Abstract
There are few studies addressing the longitudinal analysis of serum IgE levels and its impact to the development of atopic diseases in early childhood. We investigated 170 children who regularly followed up at our clinic for 4 years in a birth cohort study with at least 3 time-points of serum samples. The pattern of total serum IgE levels from 6 months to 4 years of age was clustered using K-means method in R software. Specific immunoglobulin E antibodies against food (egg white and milk) and inhalant allergens (D. pteronyssinus and D. farinae) were measured at 0.5, 1, 1.5, 2, 3 and 4 years of age. By using K-means clustering, the dynamic changes in serum IgE levels was significantly stratified into 3 clusters (cluster A, < 100 kU/L, n = 106; cluster B, 100–200 kU/L, n = 35; cluster C, ≥ 200 kU/L, n = 29). A persistent total IgE levels higher than 100 kU/L appeared to be associated with higher prevalence of sensitization to food but not mite. However, a persistent IgE levels higher than 200 kU/L was not only remarkably related to increased prevalence of mite sensitization, but also risk of eczema at age 1 and allergic rhinitis and asthma at age 2, 3 and 4. In conclusion, a persistent total serum IgE level ≥ 200 kU/L since infancy is strongly associated with the presence of food and mite sensitization, as well as the development of eczema in infants, and rhinitis and asthma later in early childhood.
Subject terms: Epidemiology, Paediatric research, Predictive markers, Asthma
Introduction
Immunoglobulin E (IgE) is widely known for its role in allergic reactions. IgE, produced by plasma cell, can recognize an allergen specifically and mediate an immune response. The immune system becomes sensitized, such that subsequent encounters with the same allergen lead to release of varies chemokines and cytokines, which results in causing symptoms of atopic diseases, for instance, local inflammation in eczema, mucous hypersecretion in rhinitis and bronchospasm in asthma1.
Several studies have presented the relationships between total serum IgE and allergen sensitization in children of different ages2,3. An increase in total serum IgE levels in infancy is associated with food sensitization, while elevated total serum IgE levels during early childhood correlate strongly with mite sensitization4. In addition, an additive effect on total serum IgE production is perceived when there is combined allergen sensitization4. However, the dynamic changes of total serum IgE levels relevant to allergen sensitization have not been well determined.
The association between allergen sensitization and atopic diseases have been well demonstrated5,6. Clinically, food sensitization appears to be associated with eczema, whereas mite sensitization is strongly related to rhinitis and asthma7,8. However, total serum IgE level is considered as a high sensitivity predictor of atopic diseases9. Elevated total serum IgE indicates high possibility of the presence of atopic diseases in children with allergy-like symptoms. Nevertheless, the longitudinal trends of total serum IgE levels and their association with allergen sensitization and atopic diseases during early childhood are still lacking.
The major aim of this study was to determine the total serum IgE levels from 6 months to 4 years of age in children from a Taiwan birth cohort study. The dynamic changes of total serum IgE levels were analyzed and their relevance to the presence of allergen sensitizations and risk for atopic diseases was also examined.
Results
Study population
In the birth cohort, initially 258 children were recruited, out of which, 182 (70.5%) children completed a 4-year follow-up at the clinic. A total of 170 children with serum samples obtained at least 3 time-points during the follow-up period were enrolled into this study. There were no significant differences in the baseline characteristics among these 170, 198, and all the 258 children studied10. At 4 years of age, atopic diseases including eczema, rhinitis, and asthma were physician-diagnosed in 20, 77, and 31 of these 170 children, respectively. The demographic characteristics of enrolled subjects, total serum IgE levels, and the diagnosis of atopic disease from 6 months to age 4 are summarized in Supplementary File S1.
Clustering analysis of total serum IgE levels
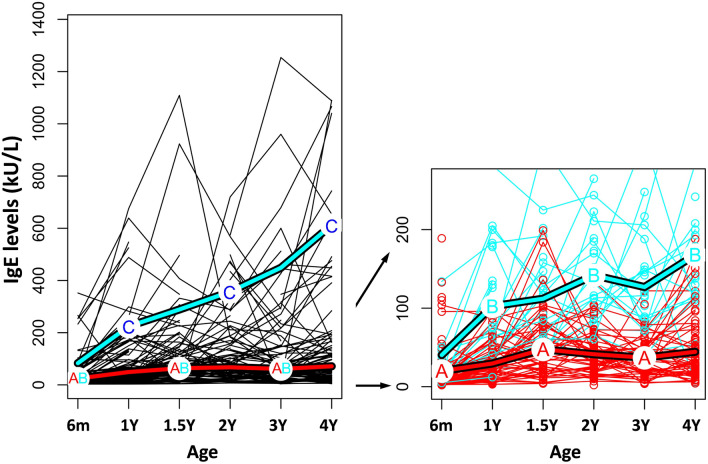
K-means clustering was performed for serum IgE levels from 6 months to 4 years of age using R software in 170 children. The dynamic changes in serum IgE levels during early childhood were stratified into three clusters notably (Fig. 1). Cluster A (n = 106) comprised children with serum IgE level persistently lower than 100 kU/L, throughout the 4-year-study period; cluster B (n = 35) comprised children with serum IgE level between 100 and 200 kU/L from age 1 to 4; and cluster C (n = 29) comprised children with increased IgE level ≥ 200 kU/L after age 1. Table 1 shows the baseline characteristics of 170 children in relation to clustering of total serum IgE levels. There were no significant differences in their characteristics among these three clusters.
Figure 1.
The pattern of total serum IgE levels from 6 month to age 4 clustering by using K-means method in R software. Cluster A (n = 106), IgE levels < 100 kU/L from birth to age 4, cluster B (n = 35), IgE levels 100–200 kU/L from age 1 to 4; cluster C (n = 29), IgE level ≥ 200 kU/L after age 1.
Table 1.
Baseline characteristics of 170 children in relation to total serum IgE clustering from 6 months to the age of 4 years.
| Characteristics | Total serum IgE levels | |||
|---|---|---|---|---|
| < 100 kU/L (A, n = 106) | 100–200 kU/L (B, n = 35) | ≥ 200 kU/L (C, n = 29) | P value | |
| Family | ||||
| Maternal atopy | 46 (43.8%) | 16 (45.7%) | 10 (34.5%) | 0.612 |
| Paternal atopy | 57 (54.3%) | 20 (57.1%) | 19 (65.5%) | 0.557 |
| Parental smoking | 58 (54.7%) | 21 (60.0%) | 13 (44.8%) | 0.470 |
| Household income | 0.213 | |||
| Low, ≤ 500,000 NTD | 41 (39.0%) | 12 (34.3%) | 12 (41.4%) | |
| Medium, 500,000–1,000,000 NTD | 49 (46.7%) | 12 (34.3%) | 13 (44.8%) | |
| High, > 1,000,000 NTD | 15 (14.3%) | 11 (31.4%) | 4 (13.8%) | |
| Infant | ||||
| Sex, male | 56 (52.8%) | 21 (60.0%) | 21 (72.4%) | 0.159 |
| Maternal age (yr) | 30.6 ± 4.6 | 31.2 ± 4.5 | 30.7 ± 4.2 | 0.798 |
| Gestational age (wk) | 37.9 ± 2.1 | 38.3 ± 1.6 | 38.5 ± 1.2 | 0.591 |
| Birth BMI (kg/m2) | 12.4 ± 1.6 | 12.7 ± 2.7 | 13.0 ± 3.2 | 0.955 |
| Breastfeeding ≥ 6 months | 0.354 | |||
| Exclusive | 39 (36.8%) | 8 (22.9%) | 12 (41.4%) | |
| Partial | 38 (35.8%) | 12 (34.3%) | 8 (27.6%) | |
| Formula | 29 (27.4%) | 15 (42.9%) | 9 (31.0%) | |
Data shown are mean ± s.d. or number (%) of patients as appropriate. NTD, new Taiwan dollar; yr, year; wk, week; BMI, body mass index.
Correlation between serum IgE levels and allergen sensitization
Table 2 summarizes the correlations of total serum IgE levels with allergen-specific IgE levels at different years of age. There was a significantly positive correlation between serum IgE levels and egg white- and milk-specific IgE levels at age 0.5, 1, 1.5 and 2. However, a significantly positive correlation was found between serum IgE levels and D. farinae- and D. pteronyssinus-specific IgE levels at 2, 3 and 4 years of age.
Table 2.
Correlations of total serum IgE levels with allergen-specific IgE levels at different years of age.
| Total IgE | Food sensitization | Mite sensitization | ||||||
|---|---|---|---|---|---|---|---|---|
| Egg white | Milk | D. pteronyssinus | D. farinae | |||||
| r | P | r | P | r | P | r | P | |
| Age 0.5 | 0.155 | 0.067 | 0.291 | < 0.001 | 0.100 | 0.236 | 0.137 | 0.106 |
| Age 1 | 0.320 | < 0.001 | 0.507 | < 0.001 | 0.083 | 0.336 | 0.078 | 0.356 |
| Age 1.5 | 0.340 | < 0.001 | 0.128 | 0.174 | 0.182 | 0.052 | 0.160 | 0.089 |
| Age 2 | 0.473 | < 0.001 | 0.313 | 0.001 | 0.604 | < 0.001 | 0.566 | < 0.001 |
| Age 3 | 0.148 | 0.137 | 0.169 | 0.089 | 0.505 | < 0.001 | 0.474 | < 0.001 |
| Age 4 | 0.298 | 0.006 | 0.161 | 0.141 | 0.664 | < 0.001 | 0.651 | < 0.001 |
The correlations between total serum IgE levels and allergen-specific IgE levels were conducted using Pearson’s rank correlation coefficient. All P values < 0.05, which is in bold, are significant.
Association between serum IgE clusters and allergen sensitization
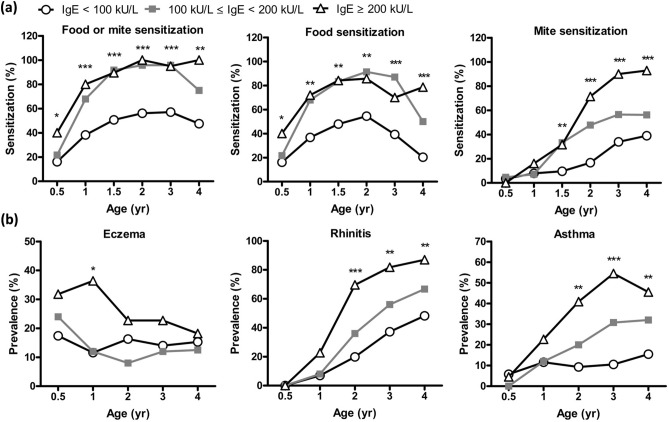
Comparisons and differences between the three clusters with respect to total serum IgE levels and allergen sensitization are shown in Fig. 2a. A significantly higher prevalence of food sensitization was found in children with IgE sensitization (> 100 kU/L, cluster B and C) compared to children grouped in cluster A at different years of age. By contrast, a significantly higher prevalence of mite sensitization was only found in children with higher serum IgE levels (≥ 200 kU/L, cluster C) in comparison with children grouped in cluster A.
Figure 2.
The relationships between total serum IgE levels clustering with allergen sensitization to food and mite (a), eczema, rhinitis and asthma (b) at different years of age. *P < 0.05; **P < 0.01; ***P < 0.001. The prevalence of food sensitization was significantly higher in children with total IgE levels ≥ 100 kU/L from 6 months to age 4, while a significantly higher prevalence of mite sensitization was only found in children with higher serum IgE levels ≥ 200 kU/L after age 1. In children with total IgE levels ≥ 200 kU/L, the prevalence of eczema was significantly higher at age 1, while the prevalence of rhinitis and asthma was significantly higher at the age of 2 to 4.
Association between serum IgE clusters and atopic diseases
Figure 2b shows the relationship between three serum IgE clusters and development of eczema, rhinitis and asthma at different ages. The prevalence of eczema decreased but allergic rhinitis and asthma increased markedly since age 2. Higher serum IgE levels (≥ 200 kU/L, cluster C) was significantly associated with higher prevalence of eczema at age 1, and allergic rhinitis and asthma at age 2, 3 and 4. After adjusting confounding factors, compared with serum IgE level < 100 kU/L in cluster A, higher serum IgE levels (≥ 200 kU/L) in cluster C appeared to show a significantly increased risk of allergic rhinitis [odds ratio (OR), 9.09; 95% confidence interval (CI), 2.20–37.53; P = 0.002] and asthma (OR, 5.94; 95% CI, 1.89–18.69; P = 0.002) at the age of 4 years.
Discussion
Total serum IgE level is commonly elevated in patients with allergic diseases. It has been suggested to be utilized in predicting the development of allergic disorders. However, the dynamic changes and values of total serum IgE levels relevant to the development of sensitization to allergens and risk of atopic diseases remains unclear, especially in children. This study provides the respective values of total serum IgE levels that have significant association with sensitization to various allergens and atopic diseases during different stages of childhood.
IgE is secreted by class-switched B cell. The class-switch recombination of a B cell requires an antigen-dependent receptor-ligand binding interaction with an activated Th2 cell. This process usually takes place within secondary lymphoid tissues. However, the somatic hypermutation and class-switch recombination of B cells are rare in infants below the age of 6 months11. The secondary lymphoid organs are not completely mature in infants, reducing the likelihood of IgE class switching12. In this study, the serum IgE levels in 6 month-old infant are generally low (< 100 kU/L) which is in agreement with previous studies that showed rarity of IgE-producing cells at 6 months of age13. Therefore, the serum IgE level of infant below the age of 6 months may not be useful in predicting the development of any diseases.
The production of IgE in infant older than 6 months starts to rise after exposing to food allergens14,15. Food sensitization in infant should be noticed early due to its essential association with eczema, a common allergic skin disease with disrupted skin barrier16,17. Eczema could be exacerbated by food allergens by initiating the immune response18,19. To prevent eczema flare ups or from getting worse, early diagnosis and further avoidance of food allergens are relevant to infant. In our study, children with serum total IgE level > 100 kU/L are demonstrated to have food sensitization. A significantly higher prevalence of eczema was also found in children with serum total IgE level ≥ 200 kU/L at the age of 1 in our study. Therefore, total serum IgE level > 100 kU/L in infants may indicate they have food sensitization and a respectively higher risk of eczema. In addition, when infant has skin symptoms and with serum total IgE level ≥ 200 kU/L, physicians should consider the diagnosis of eczema, followed by early treatment and prevention.
The increase of serum total IgE level of children after the age of 2 is more related to mite sensitization4. Furthermore, children with high house dust mite-specific IgE level is at the highest risk of rhinitis and asthma8,20, which is in consonance with our study that an increased serum total IgE level (≥ 200 kU/L) strongly correlated with mite-specific IgE levels appeared to significantly increase risk of rhinitis and asthma after age 2. Thus, in clinical practice, young children with serum total IgE level ≥ 200 kU/L might have a high possibility of the presence of mite sensitization and rhinitis and asthma should be considered in such instances with allergic symptoms.
Limitations of this birth cohort include the relatively small enrolled population of 170 children, and thus limited statistical power to detect the association for subanalyses. However, the strength of this study is manifested by its long-term, longitudinal follow-up and regular measurement of total serum and allergen-specific IgE levels which established the dynamic relationships of IgE levels with allergen sensitizations and atopic disease development over time during early childhood.
In conclusion, serum total IgE level could be predictive of allergen sensitization and atopic diseases in early childhood. In infancy, serum total IgE level > 100 kU/L may reveal the presence of food sensitization, while serum total IgE level ≥ 200 kU/L may indicate a high risk of eczema. Young children with persistent serum total IgE level ≥ 200 kU/L appear to be associated with high prevalence of mite sensitization and be at risk for allergic rhinitis and asthma. Thus, for children with serum total IgE level ≥ 200 kU/L from infancy to early childhood, there is a high chance of developing eczema in infants and rhinitis and asthma later in life, providing early diagnosis and treatment for childhood atopic diseases. However, further studies with larger sample sizes are required to validate our findings.
Methods
Patients and data collection
We enrolled children who completed a 4-year follow-up in a birth cohort study launched in Taiwan. Detailed descriptions regarding subject recruitment of this birth cohort study were reported previously4. The detailed of information regarding demographic data, child’s sex, family history of atopy, exposure to passive smoking, household income, and history of breastfeeding was collected and analyzed. Atopic diseases were evaluated and diagnosed by the same pediatric pulmonologist at the clinic. Diagnosis of atopic diseases including eczema, allergic rhinitis, and asthma was described in our previous study10,21. This study was approved by the Ethics Committee of Chang Gung Memorial Hospital (No. 103-6236A3). All experiments in this study were performed in accordance with the relevant guidelines and regulations, and written informed consent was obtained from the parents or guardians of all the study subjects.
Measurement of total serum IgE levels and clustering
Serum samples were collected and measured at 6 months, and 1, 1.5, 2, 3, and 4 years of age. As described in our previous study10, total serum IgE level was measured using ImmunoCAP (Phadia, Uppsala, Sweden) and IgE sensitization was defined as IgE levels > 100 kU/L. For clustering, total serum IgE levels at different ages were imported into R software (Version 3.6.3). K-means method was then used to group serum IgE levels into discrete and stable clusters of time series data from 6 months to 4 years of age.
Measurement of allergen-specific IgE levels
Allergen-specific IgE was determined using a commercial assay for IgE (ImmunoCAP Phadiatop Infant; Phadia) for a mix of the two most common food allergens (egg white and milk) and inhalant allergens in Taiwan (D. pteronyssinus and D. farinae) as described previously22,23. Allergen sensitization was defined as values ≥ 0.35 kU/L24.
Confounders
Confounding factors associated with atopic disease development, such as child’s gender, maternal and gestational age at birth, maternal atopy, elder siblings at birth, prenatal exposure to passive smoking, patterns of breastfeeding practices among infants, and family income, were collected and analyzed using multiple logistic regression analysis.
Statistical analysis
K-means clustering of total serum IgE levels was calculated in R software. Univariate parametric and nonparametric tests such as ANOVA, χ2, Fisher’s exact test, and Kruskal–Wallis rank sum test were used to compare baseline characteristics and allergic sensitization among serum IgE level clusters. Pearson’s correlation test was used to determine the correlation between the total serum IgE and allergen specific IgE levels. Multiple logistic regression analysis was used to determine the association between serum IgE level clusters and atopic diseases by adjusting for confounders. The Statistical Package for the Social Sciences (SPSS Statistics for Windows Version 20.0; Armonk, NY, USA) software was used for statistical analysis of data, and GraphPad Prism software (GraphPad Software Inc. Version 5.01; San Diego, CA, USA) was used to represent data graphically. Statistical hypothesis tests were two-tailed with a significance level of 0.05.
Supplementary information
Acknowledgments
This study was supported by research grant of CMRPG3E1191-5 from the Chang Gung Medical foundation, Chang Gung University, Taiwan. We are extremely grateful to all the families who took part in this study, all pediatricians for their help in recruiting them and the whole PATCH team, which includes interviewers, nurses, computer and laboratory technicians and research assistants.
Author contributions
C.-Y.W. and K.-W.Y. drafted and revised the manuscript. J.-L.H. and K.-W.S. performed experimental work and interpretation. M.-H.T. and M.-C.H. performed statistical analyses and presented the data. S.-L.L., S.-H.L. and L.-C.C. were responsible for clinical evaluation of the children and data collection. C.-Y.C. design and supervised the study. All authors discussed the results and approved the final manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available duo to the personal privacy of subjects but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chun-Ying Wong and Kuo-Wei Yeh
Supplementary information
is available for this paper at 10.1038/s41598-020-78272-8.
References
- 1.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat. Rev. Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 2.Ansotegui IJ, et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ. J. 2020;13:100080. doi: 10.1016/j.waojou.2019.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platts-Mills TAE, Schuyler AJ, Erwin EA, Commins SP, Woodfolk JA. IgE in the diagnosis and treatment of allergic disease. J. Allergy Clin. Immunol. 2016;137:1662–1670. doi: 10.1016/j.jaci.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu CY, et al. Sensitization to food and inhalant allergens in relation to atopic diseases in early childhood: a birth cohort study. PLoS ONE. 2014;9:e102809. doi: 10.1371/journal.pone.0102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasar M, Julge K, Björkstén B. Development of atopic sensitization and allergic diseases in early childhood. Acta Paediatr. 2000;89:523–527. doi: 10.1111/j.1651-2227.2000.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 6.Arshad SH, Tariq SM, Matthews S, Hakim E. Sensitization to common allergens and its association with allergic disorders at age 4 years: a whole population birth cohort study. Pediatrics. 2001;108:E33. doi: 10.1542/peds.108.2.e33. [DOI] [PubMed] [Google Scholar]
- 7.Worth A, Sheikh A. Food allergy and atopic eczema. Curr. Opin. Allergy Clin. Immunol. 2010;10:226–230. doi: 10.1097/ACI.0b013e3283387fae. [DOI] [PubMed] [Google Scholar]
- 8.Gabet S, et al. Asthma and allergic rhinitis risk depends on house dust mite specific IgE levels in PARIS birth cohort children. World Allergy Organ. J. 2019;12:100057. doi: 10.1016/j.waojou.2019.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satwani H, Rehman A, Ashraf S, Hassan A. Is serum total IgE levels a good predictor of allergies in children? J. Pak. Med. Assoc. 2009;59:698–702. [PubMed] [Google Scholar]
- 10.Chiu CY, et al. Exclusive or partial breastfeeding for 6 months is associated with reduced milk sensitization and risk of eczema in early childhood: the PATCH Birth Cohort Study. Medicine (Baltimore) 2016;95:e3391. doi: 10.1097/MD.0000000000003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridings J, Dinan L, Williams R, Roberton D, Zola H. Somatic mutation of immunoglobulin V(H)6 genes in human infants. Clin. Exp. Immunol. 1998;114:33–39. doi: 10.1046/j.1365-2249.1998.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timens W, Rozeboom T, Poppema S. Fetal and neonatal development of human spleen: an immunohistological study. Immunology. 1987;60:603–609. [PMC free article] [PubMed] [Google Scholar]
- 13.Lima JO, et al. Early expression of iepsilon, CD23 (FcepsilonRII), IL-4Ralpha, and IgE in the human fetus. J. Allergy Clin. Immunol. 2000;106:911–917. doi: 10.1067/mai.2000.110228. [DOI] [PubMed] [Google Scholar]
- 14.Luccioli S, Ross M, Labiner-Wolfe J, Fein SB. Maternally reported food allergies and other food-related health problems in infants: characteristics and associated factors. Pediatrics. 2008;122(Suppl 2):S105–112. doi: 10.1542/peds.2008-1315n. [DOI] [PubMed] [Google Scholar]
- 15.Lee SE, Kim H. Update on early nutrition and food allergy in children. Yonsei Med. J. 2016;57:542–548. doi: 10.3349/ymj.2016.57.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eller E, Kjaer HF, Host A, Andersen KE, Bindslev-Jensen C. Food allergy and food sensitization in early childhood: results from the DARC cohort. Allergy. 2009;64:1023–1029. doi: 10.1111/j.1398-9995.2009.01952.x. [DOI] [PubMed] [Google Scholar]
- 17.Hon KL, et al. Patterns of food and aeroallergen sensitization in childhood eczema. Acta Paediatr. 2008;97:1734–1737. doi: 10.1111/j.1651-2227.2008.01034.x. [DOI] [PubMed] [Google Scholar]
- 18.Sampson HA, McCaskill CC. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J. Pediatr. 1985;107:669–675. doi: 10.1016/S0022-3476(85)80390-5. [DOI] [PubMed] [Google Scholar]
- 19.Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J. Dermatol. 2016;61:645–648. doi: 10.4103/0019-5154.193673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung R, Ho P, Lam CW, Lai CK. Sensitization to inhaled allergens as a risk factor for asthma and allergic diseases in Chinese population. J. Allergy Clin. Immunol. 1997;99:594–599. doi: 10.1016/S0091-6749(97)70018-6. [DOI] [PubMed] [Google Scholar]
- 21.Chiu CY, et al. Low cord blood vitamin D levels are associated with increased milk sensitization in early childhood. Pediatr. Allergy Immunol. 2014;25:767–772. doi: 10.1111/pai.12304. [DOI] [PubMed] [Google Scholar]
- 22.Lee AJ, Thalayasingam M, Lee BW. Food allergy in Asia: how does it compare? Asia Pac. Allergy. 2013;3:3–14. doi: 10.5415/apallergy.2013.3.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yong SB, Wu CC, Tzeng YC, Hung WC, Yang KD. Different profiles of allergen sensitization in different ages and geographic areas in Changhua, Taiwan. J. Microbiol. Immunol. Infect. 2013;46:295–301. doi: 10.1016/j.jmii.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Ballardini N, Nilsson C, Nilsson M, Lilja G. ImmunoCAP Phadiatop Infant–a new blood test for detecting IgE sensitisation in children at 2 years of age. Allergy. 2006;61:337–343. doi: 10.1111/j.1398-9995.2005.00936.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available duo to the personal privacy of subjects but are available from the corresponding author on reasonable request.