Cancer, a disease caused by uncontrolled cell growth, is driven by genetic and epigenetic disruption of cell cycle, cell death, and cell state programs [1]. Anticancer therapies thus strive towards simultaneously targeting multiple critical nodes of neoplastic growth. These strategies typically include surgical and radiation-based therapies in combination with pharmacological approaches that target cancer promoting pathways, for example: targeting “driver” oncogenes, such as truncal activating mutations in specific cancers; targeting immune checkpoints, such as PD1 and CTLA4; and targeting barriers to cell death, such as anti-apoptotic proteins like BCL2 and Bcl-xL. The development of these multi-modal therapeutic strategies enables the use of lower, better tolerated, doses of individual cytotoxic agents and decreases the long-term concerns of drug resistance in cancer. Therefore, the identification and validation of therapeutic combinations, especially pharmacological agents that target oncogenic programs with desired low-toxicity profiles, is an active pursuit in cancer biology.
Cancer cell state is one such targetable oncogenic vulnerability. The altered differentiation status of tumor cells is among cancer's signature features and has been historically attributed to the activation of oncogenic master regulators, such as c-Myc, and/or the loss of tumor suppressive regulators, such as p53 [1]. Over the past two decades, though, altered cell state has acquired new meaning in tumor biology. The use of specific tumor cell markers (e.g. CD44 and CD133), the use of functional extreme-limiting dilution assays, and the ever-improving single-cell approaches to study the underlying heterogeneity of tumors, have together pointed towards the existence of a stem-like sub-population in many tumor types. The presence of such a population remains hotly debated in cancer biology; however, in several cancer contexts, these “Cancer Stem-like Cells (CSCs)” have been shown to share many biological features of normal stem cells [2]. Of particular relevance to cancer therapy, CSCs are thought to remain sheltered in a non-dividing state within a tumor mass, where they escape therapeutic cytotoxicity that is primarily directed towards eliminating the rapidly-dividing tumor cells [2]. The CSCs can then emerge, often days and weeks after clearance of the primary tumors, capable of seeding secondary drug-resistant tumors [2]. Targeting this sub-population of cells in tumors can thus have pronounced therapeutic benefit.
Is targeting cell state then a feasible anti-cancer strategy? Indeed, tumor differentiation as a therapeutic strategy is routinely used in patients with acute promyelocytic leukemia (APL), where “differentiation therapy” with all-trans Retinoic Acid (ATRA) has revolutionized the treatment of this disease [3]. Pharmacological approaches that target mutant Isocitrate Dehydrogenase (IDH) dependent tumors are perhaps another example of therapeutic “differentiation therapy”. Expression of mutant IDH is a feature of gliomas, acute myeloid leukemias (AML), and chondrosarcomas, and promotes a differentiation block in these tumors, in part, via inhibition of 2-oxoglutarate-dependent enzymes like the JumonjiC-family histone demethylases [4]. Pharmacological agents that selectively target mutant IDH (one of which has received FDA approval) promote differentiation and block tumor growth in mutant IDH driven cancers. Finally, in models of solid tumors such as the Nuclear Protein in Testis (NUT) midline carcinoma, epigenetic reprogramming with agents that target histone-modifying enzymes (such as Trichostatin A) can promote tumor differentiation and impede tumor growth [5]. Together, these observations highlight the growing evidence in support of “differentiation therapy” as a viable approach in both blood-borne and solid tumors.
Consistent with these, perhaps one other example where “differentiation therapy” has percolated into clinical practice is in the management of neuroblastoma - an extra-cranial cancer that is derived from neural crest cells. The clinical management of neuroblastomas includes surgery in combination with other therapeutic modalities (e.g. radiation and immunotherapy), and includes a maintenance regimen using a retinoic acid (RA) derivative, 13-cis-retinoic acid – a known modulator of tumor cell state. Unfortunately, despite its short-term success, this molecule not only causes many undesirable physiological effects, but also ultimately results in tumor relapse, both of which severely limits its long-term clinical use. Identifying, an equipotent substitute for these retinoic acid derivatives is of immediate relevance in treating neuroblastoma.
Recently, Dr. Elizabeth Beierle's laboratory has begun characterizing the use of the Retinoic X Receptor (RXR) agonist, UAB30, in many solid tumors [6,7]. UAB30 boasts of vastly improved off-target toxicity profile compared to other retinoic acid derivatives, and has been studied in various cancer contexts. In their latest contribution (Marayati et al. [8]), the authors use neuroblastoma patient-derived organoids to address the therapeutic utility of UAB30 as a differentiation modulator. Their findings demonstrate that in two independent patient-derived organoids, UAB30 treatment promotes tumor-suppressive features, including reduced cell proliferation, increased expression of differentiation-associated genes, and a functional decrease in a cancer stem-like cells.
UAB30 was discovered as a retinoic acid derivative nearly two decades ago and, prior to the studies by Marayati et al. in neuroblastoma, had been used in pre-clinical studies to target mammary, hepatic, and renal malignancies [7,9,10]. Importantly, besides it's canonical RA-like functions, non-canonical roles of UAB30 (e.g. Src inhibition [10]) have been associated with antiproliferative effects in other cancers. Systematic mechanistic studies of UAB30 in the future will help distinguish the relevance of such canonical and non-canonical functions in neuroblastoma. These mechanistic studies could be coupled to studies in a broader panel of patient-derived xenografts both in vitro and in vivo to begin determining the extent to which UAB30 could prove clinically beneficial. Finally, relapse to retinoic acid treatment is one of the major concerns in the clinic. It remains to be seen how sustained use of UAB30 will compare to existing RA derivatives in promoting durable responses against neuroblastoma.
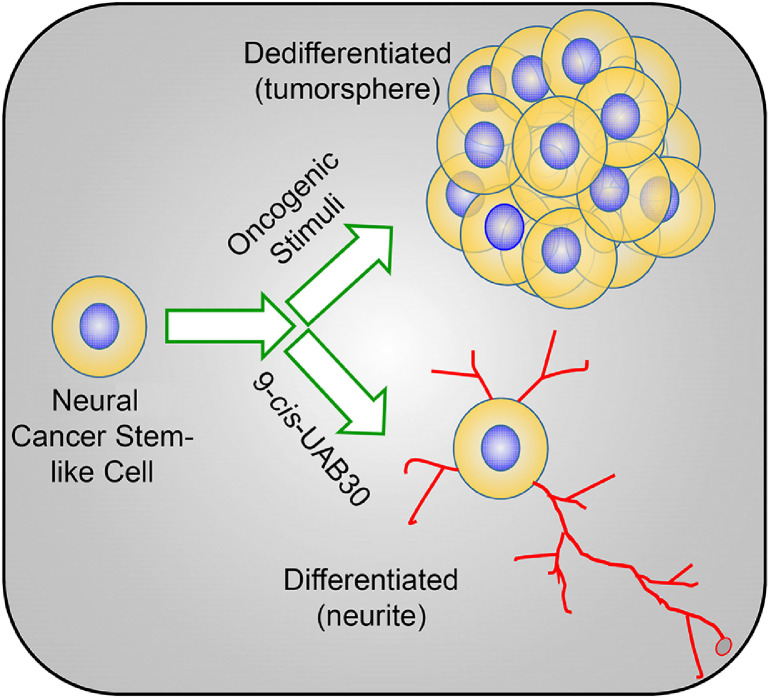
In summary, Marayati et al.’s description of the use of UAB30 in neuroblastoma now represents another potential example of “differentiation therapy” in solid tumors [8]. These findings justify further mechanistic studies in pre-clinical models and follow-up clinical studies to explore the therapeutic utility of UAB30 in neuroblastoma – and perhaps other cancers. Such studies could yield tremendous clinical impact in disease management (Fig. 1).
Fig. 1.
UAB30 promotes differentiation and reduces cancer stemness. Schema outlining the typical progression of cancer stem-like cells, in response to oncogenic and environmental stimuli, into an actively dividing population of cells that resemble ‘tumorspheres’ in vitro. Treatment with differentiation modulators, such as UAB30, promotes a neurite-like differentiated state, which is associated with reduced tumorigenic potential.
CRediT Author Statement
Abhishek A. Chakraborty: Writing, Editing, and Reviewing.
References
- 1.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Takebe N. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z.Y., Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]
- 4.Lu C. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–478. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz B.E. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res. 2011;71:2686–2696. doi: 10.1158/0008-5472.CAN-10-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams A.P. UAB30, A Novel Rexinoid Agonist, Decreases Stemness In Group 3 Medulloblastoma Human Cell Line Xenografts. Transl Oncol. 2019;12:1364–1374. doi: 10.1016/j.tranon.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waters A.M. Preclinical Evaluation of UAB30 in Pediatric Renal and Hepatic Malignancies. Mol Cancer Ther. 2016;15:911–921. doi: 10.1158/1535-7163.MCT-15-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marayati R. 9-cis-UAB30, a novel rexinoid agonist, decreases tumorigenicity and cancer cell stemness of human neuroblastoma patient-derived xenografts. Transl Oncol. 2020;14 doi: 10.1016/j.tranon.2020.100893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christov K., Grubbs C.J., Shilkaitis A., Juliana M.M., Lubet R.A. Short-term modulation of cell proliferation and apoptosis and preventive/therapeutic efficacy of various agents in a mammary cancer model. Clin Cancer Res. 2007;13:5488–5496. doi: 10.1158/1078-0432.CCR-07-0404. [DOI] [PubMed] [Google Scholar]
- 10.Kim M.S. Src is a novel potential off-target of RXR agonists, 9-cis-UAB30 and Targretin, in human breast cancer cells. Mol Carcinog. 2015;54:1596–1604. doi: 10.1002/mc.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]