Abstract
Cancer remains to be an unresolved medical challenge despite of tremendous advancement in basic science research and clinical medicine. One of the major limitations is due to the side effects of chemotherapy which remains to be palliative without offering any permanent cure for cancer. Cancer stem cells (CSCs) are the subpopulation of cells in tumors that remain viable even after surgery, chemo- and radio-therapy that eventually responsible for tumor relapse. Hence, by eliminating non-stem cancer cells and cancer stem cells from the patient, permanent cure is expected. Phytochemicals have been under the intensive study to target these CSCs effectively and permanently as they do not cause any side effects. Resveratrol (RSV) is one such compound attaining lot of interest in recent days to target CSCs either alone or in combination. RSV has been used by several researchers to target cancer cells in a variety of disease models, however its CSC targeting abilities are under intensive study at present. This review is to summarize the effects of RSV under in vitro and in vivo conditions along with advantages and disadvantages of its uses against cancer cells and cancer stem cells. From the first reports on phytochemical applications against cancer and cancer stem cells in 1997 and 2002 respectively followed by later reports, up to date observations and developments are enlisted from PubMed in this comprehensive review. RSV is shown to be a potential compound having impact on altering the signal transduction pathways in cancer cells. However, the effects are variable under in vitro and in vivo conditions, and also with its use alone or in combination with other small molecules. Past research on RSV is emphasizing the importance of in vivo experimental models and clinical trials with different prospective combinations, is a hope for future promising treatment regimen.
Keywords: Resveratrol, Cancer, Cancer stem cells, Therapeutic targeting, Signal transduction, Resistance, In vitro and in vivo studies
Graphical abstract
Highlights
-
•
Resveratrol is a potent reducing agent, and can prevent carcinogenesis due to its anti-oxidant abilities
-
•
There is effect of resveratrol with long noncoding RNAs (lncRNAs) in lung carcinogenesis
-
•
It acts as an immunomodulatory agent for treating cancer
-
•
The major drawback of is that require validation of the same effect under in vivo conditions
-
•
It should be tested with different combinations as can affect multiple pathways unlike targeted drugs
Acronyms list
- ABC
ATP-binding cassette transporters
- ALDH
Aldehyde dehydrogenase
- AML
Acute myeloid leukemia cells
- AT/RT
Atypical teratoid/rhabdoid tumor
- Bax
Bcl2 associated X protein
- Bcl2
B-cell lymphoma-2
- BNIP3
BCL2/adenovirus E1B 19 kDa protein-interacting protein-3
- CD
Cluster of differentiation
- CDK
Cyclin-dependent kinase
- CHD
Coronary heart disease
- COX
Cyclooxygenase
- CSCs
Cancer stem cells
- CYP
Cytochrome P450
- DAPK2
Death associated protein kinase-2
- EMT
Epithelial to mesenchymal transition
- eNOS
Endothelial nitric oxide synthase
- ER
Estrogen receptor
- ERK
Extracellular signal regulated kinase
- ESA
Excretory secretory antigen
- FA
Fanconi anemia
- FAS
Fatty acid synthase
- GBM
Glioblastoma multiforme
- HER-2
Human epidermal receptor-2
- HIF-1α
Hypoxia inducible factor -1α
- IL
Interleukins
- iNOS
Inducible nitric oxide synthase
- JAK
Janus kinase
- lncRNA
Long non-coding RNA
- ALT-1
Mucosa-associated lymphoid tissue lymphoma translocation protein
- MAP
Mitogen activated protein kinase
- MCP-1
Monocyte chemoattractant protein-1
- MDR1
Multi-drug resistance protein-1
- MEK
Mitogen activated protein kinase - MAPK Kinase
- MMP
Matrix metallo proteinase
- MRP1
Multidrug resistance associated protein-1
- mTOR
Mammalian target of rapamycin
- NAC
N-acetyl cysteine
- NF-κB
Nuclear factor kappa B
- nNOS
Neuronal nitric oxide synthase
- NO
Nitric oxide
- Nrf-2
Nuclear factor erythroid-2 related factor-2
- ODD
Ornithine decarboxylase
- PI3K
Phosphoinositide 3-kinase
- PPAR
Peroxisome proliferator-activated receptor
- QR2
Quinone reductase-2
- RAF
Rapidly accelerated fibrosarcoma protein kinase
- RAS
Rat sarcoma protein kinase
- RCC
Renal cell carcinoma
- ROS
Reactive oxygen species
- RSV
Resveratrol
- SCC
Squamous carcinoma cell
- SERM
Selective estrogen receptor modulator
- SIRT1
NAD-dependent deacetylase sirtuin-1
- SREBP1
Sterol regulatory element binding protein-1
- STAT
Signal transducer and activator of transcription
- TGF
Transforming growth factor
- TNBC
Triple negative breast cancer
- TRAIL
Tumor necrosis factor related apoptosis inducing ligand
- TrxR
Thioredoxin reductase
- VEGF
Vascular endothelial growth factor
1. Introduction
Resveratrol (RSV), is 3,4’,5 – trihydroxy stilbene, a phytoalexin is widely distributed in variety of plants including red grapes, berries, peanuts, etc. Highest levels of RSV are found in Japanese knotweed (Polygonum cuspidatum) and muscadine grapes (Vitis rotundifolia) (Shrikanta et al., 2015). Though its occurrence is widely distributed about more than 70 plant species, its bioavailability is challenging upon its consumption (Gambini et al., 2015). Tome-Carneiro et al. (2013) have further shown, different levels of RSV concentrations are attributed for differential health impacts. Szekeres et al. (2010) in their review demonstrated that, due to the presence of three hydroxyl groups, it was known to act as a potent anti-oxidant by interfering with intracellular redox signaling. In many studies with different model organisms, RSV is shown to increase healthy life span mediated by SIRT1 (NAD-dependent deacetylase sirtuin-1) (Bhullar and Hubbard, 2015). RSV can reduce inflammatory stress through its effects on mitochondria. It activates a group of mitochondrial proteins of sirtuin family, particularly SIRT1. Lagouge et al. (2006) had shown that activation of sirtuin family protein can in turn related to the blood sugar stabilization in the body.
RSV effects on nitric oxide cycle were well known, through which it maintains the health of immune, nervous and vascular system. Nitric oxide in the body is synthesized by the enzyme Nitric Oxide Synthase (NOS) which has a critical role in inflammation. NOS can occur in different isoforms based on its location such as endothelial NOS (eNOS), neuronal NOS (nNOS) and inducible NOS (iNOS). All the NOS isoforms (eNOS, nNOS and iNOS) have been reported to be expressed in the cardiac and endothelial cells of the blood vasculature. RSV has been proved to show its effects by acting on eNOS derived NO system thus inhibiting the damage caused due to stress-induced inflammation (Xia et al., 2014). These effects are well established functions of RSV on cardiac health. However, RSV has also been shown to exhibit broad-spectrum antimicrobial, anti-infective, anti-amyloidogenic activities and now researchers are testing for the efficacy of its anti-cancer stem cell properties.
This review is a comprehensive collection of original work and reviews to elucidate the present idea about advantage of resveratrol application particularly against CSCs. This review is also to discuss about in vitro and in vivo observations of RSV effects emphasizing its efficacy to use in future cancer therapy.
2. Resveratrol mechanisms affecting cancer cells
In the recent past, a lot of interest has been aroused in revealing the exact mechanisms of anti-cancer effects of RSV. It is a polyphenolic stilbene with an aromatic benzene bonded to three hydroxyl groups that acts as a potent anti-oxidant neutralizing the toxic effects of reactive oxygen species (ROS) in the body, thereby neoplastic transformation of cells can be prevented. However, the anti-cancer effects have been reported due to other mechanism of action as its anti-oxidant potential is not very high when compared with other biological molecules. RSV has been reported to exert its anti-cancer activity by inducing cell cycle arrest, apoptosis, differentiation and inhibiting cancer cell proliferation. Jang et al. (1997) for the first time evidenced that from topical application of RSV in an experimental skin cancer mouse model tumorigenesis found to be inhibited. RSV is shown to be effective by acting at initiation, progression and metastasis stages of tumorigenesis (Ko et al., 2017).
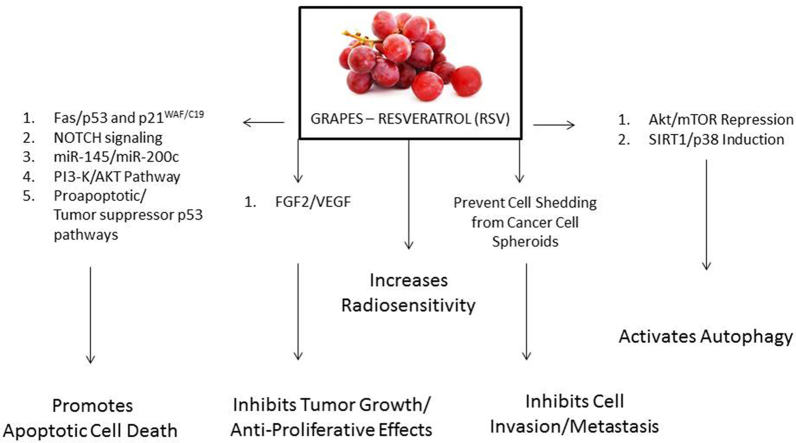
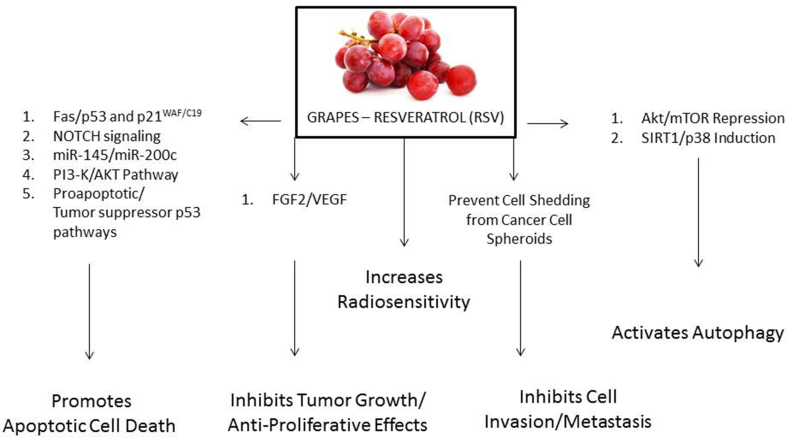
There are myriad pathways that RSV has been shown to influence on cancer cells. However, these effects are observed to be limited by the experimental conditions. Still it requires significant efforts to identify cross-talk pathway effects and to select the common key targets in cancer cells. Fig. 1, Fig. 2.
Fig. 1.
Resveratrol effects on cellular pathways and its mediated anti-cancer effects.
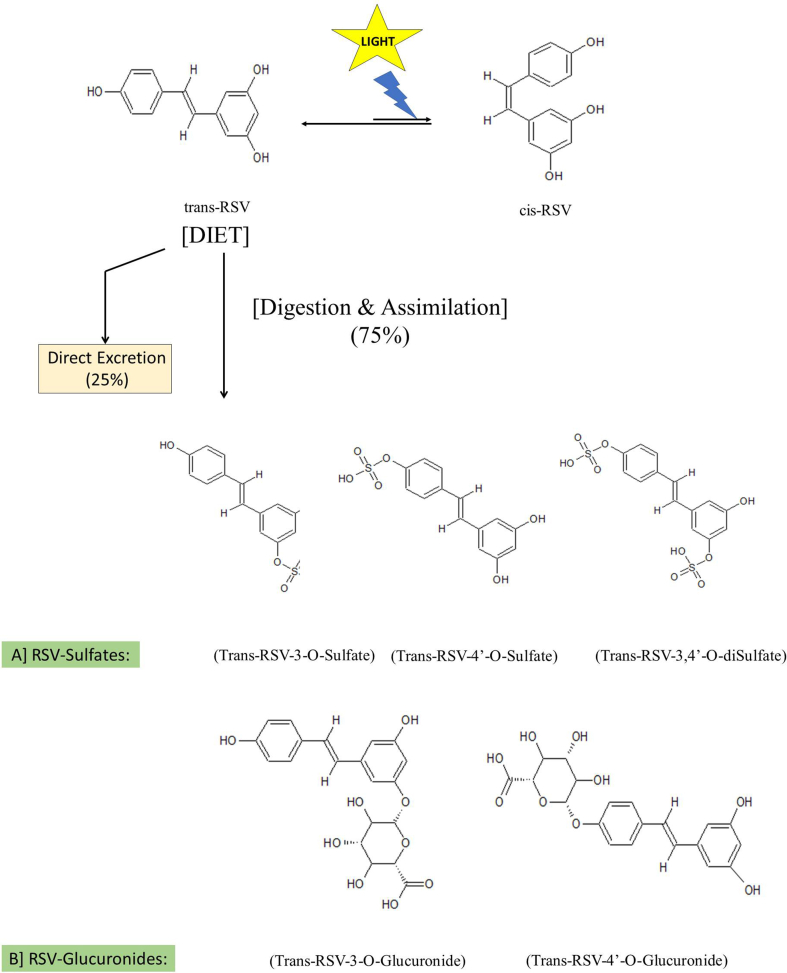
Fig. 2.
Resveratrol isomers & its immediate metabolites.
2.1. Resveratrol structure and anti-cancerous activity relevance
Resveratrol is 5-[(E)-2-(4-hydroxyphenyl)ethyl]benzene-1,3-diol with three hydroxyl groups attached to carbon atom of the two aromatic ring structures. In plants it is synthesized to provide protection against the environmental stress and to a variety of infections. The therapeutic effects of resveratrol originally started from the concept of “French paradox”, which came into an existence in the year 1992 in an epidemiological study by Renaud and de Lorgeril (1992), to understand the effect of wine consumption on coronary heart disease (CHD). Later studies have characterized different compounds in red wine with a variety of flavanols like myricetin, kaempferol, quercetin (predominant), catechin, epicatechin, oligo- and poly-meric flavan-3-ols, proanthocyanins, anthocyanins, phenolic acids such as gallic acid, caftaric acid, caffeic acid, p-coumaric acid and the resveratrol (stilbene).
The compounds with polyphenolic substituents particularly catechols or 1,4 dihydroquinone are unique in forming stable phenoxyl radical upon reaction with oxidizing agents like superoxide radicals, peroxynitrite, etc., formed in the cells posed under oxidative stress. As wine was found to be enriched with catechols, its effects initially were explained for anti-fungal, potential anti-platelet aggregation and anti-oxidant properties (Waterhouse, 2002). RSV accumulation in grape plants was found to be formed in response to Botrytis cinerea and other fungal infections. In the plants, oligomers of RSV are known as viniferins that are actual anti-fungal compounds. In wine, cis-, trans- and glucosides of both cis- and trans-resveratrol are found. Trela and Waterhouse (1996) have observed that in plants, cis-resveratrol is absent and is formed in wine due to light induced cis/trans isomerization. In dietary products, RSV occurs in glycosylated form known as “piceid” which is resistant to undergo enzyme mediated oxidation, thereby retaining its biological effects. However, intestinal cells absorb free form of RSV after the action of glycosidases there by enzyme activity is related to the absorption of RSV into the body (Fan et al., 2009).
Resveratrol in wine was explained as an important derivative of red grapes and as a constituent of biological fluid that could prevent tumor growth for the first time in 1997. Due to its structural similarity with that of the diethylstilbestrol which is a synthetic estrogen, RSV was considered as a phytoestrogen. RSV was found to inhibit the binding of radio-labeled estradiol to estrogen receptor responsible for variable effects under different test systems relating its influence on breast cancer (Gehm et al., 1997). It was reported that RSV has 16 times lower anti-oxidant potential than the α-tocopherol. But unlike other polyphenol molecules, RSV can undergo redox cycling being able to adopt quinone like structure. ROS production in the cell reported to cause activation of nuclear factor erythroid-2 related factor-2 (Nrf-2) which regulates the oxidative stress. Further, it was shown to improve recycling and cross-talk interactions with central and lipid metabolism along with modulating phase-I and II metabolism enzymes and transporters. Quinone reductase-2 (QR2), a phase-II detoxifying enzyme shown to interact directly with RSV. Inhibition of QR2 by RSV can induce other cellular anti-oxidant enzymes and thus increases cellular resistance to oxidative stress (Britton et al., 2015).
It has been shown that several methylated compounds generate formaldehyde in the cell, which was shown to be effectively being prevented by RSV. Further, the reaction products formed during the interaction between RSV and formaldehyde can act as chemopreventive factors (Tyihak et al., 1998). Thus Szende et al. (1998) reported that RSV, due to its formaldehyde capturing ability can influence cell proliferation and active cell death in a dose-dependent manner. Further, Fontecave et al. (1998) also shown that RSV can act as a potent inhibitor of ribonucleotide reductase and DNA synthesis in mammalian cells thereby controlling the cell proliferation and exhibiting chemopreventive activity.
After ingestion, RSV undergoes a variety of biotransformation in the intestinal cells, liver cells and then by the gut microbiome. From the diet, about 75% of RSV gets assimilated and metabolized rapidly and extensively to form conjugated products. Remaining 25% of ingested RSV will be excreted directly through urine. About 2% of RSV in plasma can be regenerated from the conjugated metabolites upon hydrolysis by the enzymes of microbiome. Though some biological effects have been shown to exert by RSV metabolites, in the literature the anti-cancer effects are mainly attributed only for the free form of RSV (Springer and Moco, 2019).
2.2. Anti-proliferative effects
Cancer cell proliferation is attributed due to an aberrant Mitogen Activated Protein (MAP) kinase signaling pathways. Constitutive activation of RAS/RAF/MEK/ERK (extra-cellular signal regulated kinase) pathway has significant role in the sustained cancer cell survival and proliferation. In different cancers, alterations mostly may occur at the receptor level or due to consecutively mutated downstream kinases of the respective pathways. For instance, in pancreatic cancers, epidermal growth factor (EGF) and/or human epidermal receptor-2 (HER-2) mutations are critically responsible for cancer cell proliferation (Oliverira-Cunha et al., 2011). Further, cancer cells secrete vascular endothelial growth factors (VEGF) that can induce neovascularization and that may in turn provoke cell proliferation. In renal cell carcinoma cells (RCCs) including ACHN and A498, RSV treatment is found to exert its effect on RCC proliferation, migration and invasion in a concentration dependent manner through inactivation of the Akt and ERK1/2 signaling pathways (Zhao et al., 2018). RSV is observed to have effect on VEGF expression mediated regulation of cell proliferation under in vitro condition (Liu et al., 2012). In CaCo-2 cells, treatment with 25 μM RSV has shown 70% growth inhibition due to S/G2 phase arrest. These effects were shown to be due to the inhibition of ornithine decarboxylase (ODD) activity which is enhanced in cancer cells (Schneider et al., 2000).
2.3. Cell-cycle arrest and pro-apoptotic effects
RSV is reported to be responsible for cell cycle arrest thereby inducing the cancer cells to undergo apoptosis. Singh et al. (2017) reported that the combined drug treatment of RSV and docetaxel on C4–2B and DU-145 cell lines of prostate cancer was found to be responsible for inhibited progression of G2/M phase arrest and also enhanced expression of pro-apoptotic genes of Bax, Bid and Bak. In an another study, Yuan et al. (2015) reported that A549 cells of lung cancer, upon treatment with RSV was found to arrest the cell cycle in G0/G1 phase by down regulating the expression levels of cyclin D1, cyclin-dependent kinase-4 (CDK4) and CDK6 along with an upregulated expression of CDK inhibitors, p21 and p27 in a p53 independent manner. Mitochondria in the cell have a very critical role in normal cells to decide cell survival and death fates through maintenance of an optimal Bcl2/Bax ratio. Kumar et al. (2017) shown that RSV treatment has resulted in decreased cell viability, altered cell morphology and increased apoptosis in a dose, time and caspase-independent manners in murine prostate cancer. These effects were due to the influence of the RSV via disrupted mitochondrial membrane potential and aberrant expression of Bax/Bcl-2 proteins.
2.4. Anti-metastatic effects
Metastasis is the later event of tumor progression that causes seeding of tumor cells in distant metastatic sites ultimately leading to the formation of secondary tumors. De-differentiation of cancer cells in later stages that gets induced by tumor microenvironment has been reported to be associated with enhanced metastatic abilities of cancer cells due to acquired stemness (Quail and Joyce, 2013). Cancer stemness is known to enhance the metastatic potential of several cancer types leading to aggressive secondary tumor formation at different sites (Lif et al., 2007; Li and Li, 2014). Ji et al. (2013) reported in an in vitro study, RSV treatment lead to inhibited invasion and metastasis of colorectal cancer-derived cell lines LoVo and HCT116 by suppressing the Wnt/β-catenin signaling mediated target genes of c-Myc, MMP-7, and MALT-1. At low doses, RSV is shown to be effective against breast cancer metastasis to lungs in mice by its inhibitory effect on Stat3 mediated signaling (Lee-Chang et al., 2013). The metastasis of 4T1 mouse breast cancer cells both under in vitro and in vivo conditions upon RSV pre-treatment was found to inhibit cancer cell metastasis through its inhibitory effect on MMP-9 expression (Lee et al., 2012).
3. Strategies to eliminate cancer stem cells
Most of the tumor tissues types were first discovered to contain heterogeneous cell population with distinct levels of therapeutic resistance, self-renewal capacities, low-proliferation rate and with the ability to repopulate original tumor cells (Chang, 2016). Further, these populations of cells were named as cancer stem cells, which are responsible for chemo resistance and tumor relapse (Nguyen et al., 2012). It has been concluded from different reports, that by eliminating cancer stem cells completely from the tumor site, permanent cure for cancer can be achieved. Hence, targeting the minor cancer stem cell population is a very important and prospective strategy of cancer treatment.
There have been different strategies in the literature to eliminate these CSCs such as inducing CSC differentiation and then targeting by potent apoptotic inducers, by targeting DNA damage repair enzymes, by targeting cell cycle specific regulators, by using monoclonal antibodies, by altering the drug resistance genes and recently by metabolism based therapeutic targeting. These strategies are dependent on the cancer type, specific to the stage and based on experimental system under the study (Yoshida and Saya, 2016; Jagust et al., 2019; Shibata and Hoque, 2019). RSV is reported to regulate all the major CSC signaling pathways, but exact mechanisms of its interactions are not clearly understood (Zhang et al., 2018). In spite of the promising results of CSC targeting under in vitro conditions, it require robust research to translate the observations to in vivo systems and further to the clinical settings.
3.1. Therapeutic resistance of cancer stem cells and resveratrol
Cancer stem cells are described to exhibit endogenous resistance mechanisms against radiation and chemotherapy due to preferential activation of DNA damage response, hypoxic stability, an increased activity of ABC transporters leading to efficient drug efflux, elevated expression of anti-apoptotic molecules, higher aldehyde dehydrogenase (ALDH) activity/enhanced activity of repair enzymes and quiescence or dormancy or Go – Phase (Prieto-Vila et al., 2017; Cho and Kim, 2020). Resveratrol has been shown to reverse the resistance to standard classical chemotherapeutics in non-stem cancer and cancer stem cells by sensitizing the cells in multiple ways. It is reported to cause an increased susceptibility to induce cancer cell apoptosis by interfering with pro- and anti-apoptotic factors, by regulating miRNAs, by its effect on drug- and carcinogen-metabolizing enzymes, by interfering with drug resistance gene/protein expressions and respective signaling pathways through poorly understood mechanisms (Mieszala et al., 2018; Zhang et al., 2019).
Kao et al., (2009) and Lu et al., (2009) have reported that effects of radiotherapy in RSV pretreated medulloblastoma (MB) cancer stem-like cell cultures and CD133 – positive cells derived from atypical teratoid/rhabdoid tumor (AT/RT) was reported to be significantly enhanced. Radiation combined with RSV pretreatment was observed to significantly increase the radiosensitivity in MB-CSCs. Similarly, AT/RT-CD133 (+) cells with CSC properties when treated with RSV, reported to inhibit expression of drug resistant genes and induced differentiation of AT/RT CD133(+) cells to drug-sensitive CD133(−) cells. RSV was reported to induce chemosensitization to 5-fluorouracil through inhibition of epithelial-mesenchymal transition (EMT) factors and down regulation of NF-κB regulated (inhibited IκBα kinase and IκBα phosphorylation and degradation) gene products like MMP-9, caspase-3 in colorectal cancer cells (Buhrmann et al., 2015). Choi et al. (2016) reported that RSV analog HS-1793, found to enhance radiosensitivity in mouse-derived breast cancer cells under hypoxic conditions through inhibiting the hypoxia-inducible factor-1α (HIF-1α) and VEGF protein in FM3A mouse mammary carcinoma cells. Tumor necrosis factor related apoptosis-inducing ligand (TRAIL) armed oncolytic adenovirus known as ZD55-TRAIL, reported to enhance A549 sphere cell apoptosis through mitochondrial pathway up on treatment of RSV along with small molecules embelin and LY294002 and thus shown an improved survival status of lung cancer mouse models (Yang et al., 2015a, b, c). Zhou et al. (2019) reported an increased chemotherapeutic response by RSV pretreatment which has reversed the stemness induced by gemcitabine in pancreatic cancer cells of MiaPaCa-2 and Panc-1 cells via targeting sterol regulatory element binding protein-1 (SREBP1). In SKOV3 - cancer stem cells of ovarian cancer, RSV found to potentially increase the tumoricidal effect of chemotherapeutic doxorubicin under in vitro conditions (Pouyafar et al., 2019a, Pouyafar et al., 2019b). Though couple of studies indicated role of RSV in reversing the cancer stem cell drug resistance, its mechanism of intervention has to be understood in detail in in vivo models and in human trials.
3.2. Natural products strategy
Recently, there has been a lot of attention on natural dietary product characterization with medicinal properties that can control cancer cells preventing their progression. Further, these compounds have attained importance in research and drug discovery due to their less or no toxic side effects (Rajesh et al., 2015). Panche et al. (2016) had discussed in detail about the current trends of research and developments on flavonoids as potential drug candidates. Different chemical ingredients in the diet consumed in day-to-day life have been studied for their potential benefits. Newman and Cragg (2016) have reported that from the year 1981–2006, nearly 63% of anticancer drugs used have been developed from natural products. Applications of these natural products are shown to be particularly important in cancer therapy as they do not pose any side effects.
3.3. Resveratrol strategy
RSV is one of the natural products, which was known to be responsible for cardiac health and now the same RSV has generated a lot of interest for its anti-cancerous effects. Jang et al. (1997) reported for the first time that the RSV's anticancer effect was due to its anti-initiation, anti-promotion and anti-progression activities.
RSV was reported to exhibit selective estrogen receptor modulator (SERM) activity and this observation further laid possibility of its role in breast cancers (Gehm et al., 1997). Gunther et al. (2007) found that RSV can also be used to target CSCs by observing in an attempt to test the polyphenols including RSV that could prevent the cell shedding from mouse mammary cancer spheroids inhibiting the cancer cell invasion of embryonic stem cell cultures. However, Wallenborg et al. (2009) reported that by using small amount of red wine (1–5%) containing RSV exhibited massive cell death of various cell types including neural stem cells had taken place due to increased oxidative stress mediated inhibition of thioredoxin reductase (TrxR) activity but not due to RSV. Resveratrol was shown to exert effect by the down regulation of fatty acid synthase (FAS) gene and up-regulation of pro-apoptotic genes like DAPK2 and BNIP3 in cancer stem – like cells (CD24(−)/CD44(+)/ESA(+) which were isolated from both ER+ and ER-breast cancer cell lines. These alterations were observed to cause inhibited cell viability and mammosphere formation along with induced pro-apoptotic effects (Pandey et al., 2011). There are total 160 results have been displayed which are relevant to resveratrol and cancer stem cells in the PubMed search. It is also interesting to note that, from the year 2015 there has been increasing number of reports in the same field of research. Though majority of the attempts made were with in vitro model systems, many experiments were also reported by using in vivo models. Differential effects of RSV observed in various in vitro and in vivo cancer stem cell models have been presented in Table – 1.
Table 1.
Summary of historical review of RSV effects in various CSC model systems reported.
| Ref. | CSC model system | RSV Effects |
|---|---|---|
| Gunther et al. (2007) | 4T1 Mouse mammary breast cancer cells. | Cell shedding from mouse mammary cancer spheroids ↓ Cancer cell invasion in embryonic stem cell cultures ↓ |
| Kao et al. (2009) | CD133-positive/negative cells derived from atypical teratoid/rhabdoid tumors (AT/RT-CD133(±)). |
With 200 μM treatment; in vitro proliferation and in vivo tumor relapse of CD133(+) cells ↓ With 150 μM treatment; Drug resistance genes in CD133(+) cells ↓ Differentiation of CD133(+) cells into CD133(−)↑ |
| Lu et al. (2009) | Medulloblastoma (MB)-associated 3D-spheroid forming CSCs | Proliferation and Tumorigenicity of MB-CSCs ↓ Radiosensitivity ↑ |
| Shankar et al. (2011) | Human pancreatic Cancer Stem Cells (CD133+, CD44+, CD24+, ESA+) of NOD/SCID mice, CSCs from KrasG12D transgenic mice and human pancreatic tumor derived CSCs. | Caspase 3/7 ↑ Expression of XIAP, BCL-2 and CCND1 ↓ |
| Pandey et al. (2011) | CD24(−)/CD44(+)/ESA(+) cells from estrogen receptor – ER+ and ER− breast cancer cell lines. | Lipogenesis by modulating FAS expression ↓ Apoptosis ↑ |
| Hu et al. (2012a, b) | Human promyelocytic leukemia stem cells (KG-1a) | KG-1a cells susceptible to cytokine-induced killer cell (CIK) mediated cytolysis ↑ |
| Hu et al. (2012a, b) | CD44 positive head and neck cancer (HNC) cells; HNC-Tumor Initiating Cells (TNCs) | Trans-differentiation of head and neck cancer-derived tumor-initiating cells (HNC-TICs) ↑ EMT ↓ |
| Hagiwara et al. (2012) | Orthotopic inoculation of female SCID mice with MDA-MB-231-luc-D3H2LN cells in pretreated mice with resveratrol. | Tumor suppressive miR-141 and miR-200c expression ↑ CSC phenotype ↓ |
| Sato et al. (2013) | Patient-derived Glioma Stem Cell (GSCs) cultures and Intracranial xenograft models of GSCs | p53-Nanog axis mediated Differentiation of GSCs ↑ |
| Su et al. (2013) | Human AML HL-60 cell lines and patient derived samples | Sonic hedgehog (Shh) ↓ Gli-1 nuclear translocation ↓ Cell viability ↓ IL-6 treatment induced the growth of AML cells through Shh signaling which was blocked by RSV treatment. |
| Sayd et al. (2014) | Glioblastoma Stem Cells (GSCs): Derived from Human glioblastoma tissue Normal Neural Stem Cells (NSCs): Derived from human fetal brain tissue |
GSC proliferation ↓ up to 150 μM and necrosis ↑ at higher doses. However, it has no effect on NSCs. These effects on GSCs are mediated through Sirtuin-2 which has vital enzymatic function in tumor metabolism. |
| Fu et al. (2014) | Breast cancer stem-like cells (BCSCs) isolated from MCF-7 and SUM159 | Administration of 100 mg/kg/day in NOD/SCID mice resulted xenograft tumors size ↓ BCSC cell population in tumors ↓ Autophagy in BCSCs ↑ |
| Yang et al. (2015a, b, c) | Colorectal cancer stem cells In vitro | Administration of 12.5–200 μ mol/L resulted in HCT116 CCSC proliferation ↓ in a dose-dependent manner. |
| Seino et al. (2015) | Ovarian cancer stem cells In vitro |
↑ Apoptosis of ovarian cancer stem cell A2780 independent of ROS ↓ Self-renewal capacity of A2780 stem cells depending on ROS |
| Clark et al. (2017) | Multiple patient-derived GBM stem-like cell (GSC) lines and established U87 glioma cells. | GBM and GSC growth and infiltration ↓ through modulation of AKT and p53 |
| Cilibrasi et al. (2017) | Human glioblastoma tissue derived glioma stem cells (GSCs) from different patients. | Cell proliferation ↓ Cell mortality ↑ ↓ Cell motility through modulated Wnt signaling and EMT pathway mediators. |
| Ruiz et al. (2018) | Enriched CSCs derived from cervical cancer HeLa cell lines | RAD51 expression ↓ CD49f-positive stem cell apoptosis ↑ |
| Fei et al. (2018) | Malignantly transformed dendritic cell line SU3-ihDCTC induced by glioma stem cells. | In vitro co-cultured GSC induced malignant transformed bone marrow derived dendritic cells exhibited increased sensitivity to chemotherapeutics after RSV treatment. |
| Peng and Jiang (2018) | Human osteosarcoma cell lines – MNNG/HOS, MG-63 and Osteoblast line hFOB1.19. | JAK2/STAT3 ↓ Osteosarcoma cell proliferation ↓ Tumorigenesis ↓ |
| Song et al. (2019) | LN18 and U87glioblastoma cells; U87 xenograft models | Epithelial to mesenchymal transition (EMT) of glioblastoma cell lines LN18, U87 and U87 xenografted mice models ↓ Expression of β-catenin ↓ GBM Stem cell marker expression: Twist ↓ Snail↓ Slug ↓ MMP-2 ↓ MMP-9 ↓ Smad ↓ |
| Buhrmann et al. (2019) | HCT116, RKO, SW480 colorectal cancer cell monolayer and 3D alginate cultures. | TNF-β/TNF-βR ↓ Epithelial-to-mesenchymal transition ↓ through NF-κB ↓ and focal adhesion kinase (FAK) ↓ |
| Segun et al. (2019) | Breast (MCF7), liver (HepG2), lung (A549) and prostrate (PC3) carcinoma cell lines versus normal prostrate epithelial cell (PNT2) cell lines |
Four RSV derivatives: (E)-resveratrol 3-O-rutinoside (1), 5-methoxy-(E)-resveratrol 3-O-rutinoside (2), pinostilbene (3) and 3-hydroxy-5-methoxybenzoic acid (4) isolated from the stem bark extract of C africana tested for anti-cancer stem cell activities. Except the derivative – 4, all the remaining derivatives were observed to be cytotoxic across the four cell lines. |
| Jhaveri et al. (2019) | U-87 MG: an astrocytoma grade IV cell line and LN-18: a grade IV glioblastoma cell line neurosphere cultures | Transferrin targeted liposomal formulations of Resveratrol (Tf-RES-L) used to treat GBM neurospheres. Both free RSV and RSV-formulations were found to Anchorage-independent growth of GBM neurospheres ↓ Its action exhibited through transferrin and ↑ activated caspase – 3/7. |
| Zhou et al. (2019) | MiaPaCa-2 pancreatic cancer cell lines and KPC mouse models of pancreatic ductal adenocarcinoma (PDA) | Pretreatment reversed the stemness induced by gemcitabine by targeting sterol regulatory element binding protein - 1 (SREBP1) both in vitro and in vivo. |
| Yin et al. (2020) | Patient tissue derived ‘gastric-cancer-derived-mesenchymal stem cells – GC-MSCs | IL-6, IL-8, MCP-1,VEGF expression ↓ β-catenin nuclear translocation in GC-MSCs upon pretreatment with RSV ↓ Metastasis of GC-MSCs ↓ |
| Sun et al.(2020) | ACHN and 786-O derived renal carcinoma stem cells | Size and number of tumor spheres ↓ Sonic hedgehog (Shh) pathway related proteins: SHH, SMO, Gli1, Gli2 ↓ CSC marker proteins: CD44, CD133, ALDH1A1, Oct-4, Nanog ↓ Cell proliferation ↓ Apoptosis ↑ |
3.4. Resveratrol impact on cancer stem cell signaling pathways
Cancer stemness is a spontaneous process and is mainly associated with tumor micro environmental factors that modulate the signal transduction pathways responsible for cancer stemness. The hallmark features during different types of solid tumor progression includes unregulated cell proliferation, neovascularization, hypoxia and/or intermittent hypoxia, cancer stemness and metastasis. Thus cancer stemness is presumably known to appear at the terminal stage during the tumor progression. However, there are no evidences to prove association of CSCs during the initial stages. This is another interesting area to check the stage specific effects of RSV associated with cancer stemness.
Major functional signaling pathways attributed for cancer stemness that are experimentally evidenced and are used for therapeutic targeting includes Wnt, nuclear factor-κB (NF-κB), Notch, hedgehog, janus kinase/signal transducer and activator of transcription (JAK-STAT), PI3K/AKT/mTOR (Phosphoinositide 3-Kinase/AKT/mammalian target of rapamycin), transforming growth factor (TGF)/SMAD and peroxisome proliferator-activated receptor (PPAR) pathways (Yang et al., 2020). Though some of these pathways were found to have role in cancer stemness, only anti-cancer properties of RSV were reported and its anti-cancer stemness effects are yet to be evidenced. It has been reported that in Indian triple negative breast cancers (TNBC) patients, the putative cancer stem cell marker CD133 or prominin-1 is correlated with the functional CSC signaling pathways including NOTCH-1/HES-1; Wnt/β-catenin; TGF-β III R/SMAD-7 and PTCH-1/Gli-1 (hedgehog) pathway activations (Bhaskara et al., 2019).
Phytochemicals can act as small molecular receptor blockers, kinase inhibitors, protease inhibitors, pro-apoptotic factors, spindle poisons, DNA damaging agents and cell cycle inhibitors that can influence the modulation of signaling pathways in order to impede or cure cancer. RSV effects on NOTCH signaling pathways are unique in a way that, it causes activation rather than inhibition of different proteins of NOTCH signaling leading to its anti-cancer activity (Farooqi et al., 2018). RSV has been shown to be affecting diverse cancer stemness signaling pathways that control not only the cancer stemness but also other cancer properties like cell viability, proliferation, apoptosis induction, inhibiting cell migration, etc. as reported by different researchers in various model systems enlisted in Table – 2.
Table 2.
Summary of RSV impact on cancer stem cell signaling pathways reported.
| Signaling Pathway | Experimental Model Systems | RSV Effects | Ref. |
|---|---|---|---|
| Notch signaling | Glioblastoma cell lines (A172 and T98G) | Notch-1 activation ↑ dependent p53 mediated anti-proliferative and pro-apoptotic effects | Lin et al. (2011) |
| Human GI carcinoid tumor cell lines (BON); Human pulmonary carcinoid cell lines (NC–H727) | Growth ↓ through S-phase cell cycle arrest Expression of neuroendocrine (NE) peptides/hormones chromogranin A and serotonin through activation of the Notch-2 isoform ↑ |
Pinchot et al. (2011) | |
| Anaplastic Thyroid Carcinoma (ATC) Cell Lines (HTh7 and 8505C) | Dose-dependent inhibited ATC growth ↓ Cell differentiation ↑ via activation of Notch-1 signaling ↑ |
Yu et al. (2013) | |
| Wnt signaling | Colorectal cancer cell lines (LoVo cells) | Dose-dependent inhibition of the nuclear localization of β-catenin ↓ c-Myc and MMP-7 ↓ Cell proliferation and invasion ↓ These effects of RSV are opposite to that of the long non-coding RNA-MALAT1 cell proliferation and invasion abilities |
Ji et al. (2013) |
| Human normal breast epithelial cell line (MCF10A) and breast cancer cell line (MCF-7, SUM159) | Wnt/β-catenin pathway proteins ↓ β-catenin ↑ markedly reduced RSV-induced cytotoxicity and autophagy |
Fu et al. (2014) | |
| Human normal (CCD112CoN) and colorectal cancer cell lines (HCT116, SW480, LoVo and CaCo-2) | TCF4 transcription factor expression ↓ via wnt/β-catenin pathway Phosphorylation of TCF4 ↑via ERK and P38 dependent pathways Apoptosis ↑ |
Jeong et al. (2015) | |
| Glioblastoma patient derived stem cells (GBM2, GBM7, G144, G179, G166, GliNS2, GBM04) | Wnt and EMT activator mediated GSC cell proliferation ↓ Cell mortality ↑ Cell motility↑ |
Cilibrasi et al. (2017) | |
| Squamous cell carcinoma cell line (Colo 16 cells) | RSV (100 μM) exhibited Wnt ↓ leading to Cell growth ↓ Apoptosis ↑ Transfection with β-catenin-specific siRNA enhanced RSV susceptibility |
Liu et al. (2017) | |
| GC-MSCs derived from the gastric adenocarcinoma patient tissues | RSV reversed the progress of EMT Metastasis ↓ Wnt/β-catenin pathway proteins ↓ |
Yin et al. (2020) | |
| SHH signaling (Sonic Hedgehog Pathway) | Chronic myeloid leukemia cells (K562 cells) | RSV acted as Bcr-Abl inhibitor SHH pathway proteins ↓ patched (PTCH) ↓ Smoothened (Smo) ↓ Gli-1 ↓ Viability of CML cells ↓ |
Liao et al. (2012) |
| Acute Myeloid Leukemia (AML) patient derived mononuclear cells (MNCs). | RSV blocked IL-6 stimulated growth of AML cells through SHH signaling | Su et al. (2013) | |
| Human colorectal cancer cell lines (HCT116 cells) | Cell viability and migration ↓ Apoptosis ↑ SHH pathway proteins ↓ |
Du et al. (2016) | |
| Renal cancer stem cells (ACHN and 786-O cells) | Size and number of tumorspheres ↓ via SHH signaling While purmorphamine up regulated SHH pathway and weakened the RSV effects |
Sun et al. (2020) | |
| PI3K Signaling | Human colon cancer cells (HCT116 cells) | Anti-proliferative effects ↑ via PTEN/PI3K/Akt and Wnt/β-catenin pathway protein regulation | Liu et al. (2014) |
| Glioblastoma patient derived Glioblastoma-initiating cells (GICs) | Invasion and migration of GICs ↓ via suppressing PI3K/Akt/NF-κB and MMP-2 expression ↓ | Jiao et al. (2015) | |
| Adriamycin resistant chronic myeloid leukemia cell line (K562/Adr) | Anti-proliferative activities of bestatin ↑ P-gp expression ↓ via PI3K/Akt/mTOR signaling pathway | Wang et al. (2016) | |
| Human colorectal cancer cell lines (HCT116 cells) | Anti-cancer activity ↑ PI3K/AKT signaling ↓ BMP7 ↑ Phosphorylation of Akt1/2/3 and PTEN ↑ |
Zeng et al. (2017) | |
| Human promyelocytic leukemia cells (HL-60) and ADR (Adriamycin)-resistant cell line (HL-60/ADR) | Drug resistance ↓ via PI3K/AKT/Nrf2 signaling and MRP1 expression | Li et al. (2019) | |
| Human acute promyelocytic leukemia cell lines (NB-4 and HL-60 cells) | PTEN expression ↑ PI3K/AKT pathway proteins ↓ Cell proliferation ↓ Apoptosis ↑ |
Meng et al. (2019) | |
| Human small-cell lung cancer cell lines (H446 cells) | Cell viability ↓ and apoptosis ↑via PI3K/Akt/c-Myc pathway | Li et al. (2020) | |
| Human papillary thyroid cancer cell lines (KTC-1 and TPC-1 cells); Mouse xenograft models | Anti-tumor effects ↑of rapamycin mediated by PI3K/AKT/mTOR pathway | Bian et al. (2020) | |
| Murine melanoma cell line (B16–F10), human melanoma cell line (A375) | AKT/mTOR pathway proteins ↓ Autophagy ↑ Growth, viability and migration ↓ |
Gong et al. (2020) | |
| TGF/SMAD Signaling | Human epidermoid carcinoma cell lines (A431) and mouse models | Ultraviolet B (UVB) induced malignant tumor progression ↓ in p53+/−/SKH-1 mice through Akt mediated TGF-β2 ↓ | Kim et al. (2011) |
| Colorectal cancer cell lines (LoVo cells) | Epithelial to mesenchymal transition (EMT) ↓ TGF-β1/SMAD signaling pathway ↓ |
Ji et al. (2015) | |
| Human breast cancer cell lines (MDA-MB-231) and xenograft mouse model | Migration and metastasis ↓ by reversing TGF-β1 induced EMT | Sun et al. (2019) | |
| Human glioblastoma multiforme cell lines (LN18, U87 cells) | EMT ↓ EMT-generated stem cell like properties ↓ via Smad-dependent signaling regulation |
Song et al. (2019) | |
| NF-κB Signaling | Human multiple myeloma cell lines (U266), Patient derived MM.1 or MM.1S cells | Constitutive and IL-6 induced activation of STAT3 ↓ Constitutive activation of NF-κB ↓ Cell proliferation ↓ Sensitization of bortezomib and thalidomide mediated apoptosis ↑ |
Bharadwaj et al. (2007) |
| PPAR pathway | Human colon carcinoma cell lines (SW480, HCT116, Caco2 and SW620) | Apoptosis ↑ Cell proliferation ↓ in combination with PPARγ |
Aires et al. (2014) |
| Bovine arterial endothelial cells (BAECs) and PPARα knockout mice | RSV exerted agonistic activity of PPARα as its direct target mediating long term effects of RSV under in vivo conditions | Takizawa et al. (2015) | |
| JAK/STAT Pathway | Medulloblastoma cell lines (UW228-2 and UW228-3 Cells) | Bcl-2 expression ↑ STAT3 ↓ Survivin, cyclin D1, Cox-2 and c-Myc ↓ Growth suppression ↑ Differentiation-like changes ↑ |
Yu et al. (2008) |
| Human osteosarcoma cell lines (MNNG/HOS, MG-63 cells), osteoblast cell line (hFOB1.19 cells) | Cell proliferation and tumorigenesis ↓ correlated with cytokines inhibition related JAK2/STAT3 signaling blockage | Peng et al. (2018) | |
| Human ovarian cancer cell lines (SKOV3, Caov-3, OVCAR-4 and OVCAR-8 Cells) | RSV analog – pterostilbene exhibited anti-tumor activity via anti-proliferative and pro-apoptotic mechanisms through JAK/STAT3 pathway ↓ | Wen et al. (2018) |
3.5. Resveratrol effects in combination with other molecules
The cell environment is a multi-factorial system and biologically active phytochemicals in its isolation shows differential effects due to the possible lack of secondary metabolite interaction with other molecules. Further, drug targeting by multiple strategies is one of the effective treatment regimens in cancer therapy and management, to come over the multi-drug resistance (MDR). Based on these facts, RSV treatment strategy was used in combination chemoprevention with other natural active molecules or small molecular drugs by several researchers to find the improved efficacy of RSV action. Pace-Asciak et al. (1995) reported that trans-resveratrol and quercetin combination present in red wine has shown to exhibit dose-dependent inhibition of both thrombin-induced and ADP-induced platelet aggregation preventing atherosclerosis more effectively. Initially RSV was found to be a potent anti-oxidant molecule that can prevent carcinogenesis, later several reports have indicated that it can mediate its actions through multiple ways by interacting with several molecules. Quercetin is another phytoconstituent that has been widely distributed in vegetables and fruits with many health enhancing effects along with anti-cancer effects like loss of cancer cell viability, inducing apoptosis and autophagy through PI3K, Wnt and MAPK pathway modulation (Anand David et al., 2016; Reyes-Farias and Carrasco-Pozo, 2019). Nam et al. (2016) have mentioned the various methods for nanofabrication of quercetin formulations and its applications in oncotherapy. The first combination of RSV tested was with quercetin on oral cancer cell growth and proliferation. It was reported that by treating with 50 μM RSV along with 10, 25 and 50 μM of quercetin which is another natural active component in common foods, oral squamous carcinoma cell (SCC-25) resulted in gradual significant increase in the inhibitory effect of quercetin on cell growth and DNA synthesis. Effective inhibition of SCC-25 cell growth and proliferation was reported due to enhanced activity of quercetin in presence of RSV (ElAttar and Virji, 1999). Combinational chemoprevention is only possible strategy to manage cancer cells and the same could be tested to target cancer stem cells. Different reports using various cancer and CSC models treated with RSV in combinations and their effects are presented in Table 3.
Table 3.
Summary of RSV and its combinational chemopreventive effects.
| Ref. | Experimental Models Systems | RSV Combinations | Effects |
|---|---|---|---|
| Bader & Getoff (2006) | Human Breast cancer cells – MCF7 | Mitomycin C (MMC) | Anti-tumor free radical scavenger activity under aerobic conditions in presence of mitomycin ↑ |
| Reiter et al. (2007) | Human Mast Cell line-1 (HMC-1) | Delta-Tocopherol |
Combinations of 50 μM RSV and 50 μM delta-tocopherol resulted: Protein Kinase B (PKB) Ser473-phosphorylation ↓ HMC-1 cell proliferation ↓ |
| Zhang et al. (2014) | Fanconi anemia (FA) murine models | N-acetylcysteine (NAC) | Neither RSV nor NAC could have significant chemopreventive effect in FA mouse models. |
| Yang et al. (2015a, b, c) | Human bronchial epithelial cell line BEAS-2B, 16HBE and Human lung cancer cell lines – A549 and H446. | AK001796 lncRNA |
AK001796 in lung cancer tissues and cells pretreated with RSV resulted: G0/G1 cell cycle arrest ↑ In vitro and In vivo colony formation ↓ Cell growth and proliferation ↓ |
| Li et al. (2016) | Patient derived glioblastoma-initiating cells. | Temozolamide |
Both in vitro and in vivo resulted: Apoptosis ↑ through DNA double stranded breaks, pATM/pATR/p53 pathway activation ↑ cell differentiation ↑ p-STAT3 activity ↓ |
| Hardin et al. (2016) | Anaplastic thyroid cancer cell lines – FRO, Kat18, NTHY-Ori-3, 8505C, papillary thyroid carcinoma cell line BCPAP, TPC-1 Cell line, THJ-16T and THJ-21T | Valproic acid | Stem cell marker - Aldefluor expression ↓ Proliferation ↓ Invasiveness ↓ Apoptosis ↑ Thyroid differentiation markers ↑ |
| Yuan et al. (2017) | Human ovarian carcinoma cell line – A2780 cells | Gemcitabine (GEM) along with Silver nanoparticles-RSV (AgNPs) | Combined GEM and AgNPs exhibited potent apoptotic activity ↑ |
| Dewangan et al. (2017) | Human breast cancer cells (HBCCs) - MCF-7, MCF-10A | Salinomycin | Apoptosis ↑ via reactive oxygen species (ROS) mediated mitochondrial dysfunction. Altered nuclear morphology PARP cleavage ↑, Caspase activation ↑ Modulated MAPK pathway |
| Mukherjee et al. (2018) | C57BL/6 male mice (2–4 months old); GL261 mouse glioblastoma cells | TriCurin: Curcumin, Epicatechin gallate and Resveratrol (4:1:12.5) combination |
In GL261 under In vitro: p53 ↑ apoptosis ↑ In In vivo: Repolarization of M2-like tumor (GBM) associated microglia/macrophages to the tumoricidal M1-like phenotype and intra-GBM recruitment of activated natural killer cells leading to apoptosis of tumor stem cells. |
| Pouyafar et al. (2019) | SKOV3 derived ovarian cancer stem cells In vitro | Doxorubicin (DOX) |
Treated with RSV and DOX at IC50of 55 μM and 25 ηM, respectively resulted: BAX ↑ Caspase 3 ↑ MDR1 ↓ MRP1↓ Drug resistance to doxorubicin ↓ Apoptosis ↑ |
| Pouyafar et al. (2019) | Cancer stem cells of human adenocarcinoma cell line HT-29 | Sulindac |
Transcription of autophagy signaling genes: (GALNT11) ↑ in cancer stem cells Trans-differentiation ↑ Decreased cell resistance ↓ |
| Hoca et al. (2020) | PANC-1 derived CD133+ and CD133- pancreatic cancer cells | Quercetin |
At 5,10,25,50 and 100 μM concentrations of combined treatment of CD133+ cells resulted: ACTA-2, IL-1β, and N-Cadherin ↓ TNF-α and Vimentin ↑ TNF-α and N-Cadherin ↓ in RSV alone treated CD133+ cells Quercetin could prevent EMT to a greater extent than RSV |
| Shin et al. (2020) | HeLa cervical cancer adherent and stem-like cells | Pterostilbene |
Pterostilbene exhibited better effects than RSV including: Cell cycle arrest at G2/M phase ROS-mediated Caspase-dependent apoptosis ↑ MMP-2/9 expression ↓ Tumor sphere formation and migration abilities ↓ Stemness marker expression: CD133, Oct-4, Sox2, and Nanog ↓ STAT-3 ↓ |
3.6. Limitations of resveratrol in therapy
The critical point of limitation found in RSV literature is lack of sufficient in vivo and human trial based evidences. Its observations are mainly limited due to its bioavailability under in vivo system and also due to differential effects with different RSV concentrations. Further, there is a need for clear understanding for the roles of RSV metabolites along with free form of RSV as chemotherapeutic in cancer patients. Zykova et al. (2008) reported that in human colon adenocarcinoma HT-29 cells shown inhibited Cox-1 and Cox-2 by both RSV and its metabolite RSV-4′-O-sulfate. In another study, the hydroxylated metabolites of RSV formed from gut microbiota have exerted cytotoxic properties (Bode et al., 2013). These effects in other type of cancers and in clinical studies require proper validation.
It was established for RSV effects like NF-κB activity regulation, inhibiting cytochrome P450 isoenzyme (CYP A1), cyclooxygenase (COX) activity, TP53, FAS/FASL or CD95 induced apoptosis, inhibiting the HIF-1α and VEGF expression through which its anti-cancer properties are sought. There are few clinical trials with RSV as oral administration or micronized formulations for different type of cancer patients. Few studies have indicated its advantageous effect by modulating the targeted molecules, few were inconclusive and other few studies have resulted with certain adverse effects like nausea, diarrhea, vomiting, fatigue, anemia and mainly renal toxicity in multiple myeloma patients (Popat et al., 2001).
There are controversial reports which need to be reconfirmed and studied in details. RSV was reported to promote atherosclerosis in hypercholesterolemic rabbits rather than protecting against atherosclerosis (Wilson et al., 1996). Further, RSV was shown to suppress atherosclerosis in hypercholesterolemic rabbits without affecting plasma lipid levels (Wang et al., 2005). Zhang et al. (2014) reported that tempol and N-acetylcysteine (NAC) or RSV when tested for its chemopreventive effects in tumor prone Fancd2(−/−)/Trp53(±) fanconi anemia (FA) murine models, RSV could not show effective chemopreventive effect as that of tempol. There are certain clinical trials attempted to draw conclusions for RSV as an effective chemotherapeutic is discussed in the review by Berman et al. (2017) and they reported that breast cancer and multiple myeloma patients have shown RSV as more promising molecule but limited due to adverse effects. Other clinical trials were made on prostate cancer, colorectal cancer and bladder cancer patients, but require further detailed understanding of RSV effects.
4. Conclusions and perspectives
Natural bioactive compounds in edibles with pharmacological activities have no known side effects and can have better impact by interacting with other secondary metabolites. Hence, at present potent natural bioactive compounds and their applications are on demand. RSV is a well-known compound and recently its effects of targeting CSCs have become more interesting. Being a potent reducing agent it is known to prevent carcinogenesis due to its anti-oxidant abilities, however its ability to regulate other molecules and mechanisms to target cancer cells and cancer stem cells are now attaining interest.
After the initial report in 2007, in which RSV was reported to stop cell shedding, thus inhibiting metastasis of mouse cancerous mammospheroid cells, following research on CSCs have tremendously taken a peak with most of the research groups working either with RSV alone or in combination with other molecules to test anti-cancer stem cell effects. There has been intervention of recent methods like effect of RSV and long noncoding RNAs (lncRNAs) in lung carcinogenesis (Yang et al., 2015a, b, c), inhalable resveratrol-cyclodextrin complex loaded biodegradable nanoparticles against non-small cell lung cancer (Wang et al., 2020) and as an immunomodulatory agent (Trung and An, 2018) in immunotherapy of treating cancer and cancer stem cells are some areas at the front end of modern research.
The major drawback of RSV research is that, most of the attempts include in vitro cell culture experiments that require validation of the same effect under in vivo conditions and with primary cultures of human cancer tissues along with clinical trials. As RSV is a natural bioactive compound, it should be tested with different combinations as they can affect multiple pathways unlike targeted drug molecules which make this strategy as unique therapeutic regiment to target cancer cells and cancer stem cells.
CRediT author statement
All authors contribute to Conceptualization, Methodology, Investigation, Writing- Original draft preparation, and Writing- Reviewing and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Vasanth K. Bhaskara, Email: vasanthkbhaskara@gmail.com.
Bharti Mittal, Email: genomicsbioinformatics2976@gmail.com.
Vijaya V. Mysorekar, Email: vijayamysorekar1@gmail.com.
Nagarathna Amaresh, Email: dr.nagarathnaa@gmail.com.
Jesus Simal-Gandara, Email: jsimal@uvigo.es.
References
- Aires V., Brassart B., Carlier A., Scagliarini A., Mandard S., Limagne E., Solary E., Martiny L., Tarpin M., Delmas D. A role for peroxisome proliferator-activated receptor gamma in resveratrol-induced colon cancer cell apoptosis. Mol. Nutr. Food Res. 2014;58:1785–1794. doi: 10.1002/mnfr.201300962. [DOI] [PubMed] [Google Scholar]
- Anand David A.V., Arulmoli R., Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogen Rev. 2016;10:84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader Y., Getoff N. Effect of resveratrol and mixtures of resveratrol and mitomycin C on cancer cells under irradiation. Anticancer Res. 2006;26:4403–4408. [PubMed] [Google Scholar]
- Berman A.Y., Motechin R.A., Wiesenfeld M.Y., Holz M.K. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol. 2017;1:35. doi: 10.1038/s41698-017-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara V.K., Jayaram C., Priyanga M., Nayaka N., Shivakumara A., Amaresh N., Mysorekar V.V. Aberrant signal transduction in Indian triple-negative breast cancer patients. J. Canc. Res. Therapeut. 2019;15:1162–1166. doi: 10.4103/jcrt.JCRT_803_16. [DOI] [PubMed] [Google Scholar]
- Bhullar K.S., Hubbard B.P. Lifespan and health span extension by resveratrol. Biochem Biophys Acta. 2015;1852:1209–1218. doi: 10.1016/j.bbadis.2015.01.012. [DOI] [PubMed] [Google Scholar]
- Bian P., Hu W., Liu C., Li L. Resveratrol potentiates the anti-tumor effects of rapamycin in papillary thyroid cancer: PI3K/AKT/mTOR pathway involved. Arch. Biochem. Biophys. 2020;689:108461. doi: 10.1016/j.abb.2020.108461. [DOI] [PubMed] [Google Scholar]
- Bode L.M., Bunzel D., Huch M., Cho G.S., Ruhland D., Bunzel M., Bub A., Franz C.M., Kulling S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013;97:295–309. doi: 10.3945/ajcn.112.049379. [DOI] [PubMed] [Google Scholar]
- Britton R.G., Kovoor C., Brown K. Direct molecular targets of Resveratrol: identifying key interactions to unlock complex mechanisms. Ann. N. Y. Acad. Sci. 2015;1348:124–133. doi: 10.1111/nyas.12796. [DOI] [PubMed] [Google Scholar]
- Buhrmann C., Shayan P., Kraehe P., Popper B., Goel A., Shakibaei M. Resveratrol induces chemosensitization to 5-fluorouracil through up-regulation of intercellular junctions, Epithelial-to-mesenchyal transition and apoptosis in colorectal cancer. Biochem. Pharmacol. 2015;98:51–68. doi: 10.1016/j.bcp.2015.08.105. [DOI] [PubMed] [Google Scholar]
- Buhrmann C., Yazdi M., Popper B., Kunnumakkara A.B., Aggarwal B.B., Shakibaei M. Induction of the epithelial-to-mesenchymal transition of human colorectal cancer by human TNF-β (lymphotoxin) and its reversal by resveratrol. Nutrients. 2019;11:704. doi: 10.3390/nu11030704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.C. Cancer stem cells: role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine. 2016;95:S20–S25. doi: 10.1097/MD.0000000000004766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y., Kim Y.K. Cancer stem cells as a potential target to overcome multidrug resistance. Front Oncol. 2020;10:764. doi: 10.3389/fonc.2020.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.J., Heo K., Park H.S., Yang K.M., Jeong M.H. The resveratrol analog HS-1793 enhances radiosensitivity of mouse-derived breast cancer cells under hypoxic conditions. Int. J. Oncol. 2016;49:1479–1488. doi: 10.3892/ijo.2016.3647. [DOI] [PubMed] [Google Scholar]
- Cilibrasi C., Riva G., Romano G., Cadamuro M., Bazzoni R., Butta V., Paoletta L., Dalprà L., Strazzabosco M., Lavitrano M., Giovannoni R., Bentivegna A. Resveratrol impairs glioma stem cells proliferation and motility by modulating the wnt signaling pathway. PloS One. 2017;12 doi: 10.1371/journal.pone.0169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark P.A., Bhattacharya S., Elmayan A., Darjatmoko S.R., Thuro B.A., Yan M.B., van Ginkel P.R., Polans A.S., Kuo J.S. Resveratrol targeting of AKT and p53 in glioblastoma and glioblastoma stem-like cells to suppress growth and infiltration. J. Neurosurg. 2017;126:1448–1460. doi: 10.3171/2016.1.JNS152077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewangan J., Tandon D., Srivastava S., Verma A.K., Yapuri A., Rath S.K. Novel combination of salinomycin and resveratrol synergistically enhances the anti-proliferative and pro-apoptotic effects on human breast cancer cells. Apoptosis. 2017;22:1246–1259. doi: 10.1007/s10495-017-1394-y. [DOI] [PubMed] [Google Scholar]
- Du Z., Zhou F., Jia Z., Zheng B., Han S., Cheng J., Zhu G., Huang P. The hedgehog/Gli-1 signaling pathways is involved in the inhibitory effect of resveratrol on human colorectal cancer HCT116 cells. Iran J Basic Med Sci. 2016;19:1171–1176. [PMC free article] [PubMed] [Google Scholar]
- ElAttar T.M., Virji A.S. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anti Canc. Drugs. 1999;10:187–193. doi: 10.1097/00001813-199902000-00007. [DOI] [PubMed] [Google Scholar]
- Fan P., Marston A., Hay A.E., Hostettmann K. Rapid separation of three gluosylated resveratrol analogues from the invasive plant Polygonum cuspidatum by high-speed countercurrent chromatography. J. Separ. Sci. 2009;32:2979–2984. doi: 10.1002/jssc.200900057. [DOI] [PubMed] [Google Scholar]
- Farooqi A.A., Khalid S., Ahmad A. Regulation of cell signaling pathways and miRNAs by resveratrol in different cancers. Int. J. Mol. Sci. 2018;19:652. doi: 10.3390/ijms19030652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei X., Wang A., Wang D., Meng X., Ma J., Hong L., Qin R., Wang A., Dong J., Huang Q., Wang Z. Establishment of malignantly transformed dendritic cell line SU3-ihDCTC induced by Glioma stem cells and study on its sensitivity to resveratrol. BMC Immunol. 2018;19:7. doi: 10.1186/s12865-018-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontecave M., Lepoivre M., Elleingand E., Gerez C., Guittet O. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 1998;421:277–279. doi: 10.1016/s0014-5793(97)01572-x. [DOI] [PubMed] [Google Scholar]
- Fu Y., Chang H., Peng X., Bai Q., Yi L., Zhou Y., Zhu J., Mi M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/β-catenin signaling pathway. PloS One. 2014;9 doi: 10.1371/journal.pone.0102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambini J., Ingles M., Olaso G., Lopez-Grueso R., Bonet-Costa V., Gimeno-Mallench L., Mas-Bargues C., Abdelaziz K.M., Gomez-Cabrera M.C., Vina J., Borras C. Properties of Resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev. 2015 doi: 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehm B.D., McAndrews J.M., Chien P.Y., Jameson J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C., Xia H. Resveratrol suppresses melanoma growth by promoting autophagy through inhibiting the PI3K/AKT/mTOR signaling pathway. Exp Ther Med. 2020;19:1878–1886. doi: 10.3892/etm.2019.8359. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gunther S., Ruhe C., Derikito M.G., Bose G., Sauer H., Wartenberg M. Polyphenols prevent cell shedding from mouse mammary cancer spheroids and inhibit cancer cell invasion in confrontation cultures derived from embryonic stem cells. Cancer let. 2007;250:25–35. doi: 10.1016/j.canlet.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Hagiwara K., Kosaka N., Yoshioka Y., Takahashi R.U., Takeshita F., Ochiya T. Stilbene derivatives promote Ago2-dependent tumour-suppressive microRNA activity. Sci. Rep. 2012;2:314. doi: 10.1038/srep00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin H., Yu X.M., Harrison A.D., Larrain C., Zhang R., Chen J., Chen H., Lloyd R.V. Generation of novel thyroid cancer stem-like cell clones: effects of resveratrol and valproic acid. Am. J. Pathol. 2016;186:1662–1673. doi: 10.1016/j.ajpath.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoca M., Becer E., Kabadayı H., Yücecan S., Vatansever H.S. The effect of resveratrol and quercetin on epithelial-mesenchymal transition in pancreatic cancer stem cell. Nutr. Canc. 2020;72:1231–1242. doi: 10.1080/01635581.2019.1670853. [DOI] [PubMed] [Google Scholar]
- Hu F.W., Tsai L.L., Yu C.H., Chen P.N., Chou M.Y., Yu C.C. Impairment of tumor-initiating stem-like property and reversal of epithelial-mesenchymal transdifferentiation in head and neck cancer by resveratrol treatment. Mol. Nutr. Food Res. 2012;56:1247–1258. doi: 10.1002/mnfr.201200150. [DOI] [PubMed] [Google Scholar]
- Hu L., Cao D., Li Y., He Y., Guo K. Resveratrol sensitized leukemia stem cell-like KG-1a cells to cytokine-induced killer cells-mediated cytolysis through NKG2D ligands and TRAIL receptors. Canc. Biol. Ther. 2012;13:516–526. doi: 10.4161/cbt.19601. [DOI] [PubMed] [Google Scholar]
- Jagust P., de Luxán-Delgado B., Parejo-Alonso B., Sancho P. Metabolism-based therapeutic strategies targeting cancer stem cells. Front. Pharmacol. 2019;10:203. doi: 10.3389/fphar.2019.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W., Fong H.H., Farnsworth N.R., Kinghorn A.D., Mehta R.G., Moon R.C., Pezzuto J.M. Cancer chemo preventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jeong J.B., Lee J., Lee S.H. TCF4 is a molecular target of resveratrol in the prevention of colorectal cancer. Int. J. Mol. Sci. 2015;16:10411–10425. doi: 10.3390/ijms160510411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri A., Luther E., Torchilin V. The effect of transferrin-targeted, resveratrol-loaded liposomes on neurosphere cultures of glioblastoma: implications for targeting tumour-initiating cells. J. Drug Target. 2019;27:601–613. doi: 10.1080/1061186X.2018.1550647. [DOI] [PubMed] [Google Scholar]
- Ji Q., Liu X., Fu X., Zhang L., Sui H., Zhou L., Sun J., Cai J., Qin J., Ren J., Li Q. Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated wnt/β-catenin signaling pathway. PloS One. 2013;8 doi: 10.1371/journal.pone.0078700. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ji Q., Liu X., Han Z., Zhou L., Sui H., Yan L., Jiang H., Ren J., Cai J., Li Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Canc. 2015;15:97. doi: 10.1186/s12885-015-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Li H., Liu Y., Guo A., Xu X., Qu X., Wang S., Zhao J., Li Y., Cao Y. Resveratrol inhibits the invasion of glioblastoma-initiating cells via down-regulation of the PI3K/Akt/NF-κB signaling pathway. Nutrients. 2015;7:4383–4402. doi: 10.3390/nu7064383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C.L., Huang P.I., Tsai P.H., Tsai M.L., Lo J.F., Lee Y.Y., Chen Y.J., Chen Y.W., Chiou S.H. Resveratrol-induced apoptosis and increased radiosensitivity in CD133-positive cells derived from atypical teratoid/rhabdoid tumor. Int. J. Radiat. Oncol. Biol. Phys. 2009;74:219–228. doi: 10.1016/j.ijrobp.2008.12.035. [DOI] [PubMed] [Google Scholar]
- Kim K.H., Back J.H., Zhu Y., Arbesman J., Athar M., Kopelovich L., Kim A.L., Bickers D.R. Resveratrol targets transforming growth factor-β2 signaling to block UV-induced tumor progression. J. Invest. Dermatol. 2011;131:195–202. doi: 10.1038/jid.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.H., Sethi G., Um J.Y., Shanmugam M.K., Arfuso F., Kumar A.P., Bishayee A., Ahn K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017;18:2589. doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Eroglu E., Stokes J.A., Scissum-Gunn K., Saldanha S.N., Singh U.P., Manne U., Ponnazhagan S., Mishra M.K. Resveratrol induces mitochondria-mediated, caspase-independent apoptosis in murine prostate cancer cells. Oncotarget. 2017;8:20895–20908. doi: 10.18632/oncotarget.14947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lee H.S., Ha A.W., Kim W.K. Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr Res Pract. 2012;6:294–300. doi: 10.4162/nrp.2012.6.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Chang C., Bodogai M., Martin-Montalvo A., Wejksza K., Sanghvi M., Moaddel R., de Cabo R., Biragyn A. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B-cells. J. Immunol. 2013;191:4141–4151. doi: 10.4049/jimmunol.1300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Liu Y., Jiao Y., Guo A., Xu X., Qu X., Wang S., Zhao J., Li Y., Cao Y. Resveratrol sensitizes glioblastoma-initiating cells to temozolomide by inducing cell apoptosis and promoting differentiation. Oncol. Rep. 2016;35:343–351. doi: 10.3892/or.2015.4346. [DOI] [PubMed] [Google Scholar]
- Li S., Li Q. Cancer stem cells and tumor metastasis. Int. J. Oncol. 2014;44:1806–1812. doi: 10.3892/ijo.2014.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lif F., Tiede B., Massague J., Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- Li W., Li C., Ma L., Jin F. Resveratrol inhibits viability and induces apoptosis in the small-cell lung cancer H446 cell line via the PI3K/Akt/c-Myc pathway. Oncol. Rep. 2020;44:1821–1830. doi: 10.3892/or.2020.7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Guo Y., Feng Z., Bergan R., Li B., Qin Y., Zhao L., Zhang Z., Shi M. Involvement of the PI3K/Akt/Nrf2 signaling pathway in resveratrol-mediated reversal of drug resistance in HL-60/ADR cells. Nutr. Canc. 2019;71:1007–1018. doi: 10.1080/01635581.2019.1578387. [DOI] [PubMed] [Google Scholar]
- Liao H.F., Su Y.C., Zheng Z.Y., Jhih Cai C., Hou M.H., Chao K.S., Chen Y.J. Sonic hedgehog signaling regulates Bcr-Abl expression in human chronic myeloid leukemia cells. Biomed. Pharmacother. 2012;66:378–383. doi: 10.1016/j.biopha.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Lin H., Xiong W., Zhang X., Liu B., Zhang W., Zhang Y., Cheng J., Huang H. Notch-1 activation-dependent p53 restoration contributes to resveratrol-induced apoptosis in glioblastoma cells. Oncol. Rep. 2011;26:925–930. doi: 10.3892/or.2011.1380. [DOI] [PubMed] [Google Scholar]
- Liu Y.Z., Wu K., Huang J., Liu Y., Wang X., Meng Z.J., Yuan S.X., Wang D.X., Luo J.Y., Zuo G.W., Yin L.J., Chen L., Deng Z.L., Yang J.Q., Sun W.J., He B.C. The PTEN/PI3K/Akt and Wnt/β-catenin signaling pathways are involved in the inhibitory effect of resveratrol on human colon cancer cell proliferation. Int. J. Oncol. 2014;45:104–112. doi: 10.3892/ijo.2014.2392. [DOI] [PubMed] [Google Scholar]
- Liu Z., Li Y., Yang R. Effects of resveratrol on vascular endothelial growth factor expression in osteosarcoma cells and cell proliferation. Oncol Lett. 2012;4:837–839. doi: 10.3892/ol.2012.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.L., Li H., Liu J., Wu M.L., Chen X.Y., Liu L.H., Wang Q. Inactivated Wnt signaling in resveratrol-treated epidermal squamous cancer cells and its biological implication. Oncol Lett. 2017;14:2239–2243. doi: 10.3892/ol.2017.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K.H., Chen Y.W., Tsai P.H., Tsai M.L., Lee Y.Y., Chiang C.Y., Kao C.L., Chiou S.H., Ku H.H., Lin C.H., Chen Y.J. Evaluation of radiotherapy effect in resveratrol-treated Medulloblastoma cancer stem-like cells. Childs Nerv Syst. 2009;25(5):543–550. doi: 10.1007/s00381-009-0826-6. [DOI] [PubMed] [Google Scholar]
- Meng J., Liu G.J., Song J.Y., Chen L., Wang A.H., Gao X.X., Wang Z.J. Preliminary results indicate resveratrol affects proliferation and apoptosis of leukemia cells by regulating PTEN/PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23:4285–4292. doi: 10.26355/eurrev_201905_17933. [DOI] [PubMed] [Google Scholar]
- Mieszala K., Rudewicz M., Gomulkiewicz A., Ratajczak-Wielgomas K., Grzegrzolka J., Dziegiel P., Borska S. Expression of genes and proteins of multidrug resistance in gastric cancer cells treated with resveratrol. Oncol Lett. 2018;15:5825–5832. doi: 10.3892/ol.2018.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Baidoo J., Sampat S., Mancuso A., David L., Cohen L.S., Zhou S., Banerjee P. Liposomal TriCurin, A synergistic combination of curcumin, epicatechin gallate and resveratrol, repolarizes tumor-associated microglia/macrophages, and eliminates glioblastoma (GBM) and GBM stem cells. Molecules. 2018;23:201. doi: 10.3390/molecules23010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam J.S., Sharma A.R., Nguyen L.T., Chakraborty C., Sharma G., Lee S.S. Application of bioactive quercetin in oncotherapy: from nutrition to nanomedicine. Molecules. 2016;21:E108. doi: 10.3390/molecules21010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nguyen L.V., Vanner R., Dirks P., Eaves C.J. Cancer stem cells: an evolving concept. Nat. Rev. Canc. 2012;12:133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- Oliverira-Cunha M., Newman W.G., Siriwardena A.K. Epidermal growth factor receptor in pancreatic cancer. Cancers. 2011;3:1513–1526. doi: 10.3390/cancers3021513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Asciak C.R., Hahn S., Diamandis E.P., Soleas G., Goldberg D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin. Chim. Acta. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P.R., Okuda H., Watabe M., Pai S.K., Liu W., Kobayashi A., Xing F., Fukuda K., Hirota S., Sugai T., Wakabayashi G., Koeda K., Kashiwaba M., Suzuki K., Chiba T., Endo M., Fujioka T., Tanji S., Mo Y.Y., Cao D., Wilber A.C., Watabe K. Resveratrol suppresses growth of cancer stem-like cells by inhibiting fatty acid synthase. Breast Canc. Res. Treat. 2011;130:387–398. doi: 10.1007/s10549-010-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Jiang D. Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition. PloS One. 2018;13 doi: 10.1371/journal.pone.0205918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchot S.N., Jaskula-Sztul R., Ning L., Peters N.R., Cook M.R., Kunnimalaiyaan M., Chen H. Identification and validation of Notch pathway activating compounds through a novel high-throughput screening method. Cancer. 2011;117:1386–1398. doi: 10.1002/cncr.25652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popat R., Plesner T., Davies F., Cook G., Cook M., Elliott P., Jacobson E., Gumbleton T., Oakervee H., Cavenagh J. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br. J. Haematol. 2001;160:714–717. doi: 10.1111/bjh.12154. [DOI] [PubMed] [Google Scholar]
- Pouyafar A., Rezabakhsh A., Rahbarghazi R., Heydarabad M.Z., Shokrollahi E., Sokullu E., Khaksar M., Nourazarian A., Avci Ç.B. Treatment of cancer stem cells from human colon adenocarcinoma cell line HT-29 with resveratrol and sulindac induced mesenchymal-endothelial transition rate. Cell Tissue Res. 2019;376:377–388. doi: 10.1007/s00441-019-02998-9. [DOI] [PubMed] [Google Scholar]
- Pouyafar A., Zadi Heydarabad M., Aghdam S.B., Khaksar M., Azimi A., Rahbarghazi R., Talebi M. Resveratrol potentially increased the tumoricidal effect of doxorubicin on SKOV3 cancer stem cells in vitro. J. Cell. Biochem. 2019 doi: 10.1002/jcb.28129. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Prieto-Vila M., Takahashi R.U., Usuba W., Kohama I., Ochiya T. Drug resistance driven by cancer stem cells and their niche. Int. J. Mol. Sci. 2017;18:2574. doi: 10.3390/ijms18122574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh E., Sankari L.S., Malathi L., Kruppa J.R. Naturally occurring products in cancer therapy. J. Pharm. BioAllied Sci. 2015;7:S181–S183. doi: 10.4103/0975-7406.155895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter E., Azzi A., Zingg J.M. Enhanced anti-proliferative effects of combinatorial treatment of delta-tocopherol and resveratrol in human HMC-1 cells. Biofactors. 2007;30:67–77. doi: 10.1002/biof.5520300201. [DOI] [PubMed] [Google Scholar]
- Renaud S., de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339(8808):1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- Reyes-Farias M., Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019;20:3177. doi: 10.3390/ijms20133177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz G., Valencia-Gonzalez H.A., León-Galicia I., García-Villa E., Garcia-Carrancá A., Gariglio P. Inhibition of RAD51 by siRNA and resveratrol sensitizes cancer stem cells derived from HeLa cell cultures to apoptosis. Stem Cell. Int. 2018;2018:2493869. doi: 10.1155/2018/2493869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Okada M., Shibuya K., Watanabe E., Seino S., Suzuki K., Narita Y., Shibui S., Kayama T., Kitanaka C. Resveratrol promotes proteasome-dependent degradation of Nanog via p53 activation and induces differentiation of glioma stem cells. Stem Cell Res. 2013;11:601–610. doi: 10.1016/j.scr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Sayd S., Thirant C., El-Habr E.A., Lipecka J., Dubois L.G., Bogeas A., Tahiri-Jouti N., Chneiweiss H., Junier M.P. Sirtuin-2 activity is required for glioma stem cell proliferation arrest but not necrosis induced by resveratrol. Stem Cell Rev Rep. 2014;10:103–113. doi: 10.1007/s12015-013-9465-0. [DOI] [PubMed] [Google Scholar]
- Schneider Y., Vincent F., Duranton B., Badolo L., Gosse F., Bergmann C., Seiler N., Raul F. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Canc. Lett. 2000;158:85–91. doi: 10.1016/s0304-3835(00)00511-5. [DOI] [PubMed] [Google Scholar]
- Segun P.A., Ogbole O.O., Ismail F., Nahar L., Evans A.R., Ajaiyeoba E.O., Sarker S.D. Resveratrol derivatives from Commiphora africana (A. Rich.) Endl. display cytotoxicity and selectivity against several human cancer cell lines. Phytother Res. 2019;33:159–166. doi: 10.1002/ptr.6209. [DOI] [PubMed] [Google Scholar]
- Seino M., Okada M., Shibuya K., Seino S., Suzuki S., Takeda H., Ohta T., Kurachi H., Kitanaka C. Differential contribution of ROS to resveratrol-induced cell death and loss of self-renewal capacity of ovarian cancer stem cells. Anticancer Res. 2015;35:85–96. [PubMed] [Google Scholar]
- Shankar S., Nall D., Tang S.N., Meeker D., Passarini J., Sharma J., Srivastava R.K. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PloS One. 2011;6 doi: 10.1371/journal.pone.0016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Hoque M.O. Targeting cancer stem cells: a strategy for effective eradication of cancer. Cancers. 2019;11:732. doi: 10.3390/cancers11050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H.J., Han J.M., Choi Y.S., Jung H.J. Pterostilbene suppresses both cancer cells and cancer stem-like cells in cervical cancer with superior bioavailability to resveratrol. Molecules. 2020;25:228. doi: 10.3390/molecules25010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrikanta A., Kumar A., Govindaswamy V. Resveratrol content and antioxidant properties of underutilized fruits. J. Food Sci. Technol. 2015;52:383–390. doi: 10.1007/s13197-013-0993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.K., Banerjee S., Acosta E.P., Lillard J.W., Singh R. Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/p21WAF1/CIP1 and p27KIP1 pathway. Oncotarget. 2017;8:17216–17228. doi: 10.18632/oncotarget.15303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Chen Y., Li Y., Lyu X., Cui J., Cheng Y., Zheng T., Zhao L., Zhao G. Resveratrol suppresses epithelial-mesenchymal transition in GBM by regulating smad-dependent signaling. BioMed Res. Int. 2019;2019:1321973. doi: 10.1155/2019/1321973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M., Moco S. Resveratrol and its human metabolites-effects on metabolic health and obesity. Nutrients. 2019;11:143. doi: 10.3390/nu11010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y.C., Li S.C., Wu Y.C., Wang L.M., Chao K.S., Liao H.F. Resveratrol downregulates interleukin-6-stimulated sonic hedgehog signaling in human acute myeloid leukemia. Evid Based Complement Alternat Med. 2013;2013:547430. doi: 10.1155/2013/547430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Zhang T., Liu R., Cao W., Zhang Z., Liu Z., Qian W., Wang D., Yu D., Zhong C. Resveratrol inhibition of renal cancer stem cell characteristics and modulation of the sonic hedgehog pathway. Nutr. Canc. 2020;1–11 doi: 10.1080/01635581.2020.1784966. [DOI] [PubMed] [Google Scholar]
- Sun Y., Zhou Q.M., Lu Y.Y., Zhang H., Chen Q.L., Zhao M., Su S.B. Resveratrol inhibits the migration and metastasis of MDA-MB-231 human breast cancer by reversing TGF-β1-induced epithelial-mesenchymal transition. Molecules. 2019;24:1131. doi: 10.3390/molecules24061131. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Szekeres T., Fritzer-Szekeres M., Saiko P., Jager W. Resveratrol and resveratrol analogues - structure - activity relationship. Pharm. Res. (N. Y.) 2010;27:1042–1048. doi: 10.1007/s11095-010-0090-1. [DOI] [PubMed] [Google Scholar]
- Szende B., Tyihak E., Trezl L., Szoke E., Laszlo I., Katay G., Kiraly-Veghely Z. Formaldehyde generators and capturers as influencing factors of mitotic and apoptotic processes. Acta Biol. Hung. 1998;49:323–329. [PubMed] [Google Scholar]
- Takizawa Y., Nakata R., Fukuhara K., Yamashita H., Kubodera H., Inoue H. The 4'-hydroxyl group of resveratrol is functionally important for direct activation of PPARα. PloS One. 2015;10 doi: 10.1371/journal.pone.0120865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tome-Carneiro J., Larrosa M., Gonzalez-Sarrias A., Tomas-Barbern F.A., Garcia-Conesa M.T., Espin J.C. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr. Pharmaceut. Des. 2013;19:6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trela B.C., Waterhouse A.L. Resveratrol: isomeric molar absorptives and stability. J. Agric. Food Chem. 1996;44:1253–1257. [Google Scholar]
- Trung L.Q., An D. Is resveratrol a cancer immunomodulatory molecule? Front. Pharmacol. 2018;9:1255. doi: 10.3389/fphar.2018.01255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyihak E., Albert L., Nemeth Z.I., Katay G., Kiraly-Veghely Z., Szende B. Formaldehyde cycle and the natural formaldehyde generators and captures. Acta Biol. Hung. 1998;49:225–238. [PubMed] [Google Scholar]
- Wallenborg K., Vlachos P., Eriksson S., Huijbregts L., Arner E.S.J., Joseph B., Hermanson O. Red wine triggers cell death and thioredoxin reductase inhibition: effects beyond resveratrol and SIRT1. Exp. Cell Res. 2009;315:1360–1371. doi: 10.1016/j.yexcr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang C., Jia Y., Liu Z., Shu X., Liu K. Resveratrol increases anti-proliferative activity of bestatin through downregulating P-glycoprotein expression via inhibiting PI3K/Akt/mTOR pathway in K562/ADR cells. J. Cell. Biochem. 2016;117:1233–1239. doi: 10.1002/jcb.25407. [DOI] [PubMed] [Google Scholar]
- Wang X., Parvathaneni V., Shukla S.K., Kulkarni N.S., Muth A., Kunda N.K., Gupta V. Inhalable resveratrol-cyclodextrin complex loaded biodegradable nanoparticles for enhanced efficacy against non-small cell lung cancer. Int. J. Biol. Macromol. 2020;164:638–650. doi: 10.1016/j.ijbiomac.2020.07.124. [DOI] [PubMed] [Google Scholar]
- Wang Z., Zou J., Cao K., Hsieh T.C., Huang Y., Wu J.M. Dealcoholized red wine containing known amounts of resveratrol suppresses atherosclerosis in hypercholesterolemic rabbits without affecting plasma lipid levels. Int. J. Mol. Med. 2005;16:533–540. [PubMed] [Google Scholar]
- Waterhouse A.L. Wine phenolics. Ann. N. Y. Acad. Sci. 2002;957:21–36. doi: 10.1111/j.1749-6632.2002.tb02903.x. [DOI] [PubMed] [Google Scholar]
- Wen W., Lowe G., Roberts C.M., Finlay J., Han E.S., Glackin C.A., Dellinger T.H. Pterostilbene suppresses ovarian cancer growth via induction of apoptosis and blockade of cell cycle progression involving inhibition of the STAT3 pathway. Int. J. Mol. Sci. 2018;19:1983. doi: 10.3390/ijms19071983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T., Knight T.J., Beitz D.C., Lewis D.S., Engen R.L. Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life Sci. 1996;59:PL15–PL21. doi: 10.1016/0024-3205(96)00260-3. [DOI] [PubMed] [Google Scholar]
- Xia N., Forstermann U., Li H. Resveratrol and endothelial nitric oxide. Molecules. 2014;19:16102–16121. doi: 10.3390/molecules191016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Liu J., Lyu X., Fei S. Resveratrol inhibits cell proliferation and up-regulates MICA/B expression in human colon cancer stem cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi (Chinese journal of cellular and molecular immunology) 2015;31:889–893. [PubMed] [Google Scholar]
- Yang L., Shi P., Zhao G., Xu J., Peng W., Zhang J., Zhang G., Wang X., Dong Z., Chen F., Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Xu E., Dai J., Liu B., Han Z., Wu J., Zhang S., Peng B., Zhang Y., Jiang Y. A novel long noncoding RNA AK001796 acts as an oncogene and is involved in cell growth inhibition by resveratrol in lung cancer. Toxicol. Appl. Pharmacol. 2015;285:79–88. doi: 10.1016/j.taap.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Yang Y., Xu H., Huang W., Ding M., Xiao J., Yang D., Li H., Liu X.Y., Chu L. Targeting lung cancer stem-like cells with TRAIL gene armed oncolytic adenovirus. J. Cell Mol. Med. 2015;19:915–923. doi: 10.1111/jcmm.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Zhang R., Hu Y., Li W., Wang M., Liang Z., Sun Z., Ji R., Xu W., Qian H. Gastric-cancer-derived mesenchymal stem cells: a promising target for resveratrol in the suppression of gastric cancer metastasis. Hum. Cell. 2020;33:652–662. doi: 10.1007/s13577-020-00339-5. [DOI] [PubMed] [Google Scholar]
- Yoshida G.J., Saya H. Therapeutic strategies targeting cancer stem cells. Canc. Sci. 2016;107:5–11. doi: 10.1111/cas.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L.J., Wu M.L., Li H., Chen X.Y., Wang Q., Sun Y., Kong Q.Y., Liu J. Inhibition of STAT3 expression and signaling in resveratrol-differentiated medulloblastoma cells. Neoplasia. 2008;10:736–744. doi: 10.1593/neo.08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.M., Jaskula-Sztul R., Ahmed K., Harrison A.D., Kunnimalaiyaan M., Chen H. Resveratrol induces differentiation markers expression in anaplastic thyroid carcinoma via activation of Notch1 signaling and suppresses cell growth. Mol. Canc. Therapeut. 2013;12:1276–1287. doi: 10.1158/1535-7163.MCT-12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Zhang Y., Xia J., Liu B., Zhang Q., Liu J., Luo L., Peng Z., Song Z., Zhu R. Resveratrol induces cell cycle arrest via a p53-independent pathway in A549 cells. Mol. Med. Rep. 2015;11:2459–2464. doi: 10.3892/mmr.2014.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.G., Peng Q.L., Gurunathan S. Silver nanoparticles enhance the apoptotic potential of gemcitabine in human ovarian cancer cells: combination therapy for effective cancer treatment. Int. J. Nanomed. 2017;12:6487–6502. doi: 10.2147/IJN.S135482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y.H., Zhou L.Y., Chen Q.Z., Li Y., Shao Y., Ren W.Y., Liao Y.P., Wang H., Zhu J.H., Huang M., He F., Wang J., Wu K., He B.C. Resveratrol inactivates PI3K/Akt signaling through upregulating BMP7 in human colon cancer cells. Oncol. Rep. 2017;38:456–464. doi: 10.3892/or.2017.5662. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wen X., Li M., Li S., Zhao H. Targeting cancer stem cells and signaling pathways by resveratrol and pterostilbene. Biofactors. 2018;44:61–68. doi: 10.1002/biof.1398. [DOI] [PubMed] [Google Scholar]
- Zhang Q.S., Marquez-Loza L., Sheehan A.M., Watanabe-Smith K., Eaton L., Benedetti E., Major A., Schubert K., Deater M., Joseph E., Grompe M. Evaluation of resveratrol and N-acetylcysteine for cancer chemoprevention in a Fanconi anemia murine model. Pediatr. Blood Canc. 2014;61:740–742. doi: 10.1002/pbc.24780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Jiang H., Chen Y., Ren F. Resveratrol chemosensitizes adriamycin-resistant breast cancer cells by modulating miR-122-5p. J. Cell. Biochem. 2019;120:16283–16292. doi: 10.1002/jcb.28910. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Tang H., Zeng X., Ye D., Liu J. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed. Pharmacother. 2018;11:143. doi: 10.1016/j.biopha.2017.12.029. [DOI] [PubMed] [Google Scholar]
- Zhou C., Qian W., Ma J., Cheng L., Jiang Z., Yan B., Li J., Duan W., Sun L., Cao J., Wang F., Wu E., Wu Z., Ma Q., Li X. Resveratrol enhances the chemotherapeutic response and reverses the stemness induced by gemcitabine in pancreatic cancer cells via targeting SREBP1. Cell Prolif. 2019;52 doi: 10.1111/cpr.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zykova T.A., Zhu F., Zhai X., Ma W.Y., Ermakova S.P., Lee K.W., Bode A.M., Dong Z. Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol. Carcinog. 2008;47:797–805. doi: 10.1002/mc.20437. [DOI] [PMC free article] [PubMed] [Google Scholar]