Abstract
Purpose
To evaluate the effect of the 21-gene recurrence score (RS) assay in breast cancer-specific mortality (BCSM) and decision-making for chemotherapy in older (aged ≥65 years) breast cancer.
Methods
We retrospectively included older patients with T1-2N0 and estrogen receptor-positive breast cancer in the Surveillance, Epidemiology, and End Results database. Cox regression model and competing-risks model were used for data analysis.
Results
This study included 8524 patients, 1987 (23.3%) had low RS, 5059 (59.4%) had intermediate RS, and 1478 (17.3%) had high RS. Chemotherapy was administrated in 2.0%, 8.6%, and 51.2% for low, intermediate, and high RS cohorts, respectively (P < 0.001). A total of 597 deaths were recorded, including one-quarter of breast cancer-related deaths and three-quarters as competing causes of death. The 5-year BCSM was 5.4%, 4.7%, and 9.1% for low, intermediate, and high RS cohorts, respectively (P < 0.001), using the Cox regression model, and was 0.8%, 0.9%, and 5.2% for low, intermediate, and high RS cohorts using the competing-risks regression, respectively (P < 0.001). RS was independently correlated with BCSM in both prognostic models. The stratified analysis demonstrated that chemotherapy was not correlated with a lower risk of BCSM in intermediate and high RS cohorts in both prognostic models. Sensitivity analyses replicated similar findings after stratification by the year of diagnosis and patients’ age.
Conclusions
The competing-risks model is useful in dealing with multiple end events for older breast cancer patients. 21-gene RS was independently associated with BCSM. However, chemotherapy did not significantly decrease the risk of BCSM in intermediate and high RS cohorts.
Keywords: Breast cancer, Oncotype DX, Older, Chemotherapy, Competing-risks analysis
Highlights
-
•
ODX testing should be considered in older breast cancer patients.
-
•
RS was independently correlated with BCSM in both prognostic models.
-
•
Chemotherapy was not correlated with a lower risk of BCSM in intermediate and high RS cohorts.
1. Background
Tumor genomic profile data have been integrated into prognostic assessment and decision-making of adjuvant chemotherapy for early-stage breast cancer (BC) [[1], [2], [3]]. However, there is limited evidence about the optimal treatment decisions based on genomic profile data for older women (aged ≥65 years) with BC, despite this group comprising half of all newly diagnosed cases annually [4]. Previous retrospective analyses have found the lack of benefit seen with the use of chemotherapy in unselected populations of older patients, whereas benefit was seen only in patients with hormone receptor-negative or node-positive disease. Therefore, the role of gene expression profiling to better select individuals who are most likely to benefit from chemotherapy in older patients should be investigated [5]. A higher percentage of older women with BC develop estrogen receptor (ER)-positive tumors than younger women [6]. Older patients also present with a lower rate of human epidermal growth factor receptor-2 (HER2)-positive tumors [7]. Several gene expression profiling tests, including 21-gene RS (Oncotype Dx, ODX), 50-gene (PAM 50), 70-gene (MammaPrint), 12-gene (EndoPredict) as well as Breast Cancer Index have been recommended for clinical use from the European Society for Medical Oncology and American Society of Clinical Oncology clinical practice guidelines [8,9], which are beneficial in therapy selection for ER-positive, HER2-negative, and node-negative (N0) BC patients. However, ODX is the only multigene signature recommended by the National Comprehensive Cancer Network (NCCN) for this population (category 1 evidence) [10]. Therefore, ODX testing should be considered in the majority of newly diagnosed older BC cases.
The validation studies of the ODX in early-stage BC included approximately 20–30% of older women, and age had no impact on the risk prediction of RS [3,11,12]. In particular, the first validation study indicated that age was not an independent prognostic factor when RS was included in the multivariate analysis [3]. Several studies have shown that older women were often not treated with guideline-recommended chemotherapy or were undertreated, which could lead to a higher risk of BC-related mortality compared to younger women [13,14]. Therefore, treatment should be adapted to tumor biology and general health rather than age alone. However, the effect of RS array testing in prognostication of survival and decision-making of chemotherapy in older BC patients remains controversial. Management of older BC is challenging because of the lack of high-quality evidence regarding the effect of adjuvant chemotherapy.
ODX is a gene expression profiler used to prognosticate the 10-year risk of distant metastasis in women with ER-positive, HER2-negative, and N0 BC to inform clinical decision-making of chemotherapy receipt [15]. Women with a low RS may safely omit chemotherapy and experience comparable 10-year distant metastasis rates as their counterparts who received chemotherapy. On the other hand, women with a high RS have a higher 10-year risk of distant metastasis and should therefore consider chemotherapy receipt [15]. Older women experience competing risks of death and therefore may be less motivated to undergo more aggressive treatment to reduce their 10-year risk of distant recurrence [16]. Furthermore, older women are particularly susceptible to toxicities associated with chemotherapy receipt and may prefer a higher quality of life over the possibility of reduced 10-year distant recurrence outcomes [17]. Therefore, clinical decision-making about chemotherapy receipt in the older population is necessarily different from that in younger age groups.
Competing risk refers to individual exposure to more than two courses of failure, including second malignant tumors or other causes of death. However, definitive failure can only be attributed to one of them, which indicates that the occurrence of one type of event would hinder the occurrence by other events [18]. For older patients with BC, the leading cause of death does not appear to be BC related. Studies have reported that >50% of patients died from competing events such as cardiovascular disease and a second primary tumor [16,19]. The Cox regression model has been widely accepted to describe trends of survival and determine prognostic factors, but it is limited by the risk of bias. Because this model ignores competing events as censored data, it is important to consider both the outcomes of the event of interest and the outcomes of competing risks [20]. However, to our best knowledge, no data is yet available regarding competing-risks analysis along with the RS assay for older BC patients. Accordingly, two prognostic models including the Cox regression model and the competing-risks regression model were performed for older patients in the current study to investigate the effect of RS assay in BC-specific mortality (BCSM) and decision making for treatment using a population-based cohort.
2. Materials and methods
2.1. Patients
We included ODX related variables for invasive ductal carcinoma cases diagnosed from 2004 to 2012, the ODX test orders and results were provided from the Genomic Health Clinical Laboratory. Data on ODX testing and results were linked with BC incidence data from the Surveillance, Epidemiology, and End Results (SEER) database [21]. Women aged ≥65 years, tumor size ≤5 cm (T1-2), N0, and ER-positive invasive ductal carcinoma treated with surgery from 2004 to 2012 were identified. Patients with a second primary cancer, not pathologically diagnosed, and those with missing or incomplete information regarding histological grade, progesterone receptor (PR) status, and race/ethnicity were excluded. Patients aged ≥65 years were defined as older patients because this cut-off age is regularly used in gerontology [22,23]. Because of the anonymized patient data used from the SEER program, the need for ethics approval was waived by the review board of the First Affiliated Hospital of Xiamen University.
2.2. Variables
We identified the following demographic, clinicopathological variables, and outcomes of each patient: age, race/ethnicity, T stage, histological grade, PR status, and the 21-gene RS classification. Treatment variables, including surgical procedures, radiotherapy, and chemotherapy were also included. The RS cohorts were classified into low RS (<11), intermediate RS [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25]], and high RS (>25) based on the Trial Assigning Individualized Options for Treatment (TAILORx) cut-offs [2].
2.3. Statistical analysis
The patient characteristics among the three 21-gene RS cohorts were evaluated using the chi-squared test. Independent predictors associated with the receipt of chemotherapy were determined with binomial logistic regression. The univariate analyses were performed using both the Cox regression model and the competing-risks model and used to evaluate the cumulative incidence of BCSM. The BCSM was defined as the interval from the initial diagnosis of BC to the date of death from BC. BCSM was assessed using the cause of death data recorded from the SEER registries, which was provided by the National Center for Health Statistics [24]. These analyses used SEER-derived variables that use a mapping to dichotomize causes of death as other-cause specific or breast cancer-specific [25]. Multivariate Cox regression models were used to determine the independent prognostic indicators associated with BCSM and results were presented as hazard ratios (HRs) and 95% confidence intervals (95% CIs). Moreover, competing-risks analyses with the Fine and Gray model were also used to determine the combined effect of the variables on BCSM, and results were reported as sub-distribution hazard ratios (sdHRs) and 95%CIs. Sensitivity analyses focused on the year of diagnosis (2004–2010 and 2011–2012) and patients’ age (aged 65–69, 70–74, and >74 years) were performed. All data analyses were performed with IBM SPSS 22.0 (IBM Corp., Armonk, NY) and Stata/SE version 14 (StataCorp, TX, USA). All P values < 0.05 were considered statistically significant.
3. Results
3.1. Patient characteristics
A total of 8524 patients were included (Table 1). Briefly, 87.6%, 80.2%, and 80.2% of them were PR-positive, well-moderately differentiated disease, and non-Hispanic White, respectively. In addition, approximately half of them were aged ≥70 years. Moreover, 1987 (23.3%) had low RS, 5059 (59.4%) had intermediate RS, and 1478 (17.3%) had high RS. High RS patients were more likely to show a higher histological grade, larger tumor size, and PR-negative disease compared to those in the other two RS groups.
Table 1.
Baseline characteristics of patients between 2004 and 2012 in the SEER database (n = 8524).
| Variables | n | Low (%) | Intermediate (%) | High (%) | P |
|---|---|---|---|---|---|
| Age (years) | |||||
| 65-69 | 4631 | 1020 (51.3) | 2816 (55.7) | 795 (53.8) | 0.025 |
| 70-74 | 2441 | 604 (30.4) | 1405 (27.8) | 432 (29.2) | |
| >74 | 1452 | 363 (18.3) | 838 (16.6) | 251 (17.0) | |
| Race/ethnicity | |||||
| Non-Hispanic White | 6836 | 1576 (79.3) | 4106 (81.2) | 1154 (78.1) | 0.004 |
| Non-Hispanic Black | 601 | 153 (7.7) | 326 (6.4) | 122 (8.3) | |
| Hispanic (all) | 553 | 113 (5.7) | 342 (6.8) | 98 (6.5) | |
| Other | 534 | 145 (7.3) | 285 (5.6) | 104 (6.3) | |
| Grade | |||||
| Well differentiated | 2097 | 638 (32.1) | 1337 (26.4) | 122 (8.3) | <0.001 |
| Moderately differentiated | 4737 | 1168 (58.8) | 2948 (58.3) | 621 (42.0) | |
| Poorly/undifferentiated | 1690 | 181 (9.1) | 774 (15.3) | 735 (49.7) | |
| Tumor stage | |||||
| T1 | 6477 | 1510 (76.0) | 3956 (78.2) | 1011 (68.4) | <0.001 |
| T2 | 2047 | 477 (24.0) | 1103 (21.8) | 467 (31.6) | |
| PR status | |||||
| Negative | 1056 | 41 (2.1) | 540 (10.7) | 475 (32.1) | <0.001 |
| Positive | 7468 | 1946 (97.9) | 4519 (89.3) | 1003 (67.9) | |
| Surgical procedure | |||||
| Breast-conserving surgery | 6387 | 1460 (73.5) | 3867 (76.4) | 1060 (71.7) | <0.001 |
| Mastectomy | 2137 | 527 (26.5) | 1192 (23.6) | 418 (28.3) | |
| Radiotherapy | |||||
| No | 3016 | 721 (36.3) | 1708 (33.8) | 587 (39.7) | <0.001 |
| Yes | 5508 | 1266 (63.7) | 3351 (66.2) | 891 (60.3) | |
| Chemotherapy | |||||
| No | 7295 | 1948 (98.0) | 4625 (91.4) | 722 (48.8) | <0.001 |
| Yes | 1229 | 39 (2.0) | 434 (8.6) | 756 (51.2) | |
PR, progesterone receptor; T, tumor.
3.2. The RS assay and chemotherapy decision making in older patients
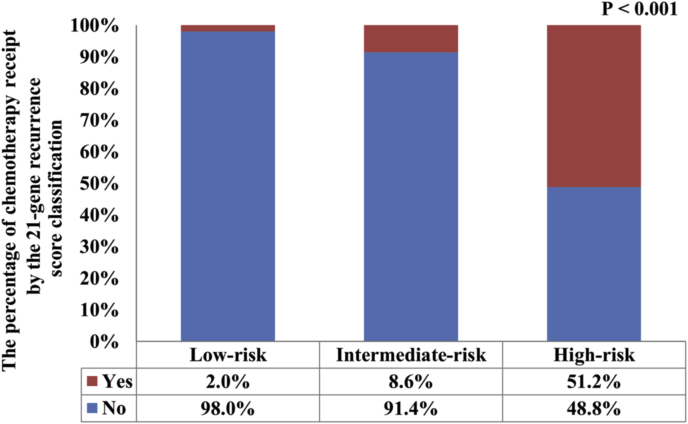
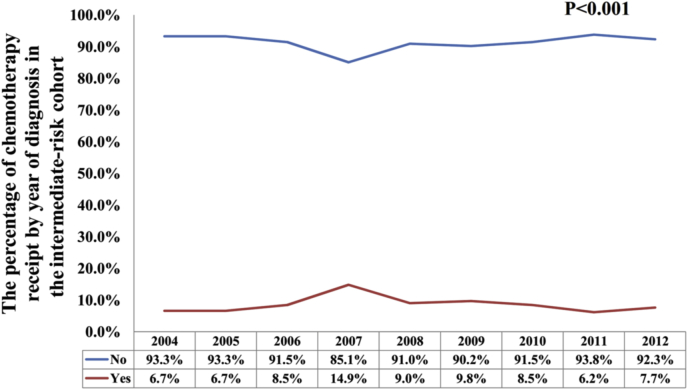
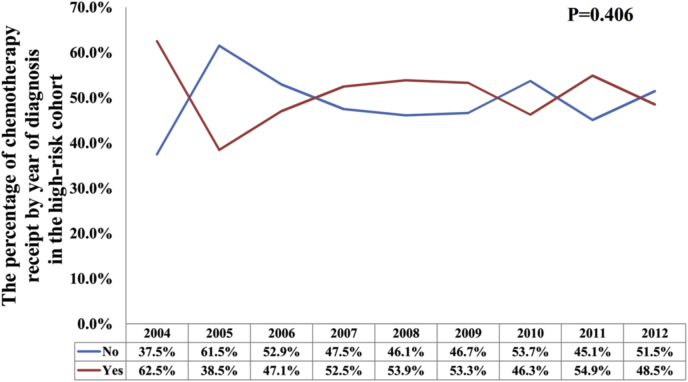
In all, 1229 (14.4%) patients received chemotherapy. Among them, 2.0%, 8.6%, and 51.2% of patients in the low, intermediate, and high RS groups were treated with chemotherapy, respectively (P < 0.001) (Fig. 1). Regarding the year of diagnosis, there was a significantly higher percentage of intermediate RS patients who received chemotherapy in the year 2007 compared to other years (P < 0.001), but had a comparable probability of chemotherapy receipt among other years (P = 0.237) (Fig. 2). In patients with a high RS cohort, the percentage of chemotherapy receipt was comparable over time (P = 0.406) (Fig. 3). The results of the binomial regression analysis indicated that RS was the most important predictor correlated with the receipt of chemotherapy. We observed that patients with intermediate RS (odds ratio [OR]: 4.207, 95% CI: 3.012–5.875, P < 0.001) and high RS (OR: 36.237, 95% CI: 25.684–51.125, P < 0.001) were more likely to receive chemotherapy than those with a low RS. Moreover, younger age, larger tumor size, higher histological grade, and PR-negative were also the independent predictors affecting receipt of chemotherapy. The year of diagnosis and race/ethnicity were not the predictors associated with the receipt of chemotherapy in the binomial regression analysis (Table 2).
Fig. 1.
The probability of chemotherapy receipt among different 21-gene recurrence score groups.
Fig. 2.
Change in use of chemotherapy during the study period in the intermediate-risk cohort.
Fig. 3.
Change in use of chemotherapy during the study period in the high-risk cohort.
Table 2.
Independent predictors associated with the receipt of chemotherapy (chemotherapy vs. no chemotherapy) between 2004 and 2012 in the SEER database (n = 8524).
| Variables | OR | 95%CI | P |
|---|---|---|---|
| Year of diagnosis | |||
| 2004 | 1 | ||
| 2005 | 0.459 | 0.136–1.555 | 0.211 |
| 2006 | 0.738 | 0.244–2.227 | 0.589 |
| 2007 | 1.255 | 0.428–3.681 | 0.679 |
| 2008 | 0.955 | 0.329–2.777 | 0.933 |
| 2009 | 0.961 | 0.331–2.785 | 0.941 |
| 2010 | 0.741 | 0.255–2.150 | 0.581 |
| 2011 | 0.755 | 0.261–2.183 | 0.604 |
| 2012 | 0.725 | 0.251–2.097 | 0.553 |
| Age (years) | |||
| 65-69 | 1 | ||
| 70-74 | 0.685 | 0.581–0.808 | <0.001 |
| >74 | 0.357 | 0.284–0.448 | <0.001 |
| Race/ethnicity | |||
| Non-Hispanic White | 1 | ||
| Non-Hispanic Black | 1.171 | 0.899–1.527 | 0.243 |
| Hispanic (all) | 1.189 | 0.902–1.568 | 0.219 |
| Other | 0.960 | 0.716–1.288 | 0.785 |
| Grade | |||
| Well differentiated | 1 | ||
| Moderately differentiated | 1.754 | 1.404–2.193 | <0.001 |
| Poorly/undifferentiated | 3.187 | 2.503–4.059 | <0.001 |
| Tumor stage | |||
| T1 | 1 | ||
| T2 | 1.361 | 1.161–1.596 | <0.001 |
| PR status | |||
| Negative | 1 | ||
| Positive | 0.782 | 0.652–0.939 | 0.008 |
| 21-gene RS classification | |||
| Low-risk | 1 | ||
| Intermediate-risk | 4.207 | 3.012–5.875 | <0.001 |
| High-risk | 36.237 | 25.684–51.125 | <0.001 |
CI, confidence interval; OR, odds ratio; PR, progesterone receptor; RS, recurrence score; T, tumor.
3.3. The cumulative incidence of BCSM
After a median follow-up time of 61 months (range: 0–142 months), a total of 597 deaths were recorded. Of these, 153 (25.6%) patients died because of BC. The top five competing events were cardiovascular diseases (n = 123), other causes of death (n = 91), chronic obstructive pulmonary disease and allied conditions (n = 43), cerebrovascular diseases (n = 42), and accidents and adverse effects (n = 21). The 5-year BCSM was 5.6% and 1.6% using the Cox regression model and competing-risks model, respectively.
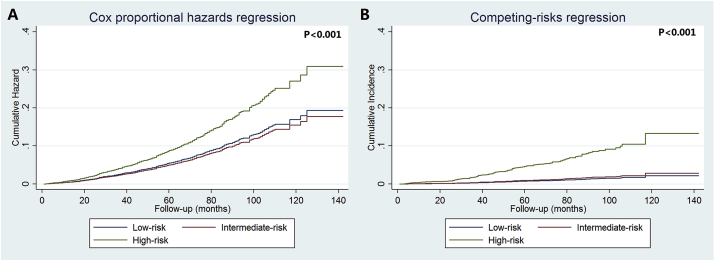
The 5-year BCSM was 5.4%, 4.7%, and 9.1% for low, intermediate, and high RS cohorts, respectively (P < 0.001), using the Cox regression (Fig. 4A). However, the competing-risks regression showed a significantly lower risk of BCSM, with a 5-year BCSM was 0.8%, 0.9%, and 5.2% for low, intermediate, and high RS cohorts, respectively (P < 0.001) (Fig. 4B).
Fig. 4.
The cumulative incidence of breast cancer-specific mortality among the three 21-gene recurrence score cohorts using Cox regression (A) and competing-risks regression (B).
3.4. Prognostic analysis
We used multivariate prognostic analysis models to determine the role of RS for BCSM, including the Cox regression model and the competing-risks model (Table 3). In the entire cohort, RS was independently correlated with BCSM in the competing-risks model. Using the low-risk cohort as a reference, we noted that the high RS cohort had a significantly higher risk of BCSM than the low RS cohort (sdHR: 4.780, 95%CI: 2.675–8.609, P < 0.001), while comparable BCSM was found between the intermediate and low RS cohorts (P = 0.445). Similar results were observed in the Cox regression model. We found similar trends of BCSM in the two multivariate prognostic analysis models that included patients without chemotherapy (Table 4).
Table 3.
Multivariate prognostic analysis for breast cancer-specific mortality using the Cox regression model and the competing-risks model in the entire cohort (n = 8524, 2004–2012 SEER database).
| Variables | Cox regression model |
Competing-risks model |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | sdHR | 95%CI | P | |
| Age (years) | ||||||
| 65-69 | 1 | 1 | ||||
| 70-74 | 1.410 | 1.153–1.725 | 0.001 | 1.232 | 0.852–1.782 | 0.267 |
| >74 | 3.070 | 2.532–3.722 | <0.001 | 1.634 | 1.088–2.453 | 0.018 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 1 | 1 | ||||
| Non-Hispanic Black | 1.209 | 0.903–1.620 | 0.202 | 1.270 | 0.736–2.190 | 0.391 |
| Hispanic (all) | 1.011 | 0.723–1.413 | 0.950 | 0.930 | 0.467–1.851 | 0.836 |
| Other | 0.775 | 0.529–1.135 | 0.191 | 0.817 | 0.400–1.670 | 0.580 |
| Grade | ||||||
| Well differentiated | 1 | 1 | ||||
| Moderately differentiated | 1.261 | 1.011–1.573 | 0.039 | 4.517 | 1.966–10.378 | <0.001 |
| Poorly/undifferentiated | 1.306 | 0.999–1.708 | 0.051 | 5.950 | 2.524–14.026 | <0.001 |
| Tumor stage | ||||||
| T1 | 1 | 1 | ||||
| T2 | 1.112 | 0.928–1.334 | 0.25 | 1.324 | 0.930–1.887 | 0.119 |
| PR status | ||||||
| Negative | 1 | 1 | ||||
| Positive | 1.005 | 0.795–1.271 | 0.968 | 1.467 | 0.946–2.277 | 0.087 |
| Surgical procedure | ||||||
| Breast-conserving surgery | 1 | 1 | ||||
| Mastectomy | 0.890 | 0.707–1.120 | 0.320 | 1.260 | 0.760–2.090 | 0.37 |
| Radiotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 0.592 | 0.478–0.734 | <0.001 | 0.897 | 0.556–1.45 | 0.654 |
| Chemotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 0.963 | 0.754–1.231 | 0.766 | 1.110 | 0.751–1.641 | 0.600 |
| 21-gene RS classification | ||||||
| Low-risk | 1 | 1 | ||||
| Intermediate-risk | 0.948 | 0.768–1.171 | 0.622 | 1.243 | 0.712–2.171 | 0.445 |
| High-risk | 1.561 | 1.182–2.062 | 0.002 | 4.780 | 2.675–8.609 | <0.001 |
CI, confidence interval; HR, hazard ratio; PR, progesterone receptor; RS, recurrence score; sdHR, sub-distribution hazard ratio; T, tumor.
Table 4.
Multivariate prognostic analysis for breast cancer-specific mortality using the Cox regression model and the competing-risks model in patients without chemotherapy (n = 7295, 2004–2012 SEER database).
| Variables | Cox regression model |
Competing-risks model |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | sdHR | 95%CI | P | |
| Age (years) | ||||||
| 65-69 | 1 | 1 | ||||
| 70-74 | 1.494 | 1.274–1.753 | <0.001 | 1.639 | 1.035–2.595 | 0.035 |
| >74 | 2.559 | 2.188–2.992 | <0.001 | 1.920 | 1.177–3.132 | 0.009 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 1 | 1 | ||||
| Non-Hispanic Black | 1.442 | 1.154–1.801 | 0.001 | 1.378 | 0.715–2.619 | 0.328 |
| Hispanic (all) | 1.019 | 0.769–1.350 | 0.897 | 1.021 | 0.446–2.336 | 0.961 |
| Other | 0.622 | 0.432–0.897 | 0.011 | 0.693 | 0.251–1.916 | 0.480 |
| Grade | ||||||
| Well differentiated | 1 | 1 | ||||
| Moderately differentiated | 1.150 | 0.976–1.354 | 0.094 | 4.170 | 1.799–9.666 | 0.001 |
| Poorly/undifferentiated | 1.128 | 0.913–1.394 | 0.263 | 4.264 | 1.714–10.609 | 0.002 |
| Tumor stage | ||||||
| T1 | 1 | 1 | ||||
| T2 | 1.239 | 1.070–1.435 | 0.004 | 1.451 | 0.935–2.249 | 0.096 |
| PR status | ||||||
| Negative | 1 | 1 | ||||
| Positive | 0.898 | 0.742–1.087 | 0.271 | 1.116 | 0.661–1.884 | 0.681 |
| Surgical procedure | ||||||
| Breast-conserving surgery | 1 | 1 | ||||
| Mastectomy | 0.810 | 0.675–0.973 | 0.025 | 1.105 | 0.615–1.984 | 0.739 |
| Radiotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 0.617 | 0.519–0.733 | <0.001 | 0.814 | 0.476–1.390 | 0.451 |
| 21-gene RS classification | ||||||
| Low-risk | 1 | 1 | ||||
| Intermediate-risk | 1.074 | 0.913–1.263 | 0.387 | 1.178 | 0.664–2.090 | 0.576 |
| High-risk | 1.657 | 1.327–2.069 | <0.001 | 4.946 | 2.718–9.000 | <0.001 |
CI, confidence interval; HR, hazard ratio; PR, progesterone receptor; RS, recurrence score; sdHR, sub-distribution hazard ratio; T, tumor.
3.5. Chemotherapy effects in intermediate and high RS patients
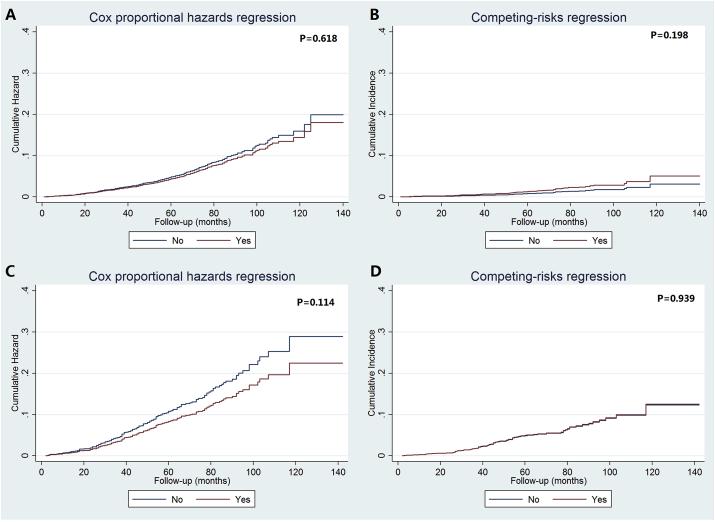
The univariate analysis using the competing-risks model showed that chemotherapy receipt was not associated with a lower risk of BCSM than non-chemotherapy cohort in both the intermediate RS (P = 0.198) and high RS (P = 0.939) patients. Similar results were found in the Cox regression model. The cumulative incidence of BCSM among the two 21-gene RS cohorts based on receipt of chemotherapy in the Cox regression model and competing-risks model are listed in Fig. 5. Only 2.0% of the low RS patients had undergone chemotherapy, thus the corresponding analysis was not performed for this patient subset.
Fig. 5.
The cumulative incidence of breast cancer-specific mortality in intermediate- and high-risk recurrence score cohorts by chemotherapy receipt using Cox regression (A, C) and competing-risks regression (B, D).
The multivariate prognostic analysis also showed that chemotherapy was not related to a lower risk of BCSM both in the two prognostic models in the intermediate and high RS cohorts (Table 5). Sensitivity analyses replicated similar findings after stratification according to the year of diagnosis and patients’ age (Table 6).
Table 5.
Multivariate prognostic analyses of chemotherapy receipt on breast cancer-specific mortality using the Cox regression model and the competing-risks model in intermediate and high-risk RS cohorts (2004–2012 SEER database).
| Variables | Cox regression model |
Competing-risks model |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | sdHR | 95%CI | P | |
| Intermediate-risk RS (n = 5059) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 1.026 | 0.687–1.532 | 0.900 | 1.449 | 0.681–3.080 | 0.335 |
| High-risk RS (n = 1478) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 0.762 | 0.558–1.051 | 0.099 | 1.039 | 0.668–1.618 | 0.864 |
CI, confidence interval; HR, hazard ratio; RS, recurrence score; sdHR, sub-distribution hazard ratio.
Table 6.
Sensitivity analyses of chemotherapy receipt on breast cancer-specific mortality using the Cox regression model and the competing-risks model (SEER database).
| Variables | Cox regression model |
Competing-risks model |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | sdHR | 95%CI | P | |
| Years 2004–2010 | ||||||
| Intermediate RS (n = 2967) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 0.824 | 0.513–1.325 | 0.425 | 1.009 | 0.395–2.578 | 0.985 |
| High RS (n = 856) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 0.923 | 0.641–1.329 | 0.668 | 1.092 | 0.665–1.791 | 0.728 |
| Years 2011–2012 | ||||||
| Intermediate RS (n = 2092) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 2.111 | 0.979–4.552 | 0.057 | 4.204 | 0.966–18.289 | 0.056 |
| High RS (n = 622) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 0.717 | 0.355–1.449 | 0.355 | 0.848 | 0.319–2.253 | 0.741 |
| Aged 65–69 years | ||||||
| Intermediate RS (n = 2816) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 1.077 | 0.610–1.901 | 0.799 | 1.94 | 0.648–5.811 | 0.236 |
| High RS (n = 795) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 1.075 | 0.659–1.753 | 0.773 | 1.602 | 0.818–3.138 | 0.17 |
| Aged 70–74 years | ||||||
| Intermediate RS (n = 1405) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 0.739 | 0.319–1.710 | 0.48 | 1.511 | 0.409–5.584 | 0.536 |
| High RS (n = 432) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 0.597 | 0.313–1.138 | 0.117 | 0.557 | 0.226–1.370 | 0.203 |
| Aged > 74 years | ||||||
| Intermediate RS (n = 838) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 1.397 | 0.634–3.078 | 0.407 | 1.187 | 0.141–10.006 | 0.875 |
| High RS (n = 251) | ||||||
| No chemotherapy | 1 | 1 | ||||
| Chemotherapy | 0.767 | 0.403–1.461 | 0.42 | 0.793 | 0.272–2.311 | 0.671 |
CI, confidence interval; HR, hazard ratio; RS, recurrence score; sdHR, sub-distribution hazard ratio.
4. Discussion
In the current study, the Cox regression model and competing-risks model were used to investigate the role of RS assay testing in prognostication of survival and decision making of chemotherapy in older women aged >65 years with BC. As expected, approximately three-quarters of deaths occurred in non-BC-related disease in our study. When investigating specific causes of death, the Cox regression model may tend to overestimate the risk of dying from BC when there are competing risks of death and patients being censored. The competing-risks model is useful in dealing with multiple end events [26]. In this study, 21-gene RS was the independent prognostic indicator for BCSM in both the Cox regression and the competing-risks models. However, chemotherapy did not significantly decrease the risk of BCSM in intermediate and high RS cohorts in both prognostic models.
Approximately half of the women diagnosed with BC were aged >65 years, but the percentage of those receiving standardized treatment among this older population was lower than of younger patients across all risk categories [4,27]. With the understanding of the biology of BC, the use of ODX testing could predict the 10-year risk of distant metastasis and predict the benefit of chemotherapy in a specific RS subgroup [2,15]. ODX is the only multigene signature recommended by the NCCN in N0 BC (category 1 evidence) and is most widely used [10]. Although ODX testing in older BC patients has gradually increased in recent years, the probability of receiving chemotherapy has also increased significantly with the increase of RS [28]. However, the percentage of older patients who underwent ODX testing was lower than theyounger cohort in a previous SEER-based study [27]. In addition, older patients were underrepresented in previous clinical trials such as TAILORx [2,29,30]. For example, 30% of patients that were enrolled in the TAILORx trial were aged between 61 and 75 years, while only 3–5% of patients were aged between 71 and 75 years; further, stratified analysis was not performed for older women, and additional information regarding comorbidities was also not collected [2]. Moreover, genomic profile data was not included in earlier trials specifically designed for older women [29,31,32]. Therefore, clinicians are unable to provide information on treatment discussions for older women owing to the scarcity of published data. Moreover, a higher risk of competing risks of death and particularly susceptible to chemotherapy-related toxicities in older BC patients may also impact the chemotherapy decision making between clinicians and patients [16,17].
A previous SEER-Medicare study indicated that the rate of chemotherapy receipt was 3.7%, 9.5%, and 58.3% in low, intermediate, and high RS of patients aged between 65 and 74 years, respectively [33], which was similar to our results. However, the rate of chemotherapy receipt was still significantly lower than that of young patients. A recent SEER analysis showed that in patients aged 18–69 years, 9%, 38%, and 73% of patients who had undergone chemotherapy for low, intermediate, and high RS cohorts, respectively [27]. Another study from the United Kingdom showed a higher chemotherapy recommendation rate for intermediate RS (51.4%, median age: 55.2 years) and high RS (85.0%, median age: 55.2 years) cohorts [34]. However, a recent study from China, which included patients aged ≥60 years, reported that 11.6%, 46.0%, and 89.5% of patients for low, intermediate, and high RS groups, respectively, were recommended chemotherapy [35]. The higher percentage of chemotherapy recommendation in this population may be because patients with tumor size >5 cm (T3) stage and node-positive disease were also included in the analysis [35]. Moreover, the included patients, patient preferences, and clinical practice of chemotherapy recommendation based on ODX testing across different countries maybe also the reasons regarding this difference.
In our study, a total of 597 deaths occurred; of these, 25.6% were BC-related deaths and three quarters were attributed to competing events. Multiple studies also confirmed that more than 50% of deaths in older women were due to non-primary cancer reasons such as cardiac disorders, second primary tumor, cerebral disorder, and other causes of death [16,19]. Non-cancer-specific mortalities were also the main cause of death in older patients in our study. In the common survival analysis, competing events were regarded as censored data. Therefore, it is necessary to consider competing risk events when evaluating the risk of BCSM, as it could overestimate the risk of BCSM [36]. The competing-risks model may better reflect the actual mortality patterns in older patients with an unbiased estimate, while death owing to other causes was not censored, rather served as a competing risk event [[37], [38], [39]]. Therefore, the competing-risks model was more suitable than the Cox regression model in assessing prognosis and decision making for treatment among older patients and avoiding common statistical analysis errors.
The cumulative incidence of BCSM among the three RS classifications was significantly lower with the competing-risks model than the Cox regression model. There have been limited studies regarding the role of ODX testing in older population. Using Kaplan–Meier analysis, Stemmer et al. indicated that the 5-year rate of distant metastasis for patients aged ≥70 years was 0.6%, 3.8%, and 8.3% for low, intermediate, and high RS cohorts, respectively [40]. After long-term follow-up, the 10-year overall mortality rate was 0.7%, 2.2%, and 9.5% for low, intermediate, and high RS cohorts respectively [15]. However, several differences should be noted in the above studies, including no age stratification (only 16.0% patients were ≥70 years old), unrecorded comorbidities, fewer patients (only 30%) in the high RS cohort not receiving adjuvant chemotherapy, and no analysis of chemotherapy effect for the high RS cohort [15,18]. The results from the TAILORx (median age: 56 years) trial also showed lower rates of distant metastasis in all the three classifications, with the 5-year rate of distant metastasis being 0.7% (endocrine therapy alone), 1.9% (2.0% in endocrine therapy plus 1.8% in chemoendocrine therapy), and 7.0% (chemoendocrine therapy alone) for low, intermediate, and high RS cohorts, respectively [2]. Although SEER does not include information regarding endocrine therapy, the cumulative incidence of BCSM in older patients by using the competing-risks model was similar to the above studies, including TAILORx, which allowed us to assume that the receipt of endocrine did not bias our risk estimates. Therefore, according to our study, integration of ODX testing results with age and comorbidity may be useful to guide discussions with older women regarding the risks and benefits of chemotherapy. However, the risk of BCSM was extremely low in our study. In addition, older persons have higher death from other causes. Moreover, older persons in the United States have different preferences for chemotherapy as they age [13,14]. Therefore, the chemotherapy effect in older patients based on the RS classification needed further exploration.
We further analyzed the effect of chemotherapy in older women. Similar to the results from TAILORx [2], we found that chemotherapy could not decrease the risk of BCSM in the intermediate RS cohort. Although half of the patients in the high RS cohort did not receive chemotherapy, it was not associated with a lower risk of BCSM according to the Cox regression model and competing-risks model. Given more complications and chemotherapy intolerance in older patients [41], specific chemotherapeutic clinical trials in older patients face challenges during recruitment. The retrospective study by Kizy et al. indicated that while the receipt of chemotherapy for the high RS cohort could improve survival in young patients, it was not correlated with a better outcome in older patients [27]. However, it should be noted that only approximately 50% of older patients received chemotherapy, while 70% of young patients received chemotherapy. Also, patients with T3 and node-positive disease were also included in the study by Kizy et al. [27]. Additionally, the effect of chemotherapy was not analyzed in the intermediate RS cohort in their study [27].
The lack of chemotherapy efficacy in intermediate and high RS cohorts could be attributed to several reasons in our study. First, comorbidities in older women may influence chemotherapy selection to favor more tolerable but less efficacious regimens [42,43]. Additionally, older patients have a higher probability of not completing chemotherapy and/or dropping out because of the associated complications [44]. Chandler et al. showed that in older women with high RS, only patients aged between 65 and 74 years with no or low-to-moderate comorbidity might have minor benefits from chemoendocrine therapy [45]. However, this study was only a simulation model to investigate the chemotherapy effects in older women, and more studies are necessary to validate the effect of this simulation model. In the current clinical practice, the recommendation of chemotherapy in older patients with high RS should balance the advantages and disadvantages of chemotherapy.
Currently, there are no results from prospective, randomized, controlled trials addressing the use of ODX testing in older patients with node-positive disease. Whether the results of our findings can be applied to older node-positive BC patients to select patients suitable for chemotherapy or to spare them the use of cytotoxic chemotherapeutic agents should be further investigated. We expect the results of the Treatment (Rx) for Positive Node, Endocrine Responsive Breast Cancer (RxPONDER) clinical trial to clarify the role of chemotherapy in older patients with RS 25 or less [46].
We included a large cohort to categorize RS distribution and evaluate independent predictors correlated with chemotherapy receipt and the chemotherapy effect by using the RS assay in older women with BC. In addition, as older women are often excluded from clinical trials, a competing-risks analysis based on population-based data allows us to generalize useful information for clinical practice. However, our study does have some limitations. First, given the retrospective nature of this study, inherent bias is expected. Second, the SEER database lacks some important information on older patients, such as the detail of comorbidities, endocrine therapy, regimen, complications, and completion of chemotherapy. Third, another limitation of the analysis is the lack of data on the use of ODX testing in an older population of patients as the cohort of this analysis includes only patients who have been offered the ODX testing. Fourth, the lack of geriatric parameters also as an important limitation for this analysis, which is crucial to better inform decision-making in this very heterogeneous population of patients [47]. In addition, we did not have detailed information on distant recurrence, which is an important factor in assessing the prognosis and chemotherapy effect based on ODX testing. Finally, the median follow-up time was relatively short in the current study (61 months), while a substantial number of recurrences occurring in ER-positive and HER2-negative BC after 5 years.
5. Conclusion
In conclusion, approximately three-quarters of deaths occurring in non-BC-related disease in older breast cancer patients. The competing-risks model is useful to deal with multiple end events for this population. 21-gene RS was an independent prognostic indicator for BCSM in both the Cox regression and the competing-risks model. However, chemotherapy did not significantly decrease the risk of BCSM in the intermediate and high RS cohorts in both prognostic models. Future studies on the utility of the ODX testing for chemotherapy decision making in older patients are needed to determine more evidence-based strategies for the management of this population.
Funding
This work was partly supported by the National Natural Science Foundation of China (No. 81872459 and 81803050), the National Natural Science Foundation of Fujian Province (No. 2020J011240), and the Commission Young and Middle-aged Talents Training Project of Fujian Health Commission (No. 2019-ZQNB-25).
Declaration of competing interest
The authors have no conflicts of interest to disclose.
Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database.
Contributor Information
Zhen-Yu He, Email: hezhy@sysucc.org.cn.
San-Gang Wu, Email: unowu12345@hotmail.com.
References
- 1.Győrffy B., Hatzis C., Sanft T. Multigene prognostic tests in breast cancer: past, present, future. Breast Cancer Res. 2015;17(1):11. doi: 10.1186/s13058-015-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sparano J.A., Gray R.J., Makower D.F. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paik S., Shak S., Tang G. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 4.Marshall S.F., Clarke C.A., Deapen D. Recent breast cancer incidence trends according to hormone therapy use: the California Teachers Study cohort. Breast Cancer Res. 2010;12(1):R4. doi: 10.1186/bcr2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battisti N.M.L., McCartney A., Biganzoli L. The conundrum of the association of chemotherapy with survival outcomes among elderly patients with curable luminal breast cancer. JAMA Oncol. 2020;6(10):1535–1537. doi: 10.1001/jamaoncol.2020.2194. [DOI] [PubMed] [Google Scholar]
- 6.Cappellani A., Di Vita M., Zanghì A. Prognostic factors in elderly patients with breast cancer. BMC Surg. 2013;13 doi: 10.1186/1471-2482-13-S2-S2. Suppl 2(Suppl 2):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blows F.M., Driver K.E., Schmidt M.K. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5) doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris L.N., Ismaila N., McShane L.M. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American society of clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34(10):1134–1150. doi: 10.1200/JCO.2015.65.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardoso F., Kyriakides S., Ohno S. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network NCCN guidelines version 1.2020 breast cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [DOI] [PubMed]
- 11.Dowsett M., Cuzick J., Wale C. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 12.Albain K.S., Barlow W.E., Shak S. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallwiener C.W., Hartkopf A.D., Grabe E. Adjuvant chemotherapy in elderly patients with primary breast cancer: are women ≥65 undertreated? J Canc Res Clin Oncol. 2016;142(8):1847–1853. doi: 10.1007/s00432-016-2194-4. [DOI] [PubMed] [Google Scholar]
- 14.Yardley D.A. vol. 7. Dove Med Press; 2015. pp. 293–301. (Taxanes in the elderly patient with metastatic breast cancer. Breast Cancer). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stemmer S.M., Steiner M., Rizel S. Ten-year clinical outcomes in N0 ER+ breast cancer patients with Recurrence Score-guided therapy. NPJ Breast Cancer. 2019;5:41. doi: 10.1038/s41523-019-0137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasif N., Neville M., Gray R. Competing risk of death in elderly patients with newly diagnosed stage I breast cancer. J Am Coll Surg. 2019;229(1):30–36. doi: 10.1016/j.jamcollsurg.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Wilson T., Dyke C., Reed H. Assessing the tolerability and efficacy of first-line chemotherapy in elderly patients with metastatic HER2-ve breast cancer. Ecancermedicalscience. 2019;13:921. doi: 10.3332/ecancer.2019.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scrucca L., Santucci A., Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40(4):381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 19.Derks M.G.M., Bastiaannet E., van de Water W. Impact of age on breast cancer mortality and competing causes of death at 10 years follow-up in the adjuvant TEAM trial. Eur J Canc. 2018;99:1–8. doi: 10.1016/j.ejca.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.T. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Canc Res. 2007;13(2 Pt 1):559–565. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 21.Surveillance, Epidemiology, and End Results (Seer) Program SEER∗Stat database: incidence - SEER 18 regs (excl AK) custom data malignant breast (with oncotype DX and additional treatment fields), nov 2017 sub (2004-2015) - linked to county attributes - total U.S., 1969-2016 counties, national cancer institute, DCCPS, surveillance research program, released april 2018, based on the november 2017 submission. www.seer.cancer.gov
- 22.Shenoy P., Harugeri A. Elderly patients’ participation in clinical trials. Perspect Clin Res. 2015;6(4):184–189. doi: 10.4103/2229-3485.167099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zizza C.A., Ellison K.J., Wernette C.M. Total water intakes of community living middle-old and oldest-old adults. J Gerontol A Biol Sci Med Sci. 2009;64(4):481–846. doi: 10.1093/gerona/gln045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surveillance, Epidemiology, and End Results (Seer) Program . National Center for Health Statistics; 2019. SEER∗Stat database: MortalityAll COD, total US (1990-2017) <Early release with vintage 2017 katrina/rita population Adjustment> linked to county AttributesTotal US, 19692017 counties. National cancer institute, division of cancer control and population sciences, surveillance research program; 2019; underlying mortality data provided by. [Google Scholar]
- 25.Howlader N., Ries L.A., Mariotto A.B. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584–1598. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Southern D.A., Faris P.D., Brant R. Kaplan-Meier methods yielded misleading results in competing risk scenarios. J Clin Epidemiol. 2006;59(10):1110–1114. doi: 10.1016/j.jclinepi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Kizy S., Altman A.M., Marmor S. 21-gene recurrence score testing in the older population with estrogen receptor-positive breast cancer. J Geriatr Oncol. 2019;10(2):322–329. doi: 10.1016/j.jgo.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Dinan M.A., Mi X., Reed S.D. Initial trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the medicare population, 2005-2009. JAMA Oncol. 2015;1(2):158–166. doi: 10.1001/jamaoncol.2015.43. [DOI] [PubMed] [Google Scholar]
- 29.Muss H.B., Berry D.A., Cirrincione C.T. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman R.A., Foster J.C., Seisler D.K. Accrual of older patients with breast cancer to alliance systemic therapy trials over time: protocol A151527. J Clin Oncol. 2017;35(4):421–431. doi: 10.1200/JCO.2016.69.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muss H.B., Berry D.A., Cirrincione C. Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25(24):3699–3704. doi: 10.1200/JCO.2007.10.9710. [DOI] [PubMed] [Google Scholar]
- 32.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Peto R., Davies C. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariotto A., Jayasekerea J., Petkov V. Expected monetary impact of oncotype DX score-concordant systemic breast cancer therapy based on the TAILORx trial. J Natl Cancer Inst. 2020;112(2):154–160. doi: 10.1093/jnci/djz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loncaster J., Armstrong A., Howell S. Impact of Oncotype DX breast Recurrence Score testing on adjuvant chemotherapy use in early breast cancer: real world experience in Greater Manchester, UK. Eur J Surg Oncol. 2017;43(5):931–937. doi: 10.1016/j.ejso.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Zeng Y., Gao W., Lin L. Impact of 21-gene recurrence score testing on adjuvant chemotherapy decision making in older patients with breast cancer. J Geriatr Oncol. 2019;11(5):843–849. doi: 10.1016/j.jgo.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 36.de Glas N.A., Kiderlen M., Vandenbroucke J.P. Performing survival analyses in the presence of competing risks: a clinical example in older breast cancer patients. J Natl Cancer Inst. 2015;108(5):djv366. doi: 10.1093/jnci/djv366. [DOI] [PubMed] [Google Scholar]
- 37.Cronin K.A., Feuer E.J. Cumulative cause-specific mortality for cancer patients in the presence of other causes: a crude analogue of relative survival. Stat Med. 2000;19(13):1729–1740. doi: 10.1002/1097-0258(20000715)19:13<1729::aid-sim484>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Schairer C., Mink P.J., Carroll L. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96(17):1311–1321. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 39.Yang L., Shen W., Sakamoto N. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer and other causes among patients with thyroid cancer. J Clin Oncol. 2013;31(4):468–474. doi: 10.1200/JCO.2012.42.4457. [DOI] [PubMed] [Google Scholar]
- 40.Stemmer S.M., Steiner M., Rizel S. Clinical outcomes in patients with node-negative breast cancer treated based on the recurrence score results: evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017;3:33. doi: 10.1038/s41523-017-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loibl S., Reinisch M. Present status of adjuvant chemotherapy for elderly breast cancer patients. Breast Care. 2012;7(6):439–444. doi: 10.1159/000345867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kornblith A.B., Lan L., Archer L. Quality of life of older patients with early-stage breast cancer receiving adjuvant chemotherapy: a companion study to cancer and leukemia group B 49907. J Clin Oncol. 2011;29(8):1022–1028. doi: 10.1200/JCO.2010.29.9859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muss H.B., Berry D.A., Cirrincione C.T. Adjuvant chemotherapy in older women with early-stage breast cancer. N Engl J Med. 2009;360(20):2055–2065. doi: 10.1056/NEJMoa0810266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaplan H.G., Malmgren J.A., Atwood M.K. Adjuvant chemotherapy and differential invasive breast cancer specific survival in elderly women. J Geriatr Oncol. 2013;4(2):148–156. doi: 10.1016/j.jgo.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Chandler Y., Jayasekera J., Schechter C. Simulation of chemotherapy effects in older breast cancer patients with high recurrence scores. J Natl Cancer Inst. 2020;112(6):574–581. doi: 10.1093/jnci/djz189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamoxifen citrate, letrozole, anastrozole Or exemestane with or without chemotherapy in treating patients with invasive RxPONDER breast cancer-full text view-ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01272037
- 47.Mohile S.G., Dale W., Somerfield M.R. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric Oncology. J Clin Oncol. 2018;36(22):2326–2347. doi: 10.1200/JCO.2018.78.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]