Abstract
Background:
Gait deficits in Parkinson disease (PD), including freezing of gait (FOG), can be among the most debilitating symptoms. Rhythmic auditory cueing has been used to alleviate some gait symptoms. However, different cue types, such as externally-generated and self-generated cues, affect gait variability differently. The differential effects of these cue types on people with PD with FOG (PD+FOG), who often have higher gait variability, and those with PD without FOG (PD-FOG) is unknown. Given the relationship of gait variability to fall risk, this is an important area to address.
Research Question:
This study aims to 1) confirm the association between falls and gait variability measures in PD-FOG, PD+FOG and age-matched Controls; 2) investigate the effects of different cue types on gait variability in PD-FOG and PD+FOG; and 3) determine whether baseline gait characteristics are associated with response to cues.
Methods:
This cross-sectional study investigated PD-FOG (n=24), PD+FOG (n=20), and Controls (n=24). Gait trials were collected during use of externally-generated and self-generated cues for all participants. Gait variability measures were the primary outcomes to assess the effects of rhythmic auditory cues.
Results:
Logistic regression models showed increased gait variability was associated with falls across groups. Repeated measures ANOVAs showed externally-generated cues increased gait variability, whereas self-generated cues did not, for all groups. Pearson’s correlations showed participants with higher baseline gait variability had greater reduction in gait variability with rhythmic auditory cueing.
Significance:
Higher gait variability is associated with falls. This study demonstrates that PD+FOG are capable of using self-generated cues without increasing gait variability measures, thereby stabilizing gait. People with higher baseline gait variability are likely to experience the largest reductions in variability with the addition of external cues.
Keywords: Parkinson disease, Gait variability, Falls, Freezing
Background
Parkinson disease (PD) is a neurodegenerative movement disorder with an increasing prevalence, and is expected to affect more than nine million people by the year 2030 [1]. Gait deficits are among the most debilitating for people with PD and lead to decreased mobility and increased risk of falls [2]. Freezing of gait (FOG), an inability to initiate or continue intended locomotion, is a disabling gait deficit that will affect more than a third of people with PD [3,4]. While the neural mechanisms are not fully understood, FOG has been associated with dysfunction in areas of the brain responsible for executive functioning and attention [5]. Common pharmacological interventions do not adequately target gait deficits, particularly gait variability [6]. It is therefore important to investigate novel forms of gait rehabilitation that target gait variability.
Typical parkinsonian gait includes decreased velocity and stride length, as well as increased variability in stride length and in step time [7]. People with PD with FOG (PD+FOG) have higher gait variability than people with PD without FOG (PD-FOG) [8]. Targeting gait variability with gait rehabilitation is likely important because higher gait variability measures are associated with an increased risk of falls among older adults and people with PD [9,10]. This association may provide insights into who may respond best to rhythmic auditory cues.
Rhythmic auditory cueing has been widely studied as a method of gait rehabilitation for people with PD [11]. This form of rehabilitation uses an auditory stimulus to which an individual matches their footfalls. Various cue types have previously been investigated, including externally-generated cues (e.g., music) and self-generated cues (e.g., singing and mental singing). Singing is associated with increased sensorimotor synchronization when linked to movement [12] and research has shown singing and mental singing affect gait velocity and stride length similarly [13]. However, externally-generated cues also increased gait variability measures, whereas self-generated cues did not [14]. These previous findings did not consider FOG status which may be an important factor to consider.
Previous research showed people with PD with and without FOG may respond differently to various cue types [15]. While PD+FOG may benefit from external rhythmic auditory cues to enhance velocity and stride length [16], the effects of self-generated cues on FOG have not been studied. A finger tapping study using a synchronization-continuation task demonstrated that PD+FOG had higher dysrhythmia of tapping during the continuation phase than PD-FOG, suggesting FOG may be associated with worse internal beat timing [17]. This would suggest PD+FOG may not be able to use self-generated cues effectively.
The present study had several aims. The first aim was to confirm that higher gait variability is associated with falls across all groups in our sample, in keeping with prior literature. The second aim was to determine the effect of externally-generated and self-generated cues on PD-FOG, PD+FOG, and Controls. We hypothesized externally-generated cues would increase gait variability for all groups, and that self-generated cues would not increase gait variability for PD-FOG and Controls only. The final aim was to determine which participants responded most to cues based on their baseline, uncued gait characteristics. We hypothesized participants with higher baseline gait variability would have the greatest response to rhythmic auditory cues.
Methods
Participants
Participants were recruited from the Movement Disorders Clinic at the medical campus of the university and the local chapter of the American Parkinson Disease Association. All participants were diagnosed with idiopathic PD. All participants met the following inclusion criteria: able to stand independently for at least 30 minutes; normal peripheral neurological function; no history of vestibular disease, no evidence of dementia (determined by a Mini Mental State Examination (MMSE) score of ≥ 24), and at least 50 years of age. Exclusion criteria included: any serious medical problem aside from PD; use of neuroleptic or other dopamine-blocking drug; evidence of abnormality on brain imaging from any previous clinical evaluation; history or evidence of other neurological deficit (e.g., previous stroke or muscle disease); history or evidence of orthopedic, muscular, psychological problem or hearing impairment; or having deep brain stimulation or any other neural implants. All participants were asked to maintain their normal medication dosage, and those taking medication were tested in their self-reported ON state for all assessments. Participants with PD were divided into two groups, people with PD without FOG (PD-FOG) and people with PD with FOG (PD+FOG). FOG status was confirmed with a score ≥ 1, during the participant’s testing visit, indicating that they answered “yes” to the first question, “Did you experience freezing episodes in the past month?”, on the New Freezing of Gait Questionnaire (N-FOGQ). Participants were asked to retrospectively report how often they have fallen in the last six months to determine fall status. Fall status was defined as non-fallers (no falls in the past six months) and fallers (one or more falls in the past six months). This study was approved by the Institutional Review Board at the university and written informed consent was obtained from all participants prior to starting the study.
Participant characteristics
Participant characteristics are summarized in Table 1. For participants with PD, motor function was assessed using the Movement Disorders Society Unified Parkinson Disease Rating Scale Part 3 (MDS-UPDRS-III) and disease stage was assessed using the Hoehn & Yahr score [18] by a trained research staff member. Freezing status and severity was determined by the New Freezing of Gait Questionnaire (NFOG-Q) [19]. Medication dosage was determined by the levodopa-equivalent daily dose (LEDD).
Table 1.
Participant characteristics.
| Controls | PD-FOG | PD+FOG | P | |
|---|---|---|---|---|
| N (female) | 24 (19) | 24 (14) | 20 (7) | - |
| Age | 66.04±7.30 | 68.79±6.92 | 67.10±8.28 | 0.44 |
| MMSE, median (range) | 29 (25, 30) | 28 (26, 30) | 29 (26, 30) | 0.18 |
| Fallers | 6 | 9 | 16 | - |
| Yrs since dx | - | 6.04±3.44 | 8.65±5.77 | 0.07 |
| LEDD | - | 777 ± 476 (22) | 1,307 ±1,032 (18) | 0.06 |
| UPDRS III | - | 24.04±8.68 | 27.55±15.09 | 0.34 |
| H&Y, median (range) | - | 2 (2, 2) | 2 (2, 3) | 0.12 |
| N-FOGQ | - | 0.00±0.00 | 16.85±8.24 | - |
Values are mean ± SD unless noted. Fallers defined as participants who experienced one or more falls in the past 6 months. T-tests and ANOVAs used for between group comparisons as appropriate. Abbreviations: Hoehn and Yahr, H&Y; Levodopa-Equivalent Daily Dose, LEDD; Mini Mental State Examination, MMSE; Movement Disorders Society Unified Parkinson Disease Rating Scale Part 3, MDS-UPDRS-III; N-FOGQ, New Freezing of Gait Questionnaire.
Gait Measures
Spatial and temporal parameters of gait were measured using a five-meter instrumented, computerized walkway (GAITRite, CIR Systems, NJ), which has been well-validated for reliably measuring gait characteristics [20]. Primary outcome measures were gait variability measures, including step time coefficient of variation (CV), stride length CV, and single support time (SST) CV. Secondary gait outcomes measures included velocity, stride length, and cadence. Velocity was normalized to average leg length, measured as the distance from the participant’s greater trochanter to their lateral malleolus (cm). For each trial, the participant walked across the walkway one time; starting and ending one meter off the walkway. This was repeated three times for each condition. There was an average of 19.9±4.0 (mean±sd) total steps per condition, which can reliably measure gait variability [21]. CV was calculated as (standard deviation/mean) x 100 for all gait variability measures. Participants wore their own comfortable pair of shoes.
A baseline measurement of each participant’s uncued walking (UNCUED) was collected first. Participants were asked to walk across the walkway at their normal, comfortable pace. Three trials were collected and averaged, and the cadence was measured by the GAITRite system. After their typical cadence was determined, the tempo of the auditory cue was set to 100% of this cadence using an open source audio editing software (The Audacity Team, audacity.sourceforge.net/) to optimize the cue rate for effects on gait variability measures [22]. The auditory cue used for all conditions was a piano arrangement of a familiar children’s song (‘Row, Row, Row, Your Boat’). This song was selected for its salient beat. Two cued conditions were then collected, in a randomized order, using this tempo. The externally-generated cue was the music condition (MUSIC). For this condition, the music was played continuously and after listening one time through, participants were asked to walk across the walkway, matching their footfalls to the beat of the music, as it continued to play. The self-generated cue was the mental singing condition (MENTAL). In this condition, the song was played through one time and after the music stopped participants were asked to sing the song in their head while matching their footfalls to the beat of their mental singing. Variability change scores, calculated as the difference in variability between each cued condition and uncued gait, were used as an indicator of response to cues.
Statistical analysis
All statistical analyses were conducted in the R statistical computing environment [23]. Between group comparisons were performed on participant characteristics using unpaired t-tests or analysis of variance models when appropriate to determine any differences between groups. Univariable and multivariable (adjusted for N-FOGQ, age, gender, LEDD, and MMSE) logistic regression models were performed to assess the association between falls (dependent variable) and gait variability measures (independent variable) for all participants. Two-way repeated measures (RM) ANOVAs were used to determine main effects of group, condition, and interaction effects of group and condition for each gait outcome measure using the afex package in R [24]. Within subject variation was accounted for in the models. Outliers were identified using the median absolute deviation and were winsorized by group and condition and violations of sphericity were corrected using the Greenhouse Geisser method. Pairwise comparisons between groups and conditions were conducted with alpha = .05 and adjusted for multiple comparisons using the Tukey method. Pearson’s correlations were performed by group to determine associations between baseline gait variability measures and change scores from auditory cueing. An initial power analysis, using gait velocity data from Harrison et al. [14], indicated a total of 63 participants were needed to detect between group differences with a moderate effect size at 80% power and alpha = .05. For all analyses, appropriate post-hoc estimates of effect sizes were included to improve model interpretations.
Results
Seventy-six participants were enrolled in the study. After enrollment and consent, two Controls (one due to MMSE < 24 and one due to pre-existing knee pain), one PD-FOG (due to inability to follow instructions), and five PD+FOG (two due to fatigue, one due to high frequency of freezing that prohibited completion of the gait tasks, and two due to medical history) were excluded. Characteristics for 68 participants who completed the study are summarized in Table 1. There were no significant differences in participant characteristics between groups.
Uncued gait characteristics between groups and faller status are summarized in Table 2. Higher step time CV, stride length CV, and SST CV were all associated with falling. Results of the univariable and multivariable logistic regression models are summarized in Table 3.
Table 2.
Uncued Gait Characteristics.
| Controls | PD-FOG | PD+FOG | ||||
|---|---|---|---|---|---|---|
| Non-fallers (n=18) |
Fallers (n=6) |
Non-fallers (n=15) |
Fallers (n=9) |
Non-fallers (n=4) |
Fallers (n=16) |
|
| Normalized Velocity (m/sec/LL) | 1.55 (0.22) | 1.61 (0.18) | 1.47 (0.19) | 1.16 (0.16) | 1.47 (0.12) | 1.30 (0.27) |
| Cadence (steps/min) | 110.69 (8.75) | 110.85 (0.21) | 109.16 (9.03 | 97.47 (8.17) | 113.27 (8.05) | 108.13 (8.84) |
| Stride Length (cm) | 127.67 (12.38) | 129.65 (17.70) | 128.37 (18.53 | 116.15 (6.07) | 125.24 (17.87) | 112.38 (20.86) |
| Step Time CV (%) | 2.36 (0.52) | 2.14 (0.24) | 2.55 (0.72 | 2.75 (1.03) | 2.35 (0.52) | 3.24 (0.73) |
| Stride Length CV (%) | 1.79 (0.77) | 1.98 (1.01) | 1.71 (0.75 | 2.46 (0.86) | 1.79 (0.70) | 2.57 (1.07) |
| SST CV (%) | 3.02 (0.85) | 2.92 (0.18) | 3.21 (0.99 | 3.64 (1.54) | 2.93 (0.76) | 4.41 (1.38) |
All values are mean (SD). Abbreviations: Leg Length, LL; Single Support Time, SST.
Table 3.
Logistic regression.
| Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Step Time CV | 3.11 | 1.45-7.45 | 0.006 | 6.45 | 1.87-33.40 | 0.009 |
| Stride Length CV | 2.15 | 1.19-4.19 | 0.016 | 3.61 | 1.32-12.14 | 0.020 |
| SST CV | 1.83 | 1.15-3.13 | 0.017 | 2.97 | 1.37-8.00 | 0.013 |
Univariable and multivariable logistic regression showing an association between falls (dependent variable) and each gait variability measure (independent variable).
Adjusted for N-FOGQ, age, gender, LEDD, and MMSE. Abbreviations: Odds Ratio, OR; Single Support Time, SST.
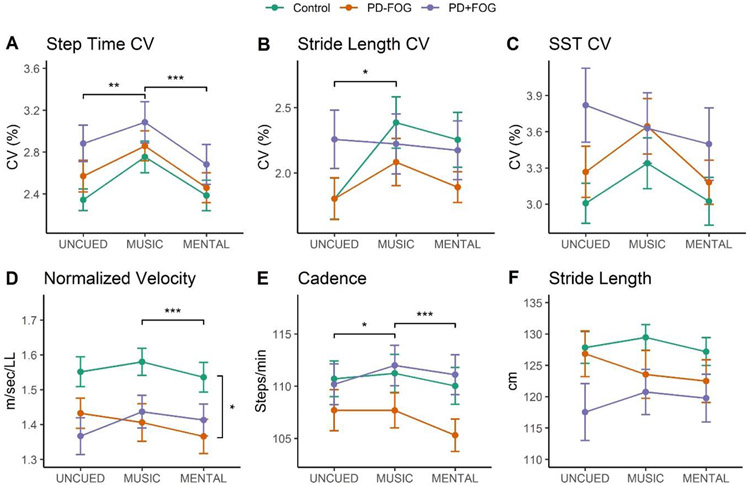
The means and standard deviations of the gait conditions by group are summarized in Table 4. For step time CV (Figure 1A), the interaction of group and condition was not significant (F3.74.121.45=.36, p=.82, ηp2=.01). There was also no main effect of group (F2.65=2.33, p=.11, ηp2=.07). There was a significant main effect of condition for step time CV (F1.87,121.45=10.47, p<.0001, ηp2=.14), with pairwise comparisons indicating a significantly higher step time CV in MUSIC compared to MENTAL (p=.0007) and UNCUED (p=.002).
Table 4.
Summary of gait performance by group and condition.
| Controls | PD-FOG | PD+FOG | |||||||
|---|---|---|---|---|---|---|---|---|---|
| UNCUED | MUSIC | MENTAL | UNCUED | MUSIC | MENTAL | UNCUED | MUSIC | MENTAL | |
| Normalized Velocity (m/sec/LL) | 1.55 (0.21) | 1.58 (0.19) | 1.54 (0.21) | 1.43 (0.21) | 1.41 (0.26) | 1.37 (0.24) | 1.37 (0.24) | 1.44 (0.21) | 1.41 (0.21) |
| Cadence (steps/min) | 110.70 (8.36) | 111.23 (8.88) | 110.03 (8.65) | 107.70 (9.61) | 107.68 (8.23) | 105.30 (7.58) | 110.19 (8.70) | 111.98 (8.69) | 111.10 (8.56) |
| Stride Length (cm) | 127.84 (12.41) | 129.46 (9.96) | 127.18 (10.98) | 126.84 (17.86) | 123.53 (18.70) | 122.48 (16.76) | 117.53 (20.28) | 120.76 (16.11) | 119.77 (17.05) |
| Step Time CV (%) | 2.34 (0.50) | 2.75 (0.74) | 2.38 (0.71) | 2.57 (0.74) | 2.86 (0.70) | 2.46 (0.70) | 2.88 (0.78) | 3.08 (0.88) | 2.68 (0.86) |
| Stride Length CV (%) | 1.80 (0.77) | 2.39 (0.96) | 2.25 (1.02) | 1.80 (0.78) | 2.08 (0.89) | 1.89 (0.57) | 2.26 (1.00) | 2.22 (1.03) | 2.17 (1.00) |
| SST CV (%) | 3.01 (0.81) | 3.34 (1.03) | 3.02 (0.97) | 3.27 (1.04) | 3.64 (1.13) | 3.18 (0.89) | 3.82 (1.37) | 3.63 (1.31) | 3.50 (1.35) |
All values are mean (SD). Abbreviations: Coefficient of Variation, CV; Single Support Time, SST.
Figure 1.
Effect of cues on gait variability measures (A-C) and spatial and temporal gait characteristics (D-F) across groups. Pairwise comparisons of the main effects of group (vertical brackets) and condition (horizontal brackets) are indicated, *p<.05, **p<.01, ***p<.001.
For stride length CV (Figure 1B), the interaction of group and condition was not significiant (F3.96,128.67=1.62, p=.17, ηp2=.05). There was no main effect of group (F2.65=.98, p=.38, ηp2=.03). There was a significant main effect of condition for stride length CV (F1.98,128.67=3.23, p=.04, ηp2=.05), with pairwise comparisons indicating a significantly higher stride length CV in MUSIC compared to UNCUED (p=.05).
For SST CV (Figure 1C), there was no interaction effect of group and condition (F3.96,128.65=.81, p=.52, ηp2=.02). There were also no main effects of group (F2.65=2.01, p=.14, ηp2=.06) or condition (F1.98,128.65=2.27, p=.11, ηp2=.03).
For normalized velocity (Figure 1D), the interaction between group and condition was significant (F3.10,100.85=4.14, p=.008, ηp2=.11). Pairwise comparisons showed MUSIC was significantly higher than MENTAL for Controls (p=.006) and PD-FOG (p=.01); MUSIC was significantly lower than UNCUED for PD-FOG (p=.02); and UNCUED was significantly higher than MENTAL for PD-FOG (p=.01), There was a signifiant main effect of group (F2,65=3.95, p=.02, ηp2=.11), with pairwise comparisons indicating higher velocity for Controls compared to PD-FOG (p=.04). There was a significant main effect of condition (F1.55,100.85=4.56, p=.02, ηp2=.07), with pairwise comparisons indicating a higher velocity in MUSIC than MENTAL (p=.0001).
For cadence (Figure 1E), there was a significant interaction between group and condition (F3.28,106.44=3.41, p=.02, ηp2=.09). Pairwise comparisons showed MUSIC was significantly higher than MENTAL for PD-FOG (p=.0008); MUSIC was significantly higher than UNCUED for PD+FOG (p=.003); and UNCUED was significantly higher than MENTAL for PD-FOG (p=.005). There was no main effect of group (F2.65=1.73, p=.18, ηp2=.05). There was a significant main effect of condition for cadence (F1.64,106.44=8.19, p=.001, ηp2=.11), with pairwise comparisons indicating higher cadence in MUSIC compared to UNCUED (p=.02) and MENTAL (p=.0004).
For stride length (Figure 1F), there was a significant interaction effect of group and condition (F2.86,92.88 =3.52, p=.02, ηp2=.10) with pairwise comparisons indicating significantly higher stride length in MUSIC compared to MENTAL for Controls (p=.04); and UNCUED was significantly higher than MENTAL for PD-FOG (p=.02). There was not a main effect of group (F2,65=1.81, p=.17, ηp2=.05) or condition (F1.43,92.88=1.48, p=.23, ηp2=.02).
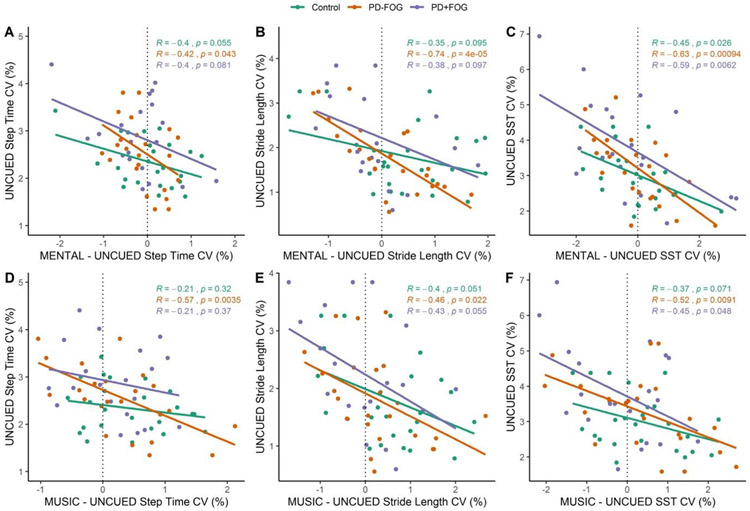
Pearson’s correlations across groups indicated significant moderate negative correlations between baseline measures and MUSIC change scores for step time CV (R=−0.50, p<.001), stride length CV (R=−0.44, p<.001), and SST CV (R=−0.60, p<.001) and for MENTAL change scores for step time CV (R=−0.50, p<.001), stride length CV (R=−0.40, p<.001), and SST CV (R=−0.52, p<.001). Pearson’s correlations by group are shown in scatter plots in Figure 2.
Figure 2.
Gait variability measure change scores from UNCUED for the MENTAL conditions (A-C) and for the MUSIC conditions (D-F). Trend lines and results of Pearson’s correlations by group are identified on the plots.
Discussion
The primary aims of the present study were to 1) confirm the association between gait variability and falls in healthy older adults and people with PD; 2) determine the effects of externally-generated and self-generated cues on gait variability in people with PD with and without FOG; and 3) determine who responded most to rhythmic auditory cues in this sample.
The results of this study first established gait variability measures are associated with falls in PD-FOG, PD+FOG, and Controls in this sample. This is consistent with previous findings of gait variability being associated with fall risk, however our sample had lower overall variability than previous work [9]. These results also established the gait variability measures as an adequate measure of gait stability in this study.
The results regarding effects of cue type on gait indicated externally-generated cues increased gait variability compared to uncued gait for all three groups, whereas self-generated cues did not. However, the self-generated cues did not reduce gait variability compared to uncued gait. This finding is consistent with previous research [13,14]; however, the present study is the first to establish these similar effects of different cue types in PD+FOG, which was contrary to our hypothesis. Previously, Tolleson et al. [17] found PD+FOG have greater dysrhythmia than PD-FOG during internally-cued movement. However, they investigated upper extremity movements and used a metronome cue, which could explain their different results. Additionally, previous research showed differences in response to different cues, such as altered cue rates, in people with PD with and without FOG [15], further supporting our initial hypothesis. Previous studies comparing PD-FOG and PD+FOG have also only investigated different externally generated cues [16], whereas the present study investigated self-generated cues. Self-generated cues may not have the same negative effect on gait variability as externally-generated cues because cues with biologically-relevant variability (e.g. adaptable, self-generated cues) may provide more benefit than cues with fixed timing (e.g. externally generated cues) [25].
Lastly, this study showed who responded most to rhythmic auditory cues with respect to gait variability. Participants with higher gait variability measures showed the greatest reduction in variability with cueing, during both externally-generated and self-generated cueing conditions. This is important as it demonstrates participants most in need of gait rehabilitation benefited from rhythmic auditory cues. This is also consistent with recent findings that found people with lesser gait deficits in the early stages of PD receive less benefit from auditory cues than people in later stages of PD [26].
Gait deficits, including increased gait variability, are associated with increased risk of falls in older adults, and particularly people with neurological disorders. Like previous research showing higher step time variability was associated with risk of falls in older adults [10], the results of the present study also demonstrated that increased gait variability is associated with falls risk in healthy older adults and people with PD. To reduce fall risk, identifying cues that will not increase gait variability measures is essential. The present study showed externally-generated cues increased gait variability, whereas self-generated cues do not for all groups. This finding is important for gait rehabilitation programs that are targeting a similar population.
Limitations
There are several limitations with this study. Participants with PD had mild to moderate disease severity, evidenced by low MDS-UPDRS-III and H&Y scores. As such, the participants in this study had relatively low uncued gait variability [16], which could have presented a floor effect with less potential for improvement from the cues. Participants were asked to retrospectively report how often they fell in the last six months, and they were not provided with a formal definition of a fall. This may have introduced bias in the measurement of falls in the study and may have impacted the reliability of this measure [27]. The present study also was not powered based on falls outcomes, and therefore may have been underpowered to provide firm conclusions from fall status compared to larger previously reported sample sizes [9,10]. Finally, the number of steps measured in the present study may have been too low to reliably measure gait variability and future studies should aim to collect gait data continuously with more steps [28].
Future Directions
Previous studies have investigated the effects of rhythmic auditory cueing interventions on incidence of falls [29] and gait variability [30]. However, these studies did not investigate the use of self-generated rhythmic auditory cues and did not consider freezing status. Future work could investigate the difference in effects of externally-generated and self-generated cues in a randomized controlled trial that includes training in use of cues over a period of months. Future studies should also aim to better optimize rhythmic auditory cues for people with PD with and without FOG, investigating parameters such as cue rate and song familiarity.
Conclusion
People with PD with and without FOG are capable of using both externally-generated and self-generated cues for gait modification. Across all groups, externally-generated cues increased gait variability, whereas self-generated cues did not. The results of the present study also confirm that higher gait variability is associated with an increased risk of falls, and that individuals with higher baseline gait variability show the most improvements in gait variability with the use of rhythmic auditory cueing.
Highlights.
External cues, but not self-generated cues, increase gait variability in all groups.
Higher baseline gait variability was associated with lower cued gait variability.
Higher gait variability is associated with falls in PD-FOG, PD+FOG, and Controls.
Acknowledgements:
The authors would like to acknowledge Martha Hessler for her contribution to participant recruitment and data collection.
Acknowledgement of Financial Support:
This study was funded by the National Institutes of Health [T32HD007434, R61AT010753] and supported by the Greater St. Louis American Parkinson Disease Association (APDA) and the APDA Advanced Center for Parkinson Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare they have no conflict of interest.
References
- [1].Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM, Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030, Neurology. 68 (2007) 384–6. 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- [2].Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silbum PA, Predictors of future falls in Parkinson disease, Neurology. 75 (2010) 116–24. 10.1212/WNL.0b013e3181e7b688. [DOI] [PubMed] [Google Scholar]
- [3].Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G, A 12-year population-based study of freezing of gait in Parkinson’s disease, Park. Relat Disord 21 (2015) 254–8. 10.1016/j.parkreldis.2014.12.020. [DOI] [PubMed] [Google Scholar]
- [4].Moore O, Peretz C, Giladi N, Freezing of gait affects quality of life of peoples with Parkinson’s disease beyond its relationships with mobility and gait, Mov Disord. 22 (2007) 2192–5. 10.1002/mds.21659. [DOI] [PubMed] [Google Scholar]
- [5].Tessitore A, Amboni M, Esposito F, Russo A, Picillo M, Marcuccio L, Pellecchia MT, Vitale C, Cirillo M, Tedeschi G, Barone P, Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait, Park. Relat Disord 18 (2012) 781–7. 10.1016/j.parkreldis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- [6].Blin O, Ferrandez AM, Pailhous J, Serratrice G, Dopa-sensitive and Dopa-resistant gait parameters in Parkinson’s disease, J. Neurol. Sci 103 (1991) 51–54. 10.1016/0022-510X(91)90283-D. [DOI] [PubMed] [Google Scholar]
- [7].Morris ME, Huxham F, McGinley J, Dodd K, Iansek R, The biomechanics and motor control of gait in Parkinson disease, Clin. Biomech 16 (2001) 459–470. 10.1016/S0268-0033(01)00035-3. [DOI] [PubMed] [Google Scholar]
- [8].Plotnik M, Giladi N, Hausdorff JM, Bilateral coordination of walking and freezing of gait in Parkinson’s disease, Eur. J. Neurosci 27 (2008) 1999–2006. 10.1111/j.1460-9568.2008.06167.x. [DOI] [PubMed] [Google Scholar]
- [9].Allah G, Launay CP, Blumen HM, Callisaya ML, De Cock A-M, Kressig RW, Srikanth V, Steinmetz J-P, Verghese J, Beauchet O, Falls, cognitive impairment, and gait performance: Results from the GOOD initiative, J. Am. Med. Dir. Assoc 18 (2017) 335–340. 10.1016/j.jamda.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Callisaya ML, Blizzard L, Schmidt MD, Martin KL, McGinley JL, Sanders LM, Srikanth VK, Gait, gait variability and the risk of multiple incident falls in older people: a population-based study, Age Ageing. 40 (2011) 481–487. 10.1093/ageing/afr055. [DOI] [PubMed] [Google Scholar]
- [11].Thaut MH, The discovery of human auditory-motor entrainment and its role in the development of neurologic music therapy, Prog Brain Res. 217 (2015) 253–66. 10.1016/bs.pbr.2014.11.030. [DOI] [PubMed] [Google Scholar]
- [12].Dalla Bella S, Berkowska M, Sowinski J, Moving to the beat and singing are linked in humans, Front Hum Neurosci. 9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Harrison EC, Horin AP, Earhart GM, Mental singing reduces gait variability more than music listening for healthy older adults and people with Parkinson disease, J. Neurol. Phys. Ther (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harrison EC, Horin AP, Earhart GM, Internal cueing improves gait more than external cueing in healthy adults and people with Parkinson disease, Sci. Rep 8 (2018) 15525 10.1038/s41598-018-33942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Willems AM, Nieuwboer A, Chavret F, Desloovere K, Dom R, Rochester L, Jones D, Kwakkel G, Van Wegen E, The use of rhythmic auditory cues to influence gait in patients with Parkinson’s disease, the differential effect for freezers and non-freezers, an explorative study, Disabil Rehabil. 28 (2006) 721–8. 10.1080/09638280500386569. [DOI] [PubMed] [Google Scholar]
- [16].Young WR, Shreve L, Quinn EJ, Craig C, Bronte-Stewart H, Auditory cueing in Parkinson’s patients with freezing of gait. What matters most: Action-relevance or cuecontinuity?, Neuropsychologia. 87 (2016) 54–62. 10.1016/j.neuropsychologia.2016.04.034. [DOI] [PubMed] [Google Scholar]
- [17].Tolleson CM, Dobolyi DG, Roman OC, Kanoff K, Barton S, Wylie SA, Kubovy M, Claassen DO, Dysrhythmia of timed movements in Parkinson׳s disease and freezing of gait, Brain Res. 1624 (2015) 222–231. 10.1016/j.brainres.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stem MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, U.R.T.F. Movement Disorder Society, Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results, Mov Disord. 23 (2008) 2129–70. 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- [19].Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N, Reliability of the new freezing of gait questionnaire: Agreement between patients with Parkinson’s disease and their carers, Gait Posture. 30 (2009) 459–463. 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- [20].Bilney B, Morris M, Webster K, Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait, Gait Posture. 17 (2003) 68–74. [DOI] [PubMed] [Google Scholar]
- [21].Kroneberg D, Elshehabi M, Meyer A-C, Doss S, Kuhn A, Maetzler W, Schmitz-Hübsch T, How many steps are enough? Assessment of gait variability in realistically confined clinical settings, Basal Ganglia. 8 (2017) 3–4. 10.1016/j.baga.2017.02.009. [DOI] [Google Scholar]
- [22].Nieuwboer A, Cueing for freezing of gait in patients with Parkinson’s disease: a rehabilitation perspective, Mov Disord. 23 Suppl 2 (2008) S475–81. 10.1002/mds.21978. [DOI] [PubMed] [Google Scholar]
- [23].R Core Team, R: A language and environment for statistical computing., (2013). http://www.R-project.org/.
- [24].Singmann H, Bolker B, Westfall J, Aust F, afex: Analysis of Factorial Experiments, R package version 0.21-2, 2018. https://CRAN.R-project.org/package=afex.
- [25].Ginis P, Nackaerts E, Nieuwboer A, Heremans E, Cueing for people with Parkinson’s disease with freezing of gait: A narrative review of the state-of-the-art and novel perspectives, Ann. Phys. Rehabil. Med 61 (2018) 407–413. 10.1016/j.rehab.2017.08.002. [DOI] [PubMed] [Google Scholar]
- [26].Lirani-Silva E, Lord S, Moat D, Rochester L, Morris R, Auditory cueing for gait impairment in persons with Parkinson disease: A pilot study of changes in response with disease progression, J. Neurol. Phys. Ther 43 (2019) 50–55. 10.1097/NPT.0000000000000250. [DOI] [PubMed] [Google Scholar]
- [27].Mackenzie L, Byles J, D’Este C, Validation of self-reported fall events in intervention studies, Clin. Rehabil 20 (2006) 331–339. 10.1191/0269215506cr947oa. [DOI] [PubMed] [Google Scholar]
- [28].Galna B, Lord S, Rochester L, Is gait variability reliable in older adults and Parkinson’s disease? Towards an optimal testing protocol, Gait Posture. 37 (2013) 580–585. 10.1016/j.gaitpost.2012.09.025. [DOI] [PubMed] [Google Scholar]
- [29].Thaut MH, Rice RR, Braun Janzen T, Hurt-Thaut CP, McIntosh GC, Rhythmic auditory stimulation for reduction of falls in Parkinson’s disease: a randomized controlled study, Clin. Rehabil (2018) 0269215518788615. 10.1177/0269215518788615. [DOI] [PubMed]
- [30].del Olmo MF, Arias P, Furio MC, Pozo MA, Cudeiro J, Evaluation of the effect of training using auditory stimulation on rhythmic movement in Parkinsonian patients—a combined motor and [18F]-FDG PET study, Parkinsonism Relat. Disord 12 (2006) 155–164. 10.1016/j.parkreldis.2005.11.002. [DOI] [PubMed] [Google Scholar]