Abstract
Objective
There is sparse evidence for the impact of gene-diet interaction on gestational diabetes mellitus (GDM) onset. Recent findings have shown that late first-trimester high adherence to a Mediterranean diet (MedDiet) pattern is associated with a GDM risk reduction. The aim of this study was to investigate if this effect could be modulated by TCF7L2 rs7903146 polymorphism.
Research design and methods: A total of 874 pregnant women participants in the St Carlos GDM prevention study, were stratified into three groups defined as “High,5–6 on targets”, “Moderate, 2–4 on targets” or “Low, 0–1 on targets” adherence to Mediterranean diet according to late first-trimester compliance with six food targets: >12 servings/week of vegetables, >12 pieces/week of fruits, <2 servings/week of juice, >3 servings/week of nuts, >6 days/week and >40 mL/day consumption of extra virgin olive oil. All patients were genotyped for rs7903146 using Taqman technology.
Results
Logistic regression analysis revealed that the risk of developing GDM in those with high adherence versus low adherence was significantly reduced only in carriers of the T-allele (CT + TT), with an adjusted odds ratio of 0.15 (95% CI:0.05–0.48). This effect was not observed in CC carriers. Interaction analysis yielded significant rs7903146-MedDiet interaction in GDM risk (p < 0.03)
Conclusions
Women carrying the rs7903146 T-allele who highly adhere to a MedDiet early in pregnancy have lower risk of developing GDM than CC carriers. This reinforces the importance of identifying patients at risk of GDM who would be especially sensitive to nutritional interventions based on their genetic characteristics.
Keywords: Gestational diabetes mellitus, Mediterranean diet, Nutritional intervention, TCF7L2 polymorphism, Gene-diet interaction
1. Introduction
Increasing evidence suggests that dietary patterns during pregnancy have an important role in the development of gestational diabetes mellitus (GDM). Our group has previously reported that adopting healthy dietary patterns early on in pregnancy reduces the risk of developing GDM. Moreover, the adherence to a Mediterranean Diet (MedDiet) supplemented with extra virgin olive oil (EVOO) and pistachios- or just emphasizing the recommendations of a daily consumption of EVOO and nuts, was associated with a 30% reduction in the incidence of GDM [[1], [2], [3]]. Furthermore, we have recently shown that GDM risk can be modified depending on the degree of adherence to a MedDiet. There seems to be an inverse linear relationship between late first-trimester (>12 gestational week-GW) degree of adherence to a MedDiet pattern and the incidence of GDM, where having a high adherence is associated with a 65% reduction GDM risk [4].
In recent years, nutrigenetic studies have provided growing evidence that genetic variants confer individual differences in response to nutritional interventions and that a gene-diet interaction may modify Type 2 diabetes mellitus (T2DM) risk. Several association studies and meta-analysis have demonstrated that GDM may share the same genetic susceptibilities with T2DM. Genome-wide association (GWA) studies have provided strong evidence that single nucleotide polymorphisms (SNPs) within Transcription Factor 7 Like 2 (TCF7L2) gene influence both T2DM and GDM risk [[5], [6], [7], [8]].
The TCF7L2 gene encodes T-cell transcription factor 4 (TCF4) that plays a key role in the Wnt signaling pathway. It is crucial in the beta-cell genesis and function and it is considered as a main regulator of glucose homeostasis. The T-allele of the rs7903146 (C/T) SNP in TCF7TL2 has been significantly associated with an increased risk of T2DM and GDM. Although it is an intronic variant it has a functional effect by modulating the transcriptional machinery that regulates the glucose response. In fact, T-allele has been associated with impaired beta-cell function and insulin secretion and lower insulin levels [9].
Some studies have reported that rs7903146 T-allele modifies the beneficial effect of specific foods, like whole-grain intake, on T2DM risk while others have not [10,11]. There are scarce data on the influence of an overall healthy food pattern, such as the MedDiet pattern, in modulating the associations between the rs7903146 polymorphism and T2DM risk. Corella et al. demonstrated that MedDiet significantly interacts with rs7903146 on fasting blood glucose (FBG) at baseline, reporting higher fasting glucose concentrations in TT carriers than CC + CT individuals when adherence to MedDiet was low. Conversely when the adherence was high this increase was not observed [12].
As far as we know, no rs7903146–diet interaction data have been published for GDM. Therefore, the aim of this study was to investigate whether the association between the degree of adherence to a MedDiet pattern ‒ based on six food targets‒ at the end of the first trimester (<12 GW) and GDM incidence is modulated by a TCF7L2 rs7903146 polymorphism.
2. Research design and methods
2.1. Study design and participants
We included 874 women entering The St. Carlos GDM prevention study [2]. This was a randomized controlled trial aimed to assess the effect of an intervention based on MedDiet supplemented with EVOO and pistachios on the incidence of GDM. This paper is based on a posterior post hoc analysis evaluating the associations of late first-trimester degrees of adherence to the six food targets (high, moderate, and low) with the risk of GDM. The trial was conducted from January 1st to December 31st, 2015 and the follow-up finished on July 2016. Details of these studies have been thoroughly described elsewhere [2,4].
The primary objective of this study is to evaluate the influence of the TCF7L2 rs7903146 polymorphism on the effect of the different degrees of adherence to the MedDiet on the incidence of GDM.
Briefly, all pregnant women who attended the first gestational visit at 8–12 GWs and had a normal fasting serum glucose level (<92 mg/dL) were invited to participate in this study. Inclusion and exclusion criteria were previously detailed [2,4]. GDM was diagnosed at 24–28 GWs with a single 2-h 75-g oral glucose tolerance test (OGTT), applying International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria.
Participants were randomized at 12–14 GWs to a control or intervention group. Both groups were followed up at baseline (visit 1); GDM screening, performed at 24–28 GWs (visit 2); 36–38 GWs (visit 3); and delivery. The two groups received dietary guidelines, based on MedDiet principles. The intervention group attended a group session where they were instructed to enhance the consumption of EVOO and nuts. Meanwhile, the control group was told to limit the consumption of all types of fats. A semi-quantitative frequency questionnaire based on the Diabetes Nutrition and Complications Trial (DNCT) study and the 14-point Mediterranean Diet Adherence Screener (MEDAS), adapted for pregnancy, were used to evaluate lifestyle patterns and applied at each follow-up visit. A detailed description of how lifestyle and diet were evaluated has been published previously [2,4].
For the present post-hoc analysis, the variables were retrieved from the main study database. The study population was treated as a cohort, independent of randomization assignment. The sample was stratified into three groups according to the degree of adherence to the MedDiet in late first trimester (from 12 to 14 to 24–28 GWs) as previously described [4]. Six food targets were chosen from the MedDiet. These were an intake of ≥40 mL/day of EVOO, an intake of EVOO >6 times/week, and an intake of >3 servings/week of nuts, >12 servings/week of vegetables, >12 pieces/week of whole fruits, <2 servings/week of juice. A high adherence was set for achieving 5–6 targets; moderate for 2–4 targets; and low for 0–1 targets. The post-hoc analysis of the data verified that the degree of compliance to the six food targets correlated positively with both the MEDAS and Nutrition scores and that the use of these six food-groups could be able to estimate an optimal adherence to MedDiet [4].
The study was approved by the Ethical Committee of the Hospital Clínico San Carlos (July 17, 2013 (CI 13/296-E)) and conducted according to the Helsinki Declaration. All patients signed informed consent.
2.2. Participant’s history, anthropometric data and biochemical analysis
A family history of T2DM and metabolic syndrome, obstetric history of GDM and miscarriages, educational status, employment, number of prior pregnancies, smoking habit, and gestational age at entry were recorded at baseline in visit 1. Blood pressure, gestational weight gain, and body mass index (BMI) were evaluated and recorded at 8–12, 24–28 and 36–38 GWs. Pre-gestational body weight (BW) was self-referred and registered at Visit 1. Laboratory tests were scheduled for each visit. Blood was drawn after an overnight fast. Serum levels of glucose, lipids and other parameters were determined using standard automated methods as previously described (4).
2.3. Genotyping
Genomic DNA was extracted with the DNAzol reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer’s recommendations. Genotyping was performed using a predesigned TaqMan assay for rs7903146 (C_29347861_10) using a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). A genotyping call rate over 95% per plate, negative sample controls and three well-differentiated genotyping clusters were required to validate results. Intra and interplate duplicates of several DNA samples were included.
2.4. Statistical analysis
Categorical variables were expressed with their frequency distribution and continuous variables as means and standard deviation (SD) or means and 95% confidence interval (CI). The Saphiro–Wilk test was used to verify normal distribution of the data. For continuous variables, the Kruskall–Wallis test or a one-way analysis of variance (ANOVA) were applied. SNPStats software was used to evaluate Hardy–Weinberg equilibrium and the association between SNPs and GDM risk under multiple inheritance models: co-dominant, dominant, recessive, over-dominant and log-additive [13]. For each polymorphism, Odds Ratio (ORs) and 95% CIs were calculated by unconditional logistic regression analysis. Dominant homozygotes were selected as reference. The Chi square for linear trends and unadjusted logistic regression analysis (women with a low adherence as the reference group) was performed, to evaluate the relationship between the different degrees of adherence and GDM and genotype. For the GDM outcomes, a subgroup analysis was conducted, introducing the interaction term between the adherence and the stratification variables. All p-values are 2-tailed at less than 0.050. Analyses were done using SPSS, version 21 (SPSS, Chicago, IL, USA).
3. Results
Table 1 summarizes the socio-demographic, clinical, biochemical, and genetic characteristics of women according to late first-trimester degree of adherence to six food targets. The cohort was ethnically heterogeneous, the majority of Caucasian origin. Most participants were primiparous. As expected, significantly different genotype frequencies between ethnic populations were observed (p < 0.001), but not between the adherence groups, considering all the sample or according the different ethnic group. The “others” group was a heterogeneous group mainly composed by African Americans and Asians. There were differences between groups in the distributions of ethnicity, family history of T2DM and metabolic syndrome, education level, and parity.
Table 1.
Characteristics of the clinical trial population at baseline according to late first-trimester degree of adherence to the six food targets.
| Variables | Groups |
p value | ||
|---|---|---|---|---|
| Low adherence (n = 136/15.6%) | Moderate adherence (n = 623/71.3%) | High adherence (n = 115/13.1%) | ||
| Age (years) | 31.2 ± 6.0 | 33.1 ± 4.9 | 33.9 ± 4.9 | 0.001 |
| Race/Ethnicity | ||||
| Caucasian | 79 (58.1) | 420 (67.4) | 88 (76.5) | 0.005 |
| Hispanic | 51 (37.5) | 188 (30.2) | 21 (18.3) | |
| Others |
6 (4.4) |
15 (2.4) |
6 (5.2) |
|
| TCF7L2 Genotype | ||||
| All | ||||
| C/C | 60 (45.5) | 300 (48.4) | 50 (47.6) | 0.603 |
| C/T | 59 (44.7) | 259 (41.8) | 49 (46.7) | |
| T/T | 13 (9.8) | 61 (9.8) | 6 (5.7) | |
| Caucasian | ||||
| C/C | 26 (34.2) | 166 (39.8) | 37 (44) | 0.291 |
| C/T | 40 (52.6) | 197 (47.2) | 42 (50) | |
| T/T | 10 (13.2) | 54 (12.9) | 5 [6] | |
| Hispanic | ||||
| C/C | 30 (60) | 125 (66.5) | 10 (58.8) | 0.671 |
| C/T | 18 [36] | 57 (30.3) | 7 (41.2) | |
| T/T | 2 (4) | 6 (3.2) | 0 (0) | |
| Others | ||||
| C/C | 4 (66.7) | 9 (60) | 3 (75) | 0.460 |
| C/T | 1 (16.7) | 5 (33.3) | 0 (0) | |
| T/T |
1 (16.7) |
1 (6.7) |
1 [25] |
|
| Family history of: | ||||
| Type 2 diabetes | 28 (20.6) | 131 (21.0) | 21 (18.3) | 0.019 |
| MetS (>2 components) |
18 (13.2) |
135 (21.7) |
30 (26.1) |
|
| Previous history of: | ||||
| Gestational diabetes | 7 (5.1) | 15 (2.4) | 3 (2.6) | 0.342 |
| Miscarriages |
45 (33.1) |
196 (31.5) |
37 (32.2) |
|
| Educational status | ||||
| Elementary education | 19 (14.0) | 54 (8.7) | 5 (4.3) | 0.001 |
| Secondary school | 49 (36.0) | 145 (23.3) | 18 (15.7) | |
| University degree | 65 (47.8) | 420 (67.4) | 92 (80.0) | |
| UNK |
3 (2.2) |
4 (0.6) |
0 (0) |
|
| Employment | 100 (73.5) | 482 (77.4) | 92 (80.0) | 0.815 |
| Number of pregnancies | ||||
| Primiparous | 50 (36.8) | 274 (44.0) | 54 (47.8) | 0.017 |
| Second pregnancy | 43 (31.6) | 196 (31.5) | 42 (36.8) | |
| >2 pregnancies |
43 (31.6) |
153 (24.5) |
19 (15.4) |
|
| Smoker | ||||
| Never | 75 (55.1) | 334 (53.6) | 68 (59.1) | 0.260 |
| Current | 16 (11.8) | 52 (8.3) | 4 (3.5) | |
| Gestational age (weeks) at baseline |
12.1 ± 0.7 |
12.1 ± 0.5 |
12.0 ± 0.5 |
0.137 |
| Body Weight (kg) | ||||
| Prepregnancy | 61.1 ± 11.8 | 61.0 ± 10.8 | 59.5 ± 9.3 | 0.383 |
| At entry | 63.3 ± 11.3 | 63.1 ± 11.1 | 61.5 ± 8.4 | 0.356 |
| Weight gain |
2.2 ± 3.2 |
2.0 ± 3.0 |
2.0 ± 2.7 |
0.707 |
| BMI (kg/m2) | ||||
| Prepregnancy | 23.4 ± 4.1 | 23.2 ± 3.8 | 22.5 ± 3.4 | 0.137 |
| At baseline |
24.3 ± 4.3 |
23.9 ± 3.9 |
23.3 ± 3.5 |
0.111 |
| Blood pressure (mm Hg): | ||||
| Systolic | 107 ± 10 | 107 ± 11 | 107 ± 10 | 0.972 |
| Diastolic |
66 ± 15 |
66 ± 9 |
66 ± 8 |
0.809 |
| Fasting blood glucose mg/dL | 82 ± 5 | 81 ± 6 | 81 ± 7 | 0.377 |
| HbA1c % | 5.2 ± 0.2 | 5.2 ± 0.3 | 5.1 ± 0.3 | 0.436 |
| Cholesterol mg/dL | 171 ± 28 | 175 ± 31 | 176 ± 25 | 0.507 |
| Triglycerides mg/dl | 82 ± 38 | 83 ± 42 | 76 ± 30 | 0.288 |
| TSH mcUI/mL | 1.9 ± 1.2 | 2.0 ± 1.3 | 2.1 ± 1.4 | 0.610 |
| T4L ng/dL | 8.6 ± 1.3 | 8.6 ± 1.6 | 8.9 ± 1.2 | 0.144 |
Data are Mean ± SD or n(%). MetS, Metabolic Syndrome. UNK, unknown. BMI, body mass index; MEDAS Score: Mediterranean Diet Adherence Screener Score. P Differences between groups analysed with ANOVA (continuous variables) and χ2 test (categorical variables). MetS, Metabolic Syndrome. UNK, unknown. BMI, body mass index).
3.1. Genotype association with GDM
Genotype frequencies did not deviate from Hardy-Weinberg equilibrium expectations. The SNPs was significantly associated with lower OR of having a GDM under codominant, dominant and overdominant models, at the expense of heterozygotes CT in the crude analysis. However, after adjustment for ethnicity, family history of metabolic disorders, parity, and BMI, only the protective effect of heterozygote CT genotype remained significant under overdominant model (Table 2).
Table 2.
TCF7L2 rs7903146 genotype association with GDM.
| Model |
Genotype | No GDM | GDM |
Crude analysis |
p trend |
Adjusted analysis∗ |
OR (95% CI) | p trend | AIC |
|---|---|---|---|---|---|---|---|---|---|
| All (n = 857) | OR (95% CI) | AIC | |||||||
| Codominant | C/C | 316 (46.1%) | 94 (54.6%) | 1.00 | 0.038 | 858.8 | 1.00 | 0.09 | 825.3 |
| C/T | 308 (45%) | 59 (34.3%) | 0.64 (0.45–0.92) | 0.69 (0.47–1.01) | |||||
| T/T | 61 (8.9%) | 19 (11.1%) | 1.05 (0.60–1.84) | 1.12 (0.62–2.02) | |||||
| Dominant | C/C | 316 (46.1%) | 94 (54.6%) | 1.00 | 0.046 | 859.4 | 1.00 | 0.13 | 825.7 |
| C/T-T/T | 369 (53.9%) | 78 (45.4%) | 0.71 (0.51–0.99) | 0.76 (0.53–1.08) | |||||
| Recessive | C/C–C/T | 624 (91.1%) | 153 (89%) | 1.00 | 0.4 | 862.6 | 1.00 | 0.33 | 827.2 |
| T/T | 61 (8.9%) | 19 (11.1%) | 1.27 (0.74–2.19) | 1.33 (0.76–2.35) | |||||
| Overdominant | C/C-T/T | 377 (55%) | 113 (65.7%) | 1.00 | 0.011 | 856.9 | 1.00 | 0.03 | 823.4 |
| C/T | 308 (45%) | 59 (34.3%) | 0.64 (0.45–0.91) | 0.68 (0.47–0.97) | |||||
| Log-additive | – | – | – | 0.86 (0.66–1.11) | 0.25 | 862 | 0.90 (0.69–1.19) | 0.47 | 827.6 |
Genotype distributions are shown as n(%) and odds ratio 95% CI (OR). CI, confidence interval; GDM, gestational diabetes mellitus; AIC, Values of Akaike’s Information Criterion; P-trend values were calculated from adjusted or unadjusted logistic regression analysis for codominant, dominant, and recessive models. ∗Adjusted logistic regression by age, ethnicity, parity, family history of DM and pregestational BMI.
TCF7L2 rs7903146-T variant was also significantly associated with mean BMI in all models except the recessive in the crude analysis (Supplementary Table S1). However, after stratifying by BMI there were no differences in GDM risk between groups (obese diabetics vs obese controls, p = 0451; non-obese diabetics vs non-obese controls, p = 0169). Moreover, when we repeated the same analysis adjusted for by age, ethnicity, parity and family history of DM, all significations were lost. Nonetheless, considering the differences found in genotype frequencies between ethnic groups and the genotype association with BMI, data were also adjusted for BMI in all the interaction analyses.
3.2. Associations and interaction between late first-trimester degrees of adherence and GDM risk
Crude logistic regression analysis of the risk of having GDM according to the degree of adherence in the whole sample and stratified by rs7903146 genotype showed that the higher the adherence to the six food targets, the lower the incidence and risk of GDM in all groups, but it seems to be only at the expense of heterozygotes CT or carriers of the T-allele (CT + TT). In carriers of the CC genotype the risk among the three groups is almost unchanged, however none of the TT carriers developed GDM. Considering this, for the following analyses the CT and TT genotypes were grouped together (Supplementary Table S2).
Table 3 shows adjusted analysis of data by age, ethnicity, parity, family history of DM and pregestational BMI. Only in carriers of the T-allele (CT + TT) the risk of developing GDM in those with moderate and high adherence was significantly reduced compared to women with a low adherence, both when taking as the reference group the low adherence group (85% risk reduction) or the low adherence plus C/C genotype group (77% risk reduction). Only a small percentage (7.4%) of carriers of the T-allele that were highly adherent developed GDM, while the majority did not (92.6%). This effect is not observed in carriers of the CC genotype. All the interaction analysis, crude or adjusted yielded significant rs7903146-MedDiet interaction in GDM risk (p < 0.04).
Table 3.
Association between late first-trimester degree of adherence to the six food targets and GDM risk stratified by TCF7L2 rs7903146 genotype. Interaction analysis between genotypes and adherence (n = 856, in crude and adjusted by age, ethnicity, parity, family history of DM and BMI).
| No GDM | GDM | ∗Crude OR (95% CI) | ∗Adjusted OR (95% CI) | †Crude OR (95% CI) | †Adjusted OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| rs7903146 C/C | Low adherence | 46 (76.6) | 14 (23.3) | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate adherence | 231 (77.0) | 69 (23.0) | 0.98 (0.51–1.89) | 0.83 (0.42–1.66) | 0.98 (0.51–1.89) | 0.83 (0.42–1.64) | |
| High adherence | 39 (78.0) | 11 (22.0) | 0.93 (0.38–2.27) | 1.06 (0.42–2.72) | 0.93 (0.38–2.27) | 1.06 (0.42–2.72) | |
| rs7903146 C/T + T/T | Low adherence | 49 (68.0) | 23 (31.9) | 1.00 | 1.00 | 1.54 (0.71–3.35) | 1.53 (0.67–3.47) |
| Moderate adherence | 269 (84.0) | 51 (15.9) | 0.40 (0.23–0.72) | 0.39 (0.21–0.741 | 0.62 (0.32–1.22) | 0.60 (0.29–1.21) | |
| High adherence | 50 (92.6) | 4 (7.4) | 0.17 (0.05–0.52) | 0.15 (0.05–0.48) | 0.26 (0.08–0.84) | 0.23 (0.07–0.77) | |
| p-interaction | 0.035 | 0.025 | 0.035 | 0.025 | |||
Data are n(%) Logistic regression analysis by genotype subgroups evaluating degree of adherence to the six food targets and risk of GDM; p-interaction: logistic regression analysis evaluating interactions between degree of adherence and genotypes. ∗ Analysis with no GDM and Low adherence as reference; †Analysis with no GDM, Low adherence and C/C genotype as reference.
We also performed the interaction analysis looking at OGTT glucose levels (Supplementary Table S3). We did not find any rs7903146 modulatory effect on the FBG, but at min 60, women with TT genotype and high adherence to the MedDiet, had lower blood glucose levels (113.67 mg/dl) than those with low adherence (136.33 mg/dl). The interaction disappeared at 120 min. Women carrying the C-allele reached similar blood glucose levels despite degrees of adherence to the MedDiet.
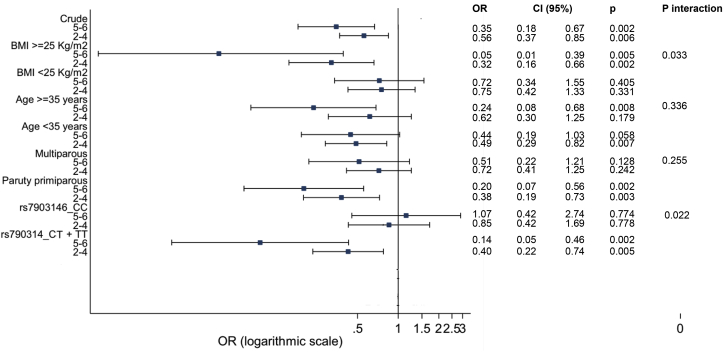
Fig. 1 shows the logistic regression analysis by subgroups for the probability of having GDM according to the level of adherence (in crude and stratified by BMI, age and parity). We have previously reported an interaction between BMI and the level of adherence (high versus low and moderate versus low) for the incidence of GDM (p = 0.033) in the way that the protective effect of having a high adherence to the six food targets was higher in women who are overweight and obese than those with a normal weight, both in the crude and adjusted analysis [4]. This effect almost overlaps with that of the T-allele. Overweight/obese women or carriers of the T-allele are those who benefit most from the protective effect of the high adherence to the six food targets (BMI OR 0.04, 95% CI 0.005–0.342, p = 0.003; rs7903146-T carriers OR 0.14, 95% CI 0.05–0.46).
Fig. 1.
Logistic regression analysis by subgroups (crude and stratified by BMI, age and parity) evaluating degree of adherence to the six food targets and risk of GDM. p: logistic regression analysis comparing ORS with the group of reference (low adherence group). p-interaction: logistic regression analysis evaluating interactions between degree of adherence and BMI (≥25 or <25 kg/m2), age (≥35 or <35 years), parity (multiparous or primiparous) and rs7903146 (CT + TT or CC). OR: odds ratio; CI: confidence interval; BMI: body mass index. High: high adherence, 5–6 targets; Moderate: moderate adherence, 2–4 targets; Low: low adherence, 0–1 targets.
4. Discussions
An inverse relationship between high-, moderate-, and low-adherence to six food targets of the MedDiet at the end of the first trimester (>12 GW) and GDM risk has been previously reported. Having a high or moderate adherence has been associated with a 65% or 44% GDM risk reduction respectively (4). The results of the current study demonstrated that TCF7L2 rs7903146 polymorphism may modulate this effect of the MedDiet on the incidence of GDM.
Some studies have reported gene-lifestyle interactions regarding T2DM incidence or GDM development [14], although the strength of evidence is still weak. TCF7L2 rs7903146 has been the most widely associated polymorphism with GDM risk across different populations. Most studies revealed that the rs7903146 T-allele was positively linked with GDM and have reported significant associations with global ORs of up to 1.65 [7,8,15], although some findings yielded controversial results [6,[16], [17], [18]]. In this study we found that the genotype TT was more frequent in GDM patients (11,1% vs 8,9%) but the difference was not statistically significant. On the contrary, carriers of CT genotypes had around 33% significant lower risk of developing GDM in both the crude and adjusted analysis Although, it cannot be ruled out that a true overdominance or advantage of heterozygotes may be the cause of the discrepancy with the published results, it may also be attributed to the fact that the sample is not large enough to detect T associated risk, or due to differences in ethnicity, the GDM diagnostic criteria applied or even the laboratory technique used for the allele detection.
In agreement with the current work, the T-allele was not significantly associated to GDM in various studies in Spanish and other Caucasian populations, with similar risks obtained to ours [[19], [20], [21], [22]]. Some authors have also found no association between rs7903146 and GDM risks in Euro-Brazilian or in Mexican population [23,24]. It is remarkable that in a recent meta-analysis from the combing results of 24 relevant publications including samples with different ethnicities, Wang et al. describe similar results to ours in the crude analysis in the whole sample, with analogous global ORs in all comparison models [15]. Apart from ethnicity, there is no apparent evidence to support that the diagnostic criteria using different OGTT could influence this association [7]. In agreement with our results, Klein et al. reported that in Caucasian women who were prospectively screened for GDM according to the IADPSG criteria, the T-allele was protective for GDM although the association was not statistically significant [25]. Curiously, Wu et al. have shown in their meta-analysis, that allelic detection of rs7903146 with another technique than the Taqman assays used in our study, was often significantly associated with increased risk of GDM because the T-allele was not properly identified in heterozygotes, consequently the homozygous genotypes were overrepresented [7]. Finally, as negative studies are less likely to be published, non-significant genetic associations might have been underreported, potentially leading to an overestimation of positive effects.
Interestingly, although our data have not been able to demonstrate an association between the presence of the T-allele and the risk of developing GDM, we found a significant gene-lifestyle interaction between rs7903146 and the degree of adherence to a MedDiet in the onset of GDM. We have shown that only in T-carriers the nutritional intervention modified the risk of developing GDM in a manner that women with moderate and high adherence had a reduced GDM risk compared to women with low adherence. This effect became significant when adjusted by age, ethnicity, parity, and gestational BMI and was not observed in carriers of the CC genotype. To the best of our knowledge this is the first time that it has been published a gene-diet interaction study investigating the modulatory influence of rs7903146 in the associations of an overall food pattern, such as the MedDiet, with GDM risk. Only a few studies of gene-lifestyle interactions and their influence on GDM development have been published. Popova et al. [22] and Grotenfelt et al. [26] found respectively an interaction effect of rs10830963-MTNR1B and rs1799884-GCK and the frequency of sausage intake on the risk of developing GDM.
Most nutrient-gene interactions studies have been conducted in T2DM. Opposite to our findings, some publications reported that the protective effect of whole-grain and fiber intake on diabetes risk was inversely associated with diabetes risk exclusively among rs7903146 CC homozygote carriers, whereas subjects carrying the T-allele seemed to benefit less or exhibit no benefit from whole-grain consumption [10,11]. However, other authors have also failed to replicate this interaction [27,28]. The majority of the studies show that T-carriers benefit most from a nutritional intervention. One publication based on data from EPIC-InterAct found a significant interaction between rs12255372, a polymorphism that is strong linkage disequilibrium with rs7903146, and coffee consumption, where the inverse association of coffee intake and T2DM was only present among participants carrying the risk-conferring T-allele [29].
Our results may be in agreement with those of Corella et al. that found a statistically significant interaction between adherence to an overall food pattern-a MedDiet supplemented with EVOO or nuts or low fat- and the rs7903146 polymorphism influencing fasting glucose concentrations. Only in TT carriers, women with low adherence to MedDiet had higher FBG levels than those with high adherence. This effect was not observed in CC + CT carriers where the FBG levels were similar regardless the MedDiet adherence score [12].
The mechanism by which rs7903146 variant alters diabetes risk remains unknown. TCF7L2, encodes a high mobility group box-containing transcription factor that plays a key role in the Wnt/β-catenin signaling pathway. Multiple studies have demonstrated that TCF7L2 rs7903146 T-variant is associated with both impaired insulin secretion due to defective β-cell genesis or function and with impaired insulinotropic effect of incretins [9,30,31]. Conversely, some authors have outlined a tendency to develop higher peripheral insulin sensitivity in carriers of the T-allele as compared with the non T-allele group [31] although a compensatory upregulation of insulin sensitivity in response to an inherent β-cell dysfunction cannot be excluded.
It has been recently proven that EVOO and other specific components of MedDiet, reduce β-cell apoptosis, normalize glucose-induced insulin secretion, delay absorption of carbohydrates, improve glucose uptake and metabolic control by lowering insulin resistance, influence incretin response and finally overcome insulin resistance [32]. Considering that maintenance of normal glucose homeostasis in pregnancy is dependent upon the capability of β-cells to increase the secretion of insulin to compensate the insulin resistance in late pregnancy, T-allele variant could affect beta cell proliferation and insulin secretion in response to specific foods and may influence GDM susceptibility. Given this, it would be rational to speculate that the effect of a healthy MedDiet would be more evident in women genetically susceptible by interacting on the same pathways and decreasing the risk of GDM.
Another possible explanation for our results with respect to obtaining higher benefits from the diet by being a carrier of the T-allele could be found in its relationship to BMI. Several authors have reported significant interactions between the MedDiet and rs7903146 influencing BMI, weight, and waist circumference. Their results are in line with ours in that a healthy diet provides a more beneficial effect in subjects carrying the T-allele. A randomized controlled trial with obese participants following a 10-wk intervention with hypoenergetic diets showed that obese individuals with the rs7903146 T-allele had better responses in weight loss and adiposity outcomes [33]. Roswall et al. showed that subjects carrying the T-allele experienced less weight gain when the adherence to the MedDiet was high [34]. Similarly, Sotos-Prieto et al. found in a Hispanic sample that subjects with CT + TT genotypes had lower weight and waist circumference when they score high to MedDiet adherence when compared with CC carriers [35]. Some authors have shown the T-allele was associated with lower BMI only in T2DM patients but not in controls [36]. Moreover, Corella et al. have suggested that the rs7903146–T2DM association had higher effect in lean compared with obese individuals [37]. Finally, other studies have found a negative impact of the rs7903146 T-allele on change in anthropometric measurements during lifestyle intervention [[38], [39], [40]].
Despite these findings, in our case it does not seem that BMI explains the result of the TCF7L2 rs7903146-diet interaction on the incidence of GDM. To explore the effect of BMI, we studied its association with rs7903146 and observed that a statistically significant association existed in all but the recessive model when we analysed the total group in crude. However, when we repeated the same analysis adjusted for age, parity and ethnicity, all significations were lost. Furthermore, there are no differences in BMI prepregnancy or at baseline nor in body weight prepregnancy, at entry or even in weight gain between the three separate groups according to MedDiet adherence. Furthermore, the interaction with adherence to the diet is maintained after adjusting for BMI and we have not found an interaction between polymorphism and weight gain according to MedDiet adherence levels.
All this suggests that in this case, it is not the interaction of polymorphism with weight that would explain the results, but rather its effect on the glucose response to nutrient intake. In this respect, we found that during OGTT women who carried the TT genotype and had a high adherence to the MedDiet reached considerably lower blood 1-h glucose levels (113.67 mg/dl) in relation to those with low adherence (136.33 mg/dl). Women carrying the C-allele reached similar glucose concentrations among them. Thus, a direct effect of the T-allele on the glucose regulation cannot be excluded.
Finally, in a previous study based on this sample we reported that the protective effect of having a high adherence to the six food targets was higher in women who were overweight and obese and who were multiparous. Interactions indicated that women BMI with ≥25 could benefit the most from improvements in healthier dietary habits in pregnancy followed by multiparous. In this study we have ascertained that women carrying the T-allele benefited from the MedDiet almost as much as overweight/obese women. Only a small percentage (7.4%) of carriers of the T-allele that were high adherents developed GDM while the majority of those did not (92.6%). This effect was not observed in carriers of the CC genotype. Our data suggest that unfavourable genetic predisposition can be compensated by a healthy diet.
There are some limitations in this study. Our results may reflect the sample is not large enough to detect T-associated risks and is heterogeneous. Although the data provided were adjusted by ethnicity, some of the results may be attributable to specific ethnic effects. However, if conducting the analysis separated by ethnicity, each of the subgroups would have even more modest statistical power to demonstrate statistically significant associations or interactions of gene variants with GDM.
5. Conclusion
In conclusion, our results suggest that women carrying the risk T-allele of the TCF7L2 rs7903146 polymorphism who highly adhere to a MedDiet early in pregnancy had a significantly lower risk of developing GDM than women who do not. We have demonstrated that a possible unfavourable genetic predisposition may be counteracted by adopting healthier diets. This reinforces the importance of identifying patients at risk of GDM based on genetic characteristics which are especially sensitive to specific types of diets and the relevance of a prompt nutritional education to pregnant women. Further studies are required to identify diet interactions with population at risk of GDM. This may help to develop more individualized intervention strategies that can contribute to precision medicine.
Funding
This research was funded by grants from Fundación para Estudios Endocrinometabolicos, IdISSC Hospital Clínico San Carlos; the Instituto de Salud Carlos III of Spain under grant number PI17/01442; (Plan Nacional de I + D + I, AES 2013–2016 subvencionado por el ISCIII y cofinanciado and Fondo Europeo de Desarrollo Regional (FEDER)). The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication are the responsibilities of the authors alone and independent of the funders.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Ana Barabash: Writing - original draft. Johanna D. Valerio: Writing - original draft. Nuria Garcia de la Torre: Supervision, helped in developing the protocol, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Inés Jimenez: Data curation, Supervision, are delivering the intervention, helped in developing the protocol, building the women and offspring database, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Laura del Valle: Data curation, Supervision, are delivering the intervention, building the women and offspring database, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Verónica Melero: Supervision, are delivering the intervention, are delivering the intervention, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Carla Assaf-Balut: Supervision, are delivering the intervention, are delivering the intervention, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Manuel Fuentes: Formal analysis, Supervision, designed the statistical analysis plan, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Elena Bordiu: Supervision, helped in developing the protocol, were in charge of GDM screening, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Alejandra Durán: Formal analysis, Investigation, Supervision, were in charge of GDM screening, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Miguel A. Herraiz: Supervision, were in charge of obstetric follow-up, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Nuria Izquierdo: Supervision, were in charge of obstetric follow-up, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. María J. Torrejón: Supervision, helped in developing the protocol, were in charge of GDM screening, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Paz de Miguel: Formal analysis, Investigation, Supervision, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Isabelle Runkle: Formal analysis, Investigation, Supervision, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Miguel A. Rubio: Supervision, helped in developing the protocol, All authors were involved in the critical revision of the manuscript for important intellectual content, material support and study supervision. All authors have seen and agreed with the content of the last version of the manuscript. Alfonso L. Calle-Pascual: Writing - original draft.
Declaration of competing interest
There are not competing interests declared.
The main investigator had the overall responsibility to ensure that the participants’ anonymity is protected. The trial staff ensured that the participants’ anonymity was maintained. The participants were be identified only by a participant ID number on the electronic database. All data collected in the study were entered onto a dedicated password-protected electronic database using a secure computer and internet connection. All documents were stored securely and only accessible by trial staff and authorized personnel. We will not publish any data that could lead to the identification of any study participants. The study complied with the Data Protection Legislation which requires data to be anonymized as soon as it is mandatory to do so.
Acknowledgements
We wish to acknowledge our deep appreciation to the administrative personnel and nurses and dieticians from the Laboratory Department (Marisol Sanchez Orta, María Dolores Hermoso Martín, María Victoria Saez de Parayuelo, Luzdivina Fernandez Muñoz) and the Pregnancy and Diabetes Unit (Maria Luisa Maroto, Reyes Merino, and Georgina Cutillas Dominguez).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2020.100069.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ruiz-Gracia T., Duran A., Fuentes M., Rubio M.A., Runkle I., Carrera E.F., Torrejon M.J., Bordiu E., Valle L.D., Garcia de la Torre N., Bedia A.R., Montanez C., Familiar C., Calle-Pascual A.L. Lifestyle patterns in early pregnancy linked to gestational diabetes mellitus diagnoses when using IADPSG criteria. The St Carlos gestational study. Clin Nutr. 2016;35:699–705. doi: 10.1016/j.clnu.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 2.Assaf-Balut C., Garcia de la Torre N., Duran A., Fuentes M., Bordiu E., Del Valle L., Familiar C., Ortola A., Jimenez I., Herraiz M.A., Izquierdo N., Perez N., Torrejon M.J., Ortega M.I., Illana F.J., Runkle I., de Miguel M.P., Montanez C., Barabash A., Cuesta M., Rubio M.A., Calle-Pascual A.L. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): a randomized controlled trial: the St. Carlos GDM prevention study. PloS One. 2017;12 doi: 10.1371/journal.pone.0185873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de la Torre N.G., Assaf-Balut C., Jimenez Varas I., Del Valle L., Duran A., Fuentes M., Del Prado N., Bordiu E., Valerio J.J., Herraiz M.A., Izquierdo N., Torrejon M.J., Cuadrado M.A., de Miguel P., Familiar C., Runkle I., Barabash A., Rubio M.A., Calle-Pascual A.L. Effectiveness of following mediterranean diet recommendations in the real world in the incidence of gestational diabetes mellitus (GDM) and adverse maternal-foetal outcomes: a prospective, universal, interventional study with a single group. The st Carlos study. Nutrients. 2019:11. doi: 10.3390/nu11061210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assaf-Balut C., Garcia de la Torre N., Fuentes M., Duran A., Bordiu E., Del Valle L., Valerio J., Jimenez I., Herraiz M.A., Izquierdo N., Torrejon M.J., de Miguel M.P., Barabash A., Cuesta M., Rubio M.A., Calle-Pascual A.L. A high adherence to six food targets of the mediterranean diet in the late first trimester is associated with a reduction in the risk of materno-foetal outcomes: the st. Carlos gestational diabetes mellitus prevention study. Nutrients. 2018;11 doi: 10.3390/nu11010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grant S.F., Thorleifsson G., Reynisdottir I., Benediktsson R., Manolescu A., Sainz J., Helgason A., Stefansson H., Emilsson V., Helgadottir A., Styrkarsdottir U., Magnusson K.P., Walters G.B., Palsdottir E., Jonsdottir T., Gudmundsdottir T., Gylfason A., Saemundsdottir J., Wilensky R.L., Reilly M.P., Rader D.J., Bagger Y., Christiansen C., Gudnason V., Sigurdsson G., Thorsteinsdottir U., Gulcher J.R., Kong A., Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 6.Ding M., Chavarro J., Olsen S., Lin Y., Ley S.H., Bao W., Rawal S., Grunnet L.G., Thuesen A.C.B., Mills J.L., Yeung E., Hinkle S.N., Zhang W., Vaag A., Liu A., Hu F.B., Zhang C. Genetic variants of gestational diabetes mellitus: a study of 112 SNPs among 8722 women in two independent populations. Diabetologia. 2018;61:1758–1768. doi: 10.1007/s00125-018-4637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L., Cui L., Tam W.H., Ma R.C., Wang C.C. Genetic variants associated with gestational diabetes mellitus: a meta-analysis and subgroup analysis. Sci Rep. 2016;6:30539. doi: 10.1038/srep30539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin P.C., Lin W.T., Yeh Y.H., Wung S.F. Transcription factor 7-like 2 (TCF7L2) rs7903146 polymorphism as a risk factor for gestational diabetes mellitus: a meta-analysis. PloS One. 2016;11 doi: 10.1371/journal.pone.0153044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin T. Current understanding on role of the Wnt signaling pathway effector TCF7L2 in glucose homeostasis. Endocr Rev. 2016;37:254–277. doi: 10.1210/er.2015-1146. [DOI] [PubMed] [Google Scholar]
- 10.Fisher E., Boeing H., Fritsche A., Doering F., Joost H.G., Schulze M.B. Whole-grain consumption and transcription factor-7-like 2 (TCF7L2) rs7903146: gene-diet interaction in modulating type 2 diabetes risk. Br J Nutr. 2009;101:478–481. doi: 10.1017/S0007114508020369. [DOI] [PubMed] [Google Scholar]
- 11.Hindy G., Sonestedt E., Ericson U., Jing X.J., Zhou Y., Hansson O., Renstrom E., Wirfalt E., Orho-Melander M. Role of TCF7L2 risk variant and dietary fibre intake on incident type 2 diabetes. Diabetologia. 2012;55:2646–2654. doi: 10.1007/s00125-012-2634-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corella D., Carrasco P., Sorli J.V., Estruch R., Rico-Sanz J., Martinez-Gonzalez M.A., Salas-Salvado J., Covas M.I., Coltell O., Aros F., Lapetra J., Serra-Majem L., Ruiz-Gutierrez V., Warnberg J., Fiol M., Pinto X., Ortega-Azorin C., Munoz M.A., Martinez J.A., Gomez-Gracia E., Gonzalez J.I., Ros E., Ordovas J.M. Mediterranean diet reduces the adverse effect of the TCF7L2-rs7903146 polymorphism on cardiovascular risk factors and stroke incidence: a randomized controlled trial in a high-cardiovascular-risk population. Diabetes Care. 2013;36:3803–3811. doi: 10.2337/dc13-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sole X., Guino E., Valls J., Iniesta R., Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22:1928–1929. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich S., Jacobs S., Zheng J.S., Meidtner K., Schwingshackl L., Schulze M.B. Gene-lifestyle interaction on risk of type 2 diabetes: a systematic review. Obes Rev. 2019;20:1557–1571. doi: 10.1111/obr.12921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang B., Xue X. Investigations of associations between seven gene polymorphisms and gestational diabetes mellitus: evidence from a meta-analysis. Gynecol Obstet Invest. 2020:1–8. doi: 10.1159/000505453. [DOI] [PubMed] [Google Scholar]
- 16.Huerta-Chagoya A., Vazquez-Cardenas P., Moreno-Macias H., Tapia-Maruri L., Rodriguez-Guillen R., Lopez-Vite E., Garcia-Escalante G., Escobedo-Aguirre F., Parra-Covarrubias A., Cordero-Brieno R., Manzo-Carrillo L., Zacarias-Castillo R., Vargas-Garcia C., Aguilar-Salinas C., Tusie-Luna T. Genetic determinants for gestational diabetes mellitus and related metabolic traits in Mexican women. PloS One. 2015;10 doi: 10.1371/journal.pone.0126408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franzago M., Fraticelli F., Nicolucci A., Celentano C., Liberati M., Stuppia L., Vitacolonna E. Molecular analysis of a genetic variants panel related to nutrients and metabolism: association with susceptibility to gestational diabetes and cardiometabolic risk in affected women. J Diabetes Res. 2017;2017:4612623. doi: 10.1155/2017/4612623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fritsche L., Sarief M., Wagner R., Stefan N., Lehmann R., Haring H.U., Grallert H., Fritsche A., Lechner A. Genetic variation in TCF7L2 rs7903146 and history of GDM negatively and independently impact on diabetes-associated metabolic traits. Diabetes Res Clin Pract. 2018;146:251–257. doi: 10.1016/j.diabres.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Vcelak J., Vejrazkova D., Vankova M., Lukasova P., Bradnova O., Halkova T., Bestak J., Andelova K., Kvasnickova H., Hoskovcova P., Vondra K., Vrbikova J., Bendlova B. T2D risk haplotypes of the TCF7L2 gene in the Czech population sample: the association with free fatty acids composition. Physiol Res. 2012;61:229–240. doi: 10.33549/physiolres.932272. [DOI] [PubMed] [Google Scholar]
- 20.Pagan A., Sabater-Molina M., Olza J., Prieto-Sanchez M.T., Blanco-Carnero J.E., Parrilla J.J., Gil A., Larque E. A gene variant in the transcription factor 7-like 2 (TCF7L2) is associated with an increased risk of gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol. 2014;180:77–82. doi: 10.1016/j.ejogrb.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Michalak-Wojnowska M., Gorczyca-Siudak D., Gorczyca T., Mosiewicz B., Kwasniewska A., Filip A., Mosiewicz J. Association between rs7901695 and rs7903146 polymorphisms of the TCF7L2 gene and gestational diabetes in the population of Southern Poland. Ginekol Pol. 2016;87:745–750. doi: 10.5603/GP.2016.0081. [DOI] [PubMed] [Google Scholar]
- 22.Popova P.V., Klyushina A.A., Vasilyeva L.B., Tkachuk A.S., Bolotko Y.A., Gerasimov A.S., Pustozerov E.A., Kravchuk E.N., Predeus A., Kostareva A.A., Grineva E.N. Effect of gene-lifestyle interaction on gestational diabetes risk. Oncotarget. 2017;8:112024–112035. doi: 10.18632/oncotarget.22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Melo S.F., Frigeri H.R., dos Santos-Weiss I.C., Rea R.R., de Souza E.M., Alberton D., Gomes de Moraes Rego F., Picheth G. Polymorphisms in FTO and TCF7L2 genes of Euro-Brazilian women with gestational diabetes. Clin Biochem. 2015;48:1064–1067. doi: 10.1016/j.clinbiochem.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Reyes-Lopez R., Perez-Luque E., Malacara J.M. Metabolic, hormonal characteristics and genetic variants of TCF7L2 associated with development of gestational diabetes mellitus in Mexican women. Diabetes Metab Res Rev. 2014;30:701–706. doi: 10.1002/dmrr.2538. [DOI] [PubMed] [Google Scholar]
- 25.Klein K., Haslinger P., Bancher-Todesca D., Leipold H., Knofler M., Handisurya A., Kautzky-Willer A., Worda C. Transcription factor 7-like 2 gene polymorphisms and gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2012;25:1783–1786. doi: 10.3109/14767058.2012.663831. [DOI] [PubMed] [Google Scholar]
- 26.Grotenfelt N.E., Wasenius N.S., Rono K., Laivuori H., Stach-Lempinen B., Orho-Melander M., Schulz C.A., Kautiainen H., Koivusalo S.B., Eriksson J.G. Interaction between rs10830963 polymorphism in MTNR1B and lifestyle intervention on occurrence of gestational diabetes. Diabetologia. 2016;59:1655–1658. doi: 10.1007/s00125-016-3989-1. [DOI] [PubMed] [Google Scholar]
- 27.Li S.X., Imamura F., Ye Z., Schulze M.B., Zheng J., Ardanaz E., Arriola L., Boeing H., Dow C., Fagherazzi G., Franks P.W., Agudo A., Grioni S., Kaaks R., Katzke V.A., Key T.J., Khaw K.T., Mancini F.R., Navarro C., Nilsson P.M., Onland-Moret N.C., Overvad K., Palli D., Panico S., Quiros J.R., Rolandsson O., Sacerdote C., Sanchez M.J., Slimani N., Sluijs I., Spijkerman A.M., Tjonneland A., Tumino R., Sharp S.J., Riboli E., Langenberg C., Scott R.A., Forouhi N.G., Wareham N.J. Interaction between genes and macronutrient intake on the risk of developing type 2 diabetes: systematic review and findings from European Prospective Investigation into Cancer (EPIC)-InterAct. Am J Clin Nutr. 2017;106:263–275. doi: 10.3945/ajcn.116.150094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Ortiz M.M., Garay-Sevilla M.E., Tejero M.E., Perez-Luque E.L. Analysis of the interaction between transcription factor 7-like 2 genetic variants with nopal and wholegrain fibre intake: effects on anthropometric and metabolic characteristics in type 2 diabetes patients. Br J Nutr. 2016;116:969–978. doi: 10.1017/S0007114516002798. [DOI] [PubMed] [Google Scholar]
- 29.InterAct C. Investigation of gene-diet interactions in the incretin system and risk of type 2 diabetes: the EPIC-InterAct study. Diabetologia. 2016;59:2613–2621. doi: 10.1007/s00125-016-4090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Florez J.C., Jablonski K.A., Bayley N., Pollin T.I., de Bakker P.I., Shuldiner A.R., Knowler W.C., Nathan D.M., Altshuler D., Diabetes Prevention Program Research G. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med. 2006;355:241–250. doi: 10.1056/NEJMoa062418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faerch K., Pilgaard K., Knop F.K., Hansen T., Pedersen O., Jorgensen T., Holst J.J. Incretin and pancreatic hormone secretion in Caucasian non-diabetic carriers of the TCF7L2 rs7903146 risk T allele. Diabetes Obes Metabol. 2013;15:91–95. doi: 10.1111/j.1463-1326.2012.01675.x. [DOI] [PubMed] [Google Scholar]
- 32.Jurado-Ruiz E., Alvarez-Amor L., Varela L.M., Berna G., Parra-Camacho M.S., Oliveras-Lopez M.J., Martinez-Force E., Rojas A., Hmadcha A., Soria B., Martin F. Extra virgin olive oil diet intervention improves insulin resistance and islet performance in diet-induced diabetes in mice. Sci Rep. 2019;9:11311. doi: 10.1038/s41598-019-47904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grau K., Cauchi S., Holst C., Astrup A., Martinez J.A., Saris W.H., Blaak E.E., Oppert J.M., Arner P., Rossner S., Macdonald I.A., Klimcakova E., Langin D., Pedersen O., Froguel P., Sorensen T.I. TCF7L2 rs7903146-macronutrient interaction in obese individuals’ responses to a 10-wk randomized hypoenergetic diet. Am J Clin Nutr. 2010;91:472–479. doi: 10.3945/ajcn.2009.27947. [DOI] [PubMed] [Google Scholar]
- 34.Roswall N., Angquist L., Ahluwalia T.S., Romaguera D., Larsen S.C., Ostergaard J.N., Halkjaer J., Vimaleswaran K.S., Wareham N.J., Bendinelli B., Palli D., Boer J.M., van der A.D., Boeing H., Loos R.J., Sorensen T.I., Tjonneland A. Association between Mediterranean and Nordic diet scores and changes in weight and waist circumference: influence of FTO and TCF7L2 loci. Am J Clin Nutr. 2014;100:1188–1197. doi: 10.3945/ajcn.114.089706. [DOI] [PubMed] [Google Scholar]
- 35.Sotos-Prieto M., Smith C.E., Lai C.Q., Tucker K.L., Ordovas J.M., Mattei J. Mediterranean diet adherence modulates anthropometric measures by TCF7L2 genotypes among Puerto Rican adults. J Nutr. 2020;150:167–175. doi: 10.1093/jn/nxz210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cauchi S., Choquet H., Gutierrez-Aguilar R., Capel F., Grau K., Proenca C., Dina C., Duval A., Balkau B., Marre M., Potoczna N., Langin D., Horber F., Sorensen T.I., Charpentier G., Meyre D., Froguel P. Effects of TCF7L2 polymorphisms on obesity in European populations. Obesity. 2008;16:476–482. doi: 10.1038/oby.2007.77. [DOI] [PubMed] [Google Scholar]
- 37.Corella D., Coltell O., Sorli J.V., Estruch R., Quiles L., Martinez-Gonzalez M.A., Salas-Salvado J., Castaner O., Aros F., Ortega-Calvo M., Serra-Majem L., Gomez-Gracia E., Portoles O., Fiol M., Diez Espino J., Basora J., Fito M., Ros E., Ordovas J.M. Polymorphism of the transcription factor 7-like 2 gene (TCF7L2) interacts with obesity on type-2 diabetes in the PREDIMED study emphasizing the heterogeneity of genetic variants in type-2 diabetes risk prediction: time for obesity-specific genetic risk scores. Nutrients. 2016;8 doi: 10.3390/nu8120793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasreddine L., Akika R., Mailhac A., Tamim H., Zgheib N.K. The interaction between genetic polymorphisms in FTO and TCF7L2 genes and dietary intake with regard to body mass and composition: an exploratory study. J Personalized Med. 2019;9 doi: 10.3390/jpm9010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haupt A., Thamer C., Heni M., Ketterer C., Machann J., Schick F., Machicao F., Stefan N., Claussen C.D., Haring H.U., Fritsche A., Staiger H. Gene variants of TCF7L2 influence weight loss and body composition during lifestyle intervention in a population at risk for type 2 diabetes. Diabetes. 2010;59:747–750. doi: 10.2337/db09-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher E., Meidtner K., Angquist L., Holst C., Hansen R.D., Halkjaer J., Masala G., Ostergaard J.N., Overvad K., Palli D., Vimaleswaran K.S., Tjonneland A., van der A.D., Wareham N.J., Sorensen T., Loos R.J., Boeing H. Influence of dietary protein intake and glycemic index on the association between TCF7L2 HapA and weight gain. Am J Clin Nutr. 2012;95:1468–1476. doi: 10.3945/ajcn.111.014670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.