Abstract
Objectives
Osteogenesis imperfecta (OI) is a heterogeneous group of genetic disorders of connective tissue that cause skeletal fragility and extra-skeletal manifestations. Classically, four different types of OI were distinguished. Type 5 OI was added due to its distinct clinical and radiographic features. In 2012, two independent groups identified a recurrent heterozygous c.-14C>T mutation in IFITM5 as the responsible genetic change for this type of OI. To our knowledge, cervical kyphosis has not been identified in the literature as a finding in type 5 OI patients. This is a retrospective review of a cohort of patients with type 5 OI and a description of associated cervical spine deformity.
Methods
After institutional review board approval, a retrospective review identified 13 patients with type 5 OI. Clinical, radiologic, and genetic data from 2002 to 2020 were reviewed.
Results
We identified 13 patients with clinical diagnosis of type 5 OI. Twelve had molecular confirmation and the classic IFITM5, c.14C>T gene mutation was identified. The remaining individual did not undergo genetic testing. Dentinogenesis imperfecta was observed in one patient, while blue sclerae or hearing loss were not present. All patients had at least one fracture and four underwent intramedullary rodding. Radiologic features included subphyseal metaphyseal radiodense line in 12/13 patients (92%), interosseous membrane calcification in seven of 13 patients (54%) (more commonly noted in the upper extremities), and hypertrophic callus in six of 13 patients (46%). Thoracolumbar spinal deformities were seen in six of 13 patients (46%) with two of these individuals requiring surgery. Cervical kyphosis was noted in nine of 13 individuals (69%) ranging in age from 3 months to 22 years. Anterior wedging of the cervical vertebral bodies was noted in the absence of any fractures. Six of nine individuals demonstrated listhesis of C2-C3 or C3-C4 segment. Magnetic resonance imaging studies were performed and reviewed in patients with cervical kyphosis and subluxation; three patients showed narrowing of spinal canal without cervical cord compression and one asymptomatic patient showed impingement of the spinal cord.
Conclusions
Cervical kyphosis appears to be a common feature of type 5 OI. It can be a presenting and apparently life-long association and does not appear to be caused by vertebral body fractures. Evaluation for cervical kyphosis should be performed in patients with a suspected or confirmed diagnosis of type 5 OI. Furthermore, if cervical kyphosis is noted in an individual with OI, type 5 OI should be considered.
Level of evidence: IV.
Keywords: Osteogenesis imperfecta type 5, Cervical kyphosis
Highlights
-
•
Cervical kyphosis appears to be a common feature of type 5 osteogenesis imperfecta.
-
•
Cervical kyphosis does not appear to be caused by vertebral body fractures.
-
•
Cervical evaluation should be consider in osteogenesis imperfecta type 5 patients.
-
•
Consider type 5 osteogenesis imperfecta individuals with cervical kyphosis.
1. Introduction
Osteogenesis imperfecta (OI) is a heterogeneous group of genetic disorders of connective tissue that cause skeletal fragility with fractures and other extra-skeletal manifestations (Forlino and Marini, 2016). Classically, four different types of OI were distinguished on the basis of clinical features with the classification based on Sillence et al.'s (1979) publication entitled “Genetic Heterogeneity in Osteogenesis Imperfecta”.
Type I demonstrates the mildest presentation and typical height, whereas type II is usually lethal in the perinatal period. Type III is the most severe form in children surviving the neonatal period. These patients have a well-defined phenotype including short stature, growth plate abnormalities, and progressive limb and spine deformities secondary to multiple fractures. Patients with a moderate to severe phenotype who do not fit into one of the above categories are classified as type IV.
In 1983, the first genetic cause of OI was identified as an internal deletion the COL1A1 gene (Chu et al., 1983). Over the subsequent years, it has been determined that most cases of OI are caused by heterozygous mutations in COL1A1 or COL1A2. These genes encode two type I procollagen alpha chains, pro-alpha 1 and pro-alpha 2. Mutations in these genes result in quantitative and/or qualitative defects in collagen production and have been detected in the four clinical types described in the original Sillence classification (Forlino and Marini, 2016; Van Dijk and Sillence, 2014; Takagi et al., 2012).
Beginning in 2004, the Sillence classification was expanded with the addition of OI types V through VII with an unknown genetic defect. These types were based on the lack of COL1A1/2 mutations and clinical or histomorphometric criteria. As the genes for these and other forms of OI began to be identified, nomenclature revisions have accordingly seen the numbers of OI types increase up to OI type XIV (Forlino et al., 2011) with the discovery of each new responsible gene. COL1A1/2 mutations still account for the large majority of OI patients, approximating 90% in populations of European origin (Van Dijk and Sillence, 2014; Van Dijk et al., 2012).
In 2011, the updated Nosology of Skeletal Disorders was published and a decision was made to group the known OI syndromes into five phenotypic groups, that is, preserving the primary four groups and adding OI type 5 (Warman et al., 2011). Arabic numerals replaced Roman numerals, as they were meant to represent a specific gene locus (Van Dijk and Sillence, 2014). The importance of the different genetic causes of the OI types was acknowledged by encapsulating the causative genes as subtypes of OI types 1–5 (Warman et al., 2012). Type 5 OI was added due to its distinct clinical and genetic pattern.
Type 5 OI, as it is defined currently, was first recognized in 1908 by Battle and Shattock (1908). They described moderate to severe skeletal fragility with progressive calcification of the inter-osseous membranes in the forearms and legs. Independently, it was identified by increased propensity to develop hyperplastic callus. The syndrome was delineated in some detail by Bauze et al. in 1975 (Bauze et al., 1975). In clinical studies it accounts for approximately 5% of individuals with OI seen in a hospital setting (Van Dijk and Sillence, 2014).
The distinguishing clinical and radiographic features include an autosomal-dominant inheritance pattern, absence of blue sclera, absence of dentinogenesis imperfecta, propensity to hyperplastic callus formation, calcification of the forearm interosseous membrane, radial-head dislocation, and a subphyseal metaphyseal radiodense line. A distinct pathogenesis is further supported by characteristic bone histomorphometry that shows coarse mesh-like lamellation (Glorieux et al., 2000). In 2012, two independent groups identified a recurrent heterozygous c.-14C>T mutation in IFITM5 as the responsible genetic change (Warman et al., 2012; Semler et al., 2012).
The clinical observation of a presence of cervical kyphosis in a known Type 5 OI patient led to a review of the literature and a review of the patterns of cervical spine deformities in a cohort of patients with Type 5 OI.
2. Materials and methods
After institutional review board approval of a retrospective cohort review, 13 type V OI patients were identified. Clinical history was reviewed from 2002 to 2020. Demographics included race, sex, date of birth, date of diagnosis, and family history. Clinical review included craniofacial features, hearing status, sclera, fracture history, ambulatory status, limb/spine deformities, and associated neurologic symptoms. In patients who had surgery, data included anatomic area, procedure performed, and diagnosis. Bisphosphonate treatment history was documented in all patients. A review of all available medical imaging was performed and included axial and appendicular radiographs, magnetic resonance imaging (MRI), computed tomography scan, and dual-energy x-ray absorptiometry. Frequencies and descriptives were used to report the data.
3. Results
Thirteen patients with a clinical diagnosis of type 5 OI were identified. Eight of 13 (61.5%) were female and five (38.5%) were male. Nine of the patients were Caucasian, two were African American, two were Hispanic, and one was Asian. Ten of the 13 patients were diagnosed with OI within the first year of life (average 4 months, range 0–12 months) and three patients after the second year (Table 1). The precise classification of type 5 OI often came later, with 12 of 13 having IFITM5 recurrent heterozygous c.-14C>T mutation. The remaining patient did not have genetic testing performed.
Table 1.
Demographic and clinical features of OI type V patients.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | M | F | M | M | F | M | F | M | F | F | F | F |
| Race | Cau | His | AA | Cau | Cau | Cau | Cau | Cau | Cau | Cau | Cau | AA | Asian |
| Age at diagnosis | 1 y | 8 m | Birth | Birth | Birth | 4 m | 2 y | 4 y | 6 m | Birth | 2 m | Birth | 1 y |
| Age at last evaluation | 1 y | 13 y | 7 y | 13 y | 14 y | 22 y | 5 y | 3 y | 19 m | 6 w | 11 m | 11 m | 12 y |
| Genetic mutation | IFITM5, c.14C>T | IFITM5, c.14C>T | ND | IFITM5, c.14C>T | IFITM5, c.14C>T | IFITM5, c.14C``T | IFITM5, c.14C>T | IFITM5, c.14C>T | IFITM5, c.14C>T | IFITM5, c.14C>T | IFITM5, c.14C>T | IFITM5, c.14C>T | IFITM5, c.14C>T |
| Inherited | No | No | No | No | No | No | Yes | Yes | Yes | Yes | No | No | Yes |
| Blue sclera | No | No | No | No | Gray | No | No | No | No | No | No | No | No |
| Dentinogenesis imperfecta | No | No | No | Yes | No | No | No | No | No | No | No | No | No |
| Hearing loss | No | No | No | No | No | No | No | No | No | No | No | No | No |
| Bisphosphonate | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
F, female; M, male; Cau, Caucasian; His, Hispanic; AA, African American; y, year(s); m, month(s); w, week(s); ND, none determined.
3.1. Clinical characteristics
Seven of the 13 (53.8%) children were delivered vaginally and the remaining six by Caesarian section. All of the Caesarian sections were performed for maternal reasons: four for maternal OI, one for high blood pressure, and one for gestational diabetes. There were no reports of hearing loss or blue sclera (one had gray sclera). One patient had dentinogenesis imperfecta. Three of 13 patients were noted to have thoracic deformities including pectus excavatum (one) and pectus carinatum (two). Seven of 13 patients (53.8%) had a calcified interosseous membrane including upper extremity (six) and lower extremity (three); one patient was not evaluable and two patients had both upper and lower extremity interosseous membrane calcification.
All patients had a history of fracture. Bisphosphonate therapy was initiated in 12 of 13 patients (92.3%). Nine of 13 patients (69.2%) had cervical kyphosis, six of these nine had associated thoracolumbar deformities such as scoliosis or kyphosis. Four patients required an orthopaedic surgical procedure (30.7%). Lower extremity intramedullary rodding was the most common surgery (four of four). In addition, two of these four individuals also underwent spinal fusion for treatment of severe thoracic kyphoscoliosis (15.3% overall) and one patient had an additional surgery to treat dislocation of a radial head. These clinical findings are summarized in Table 1, Table 2.
Table 2.
Orthopaedic manifestation of OI type V patients.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subphyseal metaphyseal radiodense line | Yes | Yes | Yes | Yes | Yes | NE | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| UE fractures/deformities | Yes/No | Yes/Yes | Yes/Yes | Yes/Yes | Yes/Yes | Yes/No | Yes/Yes | Yes/Yes | No/Yes | Yes/Yes | No/No | No/No | Yes/Yes |
| UE rodding | No | No | No | No | No | No | No | No | No | No | No | No | No |
| UE IMC | No | Yes | Yes | Yes | Yes | NE | No | No | No | No | Yes | No | Yes |
| Radial head dislocation | No | No | No | No | Yes | No | No | No | No | No | No | No | Yes |
| LE fractures/deformities | Yes/No | Yes/No | Yes/No | Yes/Yes | Yes/Yes | Yes/No | Yes/Yes | Yes/Yes | Yes/Yes | No | No/Yes | Yes/No | Yes/Yes |
| LE rodding | No | Yes | No | No | Yes | Yes | No | No | No | No | No | No | Yes |
| LE IMC | No | Yes | No | Yes | No | Yes | No | No | No | No | No | No | No |
| Thorax deformities/rib fractures | Ribs Fx | PE | No | Ribs Fx | Ribs Fx/PC | Ribs Fx | Ribs Fx | No | No | No | Ribs Fx | Ribs Fx | PC |
| Thoracolumbar spine deformities | Kyphosis | No | NE | Scoliosis/kyphosis | Scoliosis/kyphosis | No | No | No | Kyphosis | No | Scoliosis/kyphosis | No | Scoliosis/kyphosis |
| Thoracolumbar spinal surgery | No | No | No | No | Yes | No | No | No | No | No | No | No | Yes |
| Hypertrophic callus | No | Yes | No | Yes | Yes | Yes | No | No | No | Yes | No | Yes | No |
| Cervical Kyphosis | Yes | No | NE | Yes | Yes | Yes | Yes | No | Yes | NE | Yes | Yes | Yes |
NE, not evaluated; UE, upper extremities; IMC, interosseous membrane calcification; LE, lower extremities; Fx, fracture(s).
3.2. Radiographic characteristics
In 12 of 13 (92.3%) patients, subphyseal metaphyseal radiodense lines were present. The remaining patient did not have appropriate images available to review for this feature. Hyperplastic callus formation was noted in six of 13 patients (46.1%). Sagittal plane radiographs of the cervical spine demonstrated kyphosis in nine of 13 patients (69.2%). Anterior wedging and flattened vertebral bodies were seen. No vertebral body fractures were identified. In addition, anterior listhesis of C2-C3 and C3-C4 was noted in six patients (Fig. 1). No subluxation was noted in three patients. MRI studies were performed and reviewed in patients with cervical kyphosis and subluxation; three patients showed narrowing of the spinal canal without cervical cord compression and one asymptomatic patient showed impingement of the spinal cord. The youngest observation of cervical kyphosis was noted in a 3-month-old infant and the oldest observation was seen in a 22-year-old patient. Only 6 of the 13 (46.1%) patients had available bone mineral density examined by dual x-ray absorptiometry (DXA). These studies however were performed on different DXA machines at different centers and at different anatomic sites. The age of studies ranged from 17 months to 13 years.
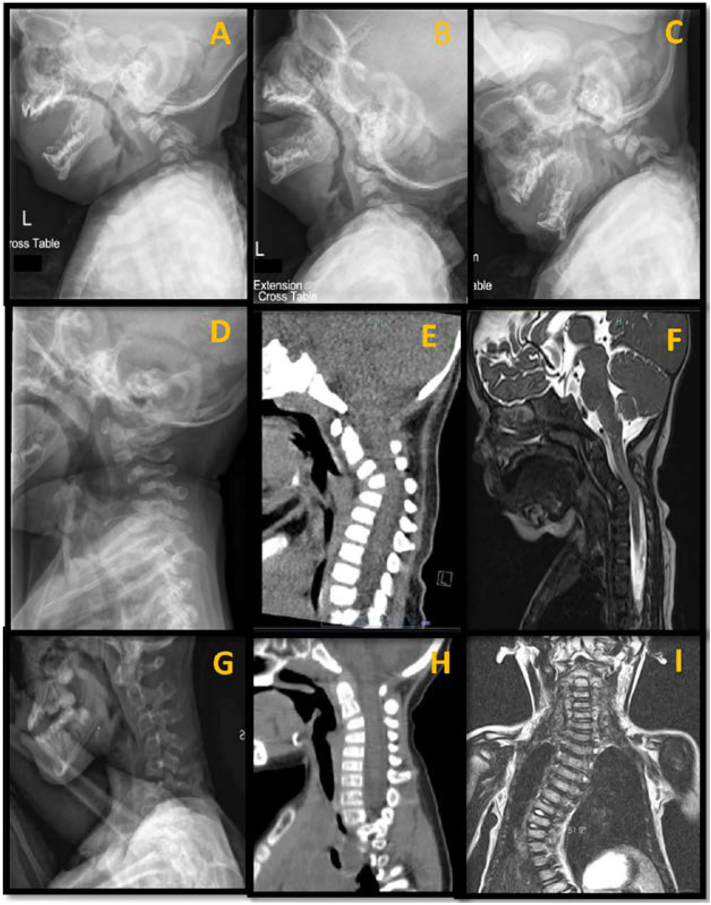
Fig. 1.
Cervical spine features of patients diagnosed with osteogenesis imperfecta type V. A, Lateral radiographic view in neutral position of cervical spine showing severe cervical kyphosis and listhesis at C2-C3; B, Lateral radiographic view in extension position of cervical spine showing persistent cervical kyphosis and listhesis at C2-C3; C, Lateral radiographic view in flexion position of cervical spine showing aggravated cervical kyphosis and listhesis at C2-C3; D, Lateral radiographic view in neutral position of cervical spine showing severe cervical kyphosis and multilevel listhesis at C2-C3 and C3-C4; E, Lateral computed tomography scan showing listhesis at C2-C3 and anterior wedge of vertebral body with narrowing of spinal canal; F, Lateral magnetic resonance image showing cervical kyphosis compromising the spinal cord (impingement C3-C4); G, Lateral radiographic view in neutral position showing cervical kyphosis with multilevel listhesis at C2-C3 and C3-C4 and vertebral bodies with anterior wedge and flattened shape; H, Lateral computed tomography scan confirming the anterior wedge and flattened vertebral body shape; I, Coronal magnetic resonance image showing associated scoliosis in a patient with cervical kyphosis.
4. Discussion
To our knowledge, this is the first case series reporting cervical kyphosis in a cohort of patients with type 5 OI. Nine of our 13 type 5 OI patients (69.2%) ranging in age from 3 months to 22 years had a cervical kyphosis. Given that cervical kyphosis falls in an intermediate frequency between the known classic radiographic features of hyperplastic callus (46.1%) and subphyseal metaphyseal radiodense lines (92.3%), in our cohort, it may represent a newly identified diagnostic feature suggesting type 5 OI.
Cervical kyphosis was noted at an early age. However, this deformity seems to persist even in adults (case 6). In our series, no patients had a history of neck trauma nor does there appear to be a relationship between the presence of kyphosis to any vertebral body fractures. Neurologic examination was unremarkable in all of these patients despite the presence of the cervical kyphosis. Listhesis, mainly centered between C2-C3 and C3-C4, and anterior wedging of vertebral bodies, appear to be the cause for the kyphotic deformity. Multiple microfractures due to low bone density or dystrophic changes of vertebral bodies could be responsible for this vertebral body shape; however, our MRI studies reported no acute bony edema rejecting fracture (Abelin et al., 2008). Dynamic images did not demonstrate cervical instability and therefore, none of these cervical kyphoses required specific surgical intervention. As this study was retrospective, no systematic longitudinal evaluations were performed. In the patients where cervical images were available over time, no changes were observed in the degree of kyphosis or in the shape of the individual vertebral bodies. This included the absence of changes during bisphosphonate therapy.
The type 5 OI population is known to have variable presentations despite identical molecular basis (Fitzgerald et al., 2013; Marini and Blissett, 2013). Other clinical and radiographic features specific to type 5 OI include calcified interosseous membrane and hyperplastic callus, which were seen in 53.8% and 46.1% of our cohort, respectively. Glorieux et al. (2000) reported seven out of seven patients had calcifications of the interosseous membrane with the upper extremity mainly affected and one case of upper/lower extremity, whereas Shapiro et al. (2013) reported 13 of 17 patients (76.5%). Hyperplastic callus had been reported by Cheung et al. (2007) in 65% of their population.
Our cohort has slightly lower rates of these features, which are known to develop over time, and our lower frequencies may represent a younger overall cohort. Nevertheless, the numbers are reasonably similar and suggest that cervical kyphosis is likely present in a broader population of patients with type 5 OI (Fitzgerald et al., 2013; Shapiro et al., 2013; Cheung et al., 2007; Hui et al., 2011). Other craniocervical abnormalities, such as basilar invagination (BI), are associated with OI (Sillence, 1994). Sillence (1994) reported BI in 25% of subjects with OI type IV and Castelein et al. (2019) reported BI in 13% of OI patients, with OI type III being the most common (39%). In our group of type 5 OI patients, we did not identify any BI.
We believe that going forward, it will be important to screen for cervical kyphosis in patients with type 5 OI and consideration be given to monitor the cervical kyphosis as we monitor other deformities of the craniocervical junction and spine in the OI population. It will be important to know if this deformity is present prior to positioning for other surgical procedures, and neuromonitoring may need to be considered (White et al., 2017).
The limitations of this study are that it is a retrospective review of 13 patients with a diagnosis of type 5 OI. It is not designed to look at the natural history or etiology of cervical kyphosis specifically, or type 5 OI in general, in these patients. Going forward, prospectively following the cervical spine in patients with type 5 OI, will be critical to better characterize the frequency and natural history of cervical kyphosis.
In conclusion, cervical kyphosis appears to be a common feature of type 5 OI. It can be a presenting and apparently life-long association. It does not appear to be caused by vertebral body fractures. Evaluation for cervical kyphosis should be performed in anyone with a suspected or confirmed diagnosis of type 5 OI. Furthermore, if cervical kyphosis is noted in an individual with OI, type 5 should be considered.
Funding source
For Jeanne M. Franzone: This work was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health, Bethesda, MD [grant number: U54-GM104941] (PI: Binder-Macleod).
CRediT authorship contribution statement
Carlos Pargas: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Jeanne M. Franzone: Conceptualization, Formal analysis, Investigation, Resources, Supervision, Validation, Visualization, Writing - review & editing. Kenneth J. Rogers: Data curation, Formal analysis, Validation, Visualization, Writing - review & editing. Frank Artinian: Data curation, Investigation, Validation, Visualization, Writing - review & editing. Adolfredo Santana: Data curation, Investigation, Validation, Visualization, Writing - review & editing. Suken A. Shah: Formal analysis, Investigation, Project administration, Validation, Visualization, Writing - review & editing. Cristina M. McGreal: Data curation, Validation, Visualization, Writing - review & editing. Richard W. Kruse: Conceptualization, Formal analysis, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing - review & editing. Michael B. Bober: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Declaration of competing interest
None.
References
- Abelin K., Vialle R., Lenoir T., Thevenin-Lemoine C., Damsin J.P., Forin V. The sagittal balance of the spine in children and adolescents with osteogenesis imperfecta. Eur. Spine J. 2008;17:1697–1704. doi: 10.1007/s00586-008-0793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle W.H., Shattock S.G. A remarkable case of difuse cancellous osteoma of the femur following a fracture, in which similar growths afterwards developed connection with other bones. Proc. R. Soc. Med. 1908;1(Pathol Sect):83–115. doi: 10.1177/003591570800101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauze R.J., Smith R., Francis M.J. A new look at osteogenesis imperfecta. A clinical, radiological and biochemical study of forty-two patients. J. Bone Joint Surg. 1975;57:2–12. [PubMed] [Google Scholar]
- Castelein R.M., Hasler C., Helenius I., Ovadia D., Yazici M., EPOS Spine Study Group Complex spine deformities in young patients with severe osteogenesis imperfecta: current concepts review. J. Child. Orthop. 2019;13:22–32. doi: 10.1302/1863-2548.13.180185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung M.S., Glorieux F.H., Rauch F. Natural history of hyperplastic callus formation in osteogenesis imperfecta type V. J. Bone Miner. Res. 2007;22:1181–1186. doi: 10.1359/jbmr.070418. https://10.1359/jbmr.070418 [DOI] [PubMed] [Google Scholar]
- Chu M.L., Williams C.J., Pepe G., Hirsch J.L., Prockop D.J., Ramirez F. Internal deletion in a collagen gene in a perinatal lethal form of osteogenesis imperfecta. Nature. 1983;304:78–80. doi: 10.1038/304078a0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J., Holden P., Wright H., Wilmot B., Hata A., Steiner R.D., Basel D. Phenotypic variability in individuals with type V osteogenesis imperfecta with identical IFITM5 mutations. J. Rare Disord. 2013;1:37–42. [PMC free article] [PubMed] [Google Scholar]
- Forlino A., Marini J.C. Osteogenesis imperfecta. Lancet. 2016;387:1657–1671. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forlino A., Cabral W.A., Barnes A.M., Marini J.C. New perspectives on osteogenesis imperfecta. Nat. Rev. Endocrinol. 2011;7:540–557. doi: 10.1038/nrendo.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorieux F.H., Rauch F., Plotkin H., Ward L., Travers R., Roughley P., Lalic L., Glorieux D.F., Fassier F., Bishop N.J. Type V osteogenesis imperfecta: a new form of brittle bone disease. J. Bone Miner. Res. 2000;15:1650–1658. doi: 10.1359/jbmr.2000.15.9.1650. [DOI] [PubMed] [Google Scholar]
- Hui P.K.T., Tung J.Y.L., Lam W.W.M., Chau M.T. Osteogenesis imperfecta type V. Skelet. Radiol. 2011;40(1609):1633. doi: 10.1007/s00256-011-1236-x. [DOI] [PubMed] [Google Scholar]
- Marini J.C., Blissett A.R. New genes in bone development: what's new in osteogenesis imperfecta. J. Clin. Endocrinol. Metab. 2013;98:3095–3103. doi: 10.1210/jc.2013-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler O., Garbes L., Keupp K., Swan D., Zimmermann K., Becker J., Iden S., Wirth B., Eysel P., Koerber F., Schoenau E., Bohlander S.K., Wollnik B., Netzer C. A mutation in the 5'-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type V with hyperplastic callus. Am. J. Hum. Genet. 2012;91:349–357. doi: 10.1016/j.ajhg.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J.R., Lietman C., Grover M., Lu J.T., Nagamani S.C., Dawson B.C., Baldridge D.M., Bainbridge M.N., Cohn D.H., Blazo M., Roberts T.T., Brennan F.S., Wu Y., Gibbs R.A., Melvin P., Campeau P.M., Lee B.H. Phenotypic variability of osteogenesis imperfecta type V caused by an IFITM5 mutation. J. Bone Miner. Res. 2013;28:1523–1530. doi: 10.1002/jbmr.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence D.O. Craniocervical abnormalities in osteogenesis imperfecta: genetic and molecular correlation. Pediatr. Radiol. 1994;24:427–430. doi: 10.1007/BF02011910. [DOI] [PubMed] [Google Scholar]
- Sillence D.O., Senn A., Danks D.M. Genetic heterogeneity in osteogenesis imperfect. J. Med. Genet. 1979;16:101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M., Ishii T., Barnes A.M., Weis M., Amano N., Tanaka M., Fukuzawa R., Nishimura G., Eyre D.R., Marini J.C., Hasegawa T. A novel mutation in LEPRE1 that eliminates only the KDEL ER- retrieval sequence causes non-lethal osteogenesis imperfecta. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk F.S., Sillence D.O. Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am. J. Med. Genet. Part A. 2014;164A:1470–1481. doi: 10.1002/ajmg.a.36545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk F.S., Byers P.H., Dalgleish R., Malfait F., Maugeri A., Rohrbach M., Symoens S., Sistermans E.A., Pals G. EMQN best practice guidelines for the laboratory diagnosis of osteogenesis imperfecta. Eur. J. Hum. Genet. 2012;20:11–19. doi: 10.1038/ejhg.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warman M.L., Cormier-Daire V., Hall C., Krakow D., Lachman R., LeMerrer M., Mortier G., Mundlos S., Nishimura G., Rimoin D.L., Robertson S., Savarirayan R., Sillence D., Spranger J., Unger S., Zabel B., Superti-Furga A. Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet A. 2011;155A(5):943–968. doi: 10.1002/ajmg.a.33909. [Epub 2011 Mar 15 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warman M.L., Cormier-Daire V., Hall C., Krakow D., Lachman R., LeMerrer M., Mortier G., Mundlos S., Nishimura G., Rimoin D.L., Robertson S., Savarirayan R., Sillence D., Cho T.J., Lee K.E., Lee S.K., Song S.J., Kim K.J., Jeon D., Lee G., Kim H.N., Lee H.R., Eom H.H., Lee Z.H., Kim O.H., Park W.Y., Park S.S., Ikegawa S., Yoo W.J., Choi I.H., Kim J.W. A single recurrent mutation in the 5′-UTR of IFITM5 causes osteogenesis imperfecta type V. Am. J. Hum. Genet. 2012;91:343–348. doi: 10.1016/j.ajhg.2012.06.005. (doi:10.1002/jbmr.1891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K.K., Bompadre V., Goldberg M.J., Bober M.B., Cho T.J., Hoover-Fong J.E., Irving M., Mackenzie W.G., Kamps S.E., Raggio C., Redding G.J., Spencer S.S., Savarirayan R., Theroux M.C., Skeletal Dysplasia Management Consortium Best practices in peri-operative management of patients with skeletal dysplasias. Am. J. Med. Genet. A. 2017;173:2584–2595. doi: 10.1002/ajmg.a.38357. [DOI] [PubMed] [Google Scholar]