Graphical abstract

Keywords: Antioxidant enzymes, Catalase, Reduced glutathione, Oxidative damage, Water-soluble fraction
Highlights
-
•
This paper evaluated effects of a WSF-UVCO on the antioxidant responses of the scallop Lima scabra.
-
•
The antioxidant defenses in L. scabra seem be highly sensitive to low doses of to WSF-UVCO.
-
•
Digestive gland and gill show stronger antioxidant responses in L. scabra exposed to WSF-UVCO.
-
•
L. scabra could be good sensor for screening pollutant impacts along the Caribbean coastline.
Abstract
Used vehicle crankcase oils are a source of contamination in Caribbean marine environments and may alter the oxidative balance of organism that inhabiting coastal ecosystems. This paper aims to evaluate effects of a water-soluble fraction of used vehicle crankcase oils (WSF-UVCO) on the antioxidant responses of the flame scallop Ctenoides scaber. The organisms were exposed to ascending sublethal concentrations 0, 0.001, 0.01 and 0.1 % of WSF-UVCO in a static system of aquaria during one week. Subsequently activities of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione-S-transferase (GST) as well as concentrations of reduced glutathione (GSH) and thiobarbituric acid reactive substances (TBARS) were determined in the digestive gland, adductor muscle and gills. SOD, CAT, GST and TBARS increased in digestive gland of organisms exposed to WSF-UVCO at medium and highest concentrations, with a concomitant decrease in GPX and GR activities. In adductor muscle CAT decreased, but GR rose with exposure to 0.01 and 0.1 % WSF-UVCO; in gills, GST rose through all WSF-UVCO concentrations, and SOD, CAT and GR increased only at 0.1 %. The fluctuations in antioxidant enzymes and GST activities point out possible adjustments to control ROS production and detoxification of xenobiotics. These biochemical responses may guarantee the oxidative balance in flame scallop during short term exposure to low concentrations of WSF-UVCO. C. scaber appears suitable as an experimental organism for evaluating biological risks of sublethal exposure to hazardous xenobiotics in tropical marine environments.
1. Introduction
The used vehicle crankcase oils (UVCO) and other petroleum derived compounds are important sources of pollution in tropical marine ecosystems [1]. UVCO are mineral-oil mixtures containing products of incomplete combustion of hydrocarbon fuels, such as poly-aromatic hydrocarbons (PAH), as well as additives such as heavy metals, chlorinated solvents and organic metal complexes [[2], [3], [4]]. Waste lubricants can enter to the marine environment through sewers, highway runoff and drainage from gas stations, car mechanics and car washes. A portion of UVCO, mainly water-soluble chemicals, is bioavailable and when absorbed and metabolized by the marine organisms, can lead to proliferation of reactive oxygen species (ROS) via redox cycling of xenobiotic biotransformation. Previous studies had demonstrated that mixture of xenobiotics present in lubricant oils may alter the antioxidants responses and other biochemical biomarkers in different species [[5], [6], [7]].
Chronic increases in ROS alter oxidative balance and can cause DNA lesions, enzyme inactivation and peroxidation of cellular and sub-cellular lipids [8]. These oxyradicals possess a high affinity for thiol groups in peptides and enzymes, impeding the regulation of cellular functions [9]. However, the cells contain a complex arsenal of molecular defenses, enzymatic and non-enzymatic, that diminish ROS proliferation to limit oxidative damage [10]. The antioxidant defense system is composed of various enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR) and glutathione peroxidase (GPx) [11]. These enzymes are scavengers of free radicals, e.g. SOD transforms superoxide anion to H2O2, which is in turn eliminated by GPx and CAT. GPx catalyzes the reduction of peroxide radicals to alcohols and oxygen, using reduced glutathione (GSH) as a substrate in a NADPH dependent reaction [[12], [13], [14]]. Another important enzyme associated to the antioxidant defense system is glutathione-S-transferase (GST). This is a phase II metabolic enzyme, which participates in the detoxification of diverse organic xenobiotics using GSH [15,16]. When the antioxidant defenses are surpassed by ROS, they could provoke to molecular perturbations, especially the formation of malondialdehyde (MDA). All these responses are early signals of oxidative damage in contaminant exposed organisms, which have been frequently used as biomarkers of pollutant exposure [[17], [18], [19]].
The mollusk-bivalves have been considered as sentinel organisms for the biomonitoring of harmful compounds in coastal areas, which have filtering lifestyle, ubiquitous distribution, easy approachability and sensitivity to a wide range of pollutants [20,21]. Ecotoxicological studies have frequently used mussels and clams as model organisms, but rarely scallops. Scallops are a commercially important shellfish in worldwide, particularly in the Caribbean region [22]. A relatively abundant scallop in Caribbean coral reef ecosystems is Ctenoides scaber (Born, 1778), commonly called the “flame scallop”, due to that they possess a red mantle and extensive tentacles [23,24]. Natural populations are found in shallow Atlantic coastal waters from North Carolina in United States of America to the northeastern coast of Venezuela, and their reproductive cycle and population dynamics as well as their potential for aquaculture are known [25,26]. As a filter feeder, C. scaber is constantly exposed to coastal waters; its distribution and habitat make it an excellent choice as a potential sentinel organism to estimate toxicological effects of marine contaminants.
C. scaber is suitable and novelty for field and laboratory studies, for this reason we chose it to evaluate physiological perturbations caused by a water-soluble fraction of used vehicle crankcase oils (WSF-UVCO) focusing on biochemical responses of the oxidative stress management system. We contrasted sub-lethal effects of this mixture of contaminants on antioxidant defenses in several soft-tissues: digestive gland, adductor muscle and gills. The digestive gland is crucial for digestion and storage of food as well as the metabolism of xenobiotics; the gills carry out gas exchange and are a potential route of entry of water-soluble contaminants; and the adductor muscle regulates shell aperture, cleanses the body cavity and powers escape responses [27]. We predicted the strongest responses to contaminants would be found in the “front-line” digestive gland and gills while responses in the adductor muscle would be weaker.
2. Materials and methods
2.1. Chemicals
All chemicals were of analytical grade (Sigma, St. Louis, MO, USA).
2.2. Scallops
One hundred sixty adult flame scallops, C. scaber (55.0–65.0 mm), were harvested by hand from crevices in the limestone habitat by SCUBA divers at 2−5 m depth from a coral reef at Gulf of Santa Fe, in the Venezuelan northeastern coast (10°22ˊ56˝ N - 64°44ˊ07˝ W). The surface seawater temperature in the moment to take the animals oscillated among 24.80–25.30 °C. To avoid spawning and stress by handling, the animals were sent to the laboratory in insulated contained (at 25 °C) with well-aerated water and recirculated with a portable electric pump. Transport from the collection site to the laboratory did not exceed an hour. To habituate the scallops to laboratory conditions, they were kept in aquaria of 100 × 60 × 50 cm (1 animal per 10 L), which contained filtered and well-aerated seawater at a salinity of 36o/oo, pH 7.8 ± 0.1 at 25 ± 1 °C for two weeks. The photoperiod was approximately 12 h light/12 h dark. These physical conditions were very similar to those at the collection site. The scallops were fed twice daily with 100 mL of cultured microalgae Tetraselmis chuii (16 × 103 cell/mL). Seventy-five percent of the seawater in the aquaria was changed every two days.
2.3. Used vehicle crankcase oil
The UVCO was obtained from a local gas station in Cumaná City (Venezuela). A stock solution of the water-soluble fraction of used vehicle crankcase oils (WSF-UVCO) was prepared by placing 0.90 g of UVCO per mL of filtered seawater into a Pyrex bottle and slowly stirring the mixture with a magnetic stirrer during 24 h at room temperature. After mixing, the oil and water phases were allowed to separate for 4 h, before the aqueous phase was siphoned off. The WSF-UVCO was filtered through a Whatman filter paper N°1 [5]. This solution was considered to be 100 % and was then diluted with filtered seawater for the preliminary (LC1, LC50) and exposure bioassays.
2.4. Experimental treatment
In a static system of four glass aquaria (40 × 50 × 50 cm), we exposed eight flame scallops to increasing sublethal concentrations of 0.001, 0.01 and 0.1% (v/v) of a WSF-UVCO, during one week. Scallops, at one per 5 L of solution were distributed in the aquaria. As a control group, a quantity similar of scallops was placed in filtered seawater, without any UVCO. This bioassay was repeated 5 times. Salinity, pH and temperature of seawater in aquaria were maintained similar to conditions during the acclimatization period. The scallops were fed daily.
The experimental exposure concentrations were chosen by below the lethal concentration LC1 estimated (0.12 % v/v), by preliminary determinations of the 96 -h LC50 (8.42 % v/v, with 95 % confidence limits of 6.22 and 14.56 %) using standard guide [28]. The aquaria were covered with glass lids to minimize evaporation of seawater and aeration occurred through the lids. During the experimental period, half of the water was renewed every day, and WSF-UVCO concentrations were reestablished. Water replacement was carried out two hours after having fed the animals to diminish accumulation of waste products. Immediately after of treatments, scallop’s soft-tissues were carefully removed from the shells using a steel spatula. The digestive gland, adductor muscle and gills (ctenidia) were dissected avoiding contamination with other tissues and frozen rapidly in liquid nitrogen. Then tissues were stored at -40 ± 1 °C for less than 2 days prior to enzymatic and molecular determinations.
The chosen exposure concentrations represent a scenario of contamination by spill or possible direct discharge of lubricant wastes into coastal-marine ecosystems where the scallops live. Moreover, the exposure period of a week using low concentrations of WSF-UVCO was the necessary minimum time to find variations in certain biochemical responses [29]. No mortality or notable behavioral change in the scallops was observed during the exposure period. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed [30,31].
2.5. Chemical analyses
For the determination of organic chemical composition, the WSF-UVCO stock was acidified to pH < 2 with concentrated sulfuric acid, and then extracted twice with methylene chloride. Then, the pH was increased to >12 with concentrated sodium hydroxide and the mixture were again extracted twice with methylene chloride. The acid and the basic extracts were dried separately over anhydrous sodium sulfate, filtered, and concentrated to 1 mL under a stream of nitrogen.
The concentrated organic extracts were analyzed by gas chromatography/mass spectrometry (GC/MS) on a Saturn 2000 Varian System equipped with a Crompact CP-sil 8 CB-MS column (50 m x0.25 mm x0.25 μm), and a Varian 1078 temperature programmable detector. The temperature program for the column included a 5 min hold time at 60 °C, temperature ramping of 10 °C/min to 320 °C, and a final hold time of 4 min. The temperature program for the injector included a hold time of 1 min, at the initial temperature of 160 °C, followed by 50 °C/min rate to 320 °C, and a hold time of 20.80 min. Helium was the carrier gas at a flow rate of 40 mL/min.
Metals were quantified in the WSF-UVCO stock by inductively coupled plasma spectrometry (Varian, ICP-AES9 model), after acidic digestion in a closed vessel using a pressure-controlled microwave heating system (CEM Corporation, model MDS-200). The metal estimations were carried out in triplicate. The detection limits (mg/L) for each metal were Mg (0.12), Zn (0.10), Ni (0.01), Pb (0.01), Al (0.20), Fe (0.08), Cu (0.01), Cd (0.02), Cr (0.02) and Ba (0.02).
2.6. Enzymes
Tissues were homogenized (1:8 w/v) in cold 20 mM Tris−HCl buffer pH 7.4, containing 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol (DTT), 500 mM sucrose, 150 mM potassium chloride (KCl) and 0.2 mM phenylmethyl-sulfonic acid (PMFS). The homogenate was centrifuged at 12,000 g for 20 min at 4.0 ± 0.1 °C. Prior to use, the supernatant was passed through a 25-G Sephadex column. This aliquot obtained was used as the sample for enzyme assays.
The enzymes were measured by spectrophotometry at 25 °C using the Lambda 2 Kinetic Package software accessory for Perkins Elmer 2 Lambda spectrophotometer with the following protocols: Superoxide dismutase (SOD, EC 1.15.1.1) activity was quantified measuring absorbance change due to the inhibitory effect of SOD on the reduction of the cytochrome c Fe+3 by the superoxide anion at 500 nm [32]. Catalase (CAT, EC 1.11.1.6) activity was determined by recording the decrease in absorbance at 240 nm using H2O2 (ξ = 40 M−1 cm−1) in 50 mM phosphate buffer pH 7.0 [33]. Glutathione peroxidase (GPx, EC 1.11.1.9) activity was recorded at 340 nm following the oxidation of NADPH (ξ = 6.22 mM−1 cm−1) during the formation of reduced glutathione (GSH) by commercial glutathione reductase (1 U/mL) using H2O2 as a substrate, and sodium azide (CAT inhibitor) in phosphate buffer pH 7.5 [34]. Glutathione reductase (GR, EC 1.11.1.9) activity was measured at 340 nm following the oxidation of NADPH by oxidized glutathione (GSSG) in phosphate buffer pH 7.5 [34]. Glutathione-S-transferase (GST, EC 2.5.1.18) activity was assayed by measuring the increase in absorbance at 340 nm of the complex of GSH and 1-chloro-2.4 dinitrobenzene (ξ=9.6 mM−1 cm−1) in phosphate buffer pH 6.5 [35]. Total enzyme activity was expressed in terms of μmoles substrate converted to product min-1 (U units) per mg of total protein. Results were expressed as mean with their respective standard deviations (SD).
2.7. Reduced glutathione (GSH)
The GSH levels were evaluated following [36], using 0.5 mM of 5,5-dithiobis-2-nitrobenzoic acid (DTNB), after precipitating proteins with trichloroacetic acid (50 % w/v). The absorbance was measured at 412 nm and GSH standard was used. The GSH concentrations were expressed in μg GSH per milligram of proteins.
2.8. Thiobarbituric acid reactive substances (TBARS) and total proteins
TBARS were estimated following the protocol of [37] using 1,1,3,3 tetraethoxypropane (TEP) as the standard. TBARS, mostly MDA, are the end product of lipid peroxidation and are widely used as a biomarker of oxidative stress. TBARS were normalized to total protein concentrations. Total proteins were quantified by the method of [38] using bovine serum albumin as standard.
2.9. Statistical analysis
Two factor analysis of variance (two-way ANOVA) was used to examine the effects of the WSF-UVCO concentrations on the activities of antioxidant enzymes, GST, GSH and TBARS concentrations in digestive gland, gills and adductor muscle. Data were tested for normality distribution (Shapiro test), homogeneity of variances (Bartlett test) and presence of outliers. Multiple comparisons among mean values used Duncan’s method [39]. The data were subjected to statistical analysis using Statgraphycs Plus 5.1 version for Windows. Statistical significance was defined as p < 0.05.
3. Results
3.1. Chemical analyses
All the chromatograms showed a large contribution of the chromatographically unresolved mixture (UCM) that is characteristic of petroleum derived products. There were resolved peaks of high abundance (>170) in the chromatograms of the acidic organic extracts. More than 80 compounds were identified from the mass spectra of the different extracts. Most could be included in six groups: phenols; benzaldehydes; benzylic alcohols, amines, carboxylic acids and esters, and miscellaneous compounds (Table 1). The exanimated solution of WSF-UVCO contained elevated concentrations of Mg, Zn, Pb, Ni, but light concentrations in other metallic elements such as Al, Fe, Ba, Cd, Cr and Cu (Table 2).
Table 1.
The organic chemicals from a water-soluble fraction of used vehicle crankcase oils (WSF-UVCO) by gas chromatography and mass spectrometry (GC/MS) (Table 1).
| Phenols | Benzaldehydes | Benzylic alcohols | Amines | Carboxylic acids and esters | Miscellaneous compounds |
|---|---|---|---|---|---|
| Organic compounds | Methyl-benzoaldehyde | Benzylic alcohols | C2 pyrines | Benzoic acid | Naphthalene |
| Phenol | Dimethyl-benzoaldehyde | C1, C2, C3 derivatives | Anilines | Methyl derivatives | Methylnaphthalene |
| 2-methyl-phenol | Trimethyl-benzoaldehyde | Hydroxyl-benzadehydes | C1, C2, and C3 | Hexanodioc acids | 2-Hydroxy-benzaldehyde |
| Dimethyl-phenol | Propyl-benzoaldehyde | Anilines | (mono and diesters) | 4-hydroxy-benzadehyde | |
| Trimethyl-phenol | C1 carbazoles | Phthalates | |||
| Tetramethyl-phenol | |||||
| Nitrophenols |
Table 2.
Metal concentration in water-soluble fraction of used vehicle crankcase oils (WSF-UVCO) by conductive plasma spectrometry.
| Metal | Mg | Zn | Pb | Ni | Al | Fe | Ba | Cd | Cr | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 843.0 | 5.95 | 0.60 | 0.14 | <0.20 | 0.09 | <0.02 | 0.02 | <0.02 | <0.01 |
3.2. Antioxidant defenses
The two-way ANOVA indicated that significant variations were observed in antioxidant defenses and oxidative damage of lipids between the several soft-tissues (Fst) and sublethal concentrations (Fsc). Additionally, there were significant interactions (Fsi) between the assessed factors for the majority of biomarkers. Fig. 1 reports the observed SOD and CAT activity in several soft-tissues. The basal activity of SOD in the tissues from control organisms was very similar, but differed significantly in organisms exposed to WSF-UVCO; this activity was highest in digestive gland and gills in comparison to adductor muscle (Fst = 9.44; p < 0.001). Specifically, SOD activity was increased in digestive gland of organisms exposed to 0.01 % and 0.1 %, and in gills of scallops at all WSF-UVCO exposure levels (Fsc = 4.70; p < 0.001). SOD levels did not change in the adductor muscle with respect to control group (Fig. 1A). The interaction between soft-tissues and exposure concentrations was not observed (Fsi = 2.51; p > 0.05).
Fig. 1.
Activity of superoxide dismutase (A) and catalase (B) from control and WSF-UVCO treated flame scallop during 7 d. Results are expressed as the mean ± SD (N = 8). The enzyme activity was expressed in terms U units (μmoles substrate converted to product min−1 per mg of protein). Letters are shown above bars when there was significant statistical difference between groups.
When CAT activity was compared among soft-tissues, the highest levels were reached in gills and digestive gland (Fst = 44.73; p < 0.001). The statistical analysis showed differences among exposure concentrations (Fsc = 3.60; p < 0.05). If contrasted independently in each organ, CAT activity is seen to rise in digestive gland of organisms exposed to 0.1 % WSF-UVCO, and in adductor muscle decreased after exposure to 0.1 %. On the other hand, CAT activity in the gills increased in organisms exposed to highest concentration of WSF-UVCO, which became 2.5 times higher than the values in control organisms (Fig. 1B). A significant interaction was observed between soft-tissues and exposure concentrations (Fsi = 4.82; p < 0.01).
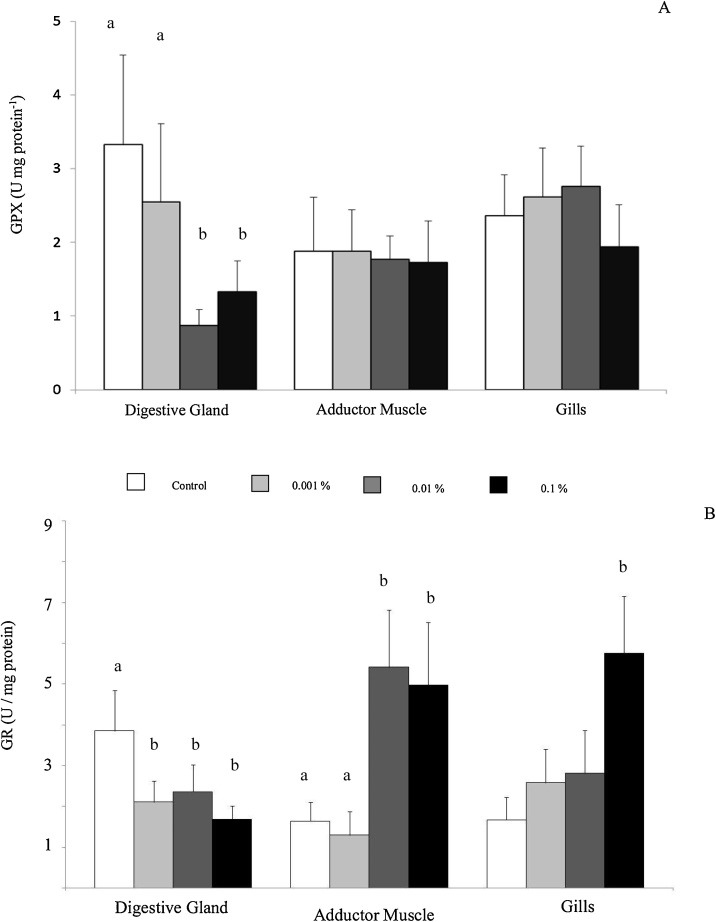
Fig. 2 shows GPx and GR activities in several soft-tissues. Digestive gland of the control organisms had the highest levels of GPx in contrast with other organs, but the inter-tissue variations were not statistically significant (Fst = 2.85; p > 0.05). GPx activity decreased markedly in digestive gland of organisms exposed to 0.01 and 0.1 % of WSF-UVCO (Fsc = 3.31; p < 0.05); but not in adductor muscle or gills (Fig. 2A). Nevertheless, GPx activity did not show statistical interaction between studied factors (Fsi = 2.21; p > 0.05). Digestive gland GR activity dropped in organisms exposed to all concentrations of WSF-UVCO (Fsc = 9.07; p < 0.001). Contrary, GR activity of the adductor muscle increased in organisms exposed to 0.01 and 0.1 %. GR activity only increased in gills of in organisms exposed 0.1 % of WSF-UVCO (Fig. 2B). Both factors showed a statistical interaction (Fsi = 5.88; p < 0.001).
Fig. 2.
Activities of and glutathione peroxidase (A) y glutathione reductase (B) and glutathione-S-transferase (B) from control and crankcase oil treated flame scallop during one week. Results are expressed as the mean ± SD (N = 8). The enzyme activity was expressed in terms U units (μmoles substrate converted to product min−1 per mg of protein). Letters are shown above bars when there was significant statistical difference between groups.
Average activities of GST activities were similar among soft-tissues (Fst = 1.67; p > 0.05). GST activity in the digestive gland increased at the highest exposure concentration (0.1 %). In the gills GST activity rose in organisms exposed to 0.01 and 0.1 % of WSF-UVCO (Fsc = 3.55; p < 0.01). Adductor muscle GST activity did not change (Fig. 3). The soft-tissues and exposure concentrations showed significant a statistical interaction (Fsi = 3.30; p < 0.05).
Fig. 3.
Activities of glutathione-S-transferase from control and crankcase oil treated flame scallop during one week. Results are expressed as the mean ± SD (N = 8). The enzyme activity was expressed in terms U units (μmoles substrate converted to product min−1 per mg of protein). Letters are shown above bars when there was significant statistical difference between groups.
Fig. 4 shows GSH and TBARS concentrations from several soft-tissues. GSH levels were higher in the digestive gland than in gills and adductor muscle (Fst = 53.99; p < 0.001). However, no changes in GSH levels occurred with exposure to WSF-UVCO (Fsc = 2.83; p > 0.05) (Fig. 4A). TBARS concentrations in the soft-tissues showed statistical difference (Fst = 13.35; p < 0.001), with highest levels in the digestive gland. The digestive gland and adductor muscle showed a slight tendency to rise, but not significant, with WSF-UVCO exposure (Fig. 4B) (Fsc = 0.40; p > 0.05).
Fig. 4.
Reduce glutathione (A) and thiobarbituric acid reactive substances (B) in control and crankcase oil treated flame scallop during one week. Letters are shown above bars when there was significant statistical difference between groups (p < 0.05).
4. Discussion
The WSF-UVCO tested contained a mixture of pro-oxidant chemicals, including several hydrocarbons and heavy metals that could be readily taken up in C. scaber by filtration. It is well known that scallops are able to accumulate rapidly significant amounts of organic and inorganic xenobiotics in their soft-tissues [[40], [41], [42]]. The metabolic transformation of most of substances present in WSF-UVCO can promote generation of ROS leading to multiple biochemical alterations [6,7,43,44]. In this study, we found the strongest responses of antioxidant defenses in digestive gland and gills, whereas the adductor muscle showed fewer changes. Each soft-tissue in C. scaber showed differential modulations, with particularly pronounced enzymatic responses (elevations or descents) to the WSF-UVCO exposure.
The digestive gland of scallops is the site of multiple oxidative reactions and can be a place of important ROS generation, particularly when the organism faces chemical stress by contaminants [45]. In this organ, several reactive molecules such as superoxide anion (●O2) and hydrogen peroxide (H2O2) can be formed through redox cycling of xenobiotics from WSF-UVCO [46]. To avoid oxidative alterations, the increased SOD and CAT activities could attenuate the excessive production of oxyradicals. In first place, SOD is capable of detoxifying ●O2 by means of their efficient dismutation to form H2O2 [47]. Although H2O2 is not a free radical, its formation can lead to the production of other free radicals and cause damage to the cell when transition metal ions are present [47]. The overproduction of H2O2 is countered by an increase of CAT activity, which it is part of biochemical adjustments against the ROS generation in the digestive gland. Similarly, other authors had demonstrated that SOD and CAT activities increase in diverse invertebrate’s species such as gastropod [48], mussels [49], clam [50] and scallops [51] exposed to heavy metals and mixtures of polyaromatic hydrocarbons under acute exposure.
The diminished activity of GPx, parallel to GR, in the digestive gland of organisms exposed to the highest concentrations of WSF-UVCO can limit the mechanisms for removal of H2O2 and lipid hydroperoxides. These changes could concomitantly reduce availability of GSH to be used directly or indirectly through enzymatic reactions in defenses against ROS [15,46,52]. The inhibition of these antioxidant enzymes may have allowed the ROS injury of membrane lipids, evidenced by the small increase of MDA concentrations in organisms exposed to the highest concentrations of WSF-UVCO; MDA is accumulating due to an oxidative stress condition [53]. The decrease of GPx and GR activities could be caused by the exposure to several types of hydrocarbons and high concentrations of heavy metals. A variety of petroleum derived xenobiotics present in the WSF-UVCO may be sequestered and tolerated in the digestive gland of bivalves [[54], [55], [56]], being able to interfere in the normal functions of antioxidant defenses [57,58,59].
Mixtures of organic and inorganic xenobiotics may lead to uncontrolled propagation of radical reactions causing harmful oxidative effects, changes in biochemical composition and energetic capacity of organisms exposed to oils [29,59,60]. It is known that significant changes of GPX and GR can decrease the levels of GSH in relation to oxidized glutathione (GSSG) in organisms under xenobiotic exposure [61,62]. In vivo studies have demonstrated that maintenance of GR activity and its final reaction product (GSH) are crucial for detoxification of ROS in mussels [63] and oysters [64]. We assume that concomitant increases of SOD and CAT activities, and overall stability of GSH levels, may represent possible compensation in the total antioxidant capacity for ROS elimination in the digestive gland; being these biochemical adjustments enough to prevent exacerbate oxidative injury.
The increased GST activity in the digestive gland of WSF-UVCO exposed organisms is clear signal of the metabolic activation and high capacity for xenobiotic detoxification [[65], [66], [67]]. The augmented activity of GST seems is a typical response in hepatopancreas of organisms exposed to different xenobiotics. In several bivalve species, the ability to metabolize and excrete polycyclic aromatic hydrocarbon through phase I and II reactions has been demonstrated [[67], [68], [69]]. The GST bind to GSH with xenobiotic metabolites derived of phase I reactions (NADPH cytochrome P450 reductase), transforming them into water soluble and easily excretable products [70]. Recently some authors suggested that GST also functions as an antioxidant enzyme by conjugating breakdown products of lipid peroxides to GSH [71], helping to avoid exacerbating MDA levels in this organ. It is well-known that MDA content of the digestive gland, in comparison to other examined organs, is due to high metabolic capacity and important role it plays in xenobiotic detoxification.
The adductor muscle facilitates opening and closing of the valves in scallops [72], having a key function to regulate the entrance of contaminant to soft-tissues. This organ exhibited weaker responses to xenobiotic exposure; apparently the ROS production and antioxidant defenses of adductor muscle in bivalve species are relatively limited [73]. In this tissue, CAT activity declined with higher levels of exposure to WSF-UVCO, which could disrupt the balance between antioxidant/prooxidant systems and increase susceptibility to oxidative stress. It is known that the rupture of H2O2 in presence of metals found in the WSF-UVCO, could force to hydroxyl radical formation (•OH) through of Fenton reaction [74]. Contrarily, GR activity rose at moderate and higher levels of exposure. We suggest that the lower CAT function to eliminate ROS could be compensated by the increase in the GR activities.
GR of adductor muscle in scallops under exposure WSF-UVCO reflects the ROS buffering function of GSH during chemical stress. GSH-dependent antioxidant reactions are likely eased when increased GR activity, which maintains the intracellular GSH concentrations to control flux of ROS [75]. The overall stability of GSH levels suggests that maintenance of this protective molecule was prioritized during exposure to the xenobiotics. GSH is considered as a primary line of defense in the detoxification of heavy metals and organic xenobiotics, and their physiological function depends on a cysteine residue which is a crucial in maintaining the reducing environment of the cell [75]. In spite of the decline in CAT activity, no oxidative damage in lipids was apparent in adductor muscle, possibly because GR and GSH may support to metabolize indeed the ROS production. These variations had been characterized in other bivalves faced to oxidative stress situations.
The exposure to WSF-UVCO led to marked responses of the antioxidant system in the gill. The activities of SOD, CAT and GR were increased at moderate and high levels of exposure, which suggest that in this tissue there is an antioxidant biochemical machinery to combat effectively to ROS under oxidative stress condition. The gills are one of the first organs that enter in contact to the mixture of pollutants, and their intracellular metabolism should provide a first line of antioxidant defense [76]. Also, the scallop gills are large leaf-like organs used for respiration and for filtering food, and during these processes large volumes of water pass through making them highly susceptible to passive assimilation of bioavailable contaminants in WSF-UVCO [41]. Similar to digestive gland, the SOD and CAT activities of gill were elevated in WSF-UVCO exposed organisms, without oxidative damage evidenced. Possibly the enhanced antioxidant enzyme activities may serve as a protective response eliminating reactive free radicals and help to resist the damage from exposure to WSF-UVCO. [50] reported stimulation in the antioxidant defenses during the detoxification in gills of clams exposed to PAH during 15 days.
Another interesting result is the increased activities of GST in the digestive gland and gills of organisms exposed to WSF-UVCO. This rise may indicate the capacity of the tissue for biotransformation and excretion during exposure to WSF-UVCO. GST carries out thiol-conjugation and neutralizes with great avidity via detoxification reactions to xenobiotics, avoiding the oxidative damage of the biological membrane. Several authors had reported elevated GST activity and increased ROS production in other species of bivalves as result of exposure to xenobiotic mixtures coming from petroleum derived compounds [51,77,78]. The not variation of GST activity in the abductor muscle could be associated to a low capacity by xenobiotic detoxification in comparison to other organs with elevated metabolic functions. In summary, we demonstrated high sensitivity of antioxidant enzymes from flame scallops exposed to complex mixtures of xenobiotics, indicating that they may be useful for detecting changes in the redox status in organisms that inhabit sites impacted by recent spills of crankcase oil. These findings should stimulate further research on toxicological risk of contamination by waste oil in marine benthic ecosystems.
Additionally, the biomarker of oxidative stress measures in C. scaber are phylogenetically conserved biochemical responses, which may be extrapolated to monitor the potential alterations in tissue redox profile of other phyla exposed to subletal concentrations of mixtures of organic and inorganic xenobiotics; the role of antioxidant responses have been correlated to effects of acute conditions that lead to disruption of tissue redox status [79].
5. Conclusion
The tissues of C. scaber show diverse metabolic and molecular defenses against oxyradical generation by redox cycling metabolism of xenobiotics. We found that exposure to chemical mixtures in WSF-UVCO resulted in more marked of antioxidant defenses in digestive gland and gill of C. scaber than in adductor muscle. The antioxidant system seems be highly sensitive to low doses of hazardous xenobiotics, which may protect to the soft-tissues against short-term effects of ROS production, and partly avoided increases in lipoperoxidation products. Given these results, we suggest C. scaber as biological sensor for screening pollutant impacts during environmental monitoring studies along the Caribbean coastline.
Data availability statement
All data, tables and figures in this manuscript used to support the findings of this study are original and are available upon request.
Declaration of Competing Interest
The authors reported no declarations of interest.
CRediT authorship contribution statement
Edgar Zapata-Vívenes: Conceptualization, Methodology, Resources, Writing - review & editing. Osmar Nusetti: Conceptualization, Writing - review & editing. Leida Marcano: Writing - review & editing. Gabriela Sánchez: Conceptualization. Helga Guderley: Conceptualization, Writing - review & editing.
Acknowledgement
The study was supported by the Science Coordination Office of Oriente University (UDO) from Venezuela.
References
- 1.Velasco-Santamaría Y.M., Corredor-Santamaría W., Torres-Tabares A. Environmental pollution by hydrocarbons in Colombia and its impact on the health of aquatic ecosystems. In: Gómez-Oliván L.M., editor. Pollution of Water Bodies in Latin America. Springer; Cham: 2019. p. 364. [DOI] [Google Scholar]
- 2.Olonisakin A., Adebayo A.O., Aremu M.O. Metal concentrations of fresh, used and treated crankcase oil. Biosci. Biotech. Res. Asia. 2005;3:187–191. http://www.biotech-asia.org/?p=4361 Available from: [Google Scholar]
- 3.Cvengros J., Liptaj T., Pónayová N. Study of polyaromatic hydrocarbons in current used motor oils. Int. J. Petrochem. Sci. Eng. 2017;2(7):219–226. doi: 10.15406/ipcse.2017.02.00060. [DOI] [Google Scholar]
- 4.Nowak P., Kucharska K., Kaminski M. Ecological and health effects of lubricant oils emitted into the environment. Intern. Int. J. Environ. Res. Public Health. 2019;16:3002. doi: 10.3390/ijerph16163002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nusetti O., Zapata-Vívenes E., Esclapés M.M., Rojas A. Antioxidant enzymes and tissue regeneration in Eurythoe complanata (Polychaeta: Amphinomidae) exposed to used vehicle crankcase oil. Arch. Environ. Contam. Toxicol. 2005;48:1–9. doi: 10.1007/s00244-004-0041-0. [DOI] [PubMed] [Google Scholar]
- 6.Jiang F., Zhang L., Yang B., Zheng L., Sun C. Biomarker responses in the bivalve Chlamys farreri to the water-soluble fraction of crude oil. Chin. J. Oceanol. Limnol. 2015;33:853–861. doi: 10.1007/s00343-015-4109-7. [DOI] [Google Scholar]
- 7.Freitas J.S., Boscolo-Pereira T.S., Pereira-Boscolo C.N., Navarro-García M., de Oliveira-Rivero C.A., De Almeida E.A. Oxidative stress, biotransformation enzymes and histopathologial alterations in nile tilapia (Orechromis niloticus) exposed to new and used automovile lubricant oil. Comp. Biochem. Physiol. 2020;234:1–11. doi: 10.1016/j.cbpc.2020.108770. [DOI] [PubMed] [Google Scholar]
- 8.Ray P.D., Huang B.W., Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris C., Hansen J.M. Oxidative stress, thiols, and redox profiles. Methods Mol. Biol. 2012;889:325–346. doi: 10.1007/978-1-61779-867-2_21. [DOI] [PubMed] [Google Scholar]
- 10.Regoli F., Giuliani M.E. Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar. Environ. Res. 2014;93:106–117. doi: 10.1016/j.marenvres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Sies H., Berndt C., Jones D.P. Oxidative stress. Annual Rev. Biochem. 2017;86(1):715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 12.Abele D., Vázquez-Medina J.P., Zenteno-Savín T. Wiley-Blackwell Publishing Ltd; London: 2011. Introduction to Oxidative Stress in Aquatic Ecosystems: Oxidative Stress in Aquatic Ecosystems. 548. [DOI] [Google Scholar]
- 13.Hellou J., Ross N.W., Moon T.W. Glutathione, glutathione S-transferase, and glutathione conjugates, complementary markers of oxidative stress in aquatic biota. Environ. Sci. Pollut. Res. 2012;19:2007–2023. doi: 10.1007/s11356-012-0909-x. [DOI] [PubMed] [Google Scholar]
- 14.Allocati N., Masulli M., Di Ilio C., Federici L. Glutathione transferases: substrates, inhibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018;7(8):1–15. doi: 10.1038/s41389-017-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aquilano K., Baldelli S., Ciriolo M.R. Glutathione: new roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014;5:196. doi: 10.3389/fphar.2014.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hea L., Hea T., Farrarb S., Jia L., Liua T., Maa X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44:532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 17.Bennedetti M., Giuliani M.E., Regoli F. Oxidative metabolism of chemical pollutants in marine organisms: molecular and biochemical biomarkers in environmental toxicology. Ann. NY Acad. Sc. 2015;13401:8–19. doi: 10.1111/nyas.12698. [DOI] [PubMed] [Google Scholar]
- 18.Breitwieser M., Viricel A., Graber M., Murillo L., Becquet V., Churlaud C., Fruitier-Arnaudin I., Huet V., Lacroix C., Pante E., Le Floch S., Thomas-Guyon H. Short-term and long-term biological effects of chronic chemical contamination on natural populations of a marine bivalve. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhagat J., Ingole B.S., Singh N. Glutathione S-transferase, catalase, superoxide dismutase, glutathione peroxidase, and lipid peroxidation as biomarkers of oxidative stress in snails: a review. Invertebrate Surviv. J. 2016;13(1):336–349. doi: 10.25431/1824-307X/isj.v13i1.336-349. [DOI] [Google Scholar]
- 20.Shahidul I.M., Tanaka M. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar. Pollut. Bull. 2004;48(7-8):624–649. doi: 10.1016/j.marpolbul.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Suárez-Ulloa V., Fernández-Tajes J., Manfrin C., Gerdol M., Venier P., Eirín-López J.M. Bivalve omics: state of the art and potential applications for the biomonitoring of harmful marine compounds. Mar. Drugs. 2013;11(11):4370–4389. doi: 10.3390/md11114370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodeiros C.J., Freites L., Alió J.J., Nuñez M., Himmelman H. Scallop aquaculture and fisheries in Venezuela. Developments in Aquaculture and Fisheries Science. 2016;40:1073–1087. doi: 10.1016/B978-0-444-62710-0.00027-4. [DOI] [Google Scholar]
- 23.Dougherty L.F., Jingchun L. Molecular phylogeny and morphological distinctions of two popular bivalves, Ctenoides scaber and Ctenoides mitis. J. Mar. Biol. 2017;1624014:1–9. doi: 10.1155/2017/1624014. [DOI] [Google Scholar]
- 24.Mikkelsen P.M., Bieler R. Systematic revision of the western Atlantic file clams, Lima and Ctenoides(Bivalvia:Limoida:Limidae) Invert. System. 2003;17:667–710. doi: 10.1071/IS03007. [DOI] [Google Scholar]
- 25.Lodeiros C.J., Himmelman J.H. Reproductive cycle of the bivalve Lima scabra(Pterioida: Limidae) and its association with environmental conditions. Rev. Biol. Trop. 1999;47:411–418. [Google Scholar]
- 26.Dukeman A.K., Blake N.J., Arnold W.S. The reproductive cycle of the flame scallop,Ctenoides scaber (Born 1778), from the lower Florida keys and its relationship with environmental. J. Shell. Res. 2005;24(2):341–351. doi: 10.2983/0730-8000(2005)24[341:TRCOTF]2.0.CO;2. [DOI] [Google Scholar]
- 27.Beninger P.G., Le Pennec M. Developments in Aquaculture and Fisheries Science. 2016. Scallop structure and function; pp. 85–159. [DOI] [Google Scholar]
- 28.Stephan C.E., Brush K.A., Smith R., Burke J., Andrew R.W. APHA 1998. Standard Methods for the Examination of Water and Waste Water. 20th ed. American Public Health Association (APHA), American Water Works Association (AWWA), AND Water Pollution Control Federation (WPCF); New York: 1978. A computer program for calculating an LC50, (1978) U.S.E.P.A, Duluth, Minnesota. [Google Scholar]
- 29.Zapata-Vívenes E., Sánchez G., Marcano L. Energetic reserves and molecular index of condition in Lima (Ctenoides) scabra exposed to crankcase oil. Zoo. Trop. 2015;33:37–45. (in Spanish with English abstract) [Google Scholar]
- 30.American Society for Testing and Materials (ASTM) West Conshohocken; Pennsylvania, USA: 2002. Standard Guide for Conducting Acute Toxicity Tests on Test Materials With Fishes, Macroinvertebrates, and Amphibians; pp. 729–796. [Google Scholar]
- 31.ASAB/ABS Guidelines for the treatment of animals in behavioral research and teaching. Animal Behav. 2018;135:1–10. doi: 10.1016/j.anbehav.2017.10.0010. [DOI] [Google Scholar]
- 32.McCord J.M., Fridovich I. Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein) J. Biol. Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 33.Aebi H. 1984. Catalase in Vitro; pp. 121–126. [DOI] [PubMed] [Google Scholar]
- 34.Nusetti O., Esclapés M.M., Nusetti S., Zapata E., Marcano L., Lodeiros C. Antioxidant defenses and oxidative stress in the marine bivalve Pinctada imbricata exposed to sublethal levels of fuel oil no 6. Interciencia. 2004;2:1–6. (In Spanish with English abstract) [Google Scholar]
- 35.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases-first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 36.Moron M.S., DePierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 37.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Rev. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 38.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 39.Sokal R., Rohlf J. fourth edition. WH Freeman and Company; San Francisco: 2012. Biometry: the Principles and Practice of Statistics in Biological Research. [DOI] [Google Scholar]
- 40.Metian M., Busimante P., Hedouin L., Warnau M. Accumulation of trace elements in the tropical scallop Comptopallium radula from coral reefs in New Caledonia. Environ. Pollut. 2008;152:543–552. doi: 10.1016/j.envpol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Marsden I.D., Cranford P.J. Scallops and marine contaminants. In: Shumway S.E., Parsons G.J., editors. Developments in Aquaculture and Fisheries Science. 3th ed. Elsevier Science; 2016. pp. 567–580. [Google Scholar]
- 42.Xiu M., Pan L., Lin P., Zheng L. The detoxification responses, damage effects and bioaccumulation in the scallop Chlamys farreri exposed to single and mixtures of benzo[a]pyrene and chrysene. Comp. Biochem. Physiol. 2016;191:36–51. doi: 10.1016/j.cbpc.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Klotz L.O., Steinbrenner H. Cellular adaptation to xenobiotics: interplay between xenosensors, reactive oxygen species and FOXO transcription factors. Redox Biol. 2017;13:646–654. doi: 10.1016/j.redox.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang F., Zhang L., Yang B., Zheng L., Sun C. Biomarker responses in the bivalve Chlamys farreri to the water-soluble fraction of crude oil. Chin. J. Oceanol. Limnol. 2015;33:853–861. doi: 10.1007/s00343-015-4109-7. [DOI] [Google Scholar]
- 45.Madkour L.H. Nanoparticles induce oxidative and endoplasmic reticulum stresses. Nanomed. Nanotoxicol. 2020 doi: 10.1007/978-3-030-. [DOI] [Google Scholar]
- 46.Abele D., Vázquez-Medina J.P., Zenteno-Savín T. Wiley-Blackwell Publishing Ltd; London: 2011. Introduction to Oxidative Stress in Aquatic Ecosystems: Oxidative Stress in Aquatic Ecosystems. 548. [DOI] [Google Scholar]
- 47.Lushchak V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011;101:13–30. doi: 10.1016/j.aquatox.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Aljahdali M.O., Alhassan A.B. Metallic Pollution and the Use of Antioxidant Enzymes as Biomarkers in Bellamya unicolor(Olivier, 1804) (Gastropoda: Bellamyinae) Water. 2020;12:202. doi: 10.3390/w12010202. [DOI] [Google Scholar]
- 49.Boudjema K., Kourdali S., Bounakous N., Meknachi A., Badis A. Catalase activity in brown mussels (Perna perna) under acute cadmium, lead, and copper exposure and depuration tests. J. Mar. Sci. 2014;830657:1–9. doi: 10.1155/2014/830657. [DOI] [Google Scholar]
- 50.Zhang H., Pan L., Tao Y. Antioxidant responses in clam Venerupis philippinarum exposed to environmental pollutant hexabromocyclododecane. Environ. Sci. Pollut. Res. 2014;21:8206–8215. doi: 10.1007/s11356-014-2801-3. [DOI] [PubMed] [Google Scholar]
- 51.Guo R., Pan L., Ji R. A multi-biomarker approach in scallop Chlamys farreri to assess the impact of contaminants in Qingdao coastal area of China. Ecotoxicol. Environ. Saf. 2017;142:399–409. doi: 10.1016/j.ecoenv.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 52.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurutas E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J. 2016;15 doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sureda A., Tejada S., Box A., Deudero S. Polycyclic aromatic hydrocarbon levels and measures of oxidative stress in the Mediterranean endemic bivalve Pinna nobilis exposed to the Don Pedro oil spill. Mar. Pollut. Bull. 2013;71(1-2) doi: 10.1016/j.marpolbul.2013.03.033. 201369-73. [DOI] [PubMed] [Google Scholar]
- 55.Sardi A.E., Sandrini-Neto L., Pereira Lda S., Silva de Assis H., Martins C.C., Lana Pda C., Camus L. Oxidative stress in two tropical species after exposure to diesel oil. Environ. Sci. Pollut. Res. 2016;23(20):20952–20962. doi: 10.1007/s11356-016-7280-2. [DOI] [PubMed] [Google Scholar]
- 56.Saavedra Y., González A., Blanco J. Anatomical distribution of heavy metals in the scallop Pecten maximus. Food Addit. Contam. 2008;25:1339–1344. doi: 10.1080/02652030802163398. [DOI] [PubMed] [Google Scholar]
- 57.Geret F., Serafim A., Barreira L., Bebianno M.J. Effect of cadmium on antioxidant enzyme activities and lipid peroxidation in the gills of the clam Ruditapes decussates. Biomarkers. 2002;7(3):242–256. doi: 10.1080/13547500210125040. [DOI] [PubMed] [Google Scholar]
- 58.Breitwieser M., Viricel A., Graber M., Murillo L., Becquet V., Churlaud C., Fruitier-Arnaudin I., Huet V., Lacroix C., Pante E., Le Floch S., Thomas-Guyon H. Short-term and long-term biological effects of chronic chemical contamination on natural populations of a marine bivalve. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0150184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trevisan R., Flesch S., Mattos J.J., Milani M.R., Bainy A.C., Dafre A.L. Zinc causes acute impairment of glutathione metabolism followed by coordinated antioxidant defenses amplification in gills of brown mussels Perna perna. Comp. Biochem. Physiol. 2013;159:22–30. doi: 10.1371/journal.pone.0150184. [DOI] [PubMed] [Google Scholar]
- 60.Sardi A.E., Renaud P.E., Morais G.C., Martins C.C., da Cunha-Lana P., Camus L. Effects of an in-situ diesel oil spill on oxidative stress in the clam Anomalocardia flexuosa. Environ. Pollut. 2017;230:891–901. doi: 10.1016/j.envpol.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 61.Sureda A., Box A., Tejada S., Blanco A., Caixach J., Deudero S. Biochemical responses of Mytilus galloprovincialis as biomarkers of acute environmental pollution caused by the Don Pedro oil spill (Eivissa Island, Spain) Aquat. Toxicol. 2011;101(3-4):540–549. doi: 10.1016/j.aquatox.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 62.Jiang F., Zhang L., Yang B., Zheng L., Sun C. Biomarker responses in the bivalve Chlamys farreri to the water-soluble fraction of crude oil. Chin. J. Oceanol. Limnol. 2015;33:853–861. doi: 10.1007/s00343-015-4109-7. [DOI] [Google Scholar]
- 63.Torres M.A., Testa C.P., Gaspari C., Masutti M.B., Panitz C.M.N., Curi-Pedrosa R., Almeida E.A., Mascio P.D., Filho D.W. Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island, Brazil. Mar. Pollut. Bull. 2002;44:923–932. doi: 10.1016/S0025-326X(02)00142-X. [DOI] [PubMed] [Google Scholar]
- 64.Trevisan R., Mello D.F., Delapedra G., Silva D.G., Arl M., Danielli N.M., Metian M., Almeida E.A., Dafre A.L. Gills as a glutathione-dependent metabolic barrier in pacific oysters Crassostrea gigas: absorption, metabolism and excretion of a model electrophile. Aquat. Toxicol. 2016;173:105–119. doi: 10.1016/j.aquatox.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 65.Henry M., Boucaud-Camou E., Lefort Y. Functional micro-anatomy of the digestive gland of the scallop Pecten maximus (L.) Aquat. Living Resour. 1991;4(3):191–202. doi: 10.1051/alr:1991021. [DOI] [Google Scholar]
- 66.Hoarau P., Garello G., Gnassia-Barelli M., Roméo M., Girard J.P. Effect of three xenobiotic compounds on Glutathione S-Transferase in the clam Ruditapes decussates. Aquat. Toxicol. 2004;68(1):87–94. doi: 10.1016/j.aquatox.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Xia B., Chen B., Sun X., Cui Y., Zhao J., Qu K. Toxicological effects of crude oil: integrated biomarker responses in the hepatopancreas of clam Ruditapes philippinarum. Asian J. Chem. 2014;26(12):3631–3638. doi: 10.14233/ajchem.2014.16736. [DOI] [Google Scholar]
- 68.Frouin H., Pellerin J., Fournier M., Pelletier E., Richard P., Pichaud N., Garnerot F. Physiological effects of polycyclic aromatic hydrocarbons on soft-shell clam Mya arenaria. Aquat. Toxicol. 2007;82:120–134. doi: 10.1016/j.aquatox.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Liu D., Pan L., Li Z., Cai Y., Miao J. Metabolites analysis, metabolic enzyme activities and bioaccumulation in the clam Ruditapes philippinarum exposed to benzo[a]pyrene. Ecotoxicol. Environ. Saf. 2014;107:251–259. doi: 10.1016/j.ecoenv.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 70.Yao L., Pan L., Guo R., Miao J. Expression profiles of different glutathione S-transferase isoforms in scallop Chlamys farreri exposed to benzo[a]pyrene and chrysene in combination and alone. Ecotoxicol. Environ. Saf. 2018;142:480–488. doi: 10.1016/j.ecoenv.2017.04.050. [DOI] [PubMed] [Google Scholar]
- 71.Singhal S.S., Singh S.P., Singhal P., Horne D., Singhal J., Awasthi S. Antioxidant role of glutathione S-transferases: 4-hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015;289:361–370. doi: 10.1016/j.taap.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chantler P.D. Developments in Aquaculture and Fisheries Science. 2006. Scallop adductor muscles: structure and function; pp. 229–316. Chapter 4. [DOI] [Google Scholar]
- 73.Guerra C., Zenteno-Savín T., Maeda-Martínez A.N., Abele D., Philipp E.E.R. The effect of predator exposure and reproduction on oxidative stress parameters in the Catarina scallop Argopecten ventricosus. Comp. Biochem. Physiol. 2013;165(1):89–96. doi: 10.1016/j.cbpa.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 74.Halliwell B., Gutteridge J.M.C. Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death. In: Halliwell B., Gutteridge J.M.C., editors. Free Radicals in Biology and Medicine. 4th ed. Oxford University Press; New York: 2007. pp. 187–267. [Google Scholar]
- 75.Dickinson D.A., Forman H.J. Cellular glutathione and thiols metabolism. Biochem. Pharmacol. 2002;64(5-6):1019–1026. doi: 10.1016/s0006-2952(02)01172-3. [DOI] [PubMed] [Google Scholar]
- 76.(a) Chi C., Giri S.S., Jun J.W., Kim S.W., Kim H.J., Kang J.W., Park S.C. Detoxification- and immune-related transcriptomic analysis of gills from bay scallops (Argopecten irradians) in response to algal toxin okadaic acid. Toxins. 2018;10(8):308. doi: 10.3390/toxins10080308. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yan F., Yang W.K., Li X.Y., Lin T.T., Lun Y.N. A trifunctional enzyme with glutathione S-transferase, glutathione peroxidase and superoxide dismutase activity. Biochim. Biophys. Acta. 2008;1780:869–872. doi: 10.1016/j.bbagen.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Liu N., Pan L., Wang J., Liu D. Application of the biomarker responses in scallop (Chlamys farreri) to assess metals and PAHs pollution in Jiaozhou Bay, China. Mar. Environ. Res. 2012;80:38–45. doi: 10.1016/j.marenvres.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Pan L., Ren J., Zheng D. Effects of benzo(a)pyrene exposure on the antioxidant enzyme activity of scallop Chlamys farreri. Chin. J. Oceanol. Limnol. 2009;27:43–53. [Google Scholar]
- 79.Veskoukis A.S., Tsatsakis A., Kouretas D. Approaching reactive species in the frame of their clinical significance: a toxicological appraisal. Food Chem. Toxicol. 2020;138 doi: 10.1016/j.fct.2020.111206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data, tables and figures in this manuscript used to support the findings of this study are original and are available upon request.