/mp.2016.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maller J. J., Broadhouse K., Rush A. J., Gordon E., Koslow S., Grieve S. M. Increased hippocampal tail volume predicts depression status and remission to anti-depressant medications in major depression. Molecular Psychiatry. 2018;23(8):1737–1744. doi: 10.1038/mp.2017.224. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y., Dai J. Advance in stress for depressive disorder. Advances in Experimental Medicine and Biology. 2019;1180:147–178. doi: 10.1007/978-981-32-9271-0_8. [DOI] [PubMed] [Google Scholar]
- 6.Lucassen P. J., Heine V. M., Muller M. B., et al. Stress, depression and hippocampal apoptosis. CNS & Neurological Disorders Drug Targets. 2006;5(5):531–546. doi: 10.2174/187152706778559273. [DOI] [PubMed] [Google Scholar]
- 7.Tamano H., Sato Y., Takiguchi M., et al. CA1 LTP attenuated by corticosterone is canceled by effusol via rescuing intracellular Zn2+ dysregulation. Cellular and Molecular Neurobiology. 2019;39(7):975–983. doi: 10.1007/s10571-019-00693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu L., Anwyl R., Rowan M. J. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387(6632):497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 9.Leuner B., Shors T. J. Stress, anxiety, and dendritic spines: what are the connections. Neuroscience. 2013;251:108–119. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Kallarackal A. J., Kvarta M. D., Cammarata E., et al. Chronic stress induces a selective decrease in AMPA receptor-mediated synaptic excitation at hippocampal temporoammonic-CA1 synapses. The Journal of Neuroscience. 2013;33(40):15669–15674. doi: 10.1523/JNEUROSCI.2588-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boku S., Nakagawa S., Toda H., Hishimoto A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry and Clinical Neurosciences. 2018;72(1):3–12. doi: 10.1111/pcn.12604. [DOI] [PubMed] [Google Scholar]
- 12.Pariante C. M. Why are depressed patients inflamed? A reflection on 20 years of research on depression, glucocorticoid resistance and inflammation. European Neuropsychopharmacology. 2017;27(6):554–559. doi: 10.1016/j.euroneuro.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Gong S., Miao Y. L., Jiao G. Z., et al. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One. 2015;10(2, article e0117503) doi: 10.1371/journal.pone.0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y., Ma R., Shen J., Su H., Xing D., Du L. A mouse model of depression induced by repeated corticosterone injections. European Journal of Pharmacology. 2008;581(1-2):113–120. doi: 10.1016/j.ejphar.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Mao R., Zhang C., Chen J., et al. Different levels of pro- and anti-inflammatory cytokines in patients with unipolar and bipolar depression. Journal of Affective Disorders. 2018;237:65–72. doi: 10.1016/j.jad.2018.04.115. [DOI] [PubMed] [Google Scholar]
- 16.Abelaira H. M., Réus G. Z., Petronilho F., Barichello T., Quevedo J. Neuroimmunomodulation in depression: a review of inflammatory cytokines involved in this process. Neurochemical Research. 2014;39(9):1634–1639. doi: 10.1007/s11064-014-1372-5. [DOI] [PubMed] [Google Scholar]
- 17.Numakawa T., Odaka H., Adachi N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. International Journal of Molecular Sciences. 2018;19(11):p. 3650. doi: 10.3390/ijms19113650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manchishi S. M., Cui R. J., Zou X. H., Cheng Z. Q., Li B. J. Effect of caloric restriction on depression. Journal of Cellular and Molecular Medicine. 2018;22(5):2528–2535. doi: 10.1111/jcmm.13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Cauwenberghe C., Vandendriessche C., Libert C., Vandenbroucke R. E. Caloric restriction: beneficial effects on brain aging and Alzheimer's disease. Mammalian Genome. 2016;27(7-8):300–319. doi: 10.1007/s00335-016-9647-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee J., Duan W., Long J. M., Ingram D. K., Mattson M. P. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. Journal of Molecular Neuroscience. 2000;15(2):99–108. doi: 10.1385/JMN:15:2:99. [DOI] [PubMed] [Google Scholar]
- 21.Yamada K., Yoshida K. Mechanical insights into the regulation of programmed cell death by p53 via mitochondria. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2019;1866(5):839–848. doi: 10.1016/j.bbamcr.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Weir H. J., Yao P., Huynh F. K., et al. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metabolism. 2017;26(6):884–896.e5. doi: 10.1016/j.cmet.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roddy D. W., Farrell C., Doolin K., et al. The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biological Psychiatry. 2019;85(6):487–497. doi: 10.1016/j.biopsych.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Kalisch R., Schubert M., Jacob W., et al. Anxiety and hippocampus volume in the rat. Neuropsychopharmacology. 2006;31(5):925–932. doi: 10.1038/sj.npp.1300910. [DOI] [PubMed] [Google Scholar]
- 25.Videbech P., Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 26.Powell T. R., Dima D., Frangou S., Breen G. Telomere length and bipolar disorder. Neuropsychopharmacology. 2018;43(2):445–453. doi: 10.1038/npp.2017.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoenfeld T. J., McCausland H. C., Morris H. D., Padmanaban V., Cameron H. A. Stress and loss of adult neurogenesis differentially reduce hippocampal volume. Biological Psychiatry. 2017;82(12):914–923. doi: 10.1016/j.biopsych.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saleh A., Potter G. G., McQuoid D. R., et al. Effects of early life stress on depression, cognitive performance and brain morphology. Psychological Medicine. 2017;47(1):171–181. doi: 10.1017/S0033291716002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gbyl K., Videbech P. Electroconvulsive therapy increases brain volume in major depression: a systematic review and meta-analysis. Acta Psychiatrica Scandinavica. 2018;138(3):180–195. doi: 10.1111/acps.12884. [DOI] [PubMed] [Google Scholar]
- 30.Chan S. W., Harmer C. J., Norbury R., O’Sullivan U., Goodwin G. M., Portella M. J. Hippocampal volume in vulnerability and resilience to depression. Journal of Affective Disorders. 2016;189:199–202. doi: 10.1016/j.jad.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Na K. S., Won E., Kang J., et al. Interaction effects of oxytocin receptor gene polymorphism and depression on hippocampal volume. Psychiatry Research: Neuroimaging. 2018;282:18–23. doi: 10.1016/j.pscychresns.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Phillips J. L., Batten L. A., Tremblay P., Aldosary F., Du L., Blier P. Impact of monoamine-related gene polymorphisms on hippocampal volume in treatment-resistant depression. Acta Neuropsychiatrica. 2015;27(6):353–361. doi: 10.1017/neu.2015.25. [DOI] [PubMed] [Google Scholar]
- 33.Mahajan G. J., Vallender E. J., Garrett M. R., et al. Altered neuro-inflammatory gene expression in hippocampus in major depressive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2018;82:177–186. doi: 10.1016/j.pnpbp.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGregor G., Irving A. J., Harvey J. Canonical JAK-STAT signaling is pivotal for long-term depression at adult hippocampal temporoammonic-CA1 synapses. The FASEB Journal. 2017;31(8):3449–3466. doi: 10.1096/fj.201601293RR. [DOI] [PubMed] [Google Scholar]
- 35.Kvarta M. D., Bradbrook K. E., Dantrassy H. M., Bailey A. M., Thompson S. M. Corticosterone mediates the synaptic and behavioral effects of chronic stress at rat hippocampal temporoammonic synapses. Journal of Neurophysiology. 2015;114(3):1713–1724. doi: 10.1152/jn.00359.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau P. Y., Katona L., Saghy P., Newton K., Somogyi P., Lamsa K. P. Long-term plasticity in identified hippocampal GABAergic interneurons in the CA1 area in vivo. Brain Structure and Function. 2017;222(4):1809–1827. doi: 10.1007/s00429-016-1309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan K. M., Qiu L. J., Ma N., et al. Acute stress facilitates LTD induction at glutamatergic synapses in the hippocampal CA1 region by activating μ-opioid receptors on GABAergic neurons. Frontiers in Neuroscience. 2019;13:p. 71. doi: 10.3389/fnins.2019.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han X., Wu H., Yin P., et al. Electroacupuncture restores hippocampal synaptic plasticity via modulation of 5-HT receptors in a rat model of depression. Brain Research Bulletin. 2018;139:256–262. doi: 10.1016/j.brainresbull.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Khoo G. H., Lin Y. T., Tsai T. C., Hsu K. S. Perineuronal nets restrict the induction of long-term depression in the mouse hippocampal CA1 region. Molecular Neurobiology. 2019;56(9):6436–6450. doi: 10.1007/s12035-019-1526-1. [DOI] [PubMed] [Google Scholar]
- 40.Sun D. G., Kang H., Tetteh H., et al. Long term potentiation, but not depression, in interlamellar hippocampus CA1. Scientific Reports. 2018;8(1):p. 5187. doi: 10.1038/s41598-018-23369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Györfi O., Nagy H., Bokor M., et al. Reduced CA2-CA3 hippocampal subfield volume is related to depression and normalized by l-DOPA in newly diagnosed Parkinson's disease. Frontiers in Neurology. 2017;8:p. 84. doi: 10.3389/fneur.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao M., Choi Y. S., Obrietan K., Dudek S. M. Synaptic plasticity (and the lack thereof) in hippocampal CA2 neurons. The Journal of Neuroscience. 2007;27(44):12025–12032. doi: 10.1523/JNEUROSCI.4094-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domínguez S., Rey C. C., Therreau L., et al. Maturation of PNN and ErbB4 signaling in area CA2 during adolescence underlies the emergence of PV interneuron plasticity and social memory. Cell Reports. 2019;29(5):1099–1112.e4. doi: 10.1016/j.celrep.2019.09.044. [DOI] [PubMed] [Google Scholar]
- 44.Leroy F., Brann D. H., Meira T., Siegelbaum S. A. Input-timing-dependent plasticity in the hippocampal CA2 region and its potential role in social memory. Neuron. 2019;102(1):260–262. doi: 10.1016/j.neuron.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi K., Ikeda Y., Haneda E., Suzuki H. Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. The Journal of Neuroscience. 2008;28(24):6272–6280. doi: 10.1523/JNEUROSCI.1656-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagena H., Manahan-Vaughan D. Learning-facilitated synaptic plasticity at CA3 mossy fiber and commissural-associational synapses reveals different roles in information processing. Cerebral Cortex. 2011;21(11):2442–2449. doi: 10.1093/cercor/bhq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagena H., Manahan-Vaughan D. Learning-facilitated long-term depression and long-term potentiation at mossy fiber-CA3 synapses requires activation of β-adrenergic receptors. Frontiers in Integrative Neuroscience. 2012;6:p. 23. doi: 10.3389/fnint.2012.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobayashi K., Imoto Y., Yamamoto F., et al. Rapid and lasting enhancement of dopaminergic modulation at the hippocampal mossy fiber synapse by electroconvulsive treatment. Journal of Neurophysiology. 2017;117(1):284–289. doi: 10.1152/jn.00740.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobayashi K., Haneda E., Higuchi M., Suhara T., Suzuki H. Chronic fluoxetine selectively upregulates dopamine D₁-like receptors in the hippocampus. Neuropsychopharmacology. 2012;37(6):1500–1508. doi: 10.1038/npp.2011.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen H., Fuchino Y., Miyamoto D., Nomura H., Matsuki N. Vagus nerve stimulation enhances perforant path-CA3 synaptic transmission via the activation of β-adrenergic receptors and the locus coeruleus. International Journal of Neuropsychopharmacology. 2012;15(4):523–530. doi: 10.1017/S1461145711000708. [DOI] [PubMed] [Google Scholar]
- 51.Qiao H., An S. C., Ren W., Ma X. M. Progressive alterations of hippocampal CA3-CA1 synapses in an animal model of depression. Behavioural Brain Research. 2014;275:191–200. doi: 10.1016/j.bbr.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 52.Gruart A., Muñoz M. D., Delgado-García J. M. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. The Journal of Neuroscience. 2006;26(4):1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li X. G., Somogyi P., Ylinen A., Buzsáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. The Journal of Comparative Neurology. 1994;339(2):181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- 54.Debanne D., Gähwiler B. H., Thompson S. M. Long-term synaptic plasticity between pairs of individual CA3 pyramidal cells in rat hippocampal slice cultures. The Journal of Physiology. 1998;507(1):237–247. doi: 10.1111/j.1469-7793.1998.237bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mishra R. K., Kim S., Guzman S. J., Jonas P. Symmetric spike timing-dependent plasticity at CA3-CA3 synapses optimizes storage and recall in autoassociative networks. Nature Communications. 2016;7(1, article 11552) doi: 10.1038/ncomms11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lucassen P. J., Müller M. B., Holsboer F., et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. The American Journal of Pathology. 2001;158(2):453–468. doi: 10.1016/S0002-9440(10)63988-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takamiya A., Plitman E., Chung J. K., et al. Acute and long-term effects of electroconvulsive therapy on human dentate gyrus. Neuropsychopharmacology. 2019;44(10):1805–1811. doi: 10.1038/s41386-019-0312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alamo C., García-Garcia P., Lopez-Muñoz F., Zaragozá C. Tianeptine, an atypical pharmacological approach to depression. Revista de Psiquiatría y Salud Mental. 2019;12(3):170–186. doi: 10.1016/j.rpsm.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Lucassen P. J., Fuchs E., Czéh B. Antidepressant treatment with tianeptine reduces apoptosis in the hippocampal dentate gyrus and temporal cortex. Biological Psychiatry. 2004;55(8):789–796. doi: 10.1016/j.biopsych.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 60.Baek S. S., Jun T. W., Kim K. J., Shin M. S., Kang S. Y., Kim C. J. Effects of postnatal treadmill exercise on apoptotic neuronal cell death and cell proliferation of maternal-separated rat pups. Brain and Development. 2012;34(1):45–56. doi: 10.1016/j.braindev.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 61.Lee H. J., Kim J. W., Yim S. V., et al. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Molecular Psychiatry. 2001;6(6):725–728. doi: 10.1038/sj.mp.4000947. [DOI] [PubMed] [Google Scholar]
- 62.Radahmadi M., Hosseini N., Nasimi A. Effect of chronic stress on short and long-term plasticity in dentate gyrus; study of recovery and adaptation. Neuroscience. 2014;280:121–129. doi: 10.1016/j.neuroscience.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Wang S. E., Ko S. Y., Jo S., et al. Downregulation of SIRT2 by chronic stress reduces expression of synaptic plasticity-related genes through the upregulation of Ehmt2. Experimental Neurobiology. 2019;28(4):537–546. doi: 10.5607/en.2019.28.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abe-Higuchi N., Uchida S., Yamagata H., et al. Hippocampal sirtuin 1 signaling mediates depression-like behavior. Biological Psychiatry. 2016;80(11):815–826. doi: 10.1016/j.biopsych.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Triviño-Paredes J., Patten A. R., Gil-Mohapel J., Christie B. R. The effects of hormones and physical exercise on hippocampal structural plasticity. Frontiers in Neuroendocrinology. 2016;41:23–43. doi: 10.1016/j.yfrne.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 66.Arancibia S., Payet O., Givalois L., Tapia-Arancibia L. Acute stress and dexamethasone rapidly increase hippocampal somatostatin synthesis and release from the dentate gyrus hilus. Hippocampus. 2001;11(4):469–477. doi: 10.1002/hipo.1061. [DOI] [PubMed] [Google Scholar]
- 67.Nakata A., Saito H., Nishiyama N. Facilitatory role of somatostatin via muscarinic cholinergic system in the generation of long-term potentiation in the rat dentate gyrus in vivo. Brain Research. 1996;723(1-2):135–140. doi: 10.1016/0006-8993(96)00233-8. [DOI] [PubMed] [Google Scholar]
- 68.Baratta M. V., Lamp T., Tallent M. K. Somatostatin depresses long-term potentiation and Ca2+ signaling in mouse dentate gyrus. Journal of Neurophysiology. 2002;88(6):3078–3086. doi: 10.1152/jn.00398.2002. [DOI] [PubMed] [Google Scholar]
- 69.Hamilton T. J., Wheatley B. M., Sinclair D. B., Bachmann M., Larkum M. E., Colmers W. F. Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proceedings of the National Academy of Sciences. 2010;107(42):18185–18190. doi: 10.1073/pnas.1011558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gruber D., Gilling K. E., Albrecht A., et al. 5-HT receptor-mediated modulation of granule cell inhibition after juvenile stress recovers after a second exposure to adult stress. Neuroscience. 2015;293:67–79. doi: 10.1016/j.neuroscience.2015.02.050. [DOI] [PubMed] [Google Scholar]
- 71.Galea L. A., Spritzer M. D., Barker J. M., Pawluski J. L. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16(3):225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- 72.Muzio L., Brambilla V., Calcaterra L., D’Adamo P., Martino G., Benedetti F. Increased neuroplasticity and hippocampal microglia activation in a mice model of rapid antidepressant treatment. Behavioural Brain Research. 2016;311:392–402. doi: 10.1016/j.bbr.2016.05.063. [DOI] [PubMed] [Google Scholar]
- 73.Shang X., Shang Y., Fu J., Zhang T. Nicotine significantly improves chronic stress-induced impairments of cognition and synaptic plasticity in mice. Molecular Neurobiology. 2017;54(6):4644–4658. doi: 10.1007/s12035-016-0012-2. [DOI] [PubMed] [Google Scholar]
- 74.Pickering M., O’Connor J. J. Pro-inflammatory cytokines and their effects in the dentate gyrus. Progress in Brain Research. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- 75.Zhang T. Y., Keown C. L., Wen X., et al. Environmental enrichment increases transcriptional and epigenetic differentiation between mouse dorsal and ventral dentate gyrus. Nature Communications. 2018;9(1):p. 298. doi: 10.1038/s41467-017-02748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Köhr G. NMDA receptor function: subunit composition versus spatial distribution. Cell and Tissue Research. 2006;326(2):439–446. doi: 10.1007/s00441-006-0273-6. [DOI] [PubMed] [Google Scholar]
- 77.García-Gallo M., Renart J., Díaz-Guerra M. The NR1 subunit of the N-methyl-D-aspartate receptor can be efficiently expressed alone in the cell surface of mammalian cells and is required for the transport of the NR2A subunit. Biochemical Journal. 2001;356(2):539–547. doi: 10.1042/bj3560539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonhaus D. W., Yeh G. C., Skaryak L., McNamara J. O. Glycine regulation of the N-methyl-D-aspartate receptor-gated ion channel in hippocampal membranes. Molecular Pharmacology. 1989;36(2):273–279. [PubMed] [Google Scholar]
- 79.Madry C., Mesic I., Bartholomäus I., Nicke A., Betz H., Laube B. Principal role of NR3 subunits in NR1/NR3 excitatory glycine receptor function. Biochemical and Biophysical Research Communications. 2007;354(1):102–108. doi: 10.1016/j.bbrc.2006.12.153. [DOI] [PubMed] [Google Scholar]
- 80.Petralia R. S., Wang Y. X., Hua F., et al. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167(1):68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roche K. W., Standley S., McCallum J., Dune Ly C., Ehlers M. D., Wenthold R. J. Molecular determinants of NMDA receptor internalization. Nature Neuroscience. 2001;4(8):794–802. doi: 10.1038/90498. [DOI] [PubMed] [Google Scholar]
- 82.El-Husseini A. E., Schnell E., Chetkovich D. M., Nicoll R. A., Bredt D. S. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290(5495):1364–1368. [PubMed] [Google Scholar]
- 83.Won S., Incontro S., Nicoll R. A., Roche K. W. PSD-95 stabilizes NMDA receptors by inducing the degradation of STEP61. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(32):E4736–E4744. doi: 10.1073/pnas.1609702113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jang S. S., Royston S. E., Xu J., et al. Regulation of STEP61 and tyrosine-phosphorylation of NMDA and AMPA receptors during homeostatic synaptic plasticity. Molecular Brain. 2015;8(1):p. 55. doi: 10.1186/s13041-015-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volianskis A., France G., Jensen M. S., Bortolotto Z. A., Jane D. E., Collingridge G. L. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Research. 2015;1621:5–16. doi: 10.1016/j.brainres.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Treccani G., Ardalan M., Chen F., et al. S-Ketamine reverses hippocampal dendritic spine deficits in Flinders Sensitive Line rats within 1 h of administration. Molecular Neurobiology. 2019;56(11):7368–7379. doi: 10.1007/s12035-019-1613-3. [DOI] [PubMed] [Google Scholar]
- 87.Yang Y., Ju W., Zhang H., Sun L. Effect of ketamine on LTP and NMDAR EPSC in hippocampus of the chronic social defeat stress mice model of depression. Frontiers in Behavioral Neuroscience. 2018;12:p. 229. doi: 10.3389/fnbeh.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nai Q., Li S., Wang S. H., et al. Uncoupling the D1-N-methyl-D-aspartate (NMDA) receptor complex promotes NMDA-dependent long-term potentiation and working memory. Biological Psychiatry. 2010;67(3):246–254. doi: 10.1016/j.biopsych.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 89.Kim Y., Jang Y. N., Kim J. Y., et al. Microtubule-associated protein 2 mediates induction of long-term potentiation in hippocampal neurons. The FASEB Journal. 2020;34(5):6965–6983. doi: 10.1096/fj.201902122RR. [DOI] [PubMed] [Google Scholar]
- 90.Makino H., Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64(3):381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ashby M. C., Maier S. R., Nishimune A., Henley J. M. Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. The Journal of Neuroscience. 2006;26(26):7046–7055. doi: 10.1523/JNEUROSCI.1235-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Penn A. C., Zhang C. L., Georges F., et al. Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature. 2017;549(7672):384–388. doi: 10.1038/nature23658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang M., Ramasamy V. S., Samidurai M., Jo J. Acute restraint stress reverses impaired LTP in the hippocampal CA1 region in mouse models of Alzheimer's disease. Scientific Reports. 2019;9(1):p. 10955. doi: 10.1038/s41598-019-47452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buonarati O. R., Hammes E. A., Watson J. F., Greger I. H., Hell J. W. Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation. Science Signaling. 2019;12(562, article eaar6889) doi: 10.1126/scisignal.aar6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cho E., Kim D. H., Hur Y. N., et al. Cyclin Y inhibits plasticity-induced AMPA receptor exocytosis and LTP. Scientific Reports. 2015;5(1, article 12624) doi: 10.1038/srep12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patterson M. A., Szatmari E. M., Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liao D., Hessler N. A., Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375(6530):400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 98.Davidson H. T., Xiao J., Dai R., Bergson C. Calcyon is necessary for activity-dependent AMPA receptor internalization and LTD in CA1 neurons of hippocampus. The European Journal of Neuroscience. 2009;29(1):42–54. doi: 10.1111/j.1460-9568.2008.06563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu Y., Huang Z., Dai C., et al. Facilitated AMPAR endocytosis causally contributes to the maternal sleep deprivation-induced impairments of synaptic plasticity and cognition in the offspring rats. Neuropharmacology. 2018;133:155–162. doi: 10.1016/j.neuropharm.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 100.Fujii S., Tanaka H., Hirano T. Suppression of AMPA receptor exocytosis contributes to hippocampal LTD. The Journal of Neuroscience. 2018;38(24):5523–5537. doi: 10.1523/JNEUROSCI.3210-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruiz A. Kainate receptors with a metabotropic signature enhance hippocampal excitability by regulating the slow after-hyperpolarization in CA3 pyramidal neurons. Advances in Experimental Medicine and Biology. 2011;717:59–68. doi: 10.1007/978-1-4419-9557-5_6. [DOI] [PubMed] [Google Scholar]
- 102.Cherubini E., Caiati M. D., Sivakumaran S. In the developing hippocampus kainate receptors control the release of GABA from mossy fiber terminals via a metabotropic type of action. Advances in Experimental Medicine and Biology. 2011;717:11–26. doi: 10.1007/978-1-4419-9557-5_2. [DOI] [PubMed] [Google Scholar]
- 103.Marques J. M., Rodrigues R. J., Valbuena S., et al. CRMP2 tethers kainate receptor activity to cytoskeleton dynamics during neuronal maturation. The Journal of Neuroscience. 2013;33(46):18298–18310. doi: 10.1523/JNEUROSCI.3136-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharma S., Darland D., Lei S., Rakoczy S., Brown-Borg H. M. NMDA and kainate receptor expression, long-term potentiation, and neurogenesis in the hippocampus of long-lived Ames dwarf mice. Age (Dordrecht, Netherlands) 2012;34(3):609–620. doi: 10.1007/s11357-011-9253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wyeth M. S., Pelkey K. A., Yuan X., et al. Neto auxiliary subunits regulate interneuron somatodendritic and presynaptic kainate receptors to control network inhibition. Cell Reports. 2017;20(9):2156–2168. doi: 10.1016/j.celrep.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kamiya H. Kainate receptor-dependent presynaptic modulation and plasticity. Neuroscience Research. 2002;42(1):1–6. doi: 10.1016/S0168-0102(01)00303-0. [DOI] [PubMed] [Google Scholar]
- 107.Sihra T. S., Rodríguez-Moreno A. Presynaptic kainate receptor-mediated bidirectional modulatory actions: mechanisms. Neurochemistry International. 2013;62(7):982–987. doi: 10.1016/j.neuint.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 108.Schiffer H. H., Heinemann S. F. Association of the human kainate receptorGluR7 gene (GRIK3) with recurrent major depressive disorder. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2007;144B(1):20–26. doi: 10.1002/ajmg.b.30374. [DOI] [PubMed] [Google Scholar]
- 109.Koromina M., Flitton M., Mellor I. R., Knight H. M. A kainate receptor GluK4 deletion, protective against bipolar disorder, is associated with enhanced cognitive performance across diagnoses in the TwinsUK cohort. The World Journal of Biological Psychiatry. 2019;20(5):393–401. doi: 10.1080/15622975.2017.1417637. [DOI] [PubMed] [Google Scholar]
- 110.Catches J. S., Xu J., Contractor A. Genetic ablation of the GluK4 kainate receptor subunit causes anxiolytic and antidepressant-like behavior in mice. Behavioural Brain Research. 2012;228(2):406–414. doi: 10.1016/j.bbr.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hunter R. G., Bellani R., Bloss E., Costa A., McCarthy K., McEwen B. S. Regulation of kainate receptor subunit mRNA by stress and corticosteroids in the rat hippocampus. PLoS One. 2009;4(1):p. e4328. doi: 10.1371/journal.pone.0004328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rodríguez-Moreno A., Sihra T. S. Metabotropic actions of kainate receptors in the control of glutamate release in the hippocampus. Advances in Experimental Medicine and Biology. 2011;717:39–48. doi: 10.1007/978-1-4419-9557-5_4. [DOI] [PubMed] [Google Scholar]
- 113.Contractor A., Swanson G., Heinemann S. F. Kainate receptors are involved in short- and long-term plasticity at mossy fiber synapses in the hippocampus. Neuron. 2001;29(1):209–216. doi: 10.1016/S0896-6273(01)00191-X. [DOI] [PubMed] [Google Scholar]
- 114.Park Y., Jo J., Isaac J. T., Cho K. Long-term depression of kainate receptor-mediated synaptic transmission. Neuron. 2006;49(1):95–106. doi: 10.1016/j.neuron.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 115.Bureau I., Bischoff S., Heinemann S. F., Mulle C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. The Journal of Neuroscience. 1999;19(2):653–663. doi: 10.1523/JNEUROSCI.19-02-00653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang L., Xu J., Nedergaard M., Kang J. A kainate receptor increases the efficacy of GABAergic synapses. Neuron. 2001;30(2):503–513. doi: 10.1016/S0896-6273(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 117.Sengupta T., Das R., Chattarji S. Chronic but not acute immobilization stress stably enhances hippocampal CA1 metabotropic glutamate receptor dependent long-term depression. Neuroscience Letters. 2016;633:101–105. doi: 10.1016/j.neulet.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 118.Chaouloff F., Hémar A., Manzoni O. Acute stress facilitates hippocampal CA1 metabotropic glutamate receptor-dependent long-term depression. The Journal of Neuroscience. 2007;27(27):7130–7135. doi: 10.1523/JNEUROSCI.1150-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fitzjohn S., Bashir Z., Farrow P. Group I mGluR induced LTD of NMDAR-synaptic transmission at the Schaffer collateral but not temperoammonic input to CA1. Current Neuropharmacology. 2016;14(5):435–440. doi: 10.2174/1570159X13666150615221502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Le Vasseur M., Ran I., Lacaille J. C. Selective induction of metabotropic glutamate receptor 1- and metabotropic glutamate receptor 5-dependent chemical long-term potentiation at oriens/alveus interneuron synapses of mouse hippocampus. Neuroscience. 2008;151(1):28–42. doi: 10.1016/j.neuroscience.2007.09.071. [DOI] [PubMed] [Google Scholar]
- 121.Le Duigou C., Kullmann D. M. Group I mGluR agonist-evoked long-term potentiation in hippocampal oriens interneurons. The Journal of Neuroscience. 2011;31(15):5777–5781. doi: 10.1523/JNEUROSCI.6265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suzuki E., Okada T. Group I metabotropic glutamate receptors are involved in TEA-induced long-term potentiation at mossy fiber-CA3 synapses in the rat hippocampus. Brain Research. 2010;1313:45–52. doi: 10.1016/j.brainres.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 123.Eng A. G., Kelver D. A., Hedrick T. P., Swanson G. T. Transduction of group I mGluR-mediated synaptic plasticity by β-arrestin2 signalling. Nature Communications. 2016;7(1):p. 13571. doi: 10.1038/ncomms13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yerna X., Schakman O., Ratbi I., et al. Role of the TRPC1 channel in hippocampal long-term depression and in spatial memory extinction. International Journal of Molecular Sciences. 2020;21(5):p. 1712. doi: 10.3390/ijms21051712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matrisciano F., Panaccione I., Zusso M., et al. Group-II metabotropic glutamate receptor ligands as adjunctive drugs in the treatment of depression: a new strategy to shorten the latency of antidepressant medication. Molecular Psychiatry. 2007;12(8):704–706. doi: 10.1038/sj.mp.4002005. [DOI] [PubMed] [Google Scholar]
- 126.O'Connor R. M., Pusceddu M. M., Dinan T. G., Cryan J. F. Impact of early-life stress, on group III mGlu receptor levels in the rat hippocampus: effects of ketamine, electroconvulsive shock therapy and fluoxetine treatment. Neuropharmacology. 2013;66:236–241. doi: 10.1016/j.neuropharm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 127.Podkowa K., Rzeźniczek S., Marciniak M., Acher F., Pilc A., Pałucha-Poniewiera A. A novel mGlu4 selective agonist LSP4-2022 increases behavioral despair in mouse models of antidepressant action. Neuropharmacology. 2015;97:338–345. doi: 10.1016/j.neuropharm.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 128.Iscru E., Goddyn H., Ahmed T., Callaerts-Vegh Z., D'Hooge R., Balschun D. Improved spatial learning is associated with increased hippocampal but not prefrontal long-term potentiation in mGluR4 knockout mice. Genes, Brain, and Behavior. 2013;12(6):615–625. doi: 10.1111/gbb.12052. [DOI] [PubMed] [Google Scholar]
- 129.Bradley S. R., Uslaner J. M., Flick R. B., Lee A., Groover K. M., Hutson P. H. The mGluR7 allosteric agonist AMN082 produces antidepressant-like effects by modulating glutamatergic signaling. Pharmacology, Biochemistry, and Behavior. 2012;101(1):35–40. doi: 10.1016/j.pbb.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 130.Mitsukawa K., Mombereau C., Lötscher E., et al. Metabotropic glutamate receptor subtype 7 ablation causes dysregulation of the HPA axis and increases hippocampal BDNF protein levels: implications for stress-related psychiatric disorders. Neuropsychopharmacology. 2006;31(6):1112–1122. doi: 10.1038/sj.npp.1300926. [DOI] [PubMed] [Google Scholar]
- 131.Stockmeier C. A., Mahajan G. J., Konick L. C., et al. Cellular changes in the postmortem hippocampus in major depression. Biological Psychiatry. 2004;56(9):640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pei G., Xu L., Huang W., Yin J. The protective role of microRNA-133b in restricting hippocampal neurons apoptosis and inflammatory injury in rats with depression by suppressing CTGF. International Immunopharmacology. 2020;78:p. 106076. doi: 10.1016/j.intimp.2019.106076. [DOI] [PubMed] [Google Scholar]
- 133.Huang X., Mao Y. S., Li C., Wang H., Ji J. L. Venlafaxine inhibits apoptosis of hippocampal neurons by up-regulating brain-derived neurotrophic factor in a rat depression model. Die Pharmazie. 2014;69(12):909–916. [PubMed] [Google Scholar]
- 134.Toda T., Parylak S. L., Linker S. B., Gage F. H. The role of adult hippocampal neurogenesis in brain health and disease. Molecular Psychiatry. 2019;24(1):67–87. doi: 10.1038/s41380-018-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hill A. S., Sahay A., Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40(10):2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yau S. Y., Li A., Tong J. B., et al. Chronic corticosterone administration reduces dendritic complexity in mature, but not young granule cells in the rat dentate gyrus. Restorative Neurology and Neuroscience. 2016;34(5):849–857. doi: 10.3233/RNN-160662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brummelte S., Galea L. A. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience. 2010;168(3):680–690. doi: 10.1016/j.neuroscience.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 138.Ikrar T., Guo N., He K., et al. Adult neurogenesis modifies excitability of the dentate gyrus. Frontiers in Neural Circuits. 2013;7:p. 204. doi: 10.3389/fncir.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Anacker C., Luna V. M., Stevens G. S., et al. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559(7712):98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Michaëlsson H., Andersson M., Svensson J., et al. The novel antidepressant ketamine enhances dentate gyrus proliferation with no effects on synaptic plasticity or hippocampal function in depressive-like rats. Acta Physiologica (Oxford, England) 2018;225(4, article e13211) doi: 10.1111/apha.13211. [DOI] [PubMed] [Google Scholar]
- 141.Pinnock S. B., Balendra R., Chan M., Hunt L. T., Turner-Stokes T., Herbert J. Interactions between nitric oxide and corticosterone in the regulation of progenitor cell proliferation in the dentate gyrus of the adult rat. Neuropsychopharmacology. 2007;32(2):493–504. doi: 10.1038/sj.npp.1301245. [DOI] [PubMed] [Google Scholar]
- 142.Terrillion C. E., Abazyan B., Yang Z., et al. DISC1 in astrocytes influences adult neurogenesis and hippocampus-dependent behaviors in mice. Neuropsychopharmacology. 2017;42(11):2242–2251. doi: 10.1038/npp.2017.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Navarrete M., Cuartero M. I., Palenzuela R., et al. Astrocytic p38α MAPK drives NMDA receptor-dependent long-term depression and modulates long-term memory. Nature Communications. 2019;10(1):p. 2968. doi: 10.1038/s41467-019-10830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hei M., Chen P., Wang S., et al. Effects of chronic mild stress induced depression on synaptic plasticity in mouse hippocampus. Behavioural Brain Research. 2019;365:26–35. doi: 10.1016/j.bbr.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 145.Paolicelli R. C., Bolasco G., Pagani F., et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 146.Riazi K., Galic M. A., Kentner A. C., Reid A. Y., Sharkey K. A., Pittman Q. J. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. The Journal of Neuroscience. 2015;35(12):4942–4952. doi: 10.1523/JNEUROSCI.4485-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tang J., Liang X., Zhang Y., et al. The effects of running exercise on oligodendrocytes in the hippocampus of rats with depression induced by chronic unpredictable stress. Brain Research Bulletin. 2019;149:1–10. doi: 10.1016/j.brainresbull.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 148.Yamazaki Y., Fujiwara H., Kaneko K., et al. Short- and long-term functional plasticity of white matter induced by oligodendrocyte depolarization in the hippocampus. GLIA. 2014;62(8):1299–1312. doi: 10.1002/glia.22681. [DOI] [PubMed] [Google Scholar]
- 149.Yamazaki Y. Oligodendrocyte physiology modulating axonal excitability and nerve conduction. Advances in Experimental Medicine and Biology. 2019;1190:123–144. doi: 10.1007/978-981-32-9636-7_9. [DOI] [PubMed] [Google Scholar]
- 150.Steadman P. E., Xia F., Ahmed M., et al. Disruption of oligodendrogenesis impairs memory consolidation in adult mice. Neuron. 2020;105(1):150–164.e6. doi: 10.1016/j.neuron.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Laine M. A., Trontti K., Misiewicz Z., et al. Genetic control of myelin plasticity after chronic psychosocial stress. eNeuro. 2018;5(4):ENEURO.0166–ENEU18.2018. doi: 10.1523/ENEURO.0166-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Monteleone P., Serritella C., Martiadis V., Maj M. Decreased levels of serum brain-derived neurotrophic factor in both depressed and euthymic patients with unipolar depression and in euthymic patients with bipolar I and II disorders. Bipolar Disorders. 2008;10(1):95–100. doi: 10.1111/j.1399-5618.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- 153.Polyakova M., Stuke K., Schuemberg K., Mueller K., Schoenknecht P., Schroeter M. L. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. Journal of Affective Disorders. 2015;174:432–440. doi: 10.1016/j.jad.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 154.Zanos P., Gould T. D. Mechanisms of ketamine action as an antidepressant. Molecular Psychiatry. 2018;23(4):801–811. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Figurov A., Pozzo-Miller L. D., Olafsson P., Wang T., Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381(6584):706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 156.Sakata K., Duke S. M. Lack of BDNF expression through promoter IV disturbs expression of monoamine genes in the frontal cortex and hippocampus. Neuroscience. 2014;260:265–275. doi: 10.1016/j.neuroscience.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 157.Diniz C., Casarotto P. C., Resstel L., Joca S. Beyond good and evil: a putative continuum-sorting hypothesis for the functional role of proBDNF/BDNF-propeptide/mBDNF in antidepressant treatment. Neuroscience and Biobehavioral Reviews. 2018;90:70–83. doi: 10.1016/j.neubiorev.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 158.Qiao H., An S. C., Xu C., Ma X. M. Role of proBDNF and BDNF in dendritic spine plasticity and depressive-like behaviors induced by an animal model of depression. Brain Research. 2017;1663:29–37. doi: 10.1016/j.brainres.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 159.Numakawa T., Suzuki S., Kumamaru E., Adachi N., Richards M., Kunugi H. BDNF function and intracellular signaling in neurons. Histology and Histopathology. 2010;25(2):237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- 160.De Vries L., Finana F., Cachoux F., Vacher B., Sokoloff P., Cussac D. Cellular BRET assay suggests a conformational rearrangement of preformed TrkB/Shc complexes following BDNF-dependent activation. Cellular Signalling. 2010;22(1):158–165. doi: 10.1016/j.cellsig.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 161.Wang J. Q., Mao L. The ERK pathway: molecular mechanisms and treatment of depression. Molecular Neurobiology. 2019;56(9):6197–6205. doi: 10.1007/s12035-019-1524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cheng C. Y., Kao S. T., Lee Y. C. Angelica sinensis extract protects against ischemia-reperfusion injury in the hippocampus by activating p38 MAPK-mediated p90RSK/p-Bad and p90RSK/CREB/BDNF signaling after transient global cerebral ischemia in rats. Journal of Ethnopharmacology. 2020;252:p. 112612. doi: 10.1016/j.jep.2020.112612. [DOI] [PubMed] [Google Scholar]
- 163.Katoh-Semba R., Kaneko R., Kitajima S., et al. Activation of p38 mitogen-activated protein kinase is required for in vivo brain-derived neurotrophic factor production in the rat hippocampus. Neuroscience. 2009;163(1):352–361. doi: 10.1016/j.neuroscience.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 164.Sakamoto K., Karelina K., Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. Journal of Neurochemistry. 2011;116(1):1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sari D., Arfian N., Tranggono U., Setyaningsih W., Romi M. M., Emoto N. <i>Centella asiatica</i> (Gotu kola) ethanol extract up-regulates hippocampal brain-derived neurotrophic factor (BDNF), tyrosine kinase B (TrkB) and extracellular signal-regulated protein kinase 1/2 (ERK1/2) signaling in chronic electrical stress model in rats. Iranian Journal of Basic Medical Sciences. 2019;22(10):1218–1224. doi: 10.22038/ijbms.2019.29012.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peng C. H., Chiou S. H., Chen S. J., et al. Neuroprotection by imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. European Neuropsychopharmacology. 2008;18(2):128–140. doi: 10.1016/j.euroneuro.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 167.Kaplan D. R., Miller F. D. Neurotrophin signal transduction in the nervous system. Current Opinion in Neurobiology. 2000;10(3):381–391. doi: 10.1016/S0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 168.Minichiello L., Calella A. M., Medina D. L., Bonhoeffer T., Klein R., Korte M. Mechanism of TrkB-mediated hippocampal long-term potentiation. Neuron. 2002;36(1):121–137. doi: 10.1016/S0896-6273(02)00942-X. [DOI] [PubMed] [Google Scholar]
- 169.Kharebava G., Makonchuk D., Kalita K. B., Zheng J. J., Hetman M. Requirement of 3-phosphoinositide-dependent protein kinase-1 for BDNF-mediated neuronal survival. The Journal of Neuroscience. 2008;28(44):11409–11420. doi: 10.1523/JNEUROSCI.2135-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nikoletopoulou V., Sidiropoulou K., Kallergi E., Dalezios Y., Tavernarakis N. Modulation of autophagy by BDNF underlies synaptic plasticity. Cell Metabolism. 2017;26(1):230–242.e5. doi: 10.1016/j.cmet.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 171.Cavalleri L., Merlo Pich E., Millan M. J., et al. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Molecular Psychiatry. 2018;23(4):812–823. doi: 10.1038/mp.2017.241. [DOI] [PubMed] [Google Scholar]
- 172.Pilar-Cuellar F., Castro E., Bretin S., Mocaer E., Pazos Á., Díaz Á. S 47445 counteracts the behavioral manifestations and hippocampal neuroplasticity changes in bulbectomized mice. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2019;93:205–213. doi: 10.1016/j.pnpbp.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 173.Sun L. J., Zhang L. M., Liu D., et al. The faster-onset antidepressant effects of hypidone hydrochloride (YL-0919) Metabolic Brain Disease. 2019;34(5):1375–1384. doi: 10.1007/s11011-019-00439-8. [DOI] [PubMed] [Google Scholar]
- 174.Wu S., Gao Q., Zhao P., et al. Sulforaphane produces antidepressant- and anxiolytic-like effects in adult mice. Behavioural Brain Research. 2016;301:55–62. doi: 10.1016/j.bbr.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 175.Zhang J. C., Yao W., Dong C., et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. The Journal of Nutritional Biochemistry. 2017;39:134–144. doi: 10.1016/j.jnutbio.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 176.Kim C. S., Chang P. Y., Johnston D. Enhancement of dorsal hippocampal activity by knockdown of HCN1 channels leads to anxiolytic- and antidepressant-like behaviors. Neuron. 2012;75(3):503–516. doi: 10.1016/j.neuron.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ni L., Xu Y., Dong S., et al. The potential role of the HCN1 ion channel and BDNF-mTOR signaling pathways and synaptic transmission in the alleviation of PTSD. Translational Psychiatry. 2020;10(1):p. 101. doi: 10.1038/s41398-020-0782-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li Y. F. A hypothesis of monoamine (5-HT) - Glutamate/GABA long neural circuit: aiming for fast-onset antidepressant discovery. Pharmacology & Therapeutics. 2020;208, article 107494 doi: 10.1016/j.pharmthera.2020.107494. [DOI] [PubMed] [Google Scholar]
- 179.Zhang H., Chi X., Pan W., et al. Antidepressant mechanism of classical herbal formula lily bulb and Rehmannia decoction: insights from gene expression profile of medial prefrontal cortex of mice with stress-induced depression-like behavior. e12649. Genes, Brain, and Behavior. 2020;19(5) doi: 10.1111/gbb.12649. [DOI] [PubMed] [Google Scholar]
- 180.Bettio L., Rajendran L., Gil-Mohapel J. The effects of aging in the hippocampus and cognitive decline. Neuroscience and Biobehavioral Reviews. 2017;79:66–86. doi: 10.1016/j.neubiorev.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 181.Sharma S., Kaur G. Neuroprotective potential of dietary restriction against kainate-induced excitotoxicity in adult male Wistar rats. Brain Research Bulletin. 2005;67(6):482–491. doi: 10.1016/j.brainresbull.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 182.Ribeiro L. C., Rodrigues L., Quincozes-Santos A., et al. Caloric restriction improves basal redox parameters in hippocampus and cerebral cortex of Wistar rats. Brain Research. 2012;1472:11–19. doi: 10.1016/j.brainres.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 183.Zeier Z., Madorsky I., Xu Y., Ogle W. O., Notterpek L., Foster T. C. Gene expression in the hippocampus: regionally specific effects of aging and caloric restriction. Mechanisms of Ageing and Development. 2011;132(1-2):8–19. doi: 10.1016/j.mad.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mladenovic Djordjevic A., Perovic M., Tesic V., et al. Long-term dietary restriction modulates the level of presynaptic proteins in the cortex and hippocampus of the aging rat. Neurochemistry International. 2010;56(2):250–255. doi: 10.1016/j.neuint.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 185.Adams M. M., Shi L., Linville M. C., et al. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Experimental Neurology. 2008;211(1):141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ding Q., Ash C., Mracek T., Merry B., Bing C. Caloric restriction increases adiponectin expression by adipose tissue and prevents the inhibitory effect of insulin on circulating adiponectin in rats. The Journal of Nutritional Biochemistry. 2012;23(8):867–874. doi: 10.1016/j.jnutbio.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 187.Platzer M., Fellendorf F. T., Bengesser S. A., et al. Adiponectin is decreased in bipolar depression. The World Journal of Biological Psychiatry. 2019;20(10):813–820. doi: 10.1080/15622975.2018.1500033. [DOI] [PubMed] [Google Scholar]
- 188.Furman J. L., Soyombo A., Czysz A. H., et al. Adiponectin moderates antidepressant treatment outcome in the combining medications to enhance depression outcomes randomized clinical trial. Personalized Medicine in Psychiatry. 2018;9-10:1–7. doi: 10.1016/j.pmip.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nicolas S., Chabry J., Guyon A., Zarif H., Heurteaux C., Petit-Paitel A. Adiponectin: an endogenous molecule with anti-inflammatory and antidepressant properties? Medical Science (Paris) 2018;34(5):417–423. doi: 10.1051/medsci/20183405014. [DOI] [PubMed] [Google Scholar]
- 190.Zhang D., Wang X., Lu X. Y. Adiponectin exerts neurotrophic effects on dendritic arborization, spinogenesis, and neurogenesis of the dentate gyrus of male mice. Endocrinology. 2016;157(7):2853–2869. doi: 10.1210/en.2015-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lecoultre V., Ravussin E., Redman L. M. The fall in leptin concentration is a major determinant of the metabolic adaptation induced by caloric restriction independently of the changes in leptin circadian rhythms. The Journal of Clinical Endocrinology and Metabolism. 2011;96(9):E1512–E1516. doi: 10.1210/jc.2011-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kraus T., Haack M., Schuld A., Hinze-Selch D., Pollmächer T. Low leptin levels but normal body mass indices in patients with depression or schizophrenia. Neuroendocrinology. 2001;73(4):243–247. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- 193.Lu X. Y., Kim C. S., Frazer A., Zhang W. Leptin: a potential novel antidepressant. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Milaneschi Y., Lamers F., Bot M., Drent M. L., Penninx B. W. Leptin dysregulation is specifically associated with major depression with atypical features: evidence for a mechanism connecting obesity and depression. Biological Psychiatry. 2017;81(9):807–814. doi: 10.1016/j.biopsych.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 195.Lord G. M. Leptin as a proinflammatory cytokine. Contributions to Nephrology. 2006;151:151–164. doi: 10.1159/000095326. [DOI] [PubMed] [Google Scholar]
- 196.Popov A., Denisov P., Bychkov M., et al. Caloric restriction triggers morphofunctional remodeling of astrocytes and enhances synaptic plasticity in the mouse hippocampus. Cell Death & Disease. 2020;11(3):p. 208. doi: 10.1038/s41419-020-2406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hori N., Hirotsu I., Davis P. J., Carpenter D. O. Long-term potentiation is lost in aged rats but preserved by calorie restriction. NeuroReport. 1992;3(12):1085–1088. doi: 10.1097/00001756-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 198.Fusco S., Ripoli C., Podda M. V., et al. A role for neuronal cAMP responsive-element binding (CREB)-1 in brain responses to calorie restriction. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(2):621–626. doi: 10.1073/pnas.1109237109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Staples M. C., Fannon M. J., Mysore K. K., et al. Dietary restriction reduces hippocampal neurogenesis and granule cell neuron density without affecting the density of mossy fibers. Brain Research. 2017;1663:59–65. doi: 10.1016/j.brainres.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Maliković J., Vuyyuru H., Koefeler H., et al. Moderate differences in common feeding diets change lipid composition in the hippocampal dentate gyrus and affect spatial cognitive flexibility in male rats. Neurochemistry International. 2019;128:215–221. doi: 10.1016/j.neuint.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 201.Poon H. F., Shepherd H. M., Reed T. T., et al. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiology of Aging. 2006;27(7):1020–1034. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 202.Chen F., Wegener G., Madsen T. M., Nyengaard J. R. Mitochondrial plasticity of the hippocampus in a genetic rat model of depression after antidepressant treatment. Synapse. 2013;67(3):127–134. doi: 10.1002/syn.21622. [DOI] [PubMed] [Google Scholar]
- 203.Li Z., Okamoto K., Hayashi Y., Sheng M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–887. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 204.Chen F., Danladi J., Ardalan M., et al. A critical role of mitochondria in BDNF-associated synaptic plasticity after one-week vortioxetine treatment. The International Journal of Neuropsychopharmacology. 2018;21(6):603–615. doi: 10.1093/ijnp/pyy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xie H., Huang H., Tang M., et al. iTRAQ-based quantitative proteomics suggests synaptic mitochondrial dysfunction in the hippocampus of rats susceptible to chronic mild stress. Neurochemical Research. 2018;43(12):2372–2383. doi: 10.1007/s11064-018-2664-y. [DOI] [PubMed] [Google Scholar]
- 206.Divakaruni S. S., van Dyke A. M., Chandra R., et al. Long-term potentiation requires a rapid burst of dendritic mitochondrial fission during induction. Neuron. 2018;100(4):860–875.e7. doi: 10.1016/j.neuron.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Seo J. H., Park H. S., Park S. S., Kim C. J., Kim D. H., Kim T. W. Physical exercise ameliorates psychiatric disorders and cognitive dysfunctions by hippocampal mitochondrial function and neuroplasticity in post-traumatic stress disorder. Experimental Neurology. 2019;322, article 113043 doi: 10.1016/j.expneurol.2019.113043. [DOI] [PubMed] [Google Scholar]
- 208.Chen F., Ardalan M., Elfving B., Wegener G., Madsen T. M., Nyengaard J. R. Mitochondria are critical for BDNF-mediated synaptic and vascular plasticity of hippocampus following repeated electroconvulsive seizures. The International Journal of Neuropsychopharmacology. 2018;21(3):291–304. doi: 10.1093/ijnp/pyx115. [DOI] [PMC free article] [PubMed] [Google Scholar]


