Abstract
Introduction
Recent consensus defines four SCLC subtypes on the basis of transcription factor expression: ASCL1, NEUROD1, POU2F3, and YAP1. The rare YAP1 subtype is associated with “neuroendocrine (NE)-low” cells among SCLC cell lines and patient samples. We evaluated YAP1 in 39 patients with phenotypically diverse circulating tumor cell–derived explant (CDX) models and revisited YAP1 in terms of prevalence, cell phenotype, and intertumor and intratumor heterogeneity.
Methods
YAP1 transcript and protein expression were assessed by RNA sequencing and immunohistochemistry or multiplexed immunofluorescence of NE and non-NE CDX subpopulations. Physically separated NE and non-NE CDX ex vivo culture lysates were Western blotted for YAP1, NE marker SYP, and AXL.
Results
RNA sequencing normalized for the four subtype transcription factors identified YAP1 expression in 14 of 39 CDX. A total of 10 CDX expressed YAP1 protein, and eight had strong YAP1 expression confined to rare non-NE cell clusters. This was confirmed in ex vivo CDX cultures in which adherent non-NE cells lacking SYP expression expressed YAP1. However, in two CDX, weaker cellular YAP1 expression was observed, widely dispersed in SYP-positive NE cells.
Conclusions
YAP1 was predominantly expressed in non-NE cell clusters in SCLC CDX, but two of 39 CDX expressed YAP1 in NE cells. CDX22P, with relatively high YAP1 expression, is an ASCL1 NE subtype with a low NE score and an outlier within this subtype in our CDX biobank. These descriptive data reveal subtly different YAP1 expression profiles, paving the way for functional studies to compare YAP1 signaling in non-NE and low NE cell contexts for potentially personalized therapeutic approaches.
Keywords: Small cell lung cancer (SCLC), YAP1, Neuroendocrine, Nonneuroendocrine, Intratumoral heterogeneity
Introduction
SCLC is an aggressive neuroendocrine (NE) cancer with poor prognosis, characterized by high circulating tumor cell (CTC) burden and early widespread metastasis.1 Treatment for SCLC remained unchanged for decades2 until the recent incorporation of immune checkpoint inhibitors into first-line treatment in the United States,3 benefiting a poorly defined subset of patients. Effective targeted therapies for patients with SCLC remain urgently required. Emerging evidence suggests that characterization of SCLC molecular heterogeneity4,5 will identify specific therapeutic vulnerabilities within patient subtypes6 to enable biomarker-driven patient stratification to improve patient outcomes. Four SCLC subtypes have been proposed on the basis of expression of key transcriptional regulators in patient tumors, patient-derived preclinical models, and human cell lines: ASCL1 and NEUROD1, both master regulators of NE phenotypes; POU2F3 that identifies a tuft cell of origin; and YAP1, a regulator of Hippo signaling.5
RNA sequencing (RNAseq) of 38 SCLC CTC–derived explants (CDXs) identified models representing three of the four consensus SCLC subtypes (ASCL1, NEUROD1, and POU2F3).4 We discovered a further subtype on the basis of the expression of the neurogenic transcription factor (TF) ATOH1,4 but our analysis did not reveal the YAP1 subtype.4 Four studies collectively reported the rarity of the YAP1 subtype, in which YAP1 was expressed in five of 126 tumors and seven of 51 SCLC cell lines with low or absent NE marker expression, a low NE score, and a variant, loosely adherent morphology.7, 8, 9, 10
The tumor-suppressive Hippo pathway controls cell proliferation, apoptosis, and organ size.11 When Hippo signaling is active, YAP1 is inactivated and sequestered in the cytoplasm for degradation. When Hippo signaling is inactive, YAP1 binds Transcriptional Enhanced Associate Domain family nuclear TFs to direct prosurvival gene expression, proliferation, and tissue growth.11,12 YAP1 nuclear activity correlates with chemoresistance, cancer stem cell renewal, and metastasis.8,11, 12, 13 YAP1 also responds to extracellular matrix stiffness and mechanical cues that promote cancer cell motility.11 Given these “hallmarks of cancer” YAP1 functions, we revisited YAP1 RNA and protein expression across 39 CDX models, evaluating the prevalence, cell phenotype, and tumor heterogeneity to provide a characterization of YAP1 at single-cell resolution.
Materials and Methods
CDX generation, RNAseq, and immunohistochemistry (IHC) were performed as described previously.4 CDX models were generated from patients’ CTCs enriched from blood samples pre-chemotherapy baseline or at posttreatment disease progression time points (designated P or PP).4
Transcriptomic analysis was performed with amendments to the previously described alignment (NF-core RNAseq pipeline with Spliced Transcripts Alignment to a Reference aligner) and annotation (mapped to eEnsembl version 99).4
Three independent tumors per CDX were used for all analyses. Antibodies for IHC were the following: (1) YAP1 (1:100, ab52771; Abcam, Cambridge, United Kingdom); (2) REST (1:150, MA5-24606; ThermoFisher Scientific, Waltham, MA); and (3) SYP (pA0299; Leica Biosystems, Wetzlar, Germany). Digitally scanned slides were analyzed using HALO software (Akoya Biosciences, Menlo Park, CA); expression levels were reported as a percentage of positive cells within each tissue slice. Multiplex immunofluorescence was performed using PerkinElmer Opal 4-Color Automation IHC Kit (NEL800001KT) (PerkinElmer, Waltham, MA) and quantified using HALO.
For ex vivo analysis, CDX tumors were disaggregated, cultured, and lysates immunoblotted as previously described14 using the aforementioned antibody to YAP1 (1:1000), REST (1:500, LS-C668231; Lifespan Biosciences, Seattle, WA), SYP (1:20,000, ab32127; Abcam, Cambridge, United Kingdom), AXL (1:500, C89E7; Cell Signalling Technology, Danvers, MA), and tubulin (1:1000, 2144S, Cell Signalling Technology). CDX cultures were treated with a titration of cisplatin concentrations for 5 days, followed by cell number analysis using Cell-Titer Glo 3D viability assay (G9683; Promega, Madison, WI).14
Results
YAP1 Is Expressed in SCLC CDX
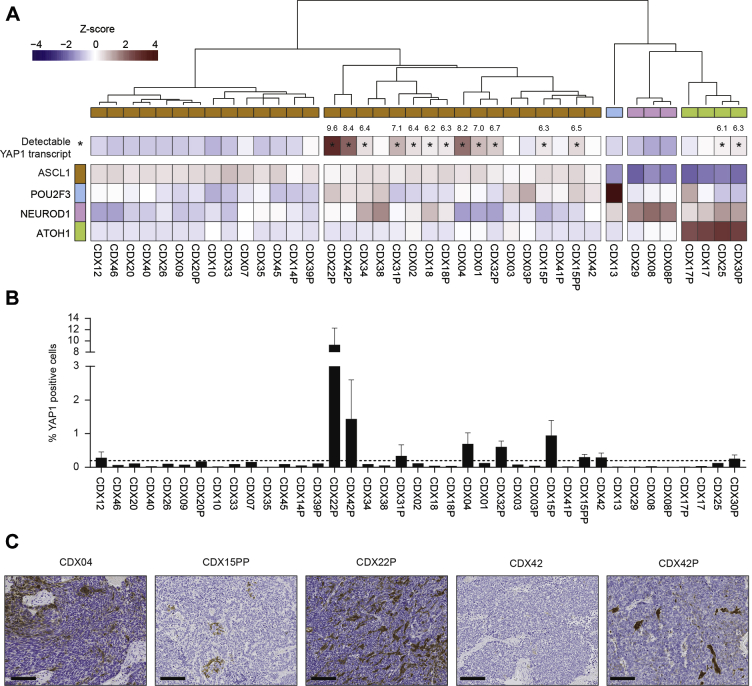
Our biobank of 38 SCLC CDX recapitulates the ASCL1, NEUROD1, and POU2F3 subtypes.4 We have generated an additional model, CDX31P, belonging to the ASCL1 subgroup (Supplementary Data 1) and derived from a patient with extensive disease, postchemotherapy. Although unbiased clustering of CDX RNAseq data did not identify the YAP1 subtype, normalization of transcript levels (variance stabilizing transform) for each TF identified 14 CDX models with detectable YAP1 transcript, albeit at relatively low levels compared with ASCL1, NEUROD1, and POU2F3 (Fig. 1A) (seven of 14 CDX variance stabilizing transforms > 6.5). Of note, our analysis revealed a bisection of the ASCL1 subtype with detectable YAP1 expression in one subset; two of the four ATOH1 CDX models expressed YAP1, and neither of the NEUROD1 or POU2F3 models were YAP1 expressers.
Figure 1.
YAP1 RNA and protein expression in CDX. (A) A heatmap illustrating relative RNA transcript expression of five SCLC TFs across the CDX biobank with YAP1-expressing models marked with an asterisk (∗) and the VST values depicted for each. The average expression is illustrated for three independent CDX tumor replicates per model. (B) Immunohistochemical analysis of YAP1 protein expression in CDX (three independent tumors per CDX). The dashed line represents a cutoff of YAP1 expression in more than 0.2% cells. (C) Representative images of YAP1 expression by IHC (brown stain) in selected CDX models. Scale bars = 100 μm. CDX, circulating tumor cell–derived explant model; IHC, immunohistochemistry; TF, transcription factor; VST, variance stabilizing transform.
YAP1 protein was expressed in more than 0.2% cells in 10 of 39 CDX models (dotted line, Fig. 1B), comprising eight of 14 models identified by RNAseq and an additional two models (Fig. 1B). Overall, there was good concordance between RNAseq and IHC; analyses were performed on independent tumor replicates, likely explaining the lack of concordance for rare cells in some models. YAP1 expression was comparable by IHC in paired CDX models generated at prechemotherapy baseline and at posttreatment disease progression (Supplementary Data 2). Concordant with RNAseq, CDX22P had the highest YAP1 expression (9% cells) (Fig. 1B), and the YAP1 expressing cells were dispersed throughout the tumor, a distribution also noted in CDX04 (Fig. 1C). In the remaining models, YAP1 was observed within cell clusters.
YAP1 Expression in Non-NE Cell Clusters
YAP1 RNA was expressed in seven of 51 SCLC cell lines, which also had low or absent NE marker expression.7, 8, 9, 10 Therefore, we evaluated YAP1 protein alongside established SCLC NE and non-NE markers by IHC on serial tissue sections (Fig. 2). Where YAP1 expression was confined to cell clusters, it colocalized with REST, a known repressor of NE differentiation in SCLC,9 concordant with reduced expression of the SCLC diagnostic NE marker SYP, indicating non-NE cell expression of YAP1 (Fig. 2A). In CDX04 and CDX22P, YAP1 was expressed in non-NE cell clusters (Fig. 2A) and throughout the tissue within NE cells (REST-low and SYP-positive) (Fig. 2B).
Figure 2.
YAP1 protein expression in non-NE cell clusters. (A) Immunohistochemical analysis in serial CDX tissue sections showing clusters of YAP1 expressing cells colocalized with non-NE marker REST and with reduced expression of NE marker SYP. (B) Diffuse YAP1 expression in regions of CDX04 and CDX22P with low or absent REST and with low expression of SYP. Representative images are depicted from the staining of three biological replicates per CDX. Scale bars = 50 μm. CDX, circulating tumor cell–derived explant model; NE, neuroendocrine.
Multiplex immunofluorescence and quantification of YAP1 and REST colocalization was carried out in seven CDX models representing a range of YAP1 and REST expression profiles (Supplementary Data 3 and Fig. 3A). In CDX models in which YAP1 expression was less than 2% and expressed in cell clusters (CDX15P, CDX15PP, CDX17, CDX30P, and CDX42P), 57% of YAP1 cells were non-NE and colocalized with REST (Fig. 3B), despite the low abundance of REST-positive (RESTpos) cells (mean 15.5%). When YAP1 expression was greater than 3% and dispersed throughout the tumor (CDX04 and CDX22P), less YAP1-REST dual positivity was observed (mean 10%), indicating that 90% of YAP1-expressing cells were NE (Fig. 3B), although non-NE YAP1–expressing cell clusters were also identified (Fig. 3A).
Figure 3.
YAP1 and REST multiplex immunofluorescence in CDX. (A) Multiplex immunofluorescence assay illustrating representative images of YAP1 (yellow) and REST (pink) expression with DAPI stained nuclei (blue). Scale bars = 10 μm. (B) Quantification of multiplex immunofluorescence showing the percentage of YAP1, REST, and dual YAP1 and REST-positive cells for each CDX. Bimodel YAP1 and REST colocalization is indicated by two means for % YAP1 cells coexpressing REST (57%, CDX15P, CDX15PP, CDX17, CDX30P, CDX42P; 10%, CDX04, CDX22P). Whole-tumor sections from three to four independent mice per CDX were analyzed and plotted individually. (C) YAP1 subcellular localization calculated for each immunofluorescence assay represented as % of cells with YAP1 expression in the nucleus and cytoplasm. The mean is plotted from three to four independent tumor replicates, and error bars represent SEM. (D) Intensity of YAP1 fluorescence in YAP1-positive and REST-negative (NE) cells versus YAP1-positive and REST-positive (non-NE cells) in CDX. Whole-tumor sections from three independent mice per CDX were analyzed. CDX, circulating tumor cell–derived explant; DAPI, 4′,6-diamidino-2-phenylindole; NE, neuroendocrine.
Only 8% of RESTpos cells were also YAP1-positive (YAP1pos) (Fig. 3B), indicating that not all non-NE cells were YAP1 lineage. The subcellular localization of YAP1 was both cytoplasmic and nuclear (Fig. 3C), in which there was more cytoplasmic expression in CDX04, CDX17, CDX15P, and CDX42P, and more nuclear expression in CDX15PP and CDX22P. There was a significant increase (1.25-fold, p = 0.0008) in the YAP1 fluorescence intensity of dual YAP1pos and RESTpos cells compared with the YAP1pos and REST-negative (RESTneg) cells (Fig. 3D); indicating that YAP1 expression was higher in non-NE cells.
YAP1 Expression and Cisplatin Response in Ex Vivo Cultures of CDX
A NE to non-NE phenotypic switch occurs in SCLC cell lines and genetically engineered mouse models5,15 and is studied through IHC with phenotype markers or differential adherence to a substrate in culture; “classical” NE cells grow as loose suspensions and “variant” non-NE cells as monolayers.5 Similarly, in six of 16 CDX ex vivo cultures tested, adherent cells resembling the variant phenotype were found (Figs. 4A and B). REST expression confirmed adherent cells were non-NE, whereas suspension cells were RESTneg and SYPpos, consistent with a NE phenotype (Fig. 4C). YAP1 was expressed in all non-NE subpopulations of CDX ex vivo cultures and by the NE cells from CDX04 and CDX22P that expressed relatively low levels of SYP (Fig. 4C). YAP1 cellular localization is dynamic,16 making a robust assessment of nuclear localization difficult in fixed tissue (Fig. 3C). However, the YAP1 downstream target AXL receptor tyrosine kinase was expressed in the YAP1pos cells only (Fig. 4C), and a YAP transcriptional target signature17 was enriched in CDX17 and CDX30P non-NE cells compared with NE cells (Fig. 4D), consistent with YAP1 nuclear activity. Concordant with a previous study,8 RNAseq of the physically separated non-NE and NE cells revealed reciprocal expression of YAP1 with three established NE markers (INSM1, SYP, CHGA) in non-NE CDX17 and CDX30P cells (Fig. 4E). In these CDX, YAP1, REST, VIM, CD44, and MYC were more highly expressed in the non-NE cell subpopulation, which also expressed the previously defined YAP1 SCLC gene signature (Supplementary Data 4).8 Overall, these data indicate that YAP1 expression is predominantly in non-NE cells but can also be present at lower levels in NE-low cells. Consistent with previous reports of increased chemoresistance in non-NE compared with NE cells in an SCLC genetically engineered mouse model,15 and the association of YAP1 expression and chemoresistance,8,13 YAP1pos non-NE cells in CDX30P and CDX31P ex vivo cultures were fivefold to 7.5-fold more resistant than YAP1neg NE cells to cisplatin (Fig. 4F).
Figure 4.
YAP1 expression in NE and non-NE CDX cells ex vivo. (A) Schematic showing generation of CDX ex vivo cultures and separation of suspension NE cells and adherent non-NE cells. (B) Representative bright-field images of suspension CDX NE cells with “classical” SCLC morphology (left panel) and adherent CDX non-NE cells with “variant” morphology (right panel). Scale bar = 200 μm. (C) Western blot analysis of REST, YAP1, AXL, and SYP expression in representative CDX NE and non-NE cell lysates, with tubulin-loading control (two replicate tumors per CDX). (D) A heatmap illustrating the relative RNA transcript expression of 24 genes associated with YAP1 activity17 in CDX17 and CDX30P NE and non-NE cells. (E) Log2 RNA fold change (non-NE versus NE) of NE and non-NE genes in CDX17 and CDX30P adherent (YAP1-positive) cells versus suspension (YAP1-negative) cells. (F) Cisplatin IC50 values (μM) of paired NE and non-NE cells derived from CDX30P and CDX31P ex vivo cultures. CDX, circulating tumor cell–derived explant; IC50, concentration that inhibits 50%; NE, neuroendocrine.
Discussion
Classification of SCLC as a recalcitrant cancer in 2012 by the U.S. government provoked a resurgence of SCLC research focused on developing and interrogating patient-derived preclinical models to more accurately reflect inter-tumoral and intra-tumoral heterogeneity.4 Identification of SCLC subtypes4,5 is beginning to reveal specific molecular vulnerabilities6 and identify novel targets and innovative biomarker-driven treatment strategies. YAP1 is a key mediator of the tumor-suppressive Hippo pathway,5 and although its expression defines one consensus SCLC subtype,5,8 there were only five studies on YAP1 in SCLC at time of writing. Although the YAP1 subtype was not represented within our published biobank of 38 CDX on the basis of unbiased hierarchical clustering of bulk RNAseq data,4 YAP1 transcript or protein was detectable in 16 CDX (Fig. 1). With the exception of CDX04 and CDX22P, YAP1-expressing CDX models had less than 2% YAP1pos cells, predominantly within clusters of the minority non-NE cell subpopulation (Figs. 2 and 3), a finding confirmed in ex vivo cultures of separated NE (suspension) and non-NE (adherent) cells (Fig. 4). For example, CDX17P was neither previously reported as a YAP1 subtype on analysis of bulk tumor by RNAseq,4 nor could we define it as such by IHC for YAP1 (Fig. 1, Supplementary Data 5A, and B). However, expansion of the rare non-NE subpopulation by culture ex vivo resulted in detectable YAP1 protein (Fig. 4).
CDX04 and CDX22P had the lowest NE score and highest YAP1 expression, and in these models, NE and non-NE cell ex vivo cultures expressed YAP1 with absent or low SYP. CDX22P is a rare ASCL1 subtype with a low NE score (0.28) (Supplementary Data 3 and Simpson et al.4), more closely matching the “NE-low” phenotype described for YAP1-expressing SCLC cell lines and tumors,5,7, 8, 9, 10 and the rest of the CDX also exhibited this correlation between YAP1 expression and lower NE score (Supplementary Data 6). Furthermore, CDX22P can be classified as a YAP1 subtype on the basis of the YAP1 50-gene signature8 (Supplemental Data 7) and provides the first example of YAP1 expression in both suspension NE-low and adherent non-NE cells (Figs. 3 and 4).
In summary, we found that high cellular YAP1 expression occurs predominantly in rare non-NE cell clusters within CDX, which explains why unbiased hierarchical clustering failed to detect a YAP1 subgroup.4 However, NE-low cells dispersed across CDX tumors can also express YAP1, albeit at lower cellular expression levels. These descriptive data from a biobank of patient-faithful CDX models reveal subtle differences within SCLC cellular phenotypes expressing YAP1. We speculate that YAP1 may fulfill different functional role(s) in NE-low versus non-NE phenotypes, warranting further study. SCLC subtyping has largely been performed using a single time point “snapshot” of intratumoral heterogeneity; yet recent evidence suggests SCLC subtypes are dynamic, and the non-NE YAP1 lineage can emerge during disease evolution.18 In future, SCLC subtyping may need to account for both temporal and spatial expressions of SCLC transcriptional drivers to fully appreciate intratumoral heterogeneity, especially as subtype-specific therapeutic vulnerabilities are discovered.19 The availability of large biobanks of patient-relevant models of SCLC, including longitudinal models, now allows the field to explore intertumoral and intratumoral heterogeneity in more detail in the search for improved and personalized treatment of this aggressive cancer.
Acknowledgments
This work was supported through Core Funding to Cancer Research UK (CRUK) Manchester Institute (grant number A27412), Manchester CRUK Centre Award (grant number A25254), the CRUK Lung Cancer Centre of Excellence (grant number A20465), and by The Christie Charitable Fund. Patient recruitment was supported by the National Institute for Healthcare Research Manchester Biomedical Research Centre and National Institute for Healthcare Research Manchester Clinical Research Facility at The Christie Hospital. Sample collection was undertaken through the molecular mechanisms underlying chemotherapy resistance, therapeutic escape, efficacy, and toxicity–improving knowledge of treatment resistance in patients with lung cancer or CHEMORES trial. Dr. Simpson and Prof. Dive supervised and devised the study. Ms. Pearsall, Dr. Simpson, and Prof. Dive co-wrote the manuscript. Ms. Pearsall, Mr. Revill, and Mr. Morgan carried out immunohistochemistry analysis, data analysis, and interpretation. Ms. Pearsall developed multiplex immunofluorescence protocols and performed immunoblotting, including data analysis and interpretation. Humphrey carried out bioinformatics analyses and interpretation and helped edit the manuscript. Dr. Frese had oversight of all patients with circulating tumor cell–derived explant models and model generation, and helped edit the manuscript. Ms. Galvin was responsible for all in vivo work described. Dr. Kerr had oversight of all bioinformatics analyses in the Cancer Biomarker Centre. Dr. Carter, Ms. Priest, and Prof. Blackhall oversaw the acquisition of ethical permission and patient consent and the collection of blood samples from patients in the CHEMORES study. Prof. Blackhall assisted with manuscript revision and is the chief investigator of the CHEMORES study. All authors read and approved the final manuscript.
Footnotes
Disclosure: The authors declare no conflict of interest.
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at https://doi.org/10.1016/j.jtho.2020.07.008.
Supplementary Data
Supplementary Data 1.
Supplementary Data 2.

Supplementary Data 4.
Supplementary Data 5.
Supplementary Data 6.

Supplementary Data 7.
References
- 1.Bunn P.A., Jr., Minna J.D., Augustyn A. Small cell lung cancer: can recent advances in biology and molecular biology be translated into improved outcomes? J Thorac Oncol. 2016;11:453–474. doi: 10.1016/j.jtho.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farago A.F., Keane F.K. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res. 2018;7:69–79. doi: 10.21037/tlcr.2018.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horn L., Mansfield A.S., Szczęsna A. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 4.Simpson K.L., Stoney R., Frese K.K. A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity. Nat Cancer. 2020;1:437–451. doi: 10.1038/s43018-020-0046-2. [DOI] [PubMed] [Google Scholar]
- 5.Rudin C.M., Poirier J.T., Byers L.A. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19:289–297. doi: 10.1038/s41568-019-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardnell R.J., Li L., Sen T. Protein expression of TTF1 and cMYC define distinct molecular subgroups of small cell lung cancer with unique vulnerabilities to aurora kinase inhibition, DLL3 targeting, and other targeted therapies. Oncotarget. 2017;8:73419–73432. doi: 10.18632/oncotarget.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horie M., Saito A., Ohshima M., Suzuki H.I., Nagase T. YAP and TAZ modulate cell phenotype in a subset of small cell lung cancer. Cancer Sci. 2016;107:1755–1766. doi: 10.1111/cas.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McColl K., Wildey G., Sakre N. Reciprocal expression of INSM1 and YAP1 defines subgroups in small cell lung cancer. Oncotarget. 2017;8:73745–73756. doi: 10.18632/oncotarget.20572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W., Girard L., Zhang Y.-A. Small cell lung cancer tumors and preclinical models display heterogeneity of neuroendocrine phenotypes. Transl Lung Cancer Res. 2018;7:32–49. doi: 10.21037/tlcr.2018.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito T., Matsubara D., Tanaka I. Loss of YAP1 defines neuroendocrine differentiation of lung tumors. Cancer Sci. 2016;107:1527–1538. doi: 10.1111/cas.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H.J., Diaz M.F., Price K.M. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat Commun. 2017;8:1–14122. doi: 10.1038/ncomms14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamar J.M., Stern P., Liu H., Schindler J.W., Jiang Z.G., Hynes R.O. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A. 2012;109:E2441–E2450. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song Y., Sun Y., Lei Y., Yang K., Tang R. YAP1 promotes multidrug resistance of small cell lung cancer by CD74-related signaling pathways. Cancer Med. 2020;9:259–268. doi: 10.1002/cam4.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lallo A., Frese K.K., Morrow C.J. The combination of the PARP inhibitor olaparib and the WEE1 inhibitor AZD1775 as a new therapeutic option for small cell lung cancer. Clin Cancer Res. 2018;24:5153–5164. doi: 10.1158/1078-0432.CCR-17-2805. [DOI] [PubMed] [Google Scholar]
- 15.Lim J.S., Ibaseta A., Fischer M.M. Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature. 2017;545:360–364. doi: 10.1038/nature22323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning S.A., Dent L.G., Kondo S., Zhao Z.W., Plachta N., Harvey K.F. Dynamic fluctuations in subcellular localization of the Hippo pathway effector Yorkie in vivo. Curr Biol. 2018;28:1651–1660.e4. doi: 10.1016/j.cub.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Xu X., Maglic D. Comprehensive molecular characterization of the Hippo signaling pathway in cancer. Cell Rep. 2018;25:1304–1317.e5. doi: 10.1016/j.celrep.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ireland A.S., Micinski A.M., Kastner D.W. MYC drives temporal evolution of small cell lung cancer subtypes by reprogramming neuroendocrine fate. Cancer Cell. 2020;38:60–78.e12. doi: 10.1016/j.ccell.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart C.A., Gay C.M., Xi Y. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat Cancer. 2020;1:423–436. doi: 10.1038/s43018-019-0020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.