Abstract
Background
Upper respiratory tract infections (URTIs) are common and burdensome infectious illness. Several trials have reported that probiotics can prevent URTIs in adults.
Objectives
To evaluate the efficacy and safety of probiotics in the prevention of URTIs in adults.
Methods
PubMed, Web of Science, Embase, and Cochrane Library were searched for reports published from database inception to May 14, 2020. Randomized controlled trials (RCTs) comparing probiotics with placebo for the prevention of URTIs in adults were included.
Results
Six RCTs with 1551 participants were included. Compared with the placebo group, the probiotics intervention group significantly reduced the incidence of URTI episodes (RR: 0.77; 95% CI: 0.68 to 0.87; P < 0.0001; I2 = 26%), the episode rate of URTIs (rate ratio: 0.72; 95% CI: 0.60 to 0.86; P = 0.0002; I2 = 99%), and the mean duration of one episode of URTI (MD: −2.66; 95% CI: −4.79 to −0.54; P = 0.01; I2 = 80%). The adverse events of probiotics were mainly mild gastrointestinal symptoms. There were no significant differences in occurrence rate of adverse effects between probiotics intervention and placebo group (rate ratio: 1.01; 95% CI: 0.80 to 1.26; P = 0.96; I2 = 99%).
Conclusion
Low-quality evidence provides support that probiotics have potential efficacy for preventing URTI episodes in adults. More trials are required to confirm this conclusion.
1. Introduction
Upper respiratory tract infections (URTIs) are the most common diseases, including the rhinitis, sinusitis, tonsillitis, pharyngitis, laryngitis, and common cold. Adults suffer from cold two to three times each year, and the global incidence of URTIs was estimated to be 17.2 billion in 2015 [1, 2]. URTIs are mainly caused by various viruses, such as influenza virus, adenovirus, rhinovirus, and respiratory syncytial virus [3]. Symptoms include stuffy nose, runny nose, cough, sore throat, headache, body aches, chills, fever, and so on. Most of URTIs are mild, but symptoms seriously affect the work and study of the infected individuals. URTIs are one of the most common reasons for seeking medical care and abusing antibiotics in some countries [4]. Therefore, it has great significance to prevent episodes of URTIs.
Probiotics are live microorganisms that promote health benefit when consumed in adequate amounts [5]. Studies have suggested that they possess the abilities of immunomodulation, intestinal epithelial barrier improvement, and pathogen inhibition [6–8]. In recent years, the effects of probiotics in the treatment and prevention of disease have been extensively studied by researchers [9]. Limited evidences showed that probiotics were beneficial for treating acute diarrhea [10], preventing eczema [11], and Clostridium difficile infection [12]. The effects of probiotics in the prevention of URTIs in adults remain controversial. The results of some randomized controlled trials (RCTs) have shown that probiotics can reduce the incidence, the number of episodes, severity, and duration of URTIs in adults [13, 14]. However, some studies reported that probiotics cannot reduce the number of URTI episodes [15, 16]. Thus, the aim of this systematic review and meta-analysis was to evaluate the effects of probiotics in the prevention of URTIs in adults.
2. Methods
2.1. Inclusion and Exclusion Criteria
Participants with the age from 18 to 65 years, RCTs comparing probiotics with placebo for the prevention of URTIs, were included. The probiotics included various strains, forms, and dosages. Studies were excluded for the following reasons: (1) they included other probiotics in placebo; (2) participants were vaccinated or took potential immune-enhancing dietary supplements during the trial process; (3) participants had congenital or acquired immune dysfunction; (4) participants had chronic allergies; and (5) participants took part in regular high-intensity physical exercise.
2.2. Outcome Assessment
The primary outcomes were the incidence of URTI episodes and the number of episodes of URTIs. Secondary outcomes included the mean duration of one episode of URTI and adverse events.
2.3. Search Strategy and Selection
A systematic search of PubMed, Web of Science, Embase, and Cochrane Library was performed for studies from database inception to 14 May 2020. Language was limited to English. The following search string was applied: (rhinit∗ OR sinusit∗ OR tonsillit∗ OR laryngit∗ OR pharyngit∗ OR “respiratory tract infection” OR “respiratory tract infections” OR “upper respiratory infection” OR “upper respiratory infections” OR “common cold” OR “common colds”) AND (probiot∗ OR prebiot∗ OR bifidobacterium∗ OR enterococ∗ OR Lactobacil∗ OR Lactococ∗ OR streptococ∗ OR saccharomyc∗) AND (random∗ OR placebo∗ OR crossover∗ OR “cross over” OR allocat∗ OR blind∗ OR Singl∗ OR doubl∗ OR trial∗). Duplicate articles were eliminated. We screened potentially eligible trials by titles and abstracts of articles obtained from the broad search, and then, full texts of these screened trials were assessed for eligibility according to the inclusion and exclusion criteria.
2.4. Data Extraction
The following data were extracted: the first author's name, year of publication, country, study design, study location, participants' characteristics, the number of participants in each group, probiotic strains, dosage, form, duration, the number of participants who experienced ≥1 URTI episode, the number of episodes of URTIs, the mean duration of one episode of URTI, and adverse events. All steps were performed independently by two researchers, and any disagreements were resolved by discussion with a third researcher.
2.5. Quality Assessment
The Cochrane risk of bias tool was used to assess the methodological quality of included trials. Two researchers evaluated each trial independently based on random sequence generation, allocation concealment, blinding of participants, blinding of outcome, incomplete outcome date, selective reporting, and other biases [17]. Discrepancies and divergence in the quality assessment were resolved by group discussion.
2.6. Statistical Analysis
In multiple arm trials, similar groups were combined to create a single pair-wise comparison according to the recommendations of Cochrane Handbook for Systematic Reviews of Intervention [18]. Dichotomous outcomes were expressed as risk ratio (RR) or rate ratio, and continuous outcomes were expressed as mean difference (MD), both with 95% confidence interval (CI). The rate ratio of the episode rate (the number of URTI episodes/person/year) of URTIs and adverse events rate between two groups and the standard error (SE) of rate ratio were calculated, and the generic inverse variance was used to pool these outcomes [4]. Statistical heterogeneity was assessed by using Cochran Q and I2 statistic. The I2 < 25%, 25–50%, and >50% were considered as low, mild, and substantial heterogeneity [19]. A random-effects model was used when the P value <0.05 or I2 ≥ 50%. In contrast, a fixed-effects model was used when the P value ≥0.05 and I2 < 50%. Sensitivity analyses were conducted by excluding each study individually to test the stability of the results. Data analyses were performed using RevMan version 5.3 provided by the Cochrane Collaboration, and P < 0.05 was considered statistically significant.
3. Results
3.1. Included Studies and Their Characteristics
The study flowchart is presented in Figure 1. A total of 6263 articles were identified by searching the databases, and 1846 duplicates were excluded. The remaining 4417 articles were screened by title and abstract, 4361 of which were excluded. We screened the remaining 56 articles carefully, and 6 articles [13, 15, 20–23] met our eligibility criteria and were ultimately included. The characteristics of included studies are presented in Table 1. These studies included 4 two-arm parallel [13, 15, 20, 21], placebo-controlled RCTs and 2 multiarm parallel [22, 23], placebo-controlled RCTs, in which one was a multicenter study [20] and the other five were single-center studies. In total, 1551 participants were involved, of whom 958 received probiotics and 593 received placebo.
Figure 1.
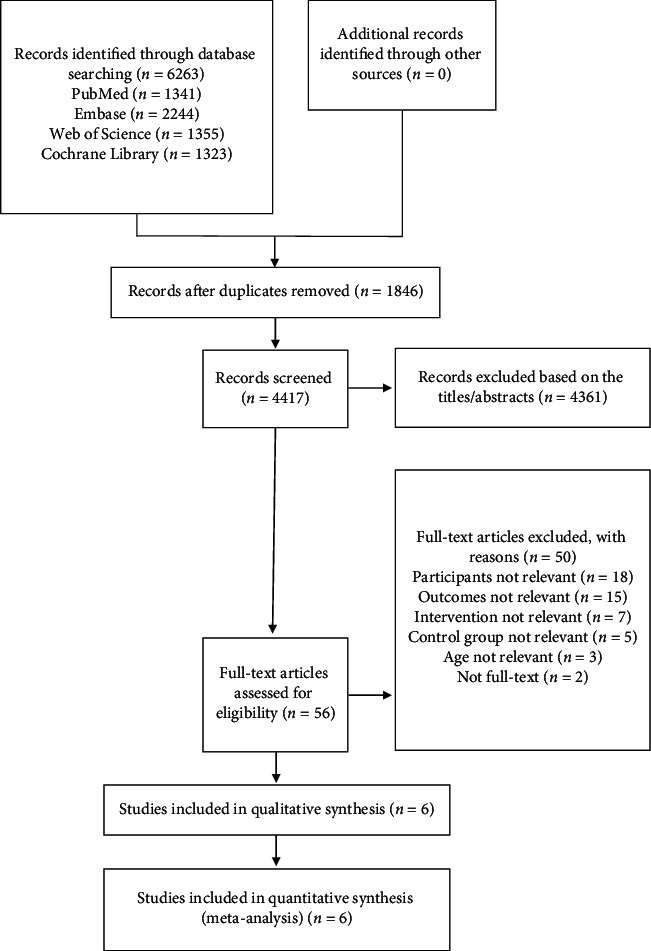
Flowchart of the literature screening.
Table 1.
Main characteristics of studies included in the meta-analysis.
| Study author/year | Country | Study type | Participants | Age, years | No. of cases∗ | Probiotics | Dose (CFU/d) | Administration form | Duration | |
|---|---|---|---|---|---|---|---|---|---|---|
| Probiotics | Placebo | |||||||||
| Berggren et al., 2011 [20] | Sweden | RCT, double-blind | Healthy adults | 18–65 | 159 | 159 | L. plantarum HEAL9 and L. paracasei 8700 : 2 | 1 × 109 | Powder | 12 w |
| Murata et al., 2018 [23] | Japan | RCT, double-blind | Healthy females (most were students) | ≥18 | 82 | 81 | Heat-killed L. paracasei MCC1849 | 1 × 1010 | Powder | 12 w |
| 78 | — | Heat-killed L. paracasei MCC1849 | 3 × 1010 | Powder | 12 w | |||||
| Shida et al., 2017 [13] | Japan | RCT | Healthy males | 30–49 | 50 | 50 | L. casei Shirota LcS-FM | 1 × 1011 | Milk | 12 w |
| Langkamp-Henken et al., 2015 [22] | USA | RCT, double-blind | Healthy students | ≥18 | 146 | 147 | L. helveticus R0052 | 3 × 109 | Capsule | 6 w |
| 142 | — | B. bifidum R0071 | 3 × 109 | Capsule | 6 w | |||||
| 148 | — | B. infantis R0033 | 3 × 109 | Capsule | 6 w | |||||
| Hirose et al., 2013 [21] | Japan | RCT, double-blind | Healthy subjects with high mental pressure | 40–64 | 39 | 39 | Heat-killed L. plantarum L-137 | NR | Tablet | 12 w |
| Smith et al., 2013 [15] | USA | RCT, double-blind | Healthy students | 18–25 | 114 | 117 | B. animalis ssp. lactis BB-12 and L. rhamnosus LGG | >1 × 109 | Powder | 12 w |
∗The number of participants in an intention-to-treat population (all the participants who were randomized to their original group, regardless of whether or not they completed the study). RCT: randomized controlled trial; NR: not reported.
3.2. Risk of Bias for the Included Studies
Four studies described adequate random sequence generation [15, 21–23], and only two studies reported adequate allocation concealment [21, 22]. Three studies did not clearly report the blinding procedure of participants and personnel [20–22], and five studies did not mention the blinding of outcome assessment [13, 20–23]. The participant dropout rates during follow-up were 0% to 16.2%, and all studies were judged as low risk of attrition rate. There was not enough information to assess the selective reporting in six studies. Three studies were judged as high risk for other biases because of the study's funding source [13, 20, 23]. The risk of bias for the included studies is shown in Figure 2.
Figure 2.
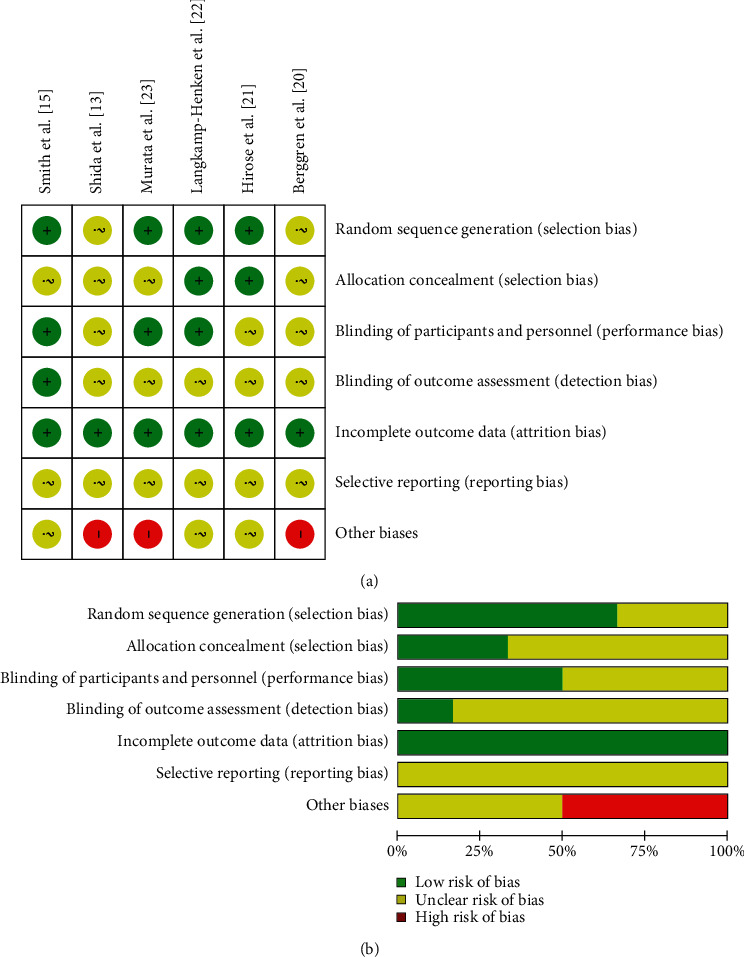
Risk of bias assessment for the included studies: (a) a summary for the risk of bias; (b) a graphic view for the risk of bias.
3.3. Incidence of URTI Episodes
Five studies reported the incidence of URTI episodes in the probiotic intervention group and placebo group [13, 20–23]. There were 791 participants in the probiotics intervention group and 438 participants in the placebo group. Pooled analyses showed that probiotics significantly reduced the incidence of URTI episodes compared with placebo (risk ratio: 0.77; 95% CI: 0.68–0.87; P < 0.0001; Figure 3(a)). A mild heterogeneity was observed (I2 = 26%; P=0.25).
Figure 3.
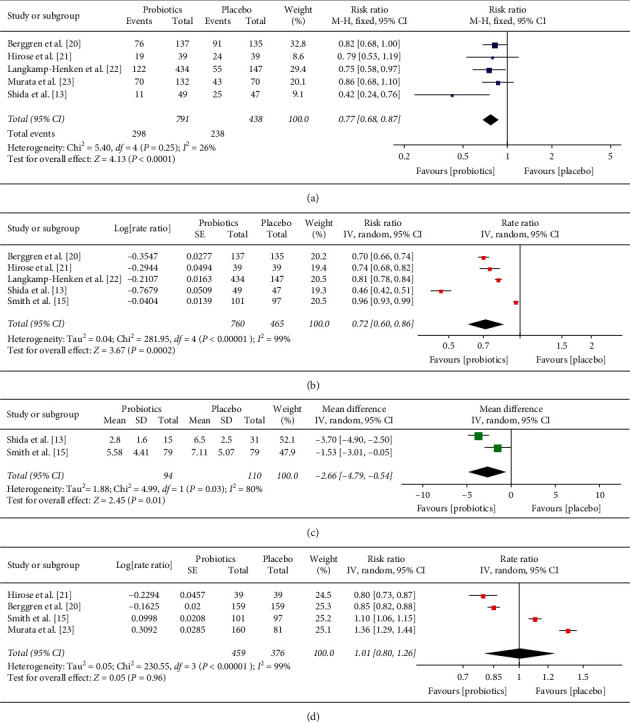
Forest plot of study results comparing probiotics with placebo groups: (a) the incidence of URTI episodes; (b) the rate ratio of episodes of URTIs; (c) the mean duration of one episode of URTI; (d) the rate ratio of the occurrence rate of adverse events.
3.4. The Number of Episodes of URTIs
Five studies reported the number of episodes of URTIs or the episode rate of URTIs [13, 15, 20–22]. There were 760 participants in the probiotics group and 465 participants in the placebo group. Pooled analyses showed that probiotics significantly reduced the episode rate of URTIs compared with placebo (rate ratio: 0.72; 95% CI: 0.60–0.86; P=0.0002; Figure 3(b)). However, there was substantial heterogeneity (I2 = 99%; P < 0.00001). We conducted sensitivity analyses by excluding each study individually. We found that this was not significantly different with the original analyses.
3.5. The Mean Duration of One Episode of URTI
Only two studies [13, 15] could be pooled with the mean duration of one episode of URTI because some studies reported the results as the mean duration of one participant's URTI episode. The result showed that probiotics significantly reduced the mean duration of one episode of URTI compared with placebo (MD: −2.66; 95% CI: −4.79 to −0.54; P = 0.01; Figure 3(c)). However, substantial heterogeneity was observed (I2 = 80%; P = 0.03).
3.6. The Adverse Events
Five studies reported adverse events, including nausea, vomiting, flatulence, abdominal pain, diarrhea, and bloating. Most of adverse events were mild [13, 15, 20, 21, 23]. Three studies suggested that none of the adverse events were associated with the trial intervention [13, 21, 23]. One study did not report the number of adverse events [13]. Four studies were pooled [15, 20, 21, 23], and the results showed that the occurrence rate of adverse events was not statistically different between two groups (rate ratio: 1.01; 95% CI: 0.80–1.26; P=0.96; Figure 3(d)). Substantial heterogeneity was observed (I2 = 99%; P < 0.00001), which was not significantly changed by using sensitivity analyses.
4. Discussion
It remains as an unsolved public health problem of finding effective prevention strategies for URTIs [24]. Recently, the number of studies that researched the potential effects of probiotics in the prevention of URTIs has increased dramatically. Previous meta-analysis showed that supplemental probiotics maybe a feasible strategy for preventing URTIs [4]. However, Hao et al. [4] included only three trials focused on adults [15, 20, 25], and most of the trials were conducted on children. Moreover, one of the included trials supplemented vitamins which were potentially immune-enhancing dietary supplements [25]. Our analysis specifically focused on adults and included recent updated RCTs.
We excluded participants who did regular high-intensity physical exercise because previous studies suggested that high-intensity physical exercise may affect immunity [26]. We also excluded participants who were vaccinated or took potential immune-enhancing dietary supplements during the trial process. In this study, only six RCTs were included according to our strict inclusion and exclusion criteria. The results in our meta-analysis indicated that probiotics could reduce the incidence of URTI episodes, the episode rate of URTIs, and the duration of one episode of URTI in adults. Furthermore, the occurrence rate of adverse effects in taking probiotics was not significantly different from taking placebo, and the adverse events of supplemental probiotics were mainly mild gastrointestinal symptoms. However, expect for the result of the incidence of URTI episodes, other results had substantial heterogeneity. Of note, these results did not significantly change during sensitivity analyses. Our results were consistent with previous findings on synbiotic, and Chan et al. [19] found that synbiotic could reduce the incidence and the episode rate of respiratory tract infections in adults.
The potential mechanism of probiotics in the prevention of URTIs is probably related to the modulation of the immune system. The study has shown that Lactobacillus casei Zhang can activate T-cells and B-cells and improve the levels of anti-inflammatory cytokines IL-4 and IL-10 [27]. Zhang et al. [28] reported that a combination of probiotics can increase the secretion of antiviral cytokines IFN-γ in blood and sIgA in the gut. In addition, natural killer (NK) cells play an important role in the prevention of URTIs. Probiotics could inhibit the reduction of NK cell activity and increase the level of salivary cortisol [13]. Besides, studies have suggested that intake of specific probiotics attenuated mental stress and reduced the risk of infection [23, 29].
This meta-analysis has some limitations. First, high heterogeneity was observed in our meta-analysis. The study population, probiotics strains, forms, and dosages varied among the included studies, which might be the reason for substantial heterogeneity. For example, we observed that the episode rate of URTIs in the B. bifidum R0071 group was lower than that in the L. helveticus R0052 group [22]. In addition, the diagnosis of URTIs was mainly through daily questionnaires, and participants might be misdiagnosed or missed diagnosis, which can affect the accuracy of the results. Third, the number of RCTs about probiotics compared with placebo for the prevention of URTIs in adults was relatively insufficient; therefore, it was not possible to conduct a subgroup analysis with age or probiotics strains groups. Finally, all included studies were in English, and selective reporting and publication bias could not be ignored.
5. Conclusion
In summary, probiotic supplementation may be an effective strategy to prevent episodes of URTI in adults. However, the quality of the evidence is low because of publication bias and substantial heterogeneity. More high-quality RCTs are required to confirm this conclusion and to assess which species of probiotics, forms, and dosages is the most efficacious in preventing URTI episodes in adults.
Acknowledgments
This work was supported by the Guangxi Natural Science Foundation (no. 2020GXNSFDA238003) and Research Project Grant of Guangxi Health Committee (no. Z20190071).
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Arroll B. Common cold. American Family Physician. 2011;84(12):1390–1391. [PubMed] [Google Scholar]
- 2.Disease GBD, Injury I, and Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furushima D., Nishimura T., Takuma N., et al. Prevention of acute upper respiratory infections by consumption of catechins in healthcare workers: a randomized, placebo-controlled trial. Nutrients. 2019;12(1):p. E4. doi: 10.3390/nu12010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao Q., Dong B. R., Wu T. Probiotics for preventing acute upper respiratory tract infections. The Cochrane Database of Systematic Reviews. 2015;(2) doi: 10.1002/14651858.CD006895.pub3.CD006895 [DOI] [PubMed] [Google Scholar]
- 5.Hill C., Guarner F., Reid G., et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 6.Thomas C. M., Versalovic J. Probiotics-host communication. Gut Microbes. 2010;1(3):148–163. doi: 10.4161/gmic.1.3.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohland C. L., MacNaughton W. K. Probiotic bacteria and intestinal epithelial barrier function. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2010;298(6):G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda S., Toh H., Hase K., et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 9.Suez J., Zmora N., Segal E., Elinav E. The pros, cons, and many unknowns of probiotics. Nature Medicine. 2019;25(5):716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 10.Ianiro G., Rizzatti G., Plomer M., et al. Bacillus clausii for the treatment of acute diarrhea in children: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10(8) doi: 10.3390/nu10081074.E1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuello-Garcia C. A., Brożek J. L., Fiocchi A., et al. Probiotics for the prevention of allergy: a systematic review and meta-analysis of randomized controlled trials. Journal of Allergy and Clinical Immunology. 2015;136(4):952–961. doi: 10.1016/j.jaci.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Shen N. T., Maw A., Tmanova L. L., et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889.e9–1900.e9. doi: 10.1053/j.gastro.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Shida K., Sato T., Iizuka R., et al. Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. European Journal of Nutrition. 2017;56(1):45–53. doi: 10.1007/s00394-015-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pu F., Guo Y., Li M., et al. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: a randomized controlled open-label trial. Clinical Interventions in Aging. 2017;12:1223–1231. doi: 10.2147/CIA.S141518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith T. J., Rigassio-Radler D., Denmark R., Haley T., Touger-Decker R. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. British Journal of Nutrition. 2013;109(11):1999–2007. doi: 10.1017/s0007114512004138. [DOI] [PubMed] [Google Scholar]
- 16.Gleeson M., Bishop N. C., Struszczak L. Effects of Lactobacillus casei Shirota ingestion on common cold infection and herpes virus antibodies in endurance athletes: a placebo-controlled, randomized trial. European Journal of Applied Physiology. 2016;116(8):1555–1563. doi: 10.1007/s00421-016-3415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor B. L., Woodfall G. E., Sheedy K. E., et al. Effect of probiotics on metabolic outcomes in pregnant women with gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2017;9(5):p. 461. doi: 10.3390/nu9050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J., Thomas J., Chandler J., et al. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.0. London, UK: Cochrane; 2019. http://www.training.cochrane.org/handbook. [Google Scholar]
- 19.Chan C. K. Y., Tao J., Chan O. S., et al. Preventing respiratory tract infections by synbiotic interventions: a systematic review and meta-analysis of randomized controlled trials. Advance in Nutrition. 2020;11(4):979–988. doi: 10.1093/advances/nmaa003.nmaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berggren A., Lazou Ahrén I., Larsson N., Önning G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. European Journal of Nutrition. 2011;50(3):203–210. doi: 10.1007/s00394-010-0127-6. [DOI] [PubMed] [Google Scholar]
- 21.Hirose Y., Yamamoto Y., Yoshikai Y., Murosaki S. Oral intake of heat-killed Lactobacillus plantarum L-137 decreases the incidence of upper respiratory tract infection in healthy subjects with high levels of psychological stress. Journal of Nutritional Science. 2013;2:p. e39. doi: 10.1017/jns.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langkamp-Henken B., Rowe C. C., Ford A. L., et al. Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: a randomised, double-blind, placebo-controlled study. British Journal of Nutrition. 2015;113(3):426–434. doi: 10.1017/s0007114514003997. [DOI] [PubMed] [Google Scholar]
- 23.Murata M., Kondo J., Iwabuchi N., et al. Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults. Beneficial Microbes. 2018;9(6):855–864. doi: 10.3920/bm2017.0197. [DOI] [PubMed] [Google Scholar]
- 24.Fuller R., Moore M. V., Lewith G., et al. Yeast-derived β-1, 3/1, 6 glucan, upper respiratory tract infection and innate immunity in older adults. Nutrition. 2017;39-40:30–35. doi: 10.1016/j.nut.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 25.de Vrese M., Winkler P., Rautenberg P., et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clinical Nutrition. 2005;24(4):481–491. doi: 10.1016/j.clnu.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Witard O. C., Turner J. E., Jackman S. R., et al. High-intensity training reduces CD8+ T-cell redistribution in response to exercise. Medicine & Science in Sports & Exercise. 2012;44(9):1689–1697. doi: 10.1249/mss.0b013e318257d2db. [DOI] [PubMed] [Google Scholar]
- 27.Hor Y.-Y., Lew L.-C., Lau A. S.-Y., et al. Probiotic Lactobacillus casei Zhang (LCZ) alleviates respiratory, gastrointestinal & RBC abnormality via immuno-modulatory, anti-inflammatory & anti-oxidative actions. Journal of Functional Foods. 2018;44:235–245. doi: 10.1016/j.jff.2018.03.017. [DOI] [Google Scholar]
- 28.Zhang H., Yeh C., Jin Z., et al. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synthetic and Systems Biotechnology. 2018;3(2):113–120. doi: 10.1016/j.synbio.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takada M., Nishida K., Kataoka-Kato A., et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterology & Motility. 2016;28(7):1027–1036. doi: 10.1111/nmo.12804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


