Abstract
Observational studies report that physical activity and metformin are associated with improved clinical outcome in patients with cancer. Inflammation is one biological mechanism hypothesized to mediate these associations. In this phase II, multi-center, 2×2 factorial trial, 139 patients with breast and colorectal cancer who completed standard therapy were randomized to one of four treatment groups for 12 weeks: exercise alone, metformin alone, exercise and metformin, or control. Inflammation outcomes included high sensitivity C-reactive protein (hs-CRP), soluble tumor necrosis factor alpha receptor two (sTNF-αR2), and interleukin 6 (IL-6). The primary modeling strategy evaluated the trial product estimand that was quantified using a generalized linear mixed model. Compared with control, exercise alone reduced hs-CRP: −30.2% (95% CI: −50.3, −1.0) and IL-6: −30.9% (95% CI: −47.3, −9.5); but did not change sTNF-αR2: 1.0% (95% CI: −10.4, 13.9). Compared with control, metformin alone did not change hs-CRP: −13.9% (95% CI: −40.0, 23.4), sTNF-αR2: −10.4% (95% CI: −21.3, 2.0), or IL-6: −22.9% (95% CI: −42.3, 2.0). Compared with control, exercise and metformin reduced sTNF-αR2: −13.1% (95% CI: −22.9, −1.0) and IL-6: −38.7% (95% CI: −52.3, −18.9); but did not change hs-CRP: −20.5% (95% CI: −44.0, 12.7). The combination of exercise and metformin was not synergistic for hs-CRP, sTNF-αR2, or IL-6. In survivors of breast and colorectal cancer with low baseline physical activity and without type 2 diabetes, exercise and metformin reduced measures of inflammation that are associated with cancer recurrence and mortality.
Keywords: breast neoplasms, colorectal neoplasms, obesity, metabolism, diabetes
INTRODUCTION
Observational studies report that physical activity and metformin after the diagnosis of early-stage cancer are associated with a 30–40% reduction in the risk of cancer recurrence and mortality (1,2). The biological processes through which physical activity and metformin may favorably impact clinical outcome remain poorly understood. Inflammation is hypothesized as a key biological mediator of these associations (3,4).
Inflammation is a hallmark of cancer and is associated with poor clinical outcome in patients with various types of solid tumors (5–7). Inflammation activates the JAK-STAT and NF-κB signaling pathways to promote cell survival, proliferation, migration, and invasion (8–10). Preclinical studies demonstrate that reducing inflammation and targeting inflammatory signaling pathways slows cell growth and delays tumor progression (11,12). Furthermore, obesity causes chronic inflammation that may promote malignant cell growth (13,14). An anti-inflammatory benefit of physical activity and metformin may occur, in part, because of reductions in adiposity (15,16).
These observations provided the scientific rationale to test the effect of exercise and metformin on pre-specified inflammation outcome measures in patients with breast and colorectal cancer. We previously reported that exercise and metformin reduced the primary endpoint of fasting plasma insulin, and secondary supportive endpoints of insulin resistance, and adiposity (17). This trial used a 2×2 factorial design, which allowed the simultaneous examination of exercise and metformin. This trial was part of the National Cancer Institute (NCI) Transdisciplinary Research on Energetics and Cancer (TREC) consortium (18).
MATERIALS AND METHODS
Study Design
The study was a 12-week, multi-center, randomized, 2×2 factorial, phase II trial. The study was conducted at three centers in the United States (Dana-Farber Cancer Institute, Duke University, and Yale University). The study was conducted in accordance with Good Clinical Practice and the ethical principles originating in the Declaration of Helsinki. The protocol and informed consent document were approved by the institutional review board for each site. All participants provided informed consent and approval from their physician prior to completing any study activities. The study was registered on Clinicaltrials.gov as NCT01340300.
Participants
Eligible participants had stage I-III breast or colorectal cancer; completed surgery, chemotherapy, and radiation ≥1 month(s) prior to enrollment (concurrent endocrine and/or trastuzumab were allowed for participants with breast cancer); were engaging in <120 min∙wk−1 of exercise; had an Eastern Cooperative Oncology Group Performance Status of 0–1; a random glucose <160 mg∙dL−1 or fasting glucose <126 mg∙dL−1; adequate renal and kidney function; were age ≥18 years; English speaking; and willing to be randomized.
Randomization and Blinding
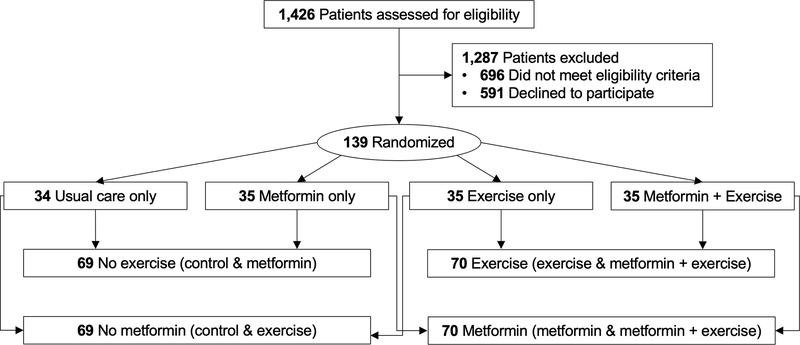
Participants were randomly assigned in an equal ratio to one of four treatment groups for 12-weeks: exercise alone, metformin alone, exercise and metformin, or control (Figure 1). Participants were stratified by body mass index (<30 kg∙m−2 vs ≥30 kg∙m−2), sex (men vs women), and cancer site (breast vs colorectal) and then randomized using a permuted block design with fixed block sizes. Participants were not blinded to treatment assignment.
Figure 1.
Flow of participants and composition of factorial groups
Exercise Treatment Plan
Exercise was performed through a combination of in-person and home-based activity. In-person exercise was supervised by an exercise physiologist. Aerobic exercise was the primary exercise type, with treadmill and outdoor walking as the most common exercise modalities. Exercise intensity was prescribed at 65–80% of the age-predicted heart rate (19). During the twice-weekly in-person exercise sessions, participants wore a heart rate monitor to learn the amount of physical exertion consistent with moderate- to vigorous-intensity exercise. Home-based exercise was monitored by self-report using exercise logs that were provided to participants. Participants progressed to the goal of 220 min∙wk−1 of exercise. This exercise dose was selected on the basis of observational studies suggesting that higher volumes of activity are associated with a lower risk of recurrence and premature mortality (20,21). Participants were encouraged to individualize their frequency (days per week), fractionation (sessions per day) and duration (minutes per session) of exercise according to a schedule that promoted optimal adherence to the prescribed exercise volume. The exercise physiologist provided behavioral support and monitored exercise adherence during the study.
Metformin Treatment Plan
Metformin was titrated over the first two weeks of the study. In week one and two, participants were instructed to consume one metformin capsule at dinner (850 mg). If no gastrointestinal distress or other adverse events were experienced after two weeks at 850 mg, participants were instructed to consume one metformin capsule at breakfast and one metformin capsule at dinner, totaling 1700 mg per day, until the end of the study. Participants who experienced adverse events at 1700 mg were allowed to continue at 850 mg for the rest of the study. Dosing of metformin for the treatment of pre-diabetes and diabetes ranges from 500–2500 mg daily, with many individuals requiring ≥1500 mg daily to achieve adequate glycemic control (22,23).
Inflammation Outcome Measures
Study participants underwent a fasting (≥10 hours) blood draw at baseline and week 12. EDTA-preserved plasma was stored at −80°C. Inflammation measures included high-sensitivity C-reactive protein (hs-CRP), soluble tumor necrosis factor alpha receptor 2 (sTNF-αR2), and interleukin 6 (IL-6). hs-CRP, sTNF-αR2, and IL-6 were selected because of their reported associations with cancer recurrence and mortality in observational studies of patients with breast and colorectal cancer (5–7). hs-CRP was measured as a marker of generalized systemic inflammation (24). sTNF-αR2 was measured as an activator of the NF-kB pathway (25); sTNF-αR2 is a surrogate marker for TNF-α that is more stable in plasma and less sensitive to diurnal variation (26). IL-6 was measured as an activator of the JAK-STAT pathway (27). hs-CRP was quantified using an immunoturbidimetric assay (Roche Diagnostics, Indianapolis, IN). sTNFα-R2 and IL-6 and were quantified using ultra-sensitive sandwich enzyme immunoassays (R&D Systems, Minneapolis, MN). Baseline and follow-up plasma samples were assayed simultaneously and in duplicate at the end of the study. Blinded quality-control samples were interspersed among cases. Coefficients of variation for all samples were ≤8%. All assays were conducted by staff who were blinded to treatment assignment.
Other Measures
Demographic characteristics including age, sex, and race were self-reported. Clinical information including type of cancer, time since cancer diagnosis, and cancer stage were abstracted from physician records. Body mass and circumferences of the waist and hip were measured in duplicate using standardized techniques.
Statistical Analysis
The sample size was selected to provide sufficient statistical power to detect change in the primary endpoint of fasting plasma insulin (17). Measures of inflammation were pre-specified as secondary outcomes. Based on estimates from the Diabetes Prevention Program (DPP) and Action for Health in Diabetes (Look AHEAD) trials (28,29), this study had sufficient statistical power to detect a standardized mean difference effect size of ≥0.48 for inflammation outcome measures.
All analyses adhered to the intention-to-treat principle. At the time this study was designed, the extent to which exercise and metformin acted independently (e.g., exercise is equally effective whether or not the participant is receiving metformin, and vice-versa) was uncertain (30). Therefore the primary inferential analysis estimated the comparative effect of each of the three intervention groups (e.g., exercise alone, metformin alone, and exercise plus metformin) with the control group (31); conceptually this contrast is a comparison of the cells within a 2×2 table (32). The primary modeling strategy evaluated the trial product estimand that was quantified using a generalized linear mixed model with observed data (i.e., no imputation) (33). This model accounts for the correlation between measures and assumes data are missing at random. The secondary modeling strategy evaluated the treatment policy estimand that was quantified using a generalized linear mixed model with predictive mean matching multiple imputation to account for missing data (33,34). Biomarker concentrations were log transformed in the inferential analysis to improve distributional normality. The baseline value of the dependent variable, randomization stratification factors, and study center were included as covariates in regression models (35). Group-by-time interaction terms were included as fixed-effects in regression models with subject-specific intercepts. A linear contrast of the four individual group means was estimated to determine if the effects of exercise and metformin were more than additive (e.g., multiplicative) (36). In the absence of evidence to suggest a multiplicative interaction, we proceeded to estimate the comparative effects of exercise vs no exercise and metformin vs no metformin, as these main effects represent the most efficient analysis of a 2×2 factorial design (37). In a 2×2 factorial design, the main effect of one independent variable (e.g., exercise vs no exercise) represents the overall effect averaged across both values of the other independent variable (e.g., metformin vs no metformin); conceptually, this contrast is a comparison of the margins of a 2×2 table (32).
Treatment effects were calculated as the treatment effect ratio, which quantifies the percent change in geometric means from baseline to 12-weeks (e.g., a treatment effect ratio of 0.75 indicates a 25% reduction), with 95% confidence intervals. Model fit was assessed using a combination of numeric and graphical techniques. Interaction terms of group, time, and randomization stratification factors were included in regression models to quantify heterogeneity of treatment effect. Exploratory analyses quantified the extent to which change in body mass and circumferences of the waist and hip mediated the observed treatment effect (38).
RESULTS
Between September 2011 and December 2015, 139 participants were recruited and randomized with primary data collection ending in May 2016. Baseline characteristics of study participants were balanced (Table 1).
Table 1.
Baseline characteristics by randomized group (N=139)
| Characteristic | Exercise & Metformin (n=35) | Exercise Only (n=35) | Metformin Only (n=35) | Control (n=34) |
|---|---|---|---|---|
| Age, yr | 53.7 (8.8) | 55.7 (10.5) | 57.0 (11.9) | 56.9 (9.2) |
| Sex, % | ||||
| Men | 6 (17.1%) | 6 (17.1%) | 6 (17.1%) | 5 (14.7%) |
| Women | 29 (82.9%) | 29 (82.9%) | 29 (82.9%) | 29 (85.3%) |
| Race, % | ||||
| White | 28 (80.0%) | 29 (82.9%) | 30 (85.7%) | 26 (76.5%) |
| Black | 3 (8.6%) | 3 (8.6%) | 1 (2.9%) | 5 (14.7%) |
| Other | 4 (11.4%) | 3 (8.6%) | 4 (11.4%) | 3 (8.8%) |
| Type of Cancer, % | ||||
| Breast | 22 (62.9%) | 22 (62.9%) | 21 (60.0%) | 22 (64.7%) |
| Colorectal | 13 (37.1%) | 13 (37.1%) | 14 (40.0%) | 12 (35.3%) |
| Time Since Diagnosis, yr | 2.8 (2.3) | 3.6 (3.3) | 3.4 (4.4) | 2.4 (2.4) |
| Cancer Stage, % | ||||
| I | 14 (40.0%) | 14 (40.0%) | 11 (31.4%) | 12 (35.3%) |
| II | 8 (22.9%) | 9 (25.7%) | 11 (31.4%) | 12 (35.3%) |
| III | 13 (37.1%) | 12 (34.3%) | 12 (34.3%) | 9 (26.5%) |
| Missing | 0 (0.0%) | 0 (0.0%) | 1 (2.9%) | 1 (2.9%) |
| Body Weight, kg | 81.3 (20.0) | 82.6 (19.9) | 84.6 (20.8) | 83.1 (22.9) |
| Waist Circumference, cm | 92.4 (14.3) | 93.6 (15.2) | 95.6 (13.3) | 95.2 (17.0) |
| Waist-to-Hip, ratio | 0.84 (0.10) | 0.85 (0.09) | 0.85 (0.09) | 0.86 (0.08) |
Data are mean ± standard deviation or n (%).
At baseline, the geometric mean (standard deviation [SD]) hs-CRP was 0.55 (1.04) mg∙L−1, sTNF-αR2 was 7.80 (0.33) pg∙mL−1, and IL-6 was 1.04 (0.82) pg∙mL−1, indicating low to moderate inflammation. Among participants randomized to exercise, 77% and 17% completed ≥50% and ≥90% of their initially prescribed exercise volume, respectively. Among participants randomized to metformin, 67% and 31% consumed ≥50% and ≥90% of their initially prescribed metformin dose, respectively. At 12-weeks, 91 (65%) participants completed their assigned intervention; reasons for premature discontinuation have been described (17). Participants who did not complete the study were more likely to be of white race [multivariable-adjusted odds ratio: 3.59 (95% CI: 1.14, 11.36)]; no other measured factors, including randomized group assignment and baseline concentrations of inflammation, were associated with study completion.
By pairwise effects analysis (e.g., contrasting the cells within the 2×2 table), compared with control, exercise alone statistically significantly reduced hs-CRP: −30.2% (95% CI: −50.3, −1.0) and IL-6: −30.9% (95% CI: −47.3, −9.5); but did not statistically significantly change sTNF-αR2: 1.0% (95% CI: −10.4, 13.9) (Table 2). Compared with control, metformin alone did not statistically significantly change hs-CRP: −13.9% (95% CI: −40.0, 23.4), sTNF-αR2: −10.4% (95% CI: −21.3, 2.0), or IL-6: −22.9% (95% CI: −42.3, 2.0). Compared with control, exercise and metformin statistically significantly reduced sTNF-αR2: −13.1% (95% CI: −22.9, −1.0) and IL-6: −38.7% (95% CI: −52.3, −18.9); but did not statistically significantly change hs-CRP: −20.5% (95% CI: −44.0, 12.7). The combination of exercise and metformin was not synergistic for hs-CRP (P=0.35), sTNF-αR2 (P=0.66), or IL-6 (P=0.69). Intervention adherence was not associated with magnitude of treatment effect; participants who adhered even minimally to either intervention achieved an inflammation lowering benefit. The correlations with exercise adherence with change in inflammation were: hs-CRP (R=−0.03, 95% CI: −0.26, 0.21); sTNF-aR2 (R=−0.12, 95% CI: −0.34, 0.12); and IL-6 (R=0.01, 95% CI: −0.24, 0.22). The correlations with metformin adherence with change in inflammation were: hs-CRP (R=0.06, 95% CI: −0.18, 0.29); sTNF-aR2 (R=0.04, 95% CI: −0.20, 0.27), and IL-6 (R=0.04, 95% CI: −0.20, 0.27). Heterogeneity of the treatment effect did not substantively differ between any randomization stratification subgroups. Results were similar using predictive mean matching multiple imputation (Supplementary Table 1).
Table 2.
Change in high sensitivity C-reactive protein (hs-CRP), soluble tumor necrosis factor alpha receptor two (sTNF-αR2) and interleukin-6 (IL-6) by randomized group
| Outcome | Randomized Group | Baseline Geometric Mean (SD) | Geometric Mean Change (SE) | Intervention Main Effect, Treatment Ratio (95% CI) |
|---|---|---|---|---|
| hs-CRP | Control | 0.80 (1.09) | 0.21 (0.17) | 1.00 (Reference) |
| Exercise | 0.69 (0.96) | −0.14 (0.14) | 0.70 (0.50, 0.99) | |
| Metformin | 0.44 (1.05) | 0.10 (0.15) | 0.86 (0.60, 1.23) | |
| Combined | 0.30 (1.07) | 0.03 (0.14) | 0.79 (0.56, 1.13) | |
| sTNF-αR2 | Control | 7.87 (0.35) | 0.03 (0.06) | 1.00 (Reference) |
| Exercise | 7.75 (0.39) | 0.06 (0.05) | 1.01 (0.89, 1.14) | |
| Metformin | 7.82 (0.26) | −0.07 (0.05) | 0.89 (0.79, 1.02) | |
| Combined | 7.77 (0.32) | −0.09 (0.05) | 0.87 (0.77, 0.99) | |
| IL-6 | Control | 1.24 (0.86) | 0.26 (0.14) | 1.00 (Reference) |
| Exercise | 1.03 (0.75) | −0.09 (0.11) | 0.69 (0.53, 0.90) | |
| Metformin | 0.90 (0.75) | 0.04 (0.12) | 0.77 (0.58, 1.02) | |
| Combined | 1.03 (0.93) | −0.20 (0.11) | 0.61 (0.47, 0.81) |
Models adjusted for the baseline value of the dependent variable, body mass index (<30 kg/m2 vs ≥30 kg/m2), sex (men vs women), cancer site (colorectal vs breast), and study center (Dana Farber Cancer Institute vs Duke University vs Yale University).
By main effects analysis (e.g., contrasting the margins of the 2×2 table), compared to no exercise, exercise statistically significantly reduced IL-6: −23.7% (95% CI: −36.9, −8.6) (Table 3); but did not statistically significantly change hs-CRP: −19.0% (95% CI: −36.6, 2.0) and sTNF-αR2: 0.0% (95% CI: −7.7, 9.4). Compared with no metformin, metformin statistically significantly reduced sTNF-αR2: −12.2% (95% CI: −18.9, −3.9); but did not statistically significantly change hs-CRP: 3.0% (95% CI: −18.1, 30.9) and IL-6: −13.9% (95% CI: −28.1, 4.0). Intervention adherence was not associated with magnitude of treatment effect. Heterogeneity of the treatment effect did not substantively differ between any randomization stratification subgroups. Results were similar using predictive mean matching multiple imputation (Supplementary Table 2).
Table 3.
Change in high sensitivity C-reactive protein (hs-CRP), soluble tumor necrosis factor alpha receptor two (sTNF-αR2), and interleukin-6 (IL-6) by exercise and metformin factorial groups
| Outcome | Overall Baseline Geometric Mean (SD) | Exercise Factorial Groups |
Metformin Factorial Groups |
||||
|---|---|---|---|---|---|---|---|
| Geometric Mean Change (SE) |
Exercise Main Effect, Treatment Ratio (95% CI) | Geometric Mean Change (SE) |
Metformin Main Effect, Treatment Ratio (95% CI) | ||||
| Exercise | No Exercise | Metformin | No Metformin | ||||
| hs-CRP | 0.55 (1.04) | −0.06 (0.10) | 0.14 (0.11) | 0.81 (0.64, 1.02) | 0.06 (0.11) | −0.01 (0.11) | 1.03 (0.82, 1.31) |
| sTNF-αR2 | 7.80 (0.33) | −0.01 (0.04) | −0.03 (0.04) | 1.00 (0.92, 1.09) | −0.08 (0.04) | 0.05 (0.04) | 0.88 (0.81, 0.96) |
| IL-6 | 1.04 (0.82) | −0.14 (0.08) | 0.13 (0.09) | 0.76 (0.63, 0.91) | −0.09 (0.08) | 0.03 (0.09) | 0.86 (0.72, 1.04) |
Models adjusted for the baseline value of the dependent variable, body mass index (<30 kg/m2 vs ≥30 kg/m2), sex (men vs women), cancer site (colorectal vs breast), and study center (Dana Farber Cancer Institute vs Duke University vs Yale University).
Change in body mass, waist circumference, or the waist-to-hip ratio did not mediate the observed treatment effect of exercise on IL-6 or the treatment effect of metformin on sTNF-αR2 (Table 4). No serious or unexpected adverse events were reported; non-serious adverse events have been described (17).
Table 4.
Change in interleukin-6 (IL-6) and soluble tumor necrosis factor alpha receptor two (sTNF-αR2) before and after adjustment for body composition change
| Before Adjustment |
After Adjustment |
|
|---|---|---|
| Intervention Main Effect, Treatment Ratio (95% CI) | Hypothesized Mediator | Intervention Main Effect, Treatment Ratio (95% CI) |
| Exercise: IL-6 | ||
| 0.76 (0.63, 0.91) | ||
| Δ Body Weight | 0.77 (0.64, 0.93) | |
| Δ Waist Circumference | 0.76 (0.64, 0.92) | |
| Δ Waist-to-Hip Ratio | 0.76 (0.63, 0.91) | |
| Metformin: sTNF-αR2 | ||
| 0.88 (0.81, 0.96) | ||
| Δ Body Weight | 0.89 (0.81, 0.96) | |
| Δ Waist Circumference | 0.89 (0.82, 0.97) | |
| Δ Waist-to-Hip Ratio | 0.89 (0.82, 0.97) |
Models adjusted for the baseline value of the dependent variable, body mass index (<30 kg/m2 vs ≥30 kg/m2), sex (men vs women), cancer site (colorectal vs breast), and study center (Dana Farber Cancer Institute vs Duke University vs Yale University).
DISCUSSION
In this randomized 2×2 factorial trial of 139 survivors of breast and colorectal cancer, exercise reduced concentrations of IL-6 and metformin reduced concentrations of sTNF-αR2 over 12 weeks. The combined effect of exercise and metformin was not multiplicative, although statistical power was limited. The observed treatment effect was consistent across randomization stratification variables including baseline body mass index, sex, and cancer type. Change in body mass, waist circumference, or the waist-to-hip ratio did not mediate the observed treatment effect of exercise and metformin on inflammation outcome measures. In pairwise effects analyses comparing each intervention group to the control group, exercise reduced hs-CRP and IL-6, and the combination of exercise and metformin reduced sTNF-αR2 and IL-6.
One of the mechanisms by which physical activity and metformin are hypothesized to exert anti-cancer effects is through their impact on the host microenvironment by reducing inflammation (3,4). Our results provide evidence that inflammation is reduced when 12-weeks of exercise or metformin are administered to patients with breast and colorectal cancer. In pairwise analysis, the combination of exercise and metformin reduced both IL-6 and sTNF-αR2 compared to control. In main effects analysis, exercise reduced IL-6 and metformin reduced sTNF-αR2. IL-6 activates the JAK/STAT pathway and TNF-α in part through its receptor, sTNF-αR2, activates the NF-kB pathway (11,12). Our results suggest that exercise and metformin inhibit distinct inflammatory processes, and the combination of exercise and metformin more comprehensively inhibit the physiology of distinct inflammation related signaling pathways than each intervention alone.
Obesity is associated with poor clinical outcome after cancer diagnosis (39). One mechanism through which obesity is hypothesized to exert pro-cancer effects is increased inflammation caused by hypertrophic metabolically active adipocytes (13,14). Exercise reduces adipose tissue and increases lean mass, despite stability of body weight (40). In patients with type 2 diabetes, metformin causes modest weight loss, preferentially through reductions in adipose tissue mass (41). We previously reported that exercise and metformin reduced body mass, waist circumference, and the waist-to-hip ratio (17). In our exploratory analysis we observed no evidence that the treatment effect of exercise or metformin on inflammation outcome measures was mediated by change in body mass or anthropometric surrogate measures of body composition (e.g., waist circumference or the waist-to-hip ratio).
The results of this trial complement the Reach for Health Trial, also conducted as part of the NCI TREC consortium (42). Reach for Health used a similar 2×2 factorial trial design to evaluate the effect of metformin or behavioral weight loss in overweight and obese patients with breast cancer. By main effects analysis, over 24-weeks, no change in hs-CRP was observed with metformin: −14.9% (95% CI: −32.9, 3.1) or behavioral weight loss: −12.4% (95% CI: −30.4, 5.5). Our study found no main effect of exercise or metformin on hs-CRP; in pairwise analysis exercise reduced hs-CRP by 30% relative to control. This observation is consistent with a meta-analysis of six randomized controlled trials demonstrating that exercise reduces hs-CRP in patients with cancer (43). However the absence of an effect of metformin on hs-CRP is in contrast to an analysis of 492 patients with breast cancer enrolled in the MA.32 trial, where metformin reduced hs-CRP by 6.7% versus placebo (44).
There are several limitations to this trial. The main limitation is the small sample size, which limited our ability to identify multiplicative interaction effects between exercise and metformin on inflammation outcome measures. The small sample size may have also limited our ability to detect small, but potentially clinically meaningful, main effects for exercise or metformin. The intervention duration was 12 weeks, which limits our ability to understand the benefits of exercise and metformin over longer time horizons. The study sample was not enrolled on the basis of having elevated biomarkers of inflammation at baseline, which limits our understanding of the treatment effect in patients with acute or chronic inflammation. Intervention adherence was modest, however adherence to exercise or metformin was not correlated with the magnitude of treatment effect. Follow-up at 12-weeks was modest, however results and conclusions of our primary analysis were robust to various missing data and statistical modeling assumptions.
There are several strengths to this trial. The randomized design and use of two distinct interventions that are both hypothesized to favorably impact inflammation outcome measures allowed for a time- and cost-efficient comparison of causal effects. Our study included patients with breast and colorectal cancer, which allowed examination of heterogeneity of the treatment effect between cancer sites. The use of three biomarker measures of inflammation allowed for a detailed physiological investigation of treatment benefit.
In one of the first randomized clinical trials evaluating two different metabolic interventions in patients with cancer, this study demonstrates that exercise and metformin reduced inflammation. The findings from this randomized trial are useful to begin to understand the biological mediators of the relationship between physical activity and metformin with clinical outcome in patients with cancer. Results from ongoing phase III randomized clinical trials with disease endpoints will inform the utilization of exercise and metformin in clinical practice, and the correlative studies embedded into these trials will offer unprecedented insight into mechanisms of treatment benefit (44,45).
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers R00-CA218603; R25-CA203650; and U54-CA155626 and the National Institute of General Medicine Sciences of the National Institutes of Health under Award Number U54-GM104940. Dr. Meyerhardt is supported by the Douglas Gray Woodruff Chair Fund, the Guo Shu Shi Fund, Anonymous Family Fund for Innovations in Colorectal Cancer, Project P Fund, and the George Stone Family Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Conflicts of Interest Disclosures: Dr. Brown reports receiving grants from the National Cancer Institute, American College of Sports Medicine, American Institute for Cancer Research, and Susan G. Komen Foundation paid to his institution. Dr. Ng has received grants from the National Cancer Institute, U.S. Department of Defense, and Cancer Research UK; institutional research funding from Genentech, Celgene, Gilead Sciences, Pharmavite, Revolution Medicines, Trovagene, Tarrex Biopharma, and Evergrande Group; and has served as an advisor or consultant to Array Biopharma, Bayer, Eli Lilly, Genentech, Seattle Genetics, and Tarrex Biopharma. Dr. Ligibel is supported by a Scholars grant from the Susan G. Komen Foundation. Dr. Meyerhardt has received institutional research funding from Boston Biomedical, has served as an advisor or consultant to Ignyta and COTA Healthcare, and served on a grant review panel for the National Comprehensive Cancer Network funded by Taiho Pharmaceutical. All other authors report no conflicts of interest.
REFERENCES
- 1.Friedenreich CM, Neilson HK, Farris MS, Courneya KS. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clin Cancer Res 2016;22(19):4766–75 doi 10.1158/1078-0432.CCR-16-0067. [DOI] [PubMed] [Google Scholar]
- 2.Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7(9):867–85 doi 10.1158/1940-6207.CAPR-13-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 2012;2(9):778–90 doi 10.1158/2159-8290.CD-12-0263. [DOI] [PubMed] [Google Scholar]
- 4.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 2012;104(11):815–40 doi 10.1093/jnci/djs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009;30(7):1073–81 doi 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 6.Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C. C-Reactive Protein Is an Important Biomarker for Prognosis Tumor Recurrence and Treatment Response in Adult Solid Tumors: A Systematic Review. PLoS One 2015;10(12):e0143080 doi 10.1371/journal.pone.0143080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med 2019;25(12):1822–32 doi 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420(6917):860–7 doi 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454(7203):436–44 doi 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 10.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431(7007):461–6 doi 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 11.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015;12(10):584–96 doi 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 12.Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol 2018;15(4):234–48 doi 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer--mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 2014;10(8):455–65 doi 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 2011;11(12):886–95 doi 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 15.McTiernan A Mechanisms linking physical activity with cancer. Nat Rev Cancer 2008;8(3):205–11 doi 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 16.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol 2016;34(35):4270–6 doi 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyerhardt JA, Irwin ML, Jones LW, Zhang S, Campbell N, Brown JC, et al. Randomized Phase II Trial of Exercise, Metformin, or Both on Metabolic Biomarkers in Colorectal and Breast Cancer Survivors. JNCI Cancer Spectr 2020;4(1):pkz096 doi 10.1093/jncics/pkz096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz KH, Gehlert S, Patterson RE, Colditz GA, Chavarro JE, Hu FB, et al. TREC to WHERE? Transdisciplinary Research on Energetics and Cancer. Clin Cancer Res 2016;22(7):1565–71 doi 10.1158/1078-0432.CCR-14-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43(7):1334–59 doi 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 20.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 2006;24(22):3535–41 doi 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 21.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA 2005;293(20):2479–86 doi 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes A. 7. Approaches to Glycemic Treatment . Diabetes Care 2016;39 Suppl 1:S52–9 doi 10.2337/dc16-S010. [DOI] [PubMed] [Google Scholar]
- 23.Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of Metformin in Type II Diabetes. The American Journal of Medicine 1997;103(6):491–7 doi 10.1016/s0002-9343(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 24.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem 2004;279(47):48487–90 doi 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez M, Cabal-Hierro L, Carcedo MT, Iglesias JM, Artime N, Darnay BG, et al. NF-kappaB signal triggering and termination by tumor necrosis factor receptor 2. J Biol Chem 2011;286(26):22814–24 doi 10.1074/jbc.M111.225631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology 2011;140(3):799–808, quiz e11 doi 10.1053/j.gastro.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sansone P, Bromberg J. Targeting the interleukin-6/Jak/stat pathway in human malignancies. J Clin Oncol 2012;30(9):1005–14 doi 10.1200/JCO.2010.31.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, et al. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes 2005;54(5):1566–72 doi 10.2337/diabetes.54.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belalcazar LM, Haffner SM, Lang W, Hoogeveen RC, Rushing J, Schwenke DC, et al. Lifestyle intervention and/or statins for the reduction of C-reactive protein in type 2 diabetes: from the look AHEAD study. Obesity (Silver Spring) 2013;21(5):944–50 doi 10.1002/oby.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boule NG, Robert C, Bell GJ, Johnson ST, Bell RC, Lewanczuk RZ, et al. Metformin and exercise in type 2 diabetes: examining treatment modality interactions. Diabetes Care 2011;34(7):1469–74 doi 10.2337/dc10-2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pocock SJ, Hughes MD. Estimation issues in clinical trials and overviews. Stat Med 1990;9(6):657–71 doi 10.1002/sim.4780090612. [DOI] [PubMed] [Google Scholar]
- 32.Montgomery AA, Peters TJ, Little P. Design, analysis and presentation of factorial randomised controlled trials. BMC Med Res Methodol 2003;3:26 doi 10.1186/1471-2288-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akacha M, Bretz F, Ruberg S. Estimands in clinical trials - broadening the perspective. Stat Med 2017;36(1):5–19 doi 10.1002/sim.7033. [DOI] [PubMed] [Google Scholar]
- 34.Rubin DB. Statistical Matching Using File Concatenation with Adjusted Weights and Multiple Imputations. Journal of Business & Economic Statistics 1986;4(1):87–94 doi 10.2307/1391390. [DOI] [Google Scholar]
- 35.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis Hoboken, NJ: John Wiley & Sons; 2012. [Google Scholar]
- 36.Ottenbacher KJ. Interpretation of interaction in factorial analysis of variance design. Stat Med 1991;10(10):1565–71 doi 10.1002/sim.4780101008. [DOI] [PubMed] [Google Scholar]
- 37.McAlister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials: a systematic review. JAMA 2003;289(19):2545–53 doi 10.1001/jama.289.19.2545. [DOI] [PubMed] [Google Scholar]
- 38.VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. New York, NY: Oxford University Press; 2015. [Google Scholar]
- 39.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol 2014;32(31):3568–74 doi 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK, et al. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009;41(2):459–71 doi 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 41.Stumvoll M, Mitrakou A, Pimenta W, Jenssen T, Yki-Jarvinen H, Van Haeften T, et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000;23(3):295–301 doi 10.2337/diacare.23.3.295. [DOI] [PubMed] [Google Scholar]
- 42.Patterson RE, Marinac CR, Sears DD, Kerr J, Hartman SJ, Cadmus-Bertram L, et al. The Effects of Metformin and Weight Loss on Biomarkers Associated With Breast Cancer Outcomes. J Natl Cancer Inst 2018;110(11):1239–47 doi 10.1093/jnci/djy040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meneses-Echavez JF, Correa-Bautista JE, Gonzalez-Jimenez E, Schmidt Rio-Valle J, Elkins MR, Lobelo F, et al. The Effect of Exercise Training on Mediators of Inflammation in Breast Cancer Survivors: A Systematic Review with Meta-analysis. Cancer Epidemiol Biomarkers Prev 2016;25(7):1009–17 doi 10.1158/1055-9965.EPI-15-1061. [DOI] [PubMed] [Google Scholar]
- 44.Goodwin PJ, Parulekar WR, Gelmon KA, Shepherd LE, Ligibel JA, Hershman DL, et al. Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J Natl Cancer Inst 2015;107(3) doi 10.1093/jnci/djv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Courneya KS, Booth CM, Gill S, O’Brien P, Vardy J, Friedenreich CM, et al. The Colon Health and Life-Long Exercise Change trial: a randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr Oncol 2008;15(6):279–85 doi 10.3747/co.v15i6.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.