Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus which causes coronavirus disease (COVID-19), has spread rapidly across the globe infecting millions of people and causing significant health and economic impacts. Authorities are exploring complimentary approaches to monitor this infectious disease at the community level. Wastewater-based epidemiology (WBE) approaches to detect SARS-CoV-2 RNA in municipal wastewater are being implemented worldwide as an environmental surveillance approach to inform health authority decision-making. Owing to the extended excretion of SARS-CoV-2 RNA in stool, WBE can surveil large populated areas with a longer detection window providing unique information on the presence of pre-symptomatic and asymptomatic cases that are unlikely to be screened by clinical testing. Herein, we analysed SARS-CoV-2 RNA in 24-h composite wastewater samples (n = 63) from three wastewater treatment plants (WWTPs) in Brisbane, Queensland, Australia from 24th of February to 1st of May 2020. A total of 21 samples were positive for SARS-CoV-2, ranging from 135 to 11,992 gene copies (GC)/100 mL of wastewater. Detections were made in a Southern Brisbane WWTP in late February 2020, up to three weeks before the first clininal case was reported there. Wastewater samples were generally positive during the period with highest caseload data. The positive SARS-CoV-2 RNA detection in wastewater while there were limited clinical reported cases demonstrates the potential of WBE as an early warning system to identify hotspots and target localised public health responses, such as increased individual testing and the provision of health warnings.
Keywords: SARS-CoV-2, COVID-19, WBE, Wastewater based epidemiology, Pandemic
Graphical abstract
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly infectious virus responsible for coronavirus disease 2019 (COVID-19). The COVID-19 global pandemic has resulted in 69,143,017 diagnosed cases and 1,576,516 deaths as of December 12, 2020 (Dong et al., 2020; WHO https://covid19.who.int/). COVID-19 patients display various symptoms including fever, dry cough, and shortness of breath. These symptoms generally appear within 2–14 days after exposure to the virus (Chen et al., 2020a, Chen et al., 2020b; He et al., 2020). However, as many as 45% of infected individuals may be asymptomatic, meaning they are infected but show no signs of illness throughout the course of the disease (Oran and Topol, 2020; Yang et al., 2020). Many clinical tests utilising reverse transcription quantitative polymerase chain reaction (RT-qPCR) assays and the enzyme-linked immunosorbent assay (ELISA) have been developed and implemented to detect SARS CoV-2 in clinical specimens such as sputum, nasopharyngeal swab, and blood (Roy et al., 2020; Wang et al., 2020).
While infection is most notable for causing respiratory illness, SARS-CoV-2 also infects gastrointestinal epithelial cells via the angiotensin-converting enzyme 2 (ACE2) receptor causing diarrhea, vomiting, and other gastrointestinal symptoms (Xiao et al., 2020). It has been estimated that 48 to 67% of infected patients shed SARS-CoV-2 in their stool (Chan et al., 2020; Cheung et al., 2020; Parasa et al., 2020; Wong et al., 2020). Consequently, SARS-CoV-2 RNA can be measured in wastewater to detect and monitor SARS-CoV-2 infection trends among the population in an approach, previously utilized for poliovirus, and now known as wastewater-based epidemiology (WBE) (Paul et al., 1940; Asghar et al., 2014). Importantly, levels of RNA shedding in stool are independent of COVID-19 severity (Zheng et al., 2020). SARS-CoV-2 RNA has also been detected in the stool despite negative clinical test results on nasopharyngeal swabs (Chen et al., 2020a, Chen et al., 2020b) and is present in patient stool for a longer period (9–16 days post symptom onset, compared to 6–11 days in nasopharyngeal swabs); hence, monitoring stool may be useful when a nasopharyngeal swab is negative (Ling et al., 2020). These observations suggest that WBE may afford a symptom agnostic means of monitoring SARS-CoV-2 infection trends within a community, for a longer period than individual clinical testing (Foladori et al., 2020).
Research groups across the globe have been mobilizing to develop and scale up a wide range of methodologies to monitor SARS-CoV-2 via WBE (Bivins et al., 2020a). Lodder and de Roda Husman (2020) detected SARS-CoV-2 RNA in wastewater in the Netherlands within four days of the first clinically diagnosed case. Detections of SARS-CoV-2 RNA in wastewater were also reported in Milan, Italy, within a few days of the first national clinical case (La Rosa et al., 2020); in Brisbane, Australia, when the number of clinical cases in the region were in the hundreds (Ahmed et al., 2020a); in secondary effluent in Yamanashi Prefecture, Japan, when reported cases were at their peak (Haramoto et al., 2020); and in the Region of Murcia, Spain, when the clinical prevalence was the lowest in the Iberian Peninsula (Randazzo et al., 2020).
Initial SARS-CoV-2 RNA detections in wastewater have also been reported in Louisiana, USA (Sherchan et al., 2020), Dubai (Albastaki et al., 2020), Gujarat, India (Kumar et al., 2020), Gothenburg, Sweden (Saguti et al., 2021), and Southeast England, UK (Martin et al., 2020). Interestingly, Medema and colleagues detected SARS-CoV-2 RNA in wastewater from a city in the Netherlands six days before the first clinical case was reported (Medema et al., 2020), and another study from the northeastern USA reported that viral titers in wastewater indicated infections were significantly greater than those that were clinically reported (Wu et al., 2020a). It is important to note that these studies measured fragments of viral RNA. While fecal transmission cannot be ruled out, SARS-CoV-2 in untreated wastewater were found to be not infective (Rimoldi et al., 2020).
Despite the flood of reported SARS-CoV-2 RNA detections in wastewater, little has been documented on the performance of virus concentration and detection methods (O'Reilly et al., 2020). A modeling exercise suggested that wastewater surveillance would theoretically be able to detect 1 shedder in a catchment of 2,000,000 people in the best case scenario, but noted limitations including uncertainties around RNA signal decay in wastewater, which has been shown to be dependent on temperatures and residence times en route to sampling points (Hart and Halden, 2020; Ahmed et al., 2020c; Ahmed et al., 2020d). Furthermore, 30–90% of infected individuals have reported shedding the virus accompanied by highly variable shedding rates (0.8 to 7.5 log10/g of feces). Therefore, predicting the number of shedders in a catchment may be difficult. A preprint has reported a detection limit of 1 shedder in 1000 people to 2 shedders per 10,000 people as estimated by monitoring hospital wastewater (Jorgensen et al., 2020). These performance specifications are functions of the underlying microbiological methods, number of viruses are shed by infected people, and interpretational frameworks used. These remain diverse, sub-optimal and unstandardized in many instances (Ahmed et al., 2020d; Lu et al., 2020; Michael-Kordatou et al., 2020; Thompson et al., 2020). Given these uncertainties, the application of WBE to inform SARS-CoV-2 management strategies and direct public health intervention outcomes remains tenuous. The Water Research Foundation describing outbreak detection as “very feasible”, outbreak tapering as “somewhat feasible”, and prevalence assessment as “may or may not be feasible” (WRF, 2020).
At present, the efficacy of WBE for SARS-CoV-2 surveillance and monitoring has yet to be fully evaluated and understood, and is limited by the short-term and cross-sectional nature of the published studies which often report RNA titers in wastewater during the exponential growth phase of an epidemic. Longitudinal observations of SARS-CoV-2 RNA shedding indicate that infected persons can shed in their stool for prolonged periods from 14 to 30 days (Cai et al., 2020; Wu et al., 2020a, Wu et al., 2020b; Xu et al., 2020), and that the levels of RNA shedding in stool decay by 5 to 7 orders of magnitude throughout the clinical progression of COVID-19 (Wölfel et al., 2020).
SARS-CoV-2 RNA is quite stable in untreated wastewater at temperatures ranging from 4 to 37 °C, permitting reliable detection following sample collection and processing (Ahmed et al., 2020b). These observations indicate that during a SARS-CoV-2 epidemic, the RNA signal in wastewater will likely build, plateau, and taper in distinct manners that will make interpretation of the signal challenging. The aim of this study was to investigate whether SARS-CoV-2 RNA can be detected and quantified in wastewater collected from three wastewater treatment plants (WWTPs), and to compare the frequency and variability of detection with COVID-19 case number data. We report a longitudinal study of SARS-CoV-2 RNA quantities in wastewater paired with reported clinical cases of COVID-19 from 24/02/2020 to 01/05/2020 in Brisbane, Queensland, Australia during both the growth, plateau, and tapering of an epidemic of COVID-19. Finally, results from this study were compared to the available data from other major Australian capital cities, Sydney and Melbourne; a Queensland regional centre, Townsville; two Queensland tourist areas, Airlie Beach of the Whitsundays and Hervey Bay; and a popular wine tourist region, the Barossa Valley in South Australia that integreated wastewater monitoring into public health responses.
2. Materials and methods
2.1. Wastewater sampling and site information
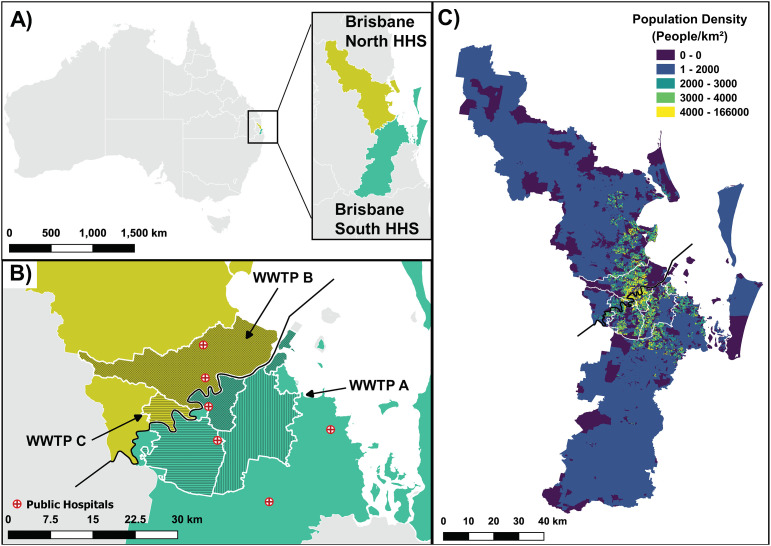
A total of 63 untreated wastewater samples were collected between 24th February and 1st May 2020 from three wastewater treatment plants (WWTPs A, B and C) representing urban catchments in Brisbane, Australia. Locations of the samples are presented in Fig. 1 . WWTP A, B, and C have representative service populations of 198,000, 505,000, and 231,000, respectively.
Fig. 1.
Sampling site locations and population density of studied catchments. A) Indicates the location within Australia where the Hospital and Health Service (HHS) areas were located, which was the main geographical location where COVID-19 cases were reported in Queensland. Queensland HHS are shown in white outline with the other state borders. B) The location of the WWTP catchments under study, layered against the Brisbane North and Brisbane South HHS in yellow and green, respectively. The main public hospitals accepting COVID-19 patients in the area are shown in red colour. Parts of WWTP B and WWTP C catchments are located in both Brisbane North and Brisbane South HHS, while WWTP A is located in Brisbane South HHS. C) Shows the population density of the 2 HHS. The main populous areas of the two HHS were within the 3 WWTP catchments under study (highlighted in white).
Samples of 500 mL to 1 L were collected using two types of automated sampling techniques – either a conventional refrigerated autosampler operating in time proportional mode (every 15 mins) or a submersible in-situ high frequency autosampler (at WWTP A, B and C) as well as grab sampling techniques (at WWTP B). Samples were transported on ice to the laboratory and stored at 4 °C until analysis. Sampling personnel wore standard personal protective equipment (PPE) for wastewater sampling, such as long pants, steel capped boots, hard hats, safety glasses and gloves to minimize potential exposure to infectious SARS-CoV-2.
2.2. COVID-19 caseload data
Case numbers in Queensland were initially published by Queensland Health by Hospital and Health Service (HHS). This was equivalent to the population coverage of 41.8% of Brisbane Metro North HHS and 50.2% of the total population of Brisbane Metro South HHS. However, WWTP B and C had part of their catchment area within both Brisbane HHS areas. Daily cases and active cases data were obtained from the Queensland Health media releases available on the Queensland Health website (https://www.health.qld.gov.au/news-events/doh-media-releases).
2.3. Virus concentration and RNA extraction
Viruses were concentrated from wastewater samples (100–200 mL based on turbidity) using adsorption-extraction with electronegative membrane as previously described (Ahmed et al., 2020a). RNA was directly extracted from the electronegative membrane using a RNeasy PowerMicrobiome Kit (Qiagen, Hilden, Germany) with slight modification. A 2-mL glass bead beating tube was replaced with a 5-mL bead tube containing garnet beads. This was done to accommodate the electronegative membrane followed by adding 990 μL of buffer PM1 and 10 μL of β-mercaptoethanol (Sigma-Aldrich, Australia). A tissue homogenizer (Precellys 24, Bertin Technologies, France) was used to homogenize the samples, in which homogenization occurred for 3 × 20 s cycles at 10,000 rpm with a 10 s pause between cycles. Homogenization did not effect RNA quality when compared to wastewater samples that were not homogenized. After homogenization, tubes were further centrifuged at 10,000 g for 5 min to pellet the membrane debris and beads. RNA was extracted from 450 μL of lysate using the RNeasy PowerMicrobiome and the QIAcube Connect platform (Qiagen) to obtain a final RNA elution volume of 100 μL. All RNA samples were stored at −80 °C and subjected to RT-qPCR analysis within 7 days of RNA extraction. Laboratory personnel wore personal protective equipment (PPE) such as gloves, coats, safety glasses, face masks, and face shields during wastewater sample processing. Filtered wastewater samples were treated with 10% bleach and discarded in the sink as per the activity risk assessment prepared for this study.
2.4. RT-qPCR analysis
Published RT-qPCR assays that target two different regions of the SARS-CoV-2 genome, specifically N (CDC N1, CDC N2) and E genes (E_Sarbeco) were used for SARS-CoV-2 RNA detection in wastewater samples (Corman et al., 2020; US CDC, 2019). The primers and probe sequences, along with qPCR cycling parameters are shown in Supplementary Table ST1. For RT-qPCR assays, double-stranded DNA gene fragment containing the assay target (gBlocks gene fragments) and 2019-nCoV_N plasmid control (Catalogue No. 10006625) were purchased from the Integrated DNA Technologies (Coralville, IA, USA) and used to generate the standard curves (copy/μL). CDC N1 and N2 standard dilutions ranged from 1 × 105 to 1 copy/μL. E_Sarbeco standard dilutions, also ranging from 1 × 105 to 1 copy/μL, were prepared from the gBlocks gene fragments as per the manufacturer's instructions. All RT-qPCR amplifications were performed in 20 μL reaction mixtures using iTaq™ Universal Probes One-Step Reaction Mix (Bio-Rad Laboratories, Richmond, CA).
Each CDC N1 and N2 RT-qPCR mixture contained 10 μL of Supermix, 2019-nCoV Kit (500 nM of forward primer, 500 nM of reverse primer and 125 nM of probe) (Catalogue No. 10006606), 0.4 μL of iScript reverse transcriptase and 3 μL of template RNA. E_Sarbeco RT-qPCR mixtures contained 10 μL of Supermix, 400 nM of forward primer, 400 nM of reverse primer, 200 nM of probe, 0.4 μL of iScript reverse transcriptase and 3 μL of template RNA. The RT-qPCR assays were performed using a Bio-Rad CFX96 thermal cycler (Bio-Rad Laboratories). All RT-qPCR reactions were performed in triplicate. For each RT-qPCR run, a series of three positive and no template controls were included.
All RT-qPCR data were generated using default settings for baseline and threshold. Data were only collected from instrument runs in which the positive control produced amplification, and no amplified product was observed in the no template control. All instrument runs passed these criteria. A master standard curve with 95% upper and lower confidence intervals was generated for each assay. The log10-linear regression of copy number and corresponding quantification cycle (Cq) values (derived from the 6-point, assay gBlock 1:10 serial dilution series) measured in triplicate from three qPCR instrument runs were used to generate the master standard curve.
For each sample replicate, the SARS-CoV-2 RNA concentration (copies/reaction) was calculated from the master standard curve and accounts for the difference in nucleic acid type between the double-stranded oligonucleotide used to generate the standard curve and the single-stranded genome of SARS-CoV-2 (i.e., divide by 2) (Bustin et al., 2009). The assay limit of detection (ALOD) for the different assays used was defined as the minimum copy number with a 95% probability of detection as previously described (Verbyla et al., 2016). The process limit of detection (PLOD) was calculated by dividing the ALOD by the RNA template volume added to the RT-qPCR well and then multiplying this number by the total volume of RNA extracted from each sample to yield the total RNA gene copies (GC). This number was then normalised to total sample volume processed to yield the PLOD of SARS-CoV-2 RNA GC/100 mL. Since an RNA sample still can yield RT-qPCR positive signal above the ALOD, a cut-off <45 quantification cycle (Cq) was used to determine positive samples.
2.5. RT-qPCR inhibition and quality control
An experiment was conducted to determine the presence of PCR inhibition in nucleic acid extracted from wastewater samples using a murine hepatitis virus (MHV) RT-qPCR assay (Besselsen et al., 2002). Known copy numbers of MHV (2 × 104 GC/reaction) were added in the RT-qPCR reactions (without sample) and the Cq values obtained acted as a reference point. The same amount of MHV GC was also added into RT-qPCR reactions in the presence of RNA extracted from wastewater samples. If the Cq value of a wastewater nucleic acid sample increases (i.e., two Cq values) the sample was considered to have PCR inhibitors (Staley et al., 2012). With respect to quality control, reagent and extraction blanks were included for each batch of RNA extraction to ensure no carryover contamination occurred during RNA extraction. No carryover contamination was observed in reagent blank samples. To minimize potential contamination, RNA extraction and RT-qPCR setup were performed in separate laboratories.
2.6. Sequencing and bioinformatics
For Miseq Illumina sequencing, six representative CDC N1 RT-qPCR products were purified with a 1× ratio of AmpureXP (BeckmanCoulter, USA) and eluted in 15 μL of DNase- and RNase-free water. Amplicons were prepared for sequencing using the NEB UltraII Total RNA kit (New England Biolabs, USA) according to the manufacturer's protocol but modified to begin at the end repair step. PCR indexing of libraries was undertaken using the NEBNExt Multiplex Oligos Unique Dual indices for Illumina using 10 cycles of PCR. Samples were pooled in equimolar amounts for sequencing and sequenced as a 75-bp paired end run using a 150 cycle v3 MiSeq kit (Illumina, USA).
Primer sequences were removed from de-multiplexed reads using cutadapt (ver. 2.9), with reads not containing primers discarded (Martin, 2011). Poor quality reads were identified and removed with trimmomatic (ver. 0.39) using a sliding window of 4 bases with an average quality of 15 (SLIDINGWINDOW:4:15) (Bolger et al., 2014). Reads were cropped to 70 bp (CROP:70), with any less than 50 bp in length discarded (MINLEN:50). Quality-controlled reads were then mapped to the reference genome (GenBank accession number NC_045512.2) using CoverM ‘make’ (ver 0.4.0, B. Woodcroft, unpublished, https://github.com/wwood/CoverM). Low quality read mappings were removed with CoverM ‘filter’ (minimum identity 95% and minimum aligned length of 90%). Read depth profiles for each sample were calculated using samtools (ver. 1.9) to confirm coverage of the targeted region (Li et al., 2009).
2.7. Ethics approval
Low risk approval as defined by the National Statement on Ethical Conduct in Human Research was obtained from CSIRO Ethics Committee (reference number 2020_035_LR).
3. Results
3.1. PCR inhibition, performance characteristics of RT-qPCR assays and ALOD
All RNA samples were free from PCR inhibition as determined by the MHV RT-qPCR assay and, were therefore, used for downstream RT-qPCR analysis without dilution (Supplementary Table ST2). The amplification efficiencies of CDC N1 and CDC N2 assays were within the prescribed range (90 to 110%) of MIQE guidelines (Bustin et al., 2009). However, the amplification efficiencies of E_Sarbeco (89.6%) were slightly outside the recommended range. The correlation coefficient (R 2) values for all assays were between 0.996 and 0.998. The slope of the standard curves, Y-intercepts, ALOD and PLOD values are shown in Supplementary Table ST3.
3.2. SARS-CoV-2 RNA in wastewater samples
Among the 63 wastewater samples tested in this study, 19/63 (30.1%) and 2/63 (3.17%) were RT-qPCR positive using CDC N1 and E_Sarbeco assays (Table 1). SARS-COV-2 RNA in remaining samples were <PLOD (Supplementary Table ST4). Of the five samples collected from WWTP A between 20/04/2020 and 11/05/2020, all samples were <PLOD for the CDC N1, CDC N2 and E_Sarbeco assays. Of the 25 wastewater samples collected from WWTP B, 3/25 (12%) samples were positive for CDC N1 assay and 1/25 (4%) sample was positive for E_Sarbeco assay. Concentration of SARS-CoV-2 were <PLOD using CDC N2 assay for all samples collected from WWTP B. The prevalence of SARS-CoV-2 RNA was much greater in wastewater samples collected from WWTP C; of the 33 samples collected, 16/33 (48.5%) were positive for CDC N1 and 1/33 (3.03%) was positive for CDC E_Sarbeco. The CDC N2 assay was not detected (i.e., <PLOD) in any wastewater samples collected from WWTP C. Of the 16 CDC N1 positive samples 15/16 (93.8%) were quantifiable with concentrations ranging from 135 to 11,992 GC/100 mL of wastewater. Similarly, of the three CDC N2 positive samples, one (33.3%) was quantifiable with a concentration of 343 GC/100 mL. Both E gene positive samples (WWTPs B and C) were quantifiable with concentrations of 113 and 222 GC/100 mL, respectively (Supplementary Table ST4). All six representative CDC N1 amplicons were confirmed through sequencing and mapping to their corresponding positions (i.e., 28,287-28,358) in the SARS-CoV-2 genome. Read depths minimum identity and aligned length to the SARS-CoV-2 reference genome NC_045512.2 are shown in Supplementary Fig. SF2.
Table 1.
Occurrence of SARS-CoV-2 in wastewater samples at three WWTPs in Southeast Queensland, Australia.
| WWTPS | Sampling period | Types of samples | Sample volume processed | Number of samples positive/number of samples collected (mean or range GC/100 mL) |
||
|---|---|---|---|---|---|---|
| CDC N1 | CDC N2 | E_Sarbeco | ||||
| WWTP A | 20/04/2020–11/05/2020 | Composite | 200 mL | 0/5 | 0/5 | 0/5 |
| WWTP B | 26/03/2020–11/05/2020 | Composite and grab | 100–200 mL | 3/25 | 0/25 | 1/25 |
| WWTP C | 24/02/2020–13/05/2020 | Composite | 100–200 mL | 16/33 | 0/33 | 1/33 |
3.3. Temporal detection of SARS-CoV-2 RNA in wastewater samples
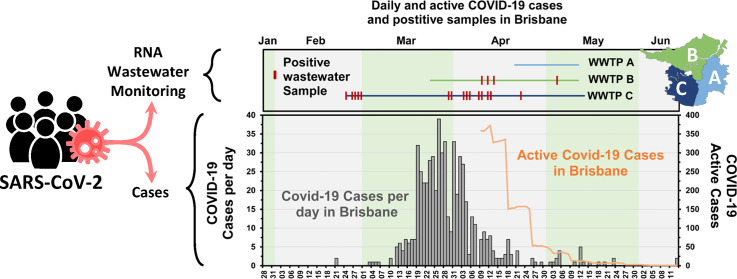
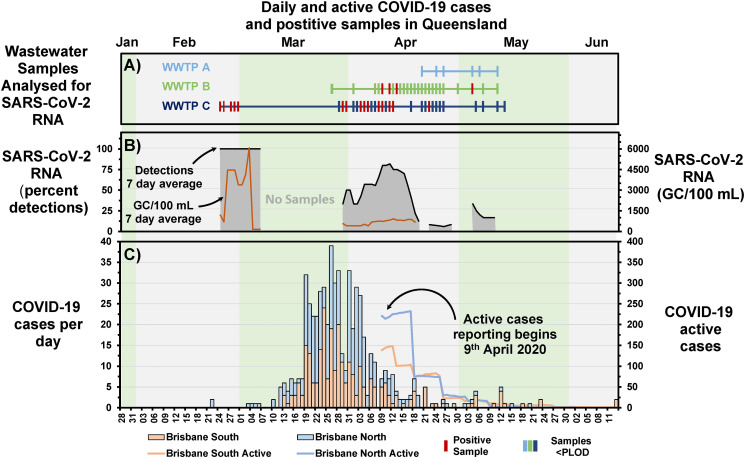
During the pandemic, cases of COVID-19 were publicly reported by the Queensland Government Department of Health by Brisbane North and South Hospital and Health Service (HHS) regions (https://www.qld.gov.au/health/conditions/health-alerts/coronavirus-covid-19). The course of the pandemic in Brisbane began with a few cases in late February to early March, then escalated from the 12th March through the peak (19th March to 4th April), and began decreasing to below five cases per day level from 22nd April, Fig. 2C. From the 9th of April the total number of active cases was reported for the HHS areas of Queensland. Over the course of the main wave of the pandemic monitored here (28th January to the 13th June) Brisbane North HHS had 317 cases and Brisbane South HHS had slightly fewer at 266 cases of COVID-19 (Fig. 2).
Fig. 2.
Comparison between the detection of SARS-CoV-2 in wastewater samples, to the number of cases observed in areas covered by the wastewater treatment plants. A) Sample timeline of samples analysed for SARS-CoV-2, with positive samples shown in red. B) The percentage of sample detections that were positive for SARS-CoV-2 RNA (black line with grey fill) for the previous 7 days (left y axis) and the GC of RNA/100 mL (yellow, plotted to right y axis) for the previous 7 days. C) Cases of COVID-19 per day in the Brisbane North and Brisbane South Hospital and Health Service (HHS) districts (stacked barplot, left y axis), as well as the number of active cases (line graph, right y axis).
Most SARS-CoV-2 RNA detections were in WWTP C wastewater, and were one month before and during the main wave of the pandemic. During the early phase of the pandemic clinical testing was limited, based on multiple criteria, including symptoms, close contacts with a positive case, or for passengers arriving from overseas with symptoms. The early detections of SARS-CoV-2 in wastewater in late February highlight that COVID-19 may have been circulating in the Brisbane South area prior to the clinical testing of individuals being available or implemented widely (Fig. 2A and C). The decay of the 7-day average detections in mid-April (9th to 21st April, Fig. 2B) align with the decrease of active cases (Fig. 2C), and may align with a 2–3 week lag in daily cases (24th to 28th March peak in daily cases vs peak of 7-day average detections, April 12th). As might be expected, the 7-day average wastewater detections tracked similarly to the 7-day average GC/100 mL; however, the GC/100 mL did not appear to agree well with daily case numbers (Supplementary Fig. SF1). Unfortunately, active case numbers were only available from 9th April, after the peak in daily cases, meaning there were only three days with data overlap with the quantified SARS-CoV-2 detections in wastewater. For those days the ratio of GC/100 mL to number of active cases were 1.7, 1.8, and 2.9.
4. Discussion
4.1. SARS-CoV-2 and COVID-19 in Brisbane, Queensland, Australia
COVID-19 was first confirmed in Melbourne, Australia, in late January 2020, with the first cases in Queensland confirmed on January 29th. The first two cases in the Brisbane study area were reported on February 22nd in Brisbane North HHS. The cases were returning residents that had flown back to Brisbane from Japan via Darwin after disembarking the Diamond Princess cruise ship that had recorded several cases of COVID-19. Over the next month in Queensland, total confirmed cases remained below 100 until March 19th when a surge in cases was observed, bringing the total cases above 1,000 by April 15th. Most cases (54%: 602/1,106, as of August 28th 2020) were confined to the Brisbane North and Brisbane South HHS areas. Several federal and state-based restrictions were imposed on residents to curb the spread of SARS-CoV-2. The Australian borders were closed on March 20th 2020 to overseas visitors, which was the beginning of a multi-stage action to contain the spread of the SARS-CoV-2 virus. By the beginning of April 2020, stage 3 restrictions were in place, which included limits on population movement from home, numbers of individuals allowed in public gatherings, closures of businesses, and additional social-distancing restrictions. Additionally, most employees were directed to work from home, there was a closure of Queensland borders, implementation of quarantine requirements following arrival, among others. Towards the end of April 2020, restrictions began to ease on a jurisdiction by jurisdiction basis.
4.2. Wastewater-based epidemiology as an early warning system for COVID-19
The current study immediately followed our proof-of-concept study, where we reported the presence of SARS-CoV-2 RNA in wastewater as a potential tool to monitor COVID-19 in the community (Ahmed et al., 2020a). Since then, several peer-reviewed studies have reported the early detection of SARS-CoV-2 RNA in wastewater before the first reported clinical diagnosis. For example, La Rosa et al. (2020) detected SARS-CoV-2 RNA in wastewater samples in Milan and Turin in December and in Bologna in January 2020. However, the first Italian case of COVID-19 was documented in February 2020. Medema et al. (2020) reported the detection of SARS-CoV-2 RNA in wastewater in the Netherlands on February 3rd – weeks before the first case was reported. The authors estimated that when they detected RNA fragments in wastewater, the COVID-19 prevalence was around or below 1 case in 100,000 people. Similarly, Randazzo et al. (2020) compared wastewater surveillance data to the declared COVID-19 cases at the municipality level in Spain. People from the community were shedding SARS-CoV-2 RNA in their stool even before the first case was reported by public health units in many of the cities where wastewaters have been sampled. In view of these research findings, we aimed to determine whether wastewater surveillance can be used as an early warning system for detecting COVID-19 at the community level in Brisbane, Australia.
In this study, we used three assays, two (CDC N1 and N2) targeting N gene and one (Sarbeco_E) targeting the E gene. The assays were chosen based on the results reported by Medema et al. (2020) who observed good agreement among these assays. In our previous study, we used the N_Sarbeco and NIID_2019-nCOV_N assays but for this study excluded these assays due to their low sensitivities (Ahmed et al., 2020a; Corman et al., 2020). However, discrepancies were still observed among the assays used. In this study, the levels of SARS-CoV-2 in RT-qPCR positive samples were near the ALOD. This may have contributed to the inconsistent results among the assays. When the concentration of the gene fragment is low, subsampling error likely to occur (Taylor et al., 2019).
For virus concentration, we used an adsorption-extraction method (pH of the sample was adjusted to 3.5) because this method is rapid (it takes <20 min to process a 100 mL volume of wastewater sample), it can concentrate viruses from both solid and liquid phases, utilizes relatively inexpensive membranes ($5 AUD per membrane), employs routine microbial laboratory equipment (filtration apparatus and a pump), and multiple samples can be processed at a time if multiple filtration units are available. In a recent study, we have shown that the mean recovery efficiency of this method is 26.7% and a modified version of the adsorption-extraction method amended with MgCl2 performed better (recovery efficiency = 65.7%) (Ahmed et al., 2020b). This modified version of the concentration method was not used in this study because we decided to use a consistent concentration method throughout the study. In this study, recovery efficiency of SARS-CoV-2 RNA in each wastewater sample using adsorption-extraction method was not determined because we did not have access to MHV (i.e., sample process control) when the study commenced. However, we obtained MHV at the end of the study, and determined the RT-qPCR inhibition by seeding MHV RNA in each wastewater RNA samples. Comparison of Cq values obtained for MHV RNA seeded wastewater samples were close to the Cq values obtained for the distilled water (i.e., benchmark) samples suggested the samples were likely free of PCR inhibitors.
The first positive detections of SARS-CoV-2 RNA in Brisbane wastewater were made in WWTP C in late February. This was at a time when the only documented clinical case of COVID-19 in Brisbane were located in Brisbane North HHS, serviced mainly by WWTP B. The two cases from the Diamond Princess cruise ship were being treated in a hospital located in the WWTP B catchment area. Therefore, the wastewater detections in WWTP C were made before any documented cases of COVID-19 were located in Brisbane South HHS, and consequently in the WWTP C catchment area. In fact, the first 3 documented cases of COVID-19 in Brisbane South HHS were not announced until the 13th of March 2020. Therefore, the wastewater detections in the last week of February at WWTP C, preceded known clinical cases by two to three weeks.
In this study, we detected RNA fragments, but not infective SARS-CoV-2 in wastewater samples. Discharge of wastewater to environmental waters was recently suggested as a potential transmission pathway for SARS-CoV-2 (Cahill and Morris, 2020). However, Rimoldi et al. (2020) reported that SARS-CoV-2 in untreated wastewater were found to be not infective (Rimoldi et al., 2020). Transmission through wastewater is unlikely to occur in Queensland, Australia, given the high efficacy of wastewater treatment processes for pathogen reduction, elevated temperature, and the fact that SARS-CoV-2 remain infectious in wastewater for a relatively shorter period of time compared to enteric viruses (Bivins et al., 2020b). However, wastewater transmission cannot be ruled out for low-income countries where the sanitation system is inadequate.
4.3. Limitations
Most positive detections of SARS-CoV-2 RNA were in wastewater samples from WWTP C in Brisbane, despite the WWTP C service area containing only one hospital accepting COVID-19 patients (three in WWTP B, none in WWTP A), and servicing a population less than half of WWTP B. There may be several reasons for this, such as the greater number of samples tested from WWTP C compared to WWTP A and B. Also, wastewater samples may still contain SARS-CoV-2 RNA but below the analytical detection limit. In this study, the Cq values of the majority of samples were near the ALOD, indicating that the SARS-CoV-2 RNA concentration was low which may have attributed to many non-detects. The recovery efficiency of the concentration method used is around 25%, and may vary from sample to sample between and across WWTPs. Furthermore, the recovery efficiency of RNA extraction kit used in this study is not known and requires further evaluation (Ahmed et al., 2020d). There are other factors, such as variable shedding rates by infected individuals, sampling frequency, dilution and mixing that may have also contributed to the low number of detects. Although, we collected 24-h composite samples, better sampling strategies (i.e., high frequency flow proportional composite sampling) may be needed to capture the SARS-CoV-2 more efficiently (Ort et al., 2010). Little is known regarding the persistence of SARS-CoV-2 RNA in wastewater collection networks. A recent study reported that SARS-CoV-2 RNA is quite stable in wastewater at temperatures ranging from 4 to 37 °C, hence decay may not play a role in the non-detections (Ahmed et al., 2020c). Overall, further methodological improvements will be required if the intended use of SARS-CoV-2 monitoring in wastewater is to provide early warning system on the presence of COVID-19 in the community.
Despite having more hospitals, WWTP B had a higher proportion of the residential Brisbane population in the less vulnerable age category; 38% of the population is aged 20–40, whereas for WWTPs A and C, this was 31%. Hence, WWTP B also had a slightly lower proportion of the population in the more vulnerable age category; with 24.2% of the population aged over 50, where WWTP A and C were 28.6 and 27.4%, respectively. WWTP B catchment was serviced mostly by areas on the north-side of Brisbane, in Brisbane North HHS, whereas WWTP A and C were servicing mainly Brisbane South HHS. WWTP B catchment is relatively large at nearly 340 km2 (as compared to WWTP C and A of approx. 200 km2). The larger sewer networks of WWTP B, and the longer sewer lines may have contributed to the lower frequency of detections if the signal and stability of the RNA fragments were influenced by residence time, length of sewer network, dilution, wastewater types (i.e., % domestic and industrial) and temperature (Ahmed et al., 2020c; Hart and Halden, 2020). The average residence time in WWTP B sewer was a maximum of 11.9 h (WWTP C = NA, WWTP A = 11.4 h), and both WWTPs A and B have longer pipe networks between pumping stations than WWTP C (WWTP A = 33.1 km longest sewer line; WWTP B = 33.9 km; WWTP C = 3.1 km), which is likely the result of catchment C WWTP being closer to the centre of the catchment area, whereas WWTPs A and B are on the catchment boundary. However, it should also be noted that the COVID-19 caseload data was not available at the resolution of the catchment areas, and this may also be influencing the poor comparison between cases and GC/100 mL. Samples were not collected due to logistics and lock down in the mid-March period when cases were escalating which may have affected interpretation and correspondence of the GC data to the number of cases.
4.4. Examples of integration of wastewater monitoring into public health responses in Australia
Pilot wastewater monitoring for SARS-CoV-2 RNA fragments in New South Wales (NSW), Australia detected positive results at Perisher, a popular winter resort ski field township on July 22nd 2020 (during the winter ski season) while there were no active cases recorded in the region (https://www.health.nsw.gov.au/news/Pages/20200730_00.aspx). At the time, NSW was experiencing a second wave in cases following an initial wave from late February to early May. After the positive detection the NSW Department of Health released this information along with public messaging for any visitors or residents in the area with even mild symptoms to come forward for testing. The media release was relatively timely, coming eight days post sample collection. While no further cases were detected, follow-up wastewater testing at the location were below detection limit for SARS-CoV-2 RNA.
In a second example, on the 3rd September 2020, the Queensland Government Department of Health released information regarding a detection of SARS-CoV-2 in wastewater analyses in a popular tourist area, Airlie Beach of the Whitsundays (https://www.health.qld.gov.au/news-events/doh-media-releases/releases/covid-19-viral-fragments-detected-in-airlie-beach-sewage). Following this announcement, pop-up COVID-19 fever clinics were set up in the area to screen residents with symptoms. However, no new cases were detected and subsequent wastewater samples were <PLOD. Similarly, on the 18th September 2020 there was a positive wastewater detection in Hervey Bay, which prompted a similar public health intervention and response by Queensland Health; follow-up wastewater testing retrieved a negative result. Similar positive detections and responses were observed for a WWTP in Townsville, Queensland, where positive wastewater results were returned in early October 2020 (https://www.abc.net.au/news/2020-10-10/coronavirus-queensland-new-cases-townsville-water-sewerage/12743082).
From these examples, these tourist regions were likely visited by asymptomatic people who were shedding into the sewer systems, possibly weeks post-infection upon recovery. In a similar example in early September, Angaston, South Australia, located in a popular wine tourist region, the Barossa Valley, had positive detections for SARS-CoV-2 RNA. The region recorded a small cluster of 34 cases linked to tourists in March (https://www.abc.net.au/news/2020-03-30/south-australian-wineries-to-close-amid-coronavirus-concerns/12103540). Similar responses were engaged: pop-up clinics and public messaging with subsequent wastewater testing returned <PLOD.
Up to the present (October 2020), only Victoria and NSW have experienced a significant second wave of COVID-19 cases, and mostly in the capital cities of Melbourne and Sydney, respectively. All other states and territories of Australia had fewer than 30 active cases at any one time during the period June to October 2020. Only Victoria observed a second wave larger than the first, which was confined mainly to the capital city, Melbourne. Gene sequencing of individual cases in Melbourne have indicated 99% of cases as of July 29th (4800 active cases at the time, close to the peak of the second wave of infections) were linked to poor compliance with quarantine of infected individuals, and from only two clusters (https://www.quarantineinquiry.vic.gov.au/sites/default/files/2020-08/20200818%20Hotel%20Quarantine%20Program%20-%20Day%204%20-%20%20FINAL.pdf). This enforces the importance of vigilance, compliance and early warning systems to detect community transmission of COVID-19 early before larger outbreaks transpire.
Based on the positive SARS-CoV-2 RNA detection in wastewater at one WWTP while there were limited clinically reported cases, WBE shows potential as an early warning system. Detection of genetic signal in wastewater could be used to marshal limited clinical testing resources to communities where cases may be newly emerging or increasing. Wastewater surveillance affords the possibility of detecting cases that are still pre-symptomatic or asymptomatic in the progression of SARS-CoV-2 infection. However, the speed of the public health response is critical to break the chain of transmission through case identification and isolation. For this reason, it is critical that wastewater sample collection and testing be performed in a timely manner and results reported to public health decision makers within hours to days.
In addition to being timely, wastewater testing must also be sensitive to minimize the probability of false negatives. The sensitivity of WBE for detecting cases is dependent on both the representativeness of the samples collected and the workflow used to detect SARS-CoV-2 RNA. False negative detection rates can be minimized by taking several approaches such as increasing the volume of wastewater analysed, analysing biological replicates, increasing the frquency of sampling, using multiple RT-qPCR assays and increasing the RT-qPCR technical replicates, including a sample process control along with other measures. During this study, the CDC N1 assay was observed to be the most sensitive of the three assays used. Nonetheless, despite the initial wave of COVID-19 cases, detections of SARS-CoV-2 RNA in samples from two WWTP catchments remained low compared to the third catchment. This experience suggests that wastewater surveillance may need to move beyond WWTP influent and into the catchment to achieve adequate population-level coverage. Given the demonstrated potential of WBE combined with traditional public health interventions and testing, further research to optimize detection workflows and sampling strategies is warranted.
5. Conclusions
-
•
In the current study, SARS-CoV-2 RNA was detected in influent from one WWTP in late February 2020, up to three weeks before the first clininal case was reported there.
-
•
Of the three RT-qPCR assays used in the study, the N1 gene target demonstrated the greatest sensitivity (19 of 63 samples positive) followed by the E gene assay (2 of 63 samples positive), with amplicons confirmed through sequencing; the N2 assay yielded negative results for all 63 samples.
-
•
The longitudinal decline of the RNA occurrence in wastewater aligned with the tapering of the first epidemic wave; however, SARS-CoV-2 RNA copy numbers in wastewater showed no correlation with daily cases numbers. However, total number of cases were much lower than experienced in other locations during the pandemic.
-
•
The observed prevalence of SARS-CoV-2 RNA in WWTP influent varied between wastewater catchments perhaps due to large differences in catchment size, pipe networks, wastewater characteristics, and subsequently, hydraulic retention times.
-
•
Early detection of SARS-CoV-2 in wastewater is particularly useful for COVID-19 management, and will aid in health messaging, warnings, setting up pop-up testing clinics of individuals to detect and minimize potential second or third waves of the pandemic.
Funding
The authors did not receive any funding for this project.
CRediT authorship contribution statement
Warish Ahmed – Study design and analysis.
Ben Tscharke – Writing, data analysis and visualisation.
Paul M. Bertsch - Study design.
Kyle Bibby – Writing.
Aaron Bivins – Writing.
Phil M. Choi – Writing.
Leah Clarke – Analysis.
Jason Dwyer – Sampling.
Janette Edson – Analysis.
Thi Minh Hong Nguyen – Analysis.
Jake W. O'Brien – Sampling.
Stuart L. Simpson – Writing and design.
Paul Sherman – Sampling.
Kevin V. Thomas - Writing and analysis.
Rory Verhagen – Sampling.
Julian Zaugg – Analysis.
Jochen F. Mueller – Study design and Sampling.
Declaration of competing interest
The authors have declared no conflicts of interest.
Acknowledgements
We thank Queensland Urban Utilities for their assistance in sampling.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.144216.
Appendix A. Supplementary data
Supplementary material
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., Brien J.W.O., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bibby K., Haramoto E., Hewitt J., Huygens F., Gyawali P., Korajkic A., Riddel S., Sherchan S.P., Simpson S.L., Sirikanchana K., Symonds E.M., Verhagen R., Vasan S.S., Kitajima M., Bivins A. Decay of SARS-CoV-2 and surrogate murine hepatitis virus RNA in untreated wastewater to inform application in wastewater-based epidemiology. Environ. Res. 2020;191:110092. doi: 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bivins A., Bertsch P.M., Bibby K., Choi P.M., Farkas K., Gyawali P., Hamilton K.A., Haramoto E., Kitajima M., Simpson S.L., Tandukar S., Thomas K., Mueller J.F. Surveillance of SARS-CoV-2 RNA in wastewater: methods optimisation and quality control are crucial for generating reliable public health information. Curr. Opin. Environ. Sci. Health. 2020;17:82–93. doi: 10.1016/j.coesh.2020.09.003. (on-line early) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albastaki A., Naji M., Lootah R., Almeheri R., Almullah H., Almarri I., Alreyami A., Aden A., Alghafr R. First confirmed detection of SARS-CoV-2 in untreated municipal wastewater and aircraft wastewater in Dubai, UAE: the use of wastewater based epidemiology as an early warning tool to monitor the prevalence of COVID-19. Sci. Total Environ. 2020 doi: 10.1016/j.scitotenv.2020.143350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar H., Diop O.M., Weldegebriel G., Malik F., Shetty S., El Bassioni L., Akande A.O., Al Maamoun E., Zaidi S., Adenijii A.J., Burns C.C., Deshpande J., Oberste M.S., Lowther S.A. Environmental surveillance for poliovirus in the global polio eradication initiative. J. Infect. Dis. 2014;210:S294–S303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besselsen D.G., Wagner A.M., Loganbill J.K. Detection of rodent coronaviruses by use of fluorogenic reverse transcriptase-polymerase chain reaction analysis. Comp. med. 2002;52(2):111–116. [PubMed] [Google Scholar]
- Bivins A., North D., Ahmad A., Ahmed W., Alm E., Been F., Bhattacharya P., Bijlsma L., Boehm A.B., Brown J., Buttiglieri G., Calabro V., Carducci A., Castiglioni S., Gurol Z.C., Chakraborty C., Costa F., Curcio S., de los Reyes F.L., III, Delgado Vela J., Farkas K., Fernazdez-Casi X., Gerba C., Gerrity D., Girones R., Gonzzalez R., Haramoto E., Harris A., Holden P.A., Ispam M.T., Jones D.L., Kasprzyk-Hordern B., Kitajima M., Kotlarz N., Kumar M., Kuroda K., La Rosa G., Malpei F., Mautus M., Mclellan S.L., Medema G., Meschke J.S., Mueller J., Newton R.J., Noble R.T., van Nuijs A., Peccia J., Perkins T.A., Pickering A.J., Rose J., Sanchez G., Smith A., Stadler L., Stauber C., Thomas K., van der Voorn T., Wiggington K., Zhu K., Bibby K. Wastewater-based epidemiology: global collaborative to maximize contributions in the fight against COVID-19. Environ. Sci. Technol. 2020;54(13):7754–7757. doi: 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Bivins A., Greaves J., Fischer R., Yinda K.C., Ahmed W., Kitajima M., Munster V.J., Bibby K. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Technol. Lett. 2020;7(12):937–942. doi: 10.1021/acs.estlett.0c00730. (on-line early) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., Vandesompele J., Wittwer C.T. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cahill N., Morris D. Recreational waters - a potential transmission route for SARS-CoV-2 to humans? Sci. Total Environ. 2020;740:140122. doi: 10.1016/j.scitotenv.2020.140122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Xu J., Lin D., Yang Z., Xu L., Qu Z., Zhang Y., Zhang H., Jia R., Liu P., Wang X., Ge Y., Xia A., Tian H., Chang H., Wang C., Li J., Wang J., Zeng M. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V.W.-S., Chiu P.K.-F., Yee C.-H., Yuan Y., Ng C.F., Teoh J.Y.-C. A systematic review on COVID-19: urological manifestations, viral RNA detection and special considerations in urological conditions. World J. Urol. 2020 doi: 10.1007/s00345-020-03246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T., Li F., Xu Q., Zhang Y., Xu S. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Lou J., Bai Y., Wang M. COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am. J. Gastroenterol. 2020;115(5):790. doi: 10.14309/ajg.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W.H., Tam A.R., Yip C.C.Y., Leung K.-H., Fung A.Y.-F., Zhang R.R., Lin Y., Cheng H.M., Zhang A.J.X., To, K.K.W, Chan K.-H., Yuen K.-Y., Leung W.K. Gastrointestinal manifestation of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brunink S., Schneider J., Schmidt L.M., Mulders D.G.J.C., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.-L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P.G., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;2020(25) doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real-time. The Lancet Infectious Disease. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review. Sci. Total Environ. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;731:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Lau E.H.Y., Wu P., Deng X., Wang J., Hao X., Lau Y.C., Wong J.Y., Guan Y., Tan X., Mo X., Chen Y., Liao B., Chen W., Hu F., Zhang Q., Zhong M., Wu Y., Zhao L., Zhang F., Cowling B.J., Li F., Leung G.M. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- Jorgensen A.D., Gamst J., Hansen L.V., Knudsen I.I.H., Jensen S.K.S. Eurofins Covid-19 sentinel TM wastewater test provide early warning of a potential COVID-19 outbreak. MedRXiv. 2020 doi: 10.1101/2020.07.10.20150573. [DOI] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic materials of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno G., Ferraro G.B., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y., Xu S.-B., Lin Y.-X., Tian D., Zhu Z.-Q., Dai F.-H., Wu F., Song Z.-G., Huang W., Chen J., Hu Bi-J., Wang S., Mao E.-Q., Zhu L., Zhang W.-H., Lu H.-Z. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilation patients. Chin. Med. J. (Engl) 2020;133(9):1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodder W., de Roda Husman A.M. SARS-CoV-2 in wastewater: potential health risk, but also data source. Lancet Gastroenterol Hepatol. 2020;5(6):533–534. doi: 10.1016/S2468-1253(20)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Huang Z., Luo J., Zhang X., ShaSha Primary concentration – the critical step in implemeting the wastewater based epidemiology for the COVID-19 pandemic: a mini-review. Sci. Total Environ. 2020;747:141245. doi: 10.1016/j.scitotenv.2020.141245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. Cutadapt Removes Adapter Sequences From High-Throughput Sequencing Reads. EMBnet. journal. 2011;17(1):10–12. [Google Scholar]
- Martin J., Dimitra K., Thomas W., Maria Z., Bentley E., Bujaki E., Fritzsche M., Mate R., Majumdar M. Tracking SARS-CoV-2 in sewage: evidence of changes in virus variant predominance during COVID-19 pandemic. Viruses. 2020;12(10):1144. doi: 10.3390/v12101144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7(7):511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Michael-Kordatou I., Karaolia P., Fatta-Kassinos D. Sewage analysis as a tool for the COVID-19 pandemic response and management: the urgent need for optimised protocols for SARS-CoV-2 detection and quantification. J Environ. Chem. Eng. 2020;8(5):104306. doi: 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oran D.P., Topol E.J. Prevalence of asymptomatic SARS-CoV-2 infection. Annals Internal Med. 2020 doi: 10.7326/M20-3012. [DOI] [PubMed] [Google Scholar]
- O’Reilly K.M., Allen D.J., Fine P., Asghar H. The challenges of informative wastewater sampling for SARS-CoV-2 must be met: lessons from polio eradication. The Lancet Microbe. 2020;1(5):E189–E190. doi: 10.1016/S2666-5247(20)30100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ort C., Lawrence M.G., Rieckermann J., Joss A. Sampling for pharmaceuticls and personal care products (PPCPs) and illicit drugs in wastewater systems: are your conclusions valid? A critical review. Environ. Sci. Technol. 2010;44(16):6024–6035. doi: 10.1021/es100779n. [DOI] [PubMed] [Google Scholar]
- Parasa S., Desai M., Chandrasekar V.T., Patel H.K., Kennedy K.F., Roesch T., Spadaccini M., Colombo M., Gabbiadini R., Artifon E.L.A., Repici A., Sharma P. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019. JAMA Netw. Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J.R., Trask J.D., Gard S. II. Poliomyelitic virus in urban sewage. J. Exp. Med. 1940;71(6):765–777. doi: 10.1084/jem.71.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Ferranfo E.C., Simon P., Allende A., Sanchez G. SARS-CoV-2 RNA titers in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Prevalence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744:140911. doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy V., Fischinger S., Atyeo C., Slein M., Loos C., Balazs A., Luedemann C., Astudillo M.G., Yang D., Wesemann D.R., Charles R., Lafrate J.A., Feldman J., Hauser B., Caradonna T., Miller T.E., Murali M.R., Baden L., Nilles E., Ryan E., Lauffenburger D., Beltran W.G., Alter G. SARS-CoV-2-specific ELISA development. J. Immunol. Methods. 2020;484:112832. doi: 10.1016/j.jim.2020.112832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saguti F., Magnil E., Enache L., Churqui M.P., Johansson A., Lumley D., Davidsson L., Dotevall L., Mattsson A., Trybala E., Lagging M., Lindh M., Gisslen M., Breczika T., Nystrom K., Norder H. Surveillance of wastewater revealed peaks of SARS-CoV-2 preceding those of hospitalized patients with COVID-19. Water Res. 2021;189:116620. doi: 10.1016/j.watres.2020.116620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmiz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley C., Gordon K.V., Schoen M.E., Harwood V.J. Performance of two quantitative PCR methods for microbial source tracking of human sewage and implications for microbial risk assessment in recreational waters. Appl. Environ. Microbiol. 2012;78(20):7317–7326. doi: 10.1128/AEM.01430-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.C., Nadeau K., Abbasi M., Lachance C., Nguyen M., Fenrich J. The ultimate qPCR experiment: producing publication quality, reproducible data for the first time. Trends Biotechnol. 2019;37(7):761–774. doi: 10.1016/j.tibtech.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Thompson J.R., Nancharaiah Y.V., Gu X., Lee W.L., Rajal B.B., Haines M.B., Girones R., Ng L.C., Alm E.J., Wuertz S. Makinf waves: wastewater surveillance of SARS-CoV-2 for population-based health management. Water Res. 2020;184:116181. doi: 10.1016/j.watres.2020.116181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Centers for Disease Control and Prevention 2019-Novel coronavirus (2019-nCoV) real-time rRT-PCR panel primers and probes. 2019. https://www.cdc.gov/coronavirus/2019-ncov/downloads/rt-pcr-panel-primer-probes.pdf
- Verbyla M.E., Symonds E.M., Kafle R.C., Cairns M.R., Irarte, Guzman A.M., Coronado O., Breitbart M., Ledo C., Mihelcic J.R. Managing microbial risks from indirect wastewater reuse for irrigation in urbanizing watersheds. Environ. Sci. Technol. 2016;50(13):6803–6813. doi: 10.1021/acs.est.5b05398. [DOI] [PubMed] [Google Scholar]
- Wang W., Xu Y., Gao R., Lu R., Han K., Wu G., Tan W. 321(18) 2020. Detection of SARS-CoV-2 in Different Types of Clinical Specimens; pp. 1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Mueller M.A., Niemeyer D., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Bruenink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized cases of coronavirus disease 2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- Wong M.C.S., Huang J., Lai C., Ng R., Chan F.L.K., Chan P.K.S. Detection of SARS CoV-2 RNA in fecal specimens of patients with confirmed COVID-19: a meta-analysis. J. Inf. Secur. 2020;81(2):e31–e38. doi: 10.1016/j.jinf.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRF . The Water Research Foundation; 2020. Wastewater Surveillance of the COVID-19 Genetic Signal in Sewersheds. Recommendations From Global Experts. [Google Scholar]
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli M., Endo N., Duvallet C., Poyet M., Moniz K., Washburne A.D., Ericson T.B., Chai P.R., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5(4) doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X., Yin H., Xiao X., Qu X., Kuang L., Fang X., Mishra N., Lu J., Shah H., Jiang J., Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Sun J., Xu Y., Li F., Huang X., Li F., Huang X., Li H., Zhao J., Hunag J., Zhao J. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg. Infect. Dis. 2020;26(8):1920–1922. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., Zhang H., Liu H., Xia H., Tang J., Zhang K., Gong S. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistene fecal viral shedding. Nature Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Gui X., Xiong Y. Comparison of clinical characteristics of patients with asymptomatic vs symptomatic coronavirus disease 2019 in Wuhan, China. JAMA Netw. Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., Chen W., Wang Q., Zhang D., Liu Y., Gong R., Ma Z., Lu S., Xiao Y., Gu Y., Zhang Z., Yao H., Xu K., Lu X., Wei G., Zhou J., Fang Q., Cai H., Qiu H., Sheng J., Chen Y., Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang Province, China, January-March 2020: retrospective cohort study. BMJ. 2020;2020(369) doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material