Abstract
OBJECTIVES:
We investigated whether the effect of smoking on the incidence of smoking-related cancers differs by HIV-infection status, if sex modifies the impact of risk factors for smoking-related cancers, and the sex-specific attributable risk of smoking on cancer incidence.
DESIGN:
Data from two large prospective studies in the United States were analyzed: 6,789 men in the Multicenter AIDS Cohort Study from 1984–2018 and 4,423 women in the Women’s Interagency HIV Study from 1994–2018.
METHODS:
Incidence rates (IRs), relative risks, and adjusted population attributable fractions (PAFs) were calculated for smoking-related cancers.
RESULTS:
During study follow-up, there were 214 incident smoking-related cancers in the men and 192 in the women. The age-adjusted IRs for smoking-related cancers were higher in the women (392/100,000) than for the men (198/100,000; p<0.01) and higher for people living with HIV (PLWH, 348/100,000) than for those without HIV (162/100,000; p<0.01). Unadjusted IRs in PLWH were higher than in those without HIV when stratifying by cumulative pack-years of smoking (all p-values<0.01). In adjusted interaction models, the effects of cumulative pack-years of smoking were significantly stronger in women. The adjusted PAFs for smoking-related cancers were non-significantly higher in the women than in the men (39% vs. 28%; p=0.35).
CONCLUSIONS:
HIV looks to be an independent risk factor for smoking-related cancers and women appear to have a greater risk than men. These results highlight the need for interventions to help PLWH, especially women, quit smoking and sustain cessation to reduce their risk of smoking-related cancers.
Keywords: cancer, HIV infection, incidence, risk factors, sex differences, smoking
INTRODUCTION
As people living with HIV (PLWH) are aging, largely due to effective ART, cancer - particularly non-AIDS defining cancer - has become a more common cause of morbidity and mortality.[1] The high prevalence of cancer risk factors among PLWH, especially tobacco use, contributes to the increased risk of cancer. In the United States (U.S.), the estimated percentage of adult PLWH who smoke was twice that of the U.S. general population and the smoking prevalence varied by HIV risk behavior groups.[2–4] A better understanding of the contribution of smoking to the development of cancer in PLWH can help promote smoking cessation among smokers living with HIV.
In 2010, approximately 29% of all cancer deaths in the U.S. were attributable to cigarette smoking.[5] The contribution of smoking to the cancer burden among PLWH is less well-defined. In a study of PLWH in Denmark, the estimated percentage of smoking-related cancer diagnoses attributable to smoking was 91%.[6]
A limitation of prior studies investigating cancer risk in PLWH has been the lack of an appropriate comparison group, which has traditionally been the general population. People LWH are very different from the general population and thus comparisons are confounded by other cancer risk factors like cigarette smoking, Human Papilloma Virus (HPV) and Hepatitis C Virus (HCV) infection, and substance use. For example, women in the Women’s Interagency HIV Study (WIHS) had a high prevalence of smoking (more than twice the national average) independent of HIV status.[7] Similarly, the prevalence of smoking in the men with and without HIV in the Multicenter AIDS Cohort Study (MACS) is almost twice as high as the national average.[8]
The present study has the unique advantage of a highly similar HIV-uninfected comparison group, as well as the ability to precisely adjust for potential confounders and examine sex-related differences. This investigation had three aims. First, to determine whether the effect of smoking on the incidence of smoking-related cancers differs by HIV-infection status among participants in the MACS and WIHS. We hypothesized that for a given smoking history, the incidence of smoking-related cancers would be higher among PLWH vs. those without HIV.[6] Second, to determine whether sex modifies the impact of cancer risk factors among WIHS and MACS participants. We hypothesized that the adjusted risk of smoking-related cancers would be higher among women (both with and without HIV) than among men.[9] Third, to estimate the sex-specific attributable risk of smoking on smoking-related cancers among women in the WIHS and men in the MACS. We hypothesized that approximately 50% of smoking-related cancers would be prevented if PLWH refrained from smoking.[10]
METHODS
Study population
Data collected through September 30, 2019, from participants in two U.S. HIV/AIDS cohort studies, the WIHS and the MACS, were used for this investigation. The WIHS is a multi-site prospective cohort study of 4,982 women with or at risk for HIV established in 1994. Women were enrolled at one of eleven centers across the U.S. and returned at 6-month intervals for a standardized interview-based questionnaire, physical examination including pelvic exam, Pap test with referral for colposcopy for any abnormality, and collection of blood for laboratory testing and storage. Detailed information about the WIHS study methodology and baseline characteristics of enrollees has been published.[11, 12]
The MACS is a multi-site prospective cohort study of 7,358 men who reported having had sex with men. Men were enrolled, starting in 1984, at four metropolitan areas in the U.S. Details about the recruitment and characteristics of the MACS cohort have been reported elsewhere.[13] Participants returned at 6-month intervals for a detailed interview, physical examination, and collection of blood for laboratory testing and storage.
Participants who had at least one follow-up visit, reported their history of tobacco use, and entered the study without a prior or prevalent (diagnosed within six months of study enrollment) smoking-related cancer were included in these analyses (6,789 men and 4,423 women). All analyses were performed using data collected through September 30, 2019, but follow-up was censored on December 31, 2018 to allow for cancer reporting delays (federal and state law requires all new cancer diagnoses to be reported to the cancer registries within 180 days [14]). The institutional review boards of each institution approved these studies; all participants provided written informed consent.
Cancer diagnosis
Cancer diagnoses were ascertained using medical records, cancer registry matches, and death certificates. Each confirmed cancer was classified according to the SEER site recoding scheme, which is based on the International Classification of Diseases for Oncology 3rd edition, [15] and all cancers with an initial diagnosis date that was at least six months after enrollment into the cohort study (to account for a lag time in diagnosis) were further classified as ‘incident’ cancers. We then identified smoking-related incident cancers: lung/bronchus, larynx, liver, colon, rectum, small intestine, kidneys, oral cavity, nose and middle ear, acute myeloid leukemia, cervix, vagina, vulva, penis, anus, pancreas, esophagus, bladder, and stomach.[16–18] We created a single combined endpoint where the first occurrence of any of these cancers was treated as an event. Those who developed a non-smoking-related cancer continued in follow-up.
Risk factors for cancer
The independent variables included several known cancer risks, such as tobacco use, alcohol consumption, illicit substance use, body mass index (BMI), and coinfection with HPV and HCV. Other variables included sociodemographic characteristics, behavioral factors, characteristics of HIV infection, geographic region, and calendar period. For further details regarding independent variables, see Appendix.
Statistical Analysis
Study participants were characterized at baseline using standard descriptive statistics. Smoking-related cancer incidence rates (IRs) were computed as the number of observed incident cancers divided by person-years of follow-up, where follow-up time was measured from six months after the baseline visit until the earliest of the first smoking-related cancer diagnosis, death, or date of the last study visit. Incidence rates of smoking-related cancers were adjusted for age and compared by cumulative pack-years smoked lagged by 10 years (since smoking-related cancers take at least 10 years to manifest)[19], years since smoking cessation, HIV status, and sex (MACS vs. WIHS). HIV serostatus was considered as a time-varying variable to allow for the possibility of HIV seroconversion during follow-up (see Appendix).
Smoking-related cancer IR comparisons were quantified using the incidence rate ratio (IRR) and performed using exact Poisson regression wherever possible. For the IRR analyses, the dependent (outcome) variable for this investigation was smoking-related cancer, yes or no. The effects of HIV-related variables were examined in two ways. First, unadjusted models were stratified by HIV serostatus. Second, pooled univariate models included indicator variables for HIV infection with and without the exposure of interest, compared to the HIV-uninfected reference group. All variables collected at each study visit (see Appendix) were evaluated using time-varying covariates. Interaction terms were added to the final multiple regression models to determine whether a covariate effect differed significantly by sex.
To explore the possibility that higher AIDS-related morbidity and mortality during the pre- and early ART era lowered the smoking-related cancer incidence rates and altered the effects of certain risk factors on cancer incidence, we repeated the multivariable analyses using only follow-up time accrued between 2001 and 2018 (the modern ART treatment era). Since several of the smoking-related cancers may also have viral etiologies, we repeated the age-adjusted IR and adjusted IRR analyses and excluded anal, cervix, gum and mouth, larynx, liver, penis, pharyngeal, salivary gland, stomach, tongue, tonsil, and vagina/vulva cancers from the definition of smoking-related cancers to see how this impacted the association between smoking history and the development of smoking-related malignancies. Last, since anal cancers were three times more common in men than women, we repeated the adjusted population attributable fraction (PAF) analyses excluding anal cancers from the definition of smoking-related cancers to assess sex differences for the remaining smoking-related malignancies.
Pooled as well as stratified (MACS and WIHS) relative risks and adjusted PAFs were calculated for 1) all incident smoking-related cancers; 2) cancers strongly associated with smoking (lung/bronchus and larynx; average PAF range in men and women combined 78-97%); 3) cancers moderately associated with smoking (esophagus, gum and mouth, lip, nose and middle ear, pharyngeal, and lower urinary tract; PAF range 31-60%); and 4) cancers weakly associated with smoking (acute myeloid leukemia, anal, cervix, colon/rectum, liver, pancreas, penis, salivary gland, small intestine, stomach, tongue, tonsil, and vagina/vulva; PAF range 8-25%).[20] For additional details regarding the PAF models and SAS macro [21], see Appendix. All analyses were performed using SAS 9.4.[22]
RESULTS
Of the potentially eligible participants enrolled in the MACS (7,358) and the WIHS (4,982), 569 men and 559 women were excluded due to either no accrual of follow-up time (473 men and 376 women), lack of information about pack-years of smoking (89 men and 148 women) or because they had a smoking-related cancer prior to or within six months of their baseline visit (7 men and 35 women).
The remaining 4,423 WIHS and 6,789 MACS participants exhibited many significant differences at baseline (Table 1). Most notably, men were enrolled in earlier years and at younger ages; were more likely to be non-Latinx white and HIV-uninfected; had more education, higher household incomes, lower BMIs, and more lifetime sex partners; and consumed more alcohol (all p<0.01). Women were more likely to smoke and had a higher prevalence of HCV, HIV, lower CD4 cell counts, higher HIV viral loads, and a greater prevalence of clinical AIDS (all p-values<0.01).
Table 1.
Baseline characteristics of participants in the MACS and WIHS.
| MACS | WIHS | P-value | |
|---|---|---|---|
| N (Percent) | |||
| Geographic region of recruitment site | < 0.01 | ||
| West coast | 1964 (28.93) | 1266 (28.62) | |
| East coast | 3264 (48.08) | 2641 (59.71) | |
| Midwest | 1561 (22.99) | 516 (11.67) | |
| Enrollment cohort | < 0.01 | ||
| 1984 - 1987 | 5302 (78.10) | 0 (0.00) | |
| 1994 - 1995 | 0 (0.00) | 2301 (52.02) | |
| 2001 - 2002 | 1149 (16.92) | 957 (21.64) | |
| 2010+ | 338 (4.98) | 1165 (26.34) | |
| Race/ethnicity | < 0.01 | ||
| Non-Latinx white | 4929 (72.62) | 591 (13.36) | |
| Non-Latinx black | 1129 (16.63) | 2763 (62.47) | |
| Other | 729 (10.74) | 1069 (24.17) | |
| Baseline educational attainment | < 0.01 | ||
| High school or less | 1234 (18.30) | 2924 (66.23) | |
| Some college | 1991 (29.52) | 1159 (26.25) | |
| College graduate | 1505 (22.31) | 233 (5.28) | |
| At least some postgraduate education | 2015 (29.87) | 99 (2.24) | |
| Annual household income | < 0.01 | ||
| < $29,999 | 1072 (71.99) | 3711 (86.50) | |
| ≥ $30,000 | 417 (28.01) | 579 (13.50) | |
| Smoking status | < 0.01 | ||
| Never | 2690 (39.62) | 1532 (34.64) | |
| Former | 1335 (19.66) | 559 (12.64) | |
| Current | 2764 (40.71) | 2332 (52.72) | |
| Alcohol use | < 0.01 | ||
| Abstainer | 574 (8.61) | 1957 (44.88) | |
| > 0 to 7 drinks/week | 3767 (56.49) | 1788 (41.00) | |
| > 7 to 12 drinks/week | 715 (10.72) | 183 (4.20) | |
| > 12 drinks/week | 1612 (24.18) | 433 (9.93) | |
| Smoked an illicit drug | < 0.01 | ||
| No | 2617 (38.55) | 2951 (66.72) | |
| Yes | 4172 (61.45) | 1472 (33.28) | |
| Injected an illicit drug | < 0.01 | ||
| No | 6255 (92.13) | 4145 (93.71) | |
| Yes | 534 (7.87) | 278 (6.29) | |
| Took an illicit drug other than through smoking/injection | < 0.01 | ||
| No | 3119 (45.94) | 4306 (97.35) | |
| Yes | 3670 (54.06) | 117 (2.65) | |
| Any HPV* | |||
| No | 1534 (50.43) | ||
| Yes | 1508 (49.57) | ||
| Oncogenic HPV* | |||
| No | 2294 (75.41) | ||
| Yes | 748 (24.59) | ||
| Hepatitis C virus | < 0.01 | ||
| No | 6375 (94.65) | 3414 (80.96) | |
| Yes | 360 (5.35) | 803 (19.04) | |
| HIV infection | < 0.01 | ||
| No | 3805 (56.05) | 1150 (26.00) | |
| Yes | 2984 (43.95) | 3273 (74.00) | |
| CD4 cell count/μl (PLWH only) | < 0.01 | ||
| < 200 | 185 (6.34) | 595 (18.61) | |
| 200 - 500 | 1073 (36.78) | 1368 (42.78) | |
| > 500 | 1659 (56.87) | 1235 (38.62) | |
| HIV viral load detectable (PLWH only) | < 0.01 | ||
| No | 481 (45.16) | 1063 (33.42) | |
| Yes | 584 (54.84) | 2118 (66.58) | |
| HIV viral load ≥ 4,000 copies/ml (PLWH only) | < 0.01 | ||
| No | 592 (55.59) | 1526 (47.91) | |
| Yes | 473 (44.41) | 1659 (52.09) | |
| Exposure to ART (PLWH only) | < 0.01 | ||
| No | 2473 (82.88) | 2327 (71.10) | |
| Yes | 511 (17.12) | 946 (28.90) | |
| History of clinical AIDS (PLWH only) | < 0.01 | ||
| No | 2969 (99.50) | 2636 (80.54) | |
| Yes | 15 (0.50) | 637 (19.46) | |
| Pre-ART nadir CD4 cell/μl count (PLWH only) | < 0.01 | ||
| < 200 | 1497 (50.37) | 1004 (43.22) | |
| ≥ 200 | 1475 (49.63) | 1319 (56.78) | |
| Post-ART nadir CD4 cell/μl count (PLWH only) | < 0.01 | ||
| < 200 | 389 (28.21) | 1098 (40.13) | |
| ≥ 200 | 990 (71.79) | 1638 (59.87) | |
| Peak HIV viral load (PLWH only) | < 0.01 | ||
| ≤ 10,000 copies/ml | 563 (20.01) | 1041 (31.81) | |
| > 10,000 copies/ml | 2251 (79.99) | 2232 (68.19) | |
| Median (IQR) | |||
| Cumulative pack-years smoked (never smokers excluded) | 14.70 (4.58-28.84) | 7.75 (3.00-15.50) | < 0.01 |
| Years since smoking cessation | 5.00 (1.00-8.25) | 1.27 (0.30-5.78) | < 0.01 |
| Age (years) | 33.37 (28.28-39.42) | 36.77 (30.63-43.52) | < 0.01 |
| BMI (kg/m2) | 23.30 (21.70-25.50) | 27.20 (23.40-33.10) | < 0.01 |
| Lifetime number of sexual partners | 102.00 (34.00-400.00) | 11.00 (5.00-35.00) | < 0.01 |
| Total number of participants | 6789 | 4423 | |
Bold indicates statistically significant.
HPV= human papilloma virus; MACS = Multicenter AIDS Cohort Study; PLWH = people living with HIV; WIHS = Women’s Interagency HIV Study.
: MACS participants not tested for HPV at baseline visit.
Due to missing values, participant frequencies within variable categories may not sum to total participant counts.
Smoking-related cancer incidence
We observed 214 smoking-related incident cancers among the MACS participants and 192 among the WIHS (see Table, Supplemental Digital Content 1). The majority of smoking-related cancers were lung/bronchus and were diagnosed in the modern ART era. Seven men and eight women had more than one primary smoking-related malignancy.
The age-adjusted IR for smoking-related cancers was higher among PLWH than participants without HIV (p<0.01) and higher among women than men (p<0.01; Table 2). Among those who smoked, incidence ratios among PLWH were higher than among those without HIV when stratifying by cumulative pack-years of smoking (all p-values<0.01). When the smoking-related cancer IRs among all women were compared to all men, the IRR was 2.0 (95% CI: 1.6-2.4). For women with HIV vs. men with HIV the IRR was 1.6 (95% CI: 1.2-2.0) and for women without HIV vs. men without HIV the IRR was 2.0 (95% CI: 1.3-3.0). Ten or more years since cessation of smoking resulted in a reduced IR of smoking-related cancers (p<0.01).
Table 2.
Smoking-related cancer incidence among participants in the MACS and WIHS.
| Baseline | Smoking-related cancer incidence | ||||||
|---|---|---|---|---|---|---|---|
| N | % | N | P-yrs | IR per 100,000* [95% CI] | P-value | ||
| HIV infection | |||||||
| Seronegative [ref] | 4955 | 44 | 118 | 63642 | 162.2 [133.6, 196.9] | ||
| Seropositive | 6257 | 56 | 271 | 75883 | 347.6 [303.1, 398.5] | < 0.01 | |
| Cohort | |||||||
| MACS [ref] | 6789 | 61 | 206 | 92047 | 197.5 [169.4, 230.3] | ||
| WIHS | 4423 | 39 | 183 | 47477 | 391.6 [333.7, 459.6] | < 0.01 | |
| Cumulative pack-years† | |||||||
| Never smokers [ref] | 6271 | 56 | 86 | 64947 | 141.7 [114.1, 175.9] | ||
| 1 - 10 | 2391 | 21 | 81 | 32684 | 266.0 [212.4, 333.1] | < 0.01 | |
| 11 - 20 | 1205 | 11 | 65 | 16740 | 373.2 [290.1, 480.0] | < 0.01 | |
| 21 - 30 | 647 | 6 | 47 | 10634 | 379.5 [282.0, 510.7] | < 0.01 | |
| > 30 | 698 | 6 | 110 | 14521 | 480.3 [388.3, 594.1] | < 0.01 | |
| Years since smoking cessation‡ | |||||||
| < 10 [ref] | 1632 | 89 | 59 | 23216 | 260.3 [192.8, 351.6] | ||
| 10 - 19 | 144 | 8 | 15 | 10909 | 100.1 [57.9, 172.9] | < 0.01 | |
| ≥ 20 | 62 | 3 | 24 | 7994 | 132.0 [81.0, 215.1] | < 0.01 | |
| Cohort by HIV serostatus | |||||||
| MACS seronegative [ref] | 3805 | 34 | 85 | 50542 | 138.3 [110.3, 173.4] | ||
| MACS seropositive | 2984 | 27 | 121 | 41505 | 279.0 [230.8, 337.4] | < 0.01 | |
| WIHS seronegative [ref] | 1150 | 10 | 33 | 13100 | 276.3 [195.5, 390.5] | ||
| WIHS seropositive | 3273 | 29 | 150 | 34377 | 434.1 [364.4, 517.2] | 0.02 | |
| Cumulative pack-years by HIV serostatus† | |||||||
| Never smokers seronegative [ref] | 2871 | 26 | 37 | 31805 | 116.6 [84.1, 161.6] | ||
| Never smokers seropositive | 3400 | 30 | 49 | 33142 | 170.6 [128.5, 226.5] | 0.08 | |
| 1 - 10 seronegative [ref] | 881 | 8 | 14 | 12591 | 122.4 [72.3, 207.1] | ||
| 1 - 10 seropositive | 1510 | 13 | 67 | 20091 | 358.8 [280.3, 459.2] | < 0.01 | |
| 11 - 20 seronegative [ref] | 505 | 5 | 13 | 6683 | 190.1 [110.1, 328.5] | ||
| 11 - 20 seropositive | 700 | 6 | 52 | 10057 | 497.6 [375.9, 658.7] | < 0.01 | |
| 21 - 30 seronegative [ref] | 310 | 3 | 11 | 4645 | 201.2 [110.9, 365.2] | ||
| 21 - 30 seropositive | 337 | 3 | 36 | 5989 | 527.8 [376.9, 739.2] | < 0.01 | |
| > 30 seronegative [ref] | 388 | 3 | 43 | 7918 | 329.8 [240.1, 453.0] | ||
| > 30 seropositive | 310 | 3 | 67 | 6602 | 668.1 [515.6, 865.7] | < 0.01 | |
| Years since smoking cessation by HIV serostatus‡ | |||||||
| < 10 seronegative [ref] | 789 | 43 | 15 | 10131 | 152.8 [90.2, 259.1] | ||
| < 10 seropositive | 843 | 46 | 44 | 13085 | 348.0 [248.3, 487.8] | < 0.01 | |
| 10 - 19 seronegative [ref] | 49 | 3 | 7 | 5354 | 93.2 [43.2, 201.5] | ||
| 10 - 19 seropositive | 95 | 5 | 8 | 5555 | 106.7 [51.8, 220.0] | 0.79 | |
| ≥ 20 seronegative [ref] | 24 | 1 | 5 | 4171 | 48.3 [19.1, 121.8] | ||
| ≥ 20 seropositive | 38 | 2 | 19 | 3823 | 228.6 [135.2, 386.4] | < 0.01 | |
| Total | 11212 | 100 | 389 | 139524 | 278.8 [252.4, 307.9] | ||
Bold indicates statistically significant.
MACS = Multicenter AIDS Cohort Study; WIHS = Women’s Interagency HIV Study; P-years = person-years; IR = incidence rate; CI = confidence interval.
: All estimates adjusted for age.
: Lagged by 10 years.
: Study population limited to former smoking participants.
In unadjusted analysis and for participants without HIV, IRRs for smoking-related cancers were higher for older ages, non-Latinx blacks compared to non-Latinx whites, increasing cumulative pack-years of smoking compared to never smokers, those with a history of injecting drugs, and those with HCV at baseline (Table 3). People without HIV with more years of education also had lower unadjusted IRRs for smoking-related cancers. Similar results were seen for PLWH, with higher unadjusted IRRs for smoking-related cancers associated with older age, non-Latinx blacks, higher cumulative pack-years of smoking, history of injecting drugs, and HCV infection at baseline. Lower IRRs were associated with more years of education, as well as greater household income. In PLWH, higher IRRs were associated with having a prior AIDS diagnosis and exposure to ART, and lower IRRs associated with having CD4 cell counts ≥200.
Table 3.
Unadjusted smoking-related cancer incidence rates among participants in the MACS and WIHS, stratified by HIV serostatus.
| HIV Seronegative | HIV Seropositive | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. cancers | P-yrs | IR per 100,000 | IRR [95% CI] | P-value | No. cancers | P-yrs | IR per 100,000 | IRR [95% CI] | P-value | ||
| Geographic region of recruitment site | |||||||||||
| West coast [ref] | 32 | 18801 | 170.2 | 91 | 24545 | 370.7 | |||||
| East coast | 76 | 34552 | 220.0 | 1.3 [0.8, 2.0] | 0.26 | 140 | 36518 | 383.4 | 1.0 [0.8, 1.4] | 0.86 | |
| Midwest | 10 | 10288 | 97.2 | 0.6 [0.3, 1.2] | 0.15 | 40 | 14819 | 269.9 | 0.7 [0.5, 1.1] | 0.11 | |
| Calendar period | |||||||||||
| Before 1996 [ref] | 22 | 24454 | 90.0 | 21 | 16983 | 123.7 | |||||
| 1996 - 1999 | 4 | 4046 | 98.9 | 1.1 [0.3, 3.2] | 1.00 | 27 | 9632 | 280.3 | 2.3 [1.2, 4.2] | < 0.01 | |
| 2000 - 2003 | 8 | 7921 | 101.0 | 1.1 [0.4, 2.6] | 0.92 | 29 | 9288 | 312.2 | 2.5 [1.4, 4.7] | < 0.01 | |
| 2004 - 2007 | 15 | 8180 | 183.4 | 2.0 [1.0, 4.1] | 0.06 | 47 | 11664 | 402.9 | 3.3 [1.9, 5.7] | < 0.01 | |
| 2008 - 2011 | 30 | 7279 | 412.1 | 4.6 [2.6, 8.3] | < 0.01 | 60 | 10275 | 583.9 | 4.7 [2.8, 8.2] | < 0.01 | |
| 2012 - 2015 | 23 | 6762 | 340.1 | 3.8 [2.0, 7.1] | < 0.01 | 47 | 10017 | 469.2 | 3.8 [2.2, 6.7] | < 0.01 | |
| 2016 - 2018 | 16 | 5001 | 319.9 | 3.6 [1.7, 7.1] | < 0.01 | 40 | 8024 | 498.5 | 4.0 [2.3, 7.2] | < 0.01 | |
| Age (years) | |||||||||||
| < 40 [ref] | 8 | 20781 | 38.5 | 22 | 24679 | 89.1 | |||||
| 40 - 49 | 18 | 19444 | 92.6 | 2.4 [1.0, 6.4] | 0.05 | 74 | 26996 | 274.1 | 3.1 [1.9, 5.2] | < 0.01 | |
| 50 - 59 | 44 | 14239 | 309.0 | 8.0 [3.7, 19.7] | < 0.01 | 117 | 17876 | 654.5 | 7.3 [4.6, 12.2] | < 0.01 | |
| ≥ 60 | 48 | 9178 | 523.0 | 13.6 [6.4, 33.3] | < 0.01 | 58 | 6332 | 916.0 | 10.3 [6.2, 17.6] | < 0.01 | |
| Race/ethnicity | |||||||||||
| Non-Latinx white [ref] | 67 | 43512 | 154.0 | 107 | 34094 | 313.8 | |||||
| Non-Latinx black | 39 | 12910 | 302.1 | 2.0 [1.3, 3.0] | < 0.01 | 122 | 27476 | 444.0 | 1.4 [1.1, 1.9] | 0.01 | |
| Other | 12 | 7207 | 166.5 | 1.1 [0.5, 2.0] | 0.90 | 42 | 14312 | 293.5 | 0.9 [0.6, 1.3] | 0.79 | |
| Baseline educational attainment | |||||||||||
| High school or less [ref] | 46 | 14332 | 321.0 | 146 | 30342 | 481.2 | |||||
| Some college | 26 | 16147 | 161.0 | 0.5 [0.3, 0.8] | < 0.01 | 74 | 22555 | 328.1 | 0.7 [0.5, 0.9] | < 0.01 | |
| College graduate | 15 | 13132 | 114.2 | 0.4 [0.2, 0.6] | < 0.01 | 22 | 10979 | 200.4 | 0.4 [0.3, 0.7] | < 0.01 | |
| At least some postgraduate education | 31 | 19770 | 156.8 | 0.5 [0.3, 0.8] | < 0.01 | 29 | 11879 | 244.1 | 0.5 [0.3, 0.8] | < 0.01 | |
| Annual household income | |||||||||||
| < $29,999 [ref] | 35 | 19640 | 178.2 | 147 | 39335 | 373.7 | |||||
| ≥ $30,000 | 33 | 25318 | 130.3 | 0.7 [0.4, 1.2] | 0.24 | 42 | 20961 | 200.4 | 0.5 [0.4, 0.8] | < 0.01 | |
| BMI (kg/m2) | |||||||||||
| Under or normal weight (< 25) [ref] | 24 | 20864 | 115.0 | 76 | 26627 | 285.4 | |||||
| Overweight (25 - 29) | 18 | 19427 | 92.7 | 0.8 [0.4, 1.5] | 0.59 | 51 | 20883 | 244.2 | 0.9 [0.6, 1.2] | 0.44 | |
| Obese (≥ 30) | 17 | 13877 | 122.5 | 1.1 [0.5, 2.1] | 0.96 | 29 | 16224 | 178.7 | 0.6 [0.4, 1.0] | 0.04 | |
| Smoking status | |||||||||||
| Never [ref] | 16 | 20340 | 78.7 | 20 | 22155 | 90.3 | |||||
| Former | 27 | 21242 | 127.1 | 1.6 [0.8, 3.2] | 0.17 | 57 | 22372 | 254.8 | 2.8 [1.7, 5.0] | < 0.01 | |
| Current | 24 | 18163 | 132.1 | 1.7 [0.9, 3.4] | 0.14 | 92 | 26447 | 347.9 | 3.9 [2.4, 6.6] | < 0.01 | |
| Cumulative pack-years* | |||||||||||
| Never smokers [ref] | 37 | 31805 | 116.3 | 49 | 33141 | 147.9 | |||||
| 1 - 10 | 14 | 12592 | 111.2 | 1.0 [0.5, 1.8] | 1.00 | 67 | 20092 | 333.5 | 2.3 [1.5, 3.3] | < 0.01 | |
| 11 - 20 | 13 | 6682 | 194.6 | 1.7 [0.8, 3.2] | 0.16 | 52 | 10057 | 517.1 | 3.5 [2.3, 5.3] | < 0.01 | |
| 21 - 30 | 11 | 4644 | 236.9 | 2.0 [0.9, 4.1] | 0.07 | 36 | 5989 | 601.1 | 4.1 [2.6, 6.4] | < 0.01 | |
| > 30 | 43 | 7918 | 543.1 | 4.7 [2.9, 7.5] | < 0.01 | 67 | 6603 | 1014.7 | 6.9 [4.7, 10.1] | < 0.01 | |
| Years since smoking cessation† | |||||||||||
| < 10 [ref] | 15 | 10131 | 148.1 | 44 | 13085 | 336.3 | |||||
| 10 - 19 | 7 | 5354 | 130.7 | 0.9 [0.3, 2.3] | 0.98 | 8 | 5555 | 144.0 | 0.4 [0.2, 0.9] | 0.03 | |
| ≥ 20 | 5 | 4171 | 119.9 | 0.8 [0.2, 2.3] | 0.90 | 19 | 3823 | 497.0 | 1.5 [0.8, 2.6] | 0.20 | |
| Alcohol use | |||||||||||
| Abstainer [ref] | 14 | 12615 | 111.0 | 89 | 25881 | 343.9 | |||||
| > 0 to 7 drinks/week | 31 | 32542 | 95.3 | 0.9 [0.4, 1.7] | 0.74 | 54 | 33666 | 160.4 | 0.5 [0.3, 0.7] | < 0.01 | |
| > 7 to 12 drinks/week | 10 | 6883 | 145.3 | 1.3 [0.5, 3.2] | 0.65 | 9 | 4644 | 193.8 | 0.6 [0.2, 1.1] | 0.12 | |
| > 12 drinks/week | 10 | 7467 | 133.9 | 1.2 [0.5, 2.9] | 0.80 | 16 | 6018 | 265.9 | 0.8 [0.4, 1.3] | 0.41 | |
| Smoked an illicit drug | |||||||||||
| No [ref] | 108 | 44170 | 244.5 | 210 | 51565 | 407.3 | |||||
| Yes | 10 | 19471 | 51.4 | 0.2 [0.1, 0.4] | < 0.01 | 61 | 24317 | 250.9 | 0.6 [0.5, 0.8] | < 0.01 | |
| Injected an illicit drug | |||||||||||
| No [ref] | 118 | 63013 | 187.3 | 265 | 74212 | 357.1 | |||||
| Yes | 0 | 629 | 110.5 | 0.6 [−∞, 2.6] | 0.62 | 6 | 1670 | 359.3 | 1.0 [0.4, 2.2] | 1.00 | |
| Took illicit drugs other than through smoking/injection | |||||||||||
| No [ref] | 112 | 50417 | 222.1 | 257 | 62667 | 410.1 | |||||
| Yes | 6 | 13225 | 45.4 | 0.2 [0.1, 0.5] | < 0.01 | 14 | 13215 | 105.9 | 0.3 [0.1, 0.4] | < 0.01 | |
| History of injection drug use | |||||||||||
| No [ref] | 100 | 59434 | 168.3 | 204 | 65911 | 309.5 | |||||
| Yes | 18 | 4207 | 427.9 | 2.5 [1.4, 4.2] | < 0.01 | 67 | 9964 | 672.4 | 2.2 [1.6, 2.9] | < 0.01 | |
| Any HPV | |||||||||||
| No [ref] | 0 | 457 | 151.7 | 0 | 836 | 82.9 | |||||
| Yes | 3 | 1240 | 215.1 | 1.4 [0.2, ∞] | 0.78 | 4 | 2388 | 153.4 | 1.9 [0.3, ∞] | 0.60 | |
| Oncogenic HPV | |||||||||||
| No [ref] | 1 | 1117 | 89.5 | 1 | 1864 | 53.6 | |||||
| Yes | 2 | 581 | 344.2 | 3.8 [0.2, 226.8] | 0.54 | 3 | 1360 | 220.6 | 4.1 [0.3, 215.9] | 0.41 | |
| Lifetime number of sexual partners | |||||||||||
| < 30 [ref] | 33 | 10845 | 304.3 | 86 | 21916 | 392.4 | |||||
| 30 - 59 | 12 | 10328 | 116.2 | 0.4 [0.2, 0.8] | < 0.01 | 45 | 11518 | 390.7 | 1.0 [0.7, 1.4] | 1.00 | |
| 60 - 89 | 9 | 6458 | 139.4 | 0.5 [0.2, 1.0] | 0.04 | 26 | 5452 | 476.9 | 1.2 [0.8, 1.9] | 0.44 | |
| ≥ 90 | 64 | 36010 | 177.7 | 0.6 [0.4, 0.9] | 0.02 | 114 | 36996 | 308.1 | 0.8 [0.6, 1.1] | 0.11 | |
| Hepatitis C virus at baseline | |||||||||||
| No [ref] | 99 | 60196 | 164.5 | 188 | 64552 | 291.2 | |||||
| Yes | 19 | 3060 | 620.9 | 3.8 [2.2, 6.2] | < 0.01 | 75 | 10185 | 736.4 | 2.5 [1.9, 3.3] | < 0.01 | |
| CD4 cell/μl count (HIV seropositive only) | |||||||||||
| < 200 [ref] | - | 37 | 9455 | 391.3 | |||||||
| 200 - 500 | - | 72 | 25197 | 285.7 | 0.7 [0.5, 1.1] | 0.15 | |||||
| > 500 | - | 59 | 31825 | 185.4 | 0.5 [0.3, 0.7] | < 0.01 | |||||
| HIV viral load detectable (HIV seropositive only) | |||||||||||
| No [ref] | - | 104 | 33789 | 307.8 | |||||||
| Yes | - | 55 | 24050 | 228.7 | 0.7 [0.5, 1.0] | 0.09 | |||||
| HIV viral load ≥ 4,000 copies/ml (HIV seropositive only) | |||||||||||
| No [ref] | - | 119 | 39778 | 299.2 | |||||||
| Yes | - | 40 | 18074 | 221.3 | 0.7 [0.5, 1.1] | 0.11 | |||||
| Exposure to ART (HIV seropositive only) | |||||||||||
| No [ref] | - | 85 | 29323 | 289.9 | |||||||
| Yes | - | 186 | 46559 | 399.5 | 1.4 [1.1, 1.8] | 0.02 | |||||
| History of clinical AIDS (HIV seropositive only) | |||||||||||
| No [ref] | - | 132 | 55726 | 236.9 | |||||||
| Yes | - | 139 | 20156 | 689.6 | 2.9 [2.3, 3.7] | < 0.01 | |||||
| Pre-ART nadir CD4 cell/μl count (HIV seropositive only) | |||||||||||
| < 200 [ref] | - | 120 | 28689 | 418.3 | |||||||
| ≥ 200 | - | 139 | 40954 | 339.4 | 0.8 [0.6, 1.0] | 0.11 | |||||
| Post-ART nadir CD4 cell/μl count (HIV seropositive only) | |||||||||||
| < 200 [ref] | - | 124 | 23331 | 531.5 | |||||||
| ≥ 200 | - | 82 | 37673 | 217.7 | 0.4 [0.3, 0.5] | < 0.01 | |||||
| Peak HIV viral load (copies/ml, HIV seropositive only) | |||||||||||
| ≤ 10,000 [ref] | - | 55 | 14776 | 372.2 | |||||||
| > 10,000 | - | 207 | 59889 | 345.6 | 0.9 [0.7, 1.3] | 0.67 | |||||
| Total | 118 | 63642 | 185.4 | 271 | 75882 | 357.1 | |||||
Bold indicates statistically significant.
MACS = Multicenter AIDS Cohort Study; WIHS = Women’s Interagency HIV Study; P-years = person-years; IR = incidence rate; IRR = incidence rate ratio; CI = confidence interval
: Lagged by 10 years.
: Study population limited to former smoking participants.
In adjusted analyses combining participants with and without HIV, the variables that remained significantly associated with an increased IRR for smoking-related cancer were ages 40 and above, 11 or more cumulative pack-years of smoking, and having HIV with or without a prior AIDS diagnosis (Table 4, column 2). Factors associated with a decreased IRR for smoking-related cancers were annual household income ≥$30,000 and for PLWH, having CD4 cell counts >200. Significant cohort interactions (Table 4, column 3) were observed for age 40-49 years (effect stronger in the WIHS), other race (effect stronger in the MACS), smoking 1-20 cumulative pack-years (effect stronger in the WIHS), and among PLWH for CD4 cell counts of 200-500 cells (effect stronger in the MACS). In analyses that were restricted to only those alive in the modern ART era (year ≥2001), exposures significantly associated with an increased or decreased risk of smoking-related cancers (Table 4, columns 4 and 5) were highly similar to those for all years (Table 4, columns 2 and 3). Additionally, PLWH in the modern ART era who had a detectable HIV viral load had a lower risk for these cancers (Table 4, column 4).
Table 4.
Adjusted smoking-related cancer incidence rate ratios among participants in the MACS and WIHS.
| All years (1984-2018) | Modern ART years (2001-2018) | |||
|---|---|---|---|---|
| IRR [95% CI] | P-value for cohort interaction* | IRR [95% CI] | P-value for cohort interaction* | |
| Age (years) | ||||
| < 40 [ref] | ||||
| 40 - 49 | 2.0 [1.1, 3.6] | 0.05 | 1.9 [0.8, 4.2] | 0.24 |
| 50 - 59 | 4.9 [2.8, 8.7] | 0.11 | 3.9 [1.8, 8.4] | 0.37 |
| ≥ 60 | 6.6 [3.6, 12.1] | 0.18 | 5.7 [2.6, 12.7] | 0.56 |
| Race/ethnicity | ||||
| Non-Latinx white [ref] | ||||
| Non-Latinx black | 1.2 [0.8, 1.8] | 0.74 | 1.2 [0.8, 1.9] | 0.89 |
| Other | 0.6 [0.3, 1.2] | 0.04 | 0.6 [0.3, 1.3] | 0.04 |
| Annual household income | ||||
| < $29,999 [ref] | ||||
| ≥ $30,000 | 0.6 [0.4, 0.8] | 0.58 | 0.6 [0.4, 0.9] | 0.44 |
| Cumulative pack-years† | ||||
| Never smokers [ref] | ||||
| 1 - 10 | 1.3 [0.8, 2.1] | 0.02 | 1.3 [0.8, 2.2] | 0.02 |
| 11 - 20 | 2.0 [1.2, 3.2] | 0.04 | 1.7 [0.9, 2.9] | 0.03 |
| 21 - 30 | 2.7 [1.7, 4.4] | 0.20 | 2.4 [1.4, 4.1] | 0.28 |
| > 30 | 3.4 [2.1, 5.3] | 0.06 | 3.3 [2.0, 5.3] | 0.08 |
| Alcohol use | ||||
| Abstainer [ref] | ||||
| > 0 to 7 drinks/week | 0.7 [0.5, 1.1] | 0.17 | 0.8 [0.6, 1.2] | 0.22 |
| > 7 to 12 drinks/week | 0.9 [0.4, 1.7] | 0.53 | 0.6 [0.3, 1.5] | 0.63 |
| > 12 drinks/week | 1.2 [0.8, 2.0] | 0.68 | 1.4 [0.8, 2.3] | 0.73 |
| History of injection drug use | ||||
| No [ref] | ||||
| Yes | 1.2 [0.8, 1.7] | 0.38 | 1.2 [0.8, 1.8] | 0.75 |
| Hepatitis C virus at baseline | ||||
| No [ref] | ||||
| Yes | 1.1 [0.7, 1.7] | 0.21 | 1.2 [0.8, 1.9] | 0.26 |
| HIV status | ||||
| Seronegative [ref] | ||||
| Seropositive and no history of clinical AIDS | 1.5 [1.1, 2.2] | 0.67 | 1.5 [1.0, 2.2] | 0.75 |
| Seropositive and history of clinical AIDS | 3.0 [2.0, 4.4] | 0.92 | 2.7 [1.7, 4.2] | 0.62 |
| CD4 cell count/μl (PLWH only) | ||||
| < 200 [ref] | ||||
| 200 - 500 | 0.5 [0.3, 0.9] | 0.03 | 0.5 [0.3, 0.9] | 0.16 |
| > 500 | 0.3 [0.2, 0.6] | 0.20 | 0.3 [0.2, 0.6] | 0.65 |
| HIV viral load detectable (PLWH only) | ||||
| No [ref] | ||||
| Yes | 0.8 [0.5, 1.2] | 0.43 | 0.4 [0.2, 0.9] | 0.05 |
| Exposure to ART (PLWH only) | ||||
| No [ref] | ||||
| Yes | 1.1 [0.7, 1.9] | 0.67 | 0.8 [0.4, 1.6] | 0.61 |
Bold indicates statistically significant.
MACS = Multicenter AIDS Cohort Study; PLWH = people living with HIV; WIHS = Women’s Interagency HIV Study; IRR = incidence rate ratio; CI = confidence interval.
: Tests whether the effect of the given covariate differs between MACS and WIHS.
: Lagged by 10 years.
Excluding virus-associated cancers from the definition of smoking-related cancers did not affect the association between smoking and smoking-related cancer risk, but did affect the observed differences between PLWH and people without HIV (data not shown). This suggests that PLWH are more likely to develop virus-associated cancers than people without HIV, and that those cancers may have contributed to the higher incidence of smoking-related cancers in PLWH. Excluding just anal cancer from the definition of smoking-related cancers strengthened the association between smoking and smoking-related cancer incidence among both the men and the women equally (data not shown).
Based on adjusted PAF models with cumulative pack-years groups of 0-5 pack-years (reference), 6-20 pack-years, and >20 pack-years, an estimated 34% (95% CI: 21–46%) of all smoking-related cancers were attributable to smoking cigarettes among all participants, 31% (95% CI: 15–45%) among PLWH, and 41% (95% CI: 13–60%) among participants without HIV (Figure 1). The adjusted PAFs were non-significantly higher in the women than in men: 39% vs. 28% for all participants (p=0.35), 34% vs. 25% among PLWH (p=0.58), and 67% vs. 27% among those without HIV (p=0.11). When virus-associated cancers were removed from these analyses, the point estimates were higher in all groups but still well within the 95% CI of the full sample results (data not shown).
Figure 1. Adjusted population attributable fractions of smoking on smoking-related cancer incidence in the MACS and WIHS.
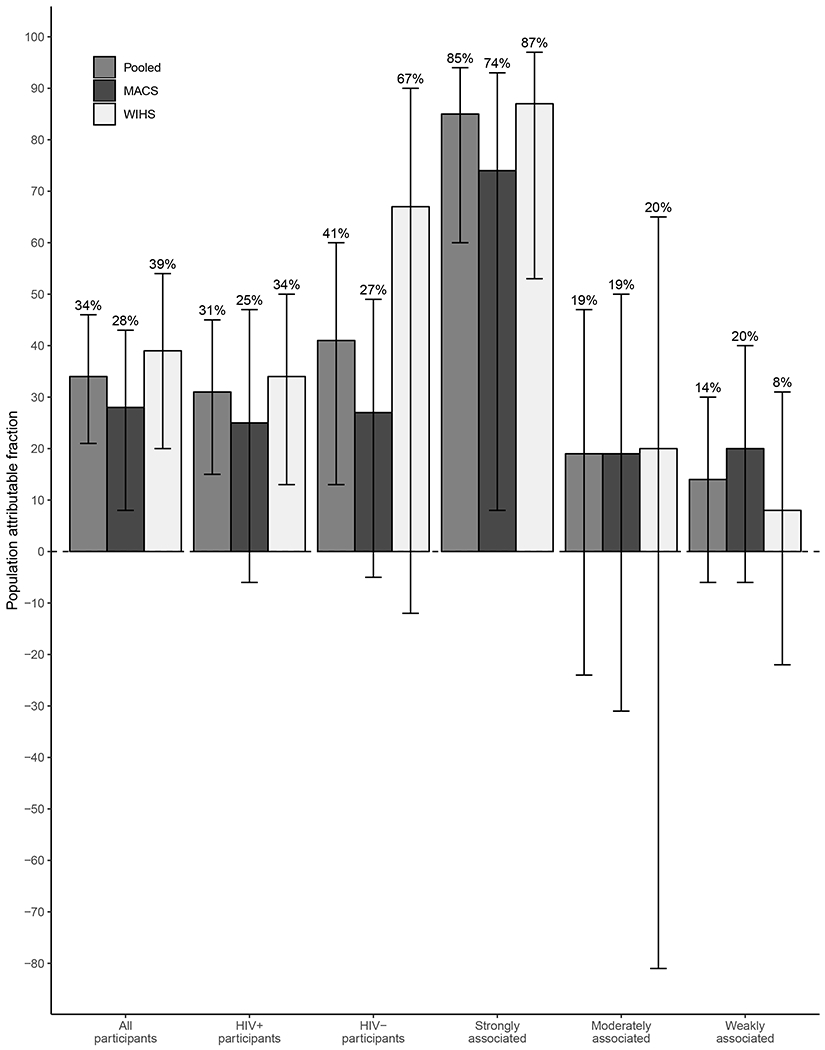
PAFs were calculated among all participants for all smoking-related cancers (“All participants”), among HIV seropositive participants for all smoking-related cancers (“HIV+ participants”), among HIV seronegative participants for all smoking-related cancers (“HIV- participants”), and among all participants for smoking-related cancers strongly, moderately, or weakly associated with smoking (“Strongly associated”, “Moderately associated”, and “Weakly associated”, respectively). Bars indicate 95% CIs.
MACS = Multicenter AIDS Cohort Study; WIHS = Women’s Interagency HIV Study CI = confidence interval; PAF = population attributable fraction
When cancer types were stratified by the strength of their association with smoking, the adjusted PAF estimates were 85% (95% CI: 60–94%), 19% (95% CI: −24–47%), and 14% (95% CI: −6–30%) for cancers strongly, moderately, and weakly associated with smoking, respectively (Figure 1). In these stratified analyses, the adjusted PAFs were not significantly different for women compared to men. The removal of virus-associated malignancies increased the point estimate for cancers strongly associated with smoking while the point estimates for cancers moderately and weakly associated with smoking fluctuated but all were still within the 95% CI of the total sample (data not shown).
DISCUSSION
With over 139,500 person-years of follow-up, this is one of the largest cohort studies to examine the contribution of smoking on the cancer burden among PLWH relative to highly similar people without HIV. Among those with a history of smoking, the observed incidence of smoking-related cancers was significantly higher among PLWH than participants without HIV, as hypothesized in our aim 1. This reaffirms that HIV infection is associated with a higher risk of smoking-related cancers beyond what would be expected from cigarette smoking alone, a finding reported in previous studies.[6, 23–25] However, these prior studies did not have a highly similar comparison group of participants without HIV or detailed smoking and other behavioral histories, as in the present study.
In both minimally and fully adjusted analyses, we observed that women have a higher risk of smoking-related cancers than men. The reasons for this are unclear and the exact mechanism may be confounded by unmeasured factors that might differentially and adversely affect women - such as the environment (air pollution and second-hand smoke), genetics, physiology, and hormones. The majority of the women in this study were African American and had fewer pack-years of smoking than the men. Studies indicate that African American smokers have a higher risk for lung cancer compared to whites, [26] and this racial difference is most pronounced at lower levels of daily cigarette consumption.[27] In addition, women appear to have a higher incidence of lung cancer than men. [28–30] This may be due to an increased susceptibility to tobacco carcinogens among women, smaller lung size in women, or the effect of hormones in carcinogenesis, such as the role of estrogens as promoters of lung cancer.[29, 31, 32]
In our PAF models that pooled men and women, an estimated 31% (95% CI: 15–45%) of all smoking-related cancers were attributed to smoking more than 5 pack-years in a lifetime among PLWH. This is within the range reported by other studies of PLWH[10, 33, 34], although we found higher estimates for women than for men. Our investigation has the added advantage of a more precise adjustment for smoking, as well as adjustment for other important confounders such as CD4 cell count, HIV viral load detectability, and exposure to ART. Varying degrees of association between smoking and smoking-related cancers likely influenced our PAF estimates. When smoking-related cancers were stratified by strength of association, the estimated PAF for cancers strongly associated with smoking (lung, bronchus, and larynx) was 74% among men and 87% among women. When we removed laryngeal cancers from this group (a virus-associated cancer[35]), the PAF was 86% for men and 93% for women suggesting that inclusion of virus-related cancers may dilute the effect of smoking on these cancers. Our estimates for lung and bronchial cancers are higher than estimates reported in other studies of the general population (men 75–84% and women 70–79%).[16] Our estimates were similar to another study of PLWH (82–98% for lung cancer) [10] which supports the interpretation that HIV is a risk factor for cancers that are strongly associated with smoking.
There is a substantial burden of smoking-related cancers among PLWH and unless the high prevalence of cigarette smoking is reduced, this will likely increase over time due to increasing longevity among PLWH on ART. One study reported that among PLWH who adhere to ART, smoking is projected to be a much greater health threat than HIV.[36] Another study assessed the impact of smoking cessation on cancer risk among PLWH and observed that the lung cancer IR was reduced by more than half but was still elevated five years after cessation (from an adjusted IRR of 19.08 to 8.69), while the incidence of other smoking-related cancers was found to decline to that of non-smokers after 1–2 years of cessation (from an adjusted IRR of 2.06 to 1.32).[37]
In addition to smoking-related cancers, preventing the initiation of smoking and smoking cessation lessens the burden for all smoking-related diseases. For PLWH this is especially important given the elevated risk for several other diseases that can be exacerbated by smoking, including CVD, pulmonary infections, COPD, and pneumonia.[33, 38–40]
This investigation was not without limitations. First, certain smoking-related cancers, such as gum and mouth, had very few events – perhaps due to competing causes of death among PLWH. Our risk estimates for smoking-related cancers may therefore be biased towards the null. Despite this limitation, we were able to demonstrate significantly elevated risk of these cancers among PLWH compared to highly similar people without HIV. Second, these two cohorts of men and women differed in other ways besides sex and we were unable to adjust for unmeasured risk factors such as the living environment (second hand smoke, air pollution, etc.), which may have contributed to their cancer burden. Nevertheless, we were able to adjust our analyses for many other potential risk factors – far more than most other studies that have investigated the cancer burden in PLWH. Last, as per WIHS protocol the women received screening for cervical, vaginal, and vulvar cancers. Screening may have led to treatment of pre-cancers and reduced the cancer burden for these malignancies. Earlier cancer detection from screening may have also resulted in those cancers occurring at lower cumulative pack-years of smoking and therefore attenuated the effect estimates between smoking and smoking-related cancer incidence.
The strengths of this longitudinal study include a large and diverse sample with many person-years of follow-up, a highly similar comparison group of people without HIV infection, detailed baseline and follow-up social and clinical histories, and a study period that spans the pre-ART, early ART, and current ART era. Notably, the adjusted IRR point estimates for smoking-related cancer risk factors among participants in the modern ART era were virtually identical to the estimates among participants across all years, indicating that these have not dramatically changed since the introduction of potent therapy. Because of these strengths, we were able to do what few, if any, have been able to do before us: compare men to women and those with and without HIV, as well as to fully adjust our models for a multitude of potential cancer-causing risk factors.
Our results, along with those from prior well-designed observational studies, provide strong evidence of the excess risk of smoking-related cancers among PLWH who smoke compared to smokers without HIV. What is novel in our study are the sex differences, with women LWH having a greater risk of smoking-related cancers than men LWH. These data lend strong support for integrating smoking cessation interventions into ongoing HIV programs and educating PLWH, especially women, about the harm of smoking and the benefits of quitting to reduce their risk of smoking-related cancers. There is indisputable evidence of the harm caused by smoking in PLWH and an equally clear opportunity for health care providers to intervene as part of comprehensive care of PLWH.
Supplementary Material
ACKNOWLEDGEMENTS
Data in this manuscript were collected by the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Connie Wofsy Women’s HIV Study, Northern California CRS (Bradley Aouizerat and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (Johns Hopkins Institute for Clinical and Translational Research (ICTR), P30-AI-050409 (Atlanta CFAR), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
We would like to acknowledge the National Program of Cancer Registries of the Centers for Disease Control and Prevention (CDC) for the funds that helped support the collection and availability of the cancer registry data and thank the following state cancer registries for their help: AL, CA, FL, GA, IL, MD, MS, NY, NC, OH, PA, and VA. The authors assume full responsibility for analyses and interpretations of these data.
Sources of funding: National Institutes of Health
APPENDIX
Pack-years of smoking were calculated by multiplying the number of packs of cigarettes smoked per day by the number of years the person has smoked. Cumulative pack-years of smoking was constructed by summing collected smoking pack-year data. In cases of missing pack-year data, values of cumulative pack-years were interpolated using per-person observed smoking trends. Cumulative pack-years of smoking was then lagged by 10 years for use in analyses. Data values for lagged cumulative pack-years that were within the 10 year lag window were interpolated using per-person historical smoking data collected by the studies, such as age started smoking, age quit smoking, and average number of cigarette packs smoked per day. Smoking status at a study visit was coded as never, former, and current. Years since smoking cessation was calculated only for former smokers, and the year count reset if an individual quit smoking for a period but then restarted.
Age was categorized as <40 years, 40–49 years, 50–59 years, and ≥60 years. Alcohol consumption since last study visit was categorized as abstainer, zero to seven drinks/week, seven to 12 drinks/week, and greater than 12 drinks/week. Illicit substance use since last study visit was subdivided into smoking, injecting, or taking an illicit drug not through smoking/injection. BMI was measured per visit. Coinfection with HPV at a study visit was divided into any HPV and oncogenic HPV. WIHS participants were tested far more frequently for HPV than MACS participants.
Race/ethnicity was recorded at study enrollment, and categorized as non-Latinx white, non-Latinx black, and other. Educational attainment was also recorded at enrollment, and coded into high school or less, some college, college graduate, and at least some postgraduate education. Household income was measured at each study visit, and categorized as less than $29,999 and $30,000 and above. Missing values of household income were interpolated with data collected within six months of the missing value.
Geographic region was categorized as west coast, east coast, and Midwest and based on the location of study recruitment. Calendar period was categorized as before 1996 and with three year intervals from then on. History of injection drug use was coded as positive if an individual reported to ever having used injection drugs. Lifetime number of sexual partners was treated as time-varying, and updated with each round of newly collected data at a study visit.
HIV serostatus was assessed at each study visit. CD4 cell count and HIV RNA level were measured at each study visit. CD4 cell count was explored as cell count at a visit, pre-ART nadir CD4 cell count, and post-ART nadir CD4 cell count. HIV RNA level was examined with a variable indicating if the HIV viral load was detectable (defined as > 500 copies/ml, as this was the common cutoff among all HIV viral load assays used throughout study follow-up), if the viral load was below or above 4,000 copies/ml, and the peak HIV viral load. ART use was collected at each study visit, and analyzed as a variable indicating exposure to ART. Similarly, a prior history of clinical AIDS (using the CDC case definition,[41] and excluding those individuals whose AIDS diagnosis was based solely on CD4 cell count) was collected at each study visit, and analyzed as a time-varying covariate.
An individual contributed person-time to the HIV serostatus group they belonged to at a given study visit. In the event of a seroconversion during follow-up (443 men and 17 women), the date of seroconversion was defined as the midpoint between the last HIV seronegative test and the first HIV seropositive test. Person-time occurring before the date of seroconversion was added to the HIV seronegative group and person-time occurring after the date of seroconversion was applied to the HIV seropositive group.
PAFs were calculated relative to a model with all cumulative pack-year values (lagged by 10 years) set to 0–5 pack-years, and were adjusted for age, race/ethnicity, annual household income, alcohol use, history of injection drug use, baseline hepatitis C infection status, HIV status, and study cohort. The HIV seropositive-specific model did not adjust for history of clinical AIDS, and instead adjusted for CD4 cell count, detectable HIV viral load, and exposure to ART. To assess the contribution of smoking-related cancers that also had viral etiologies, we repeated the PAF analyses and removed this subset of malignancies. The PAFs were calculated using a SAS macro provided by Laaksonen and colleagues.[21]
Footnotes
Disclosure statement: The authors have no conflicts of interest to disclose.
Human Participant Protection
Study protocols and consent materials were reviewed and approved by the institutional review boards at each of the collaborating institutions and informed consent was obtained from the participants.
Supplemental Digital Content 1. Table that lists the incident cancers diagnosed in the MACS and WIHS. Pdf
REFERENCES
- 1.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103(9):753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med 2015; 162(5):335–344. [DOI] [PubMed] [Google Scholar]
- 3.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current Cigarette Smoking Among Adults - United States, 2005–2015. MMWR Morb Mortal Wkly Rep 2016; 65(44):1205–1211. [DOI] [PubMed] [Google Scholar]
- 4.Lifson AR, Lando HA. Smoking and HIV: prevalence, health risks, and cessation strategies. Curr HIV/AIDS Rep 2012; 9(3):223–230. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs EJ, Newton CC, Carter BD, Feskanich D, Freedman ND, Prentice RL, et al. What proportion of cancer deaths in the contemporary United States is attributable to cigarette smoking? Ann Epidemiol 2015; 25(3):179–182 e171. [DOI] [PubMed] [Google Scholar]
- 6.Helleberg M, Gerstoft J, Afzal S, Kronborg G, Larsen CS, Pedersen C, et al. Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV. AIDS 2014; 28(10):1499–1508. [DOI] [PubMed] [Google Scholar]
- 7.Hessol NA, Weber KM, D’Souza G, Burton D, Young M, Milam J, et al. Smoking Cessation and Recidivism in the Women’s Interagency Human Immunodeficiency Virus Study. Am J Prev Med 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akhtar-Khaleel WZ, Cook RL, Shoptaw S, Surkan P, Stall R, Beyth RJ, et al. Trends and Predictors of Cigarette Smoking Among HIV Seropositive and Seronegative Men: The Multicenter Aids Cohort Study. AIDS Behav 2016; 20(3):622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hessol NA, Martinez-Maza O, Levine AM, Morris A, Margolick JB, Cohen MH, et al. Lung cancer incidence and survival among HIV-infected and uninfected women and men. AIDS 2015; 29(10):1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altekruse SF, Shiels MS, Modur SP, Land SR, Crothers KA, Kitahata MM, et al. Cancer burden attributable to cigarette smoking among HIV-infected people in North America. AIDS 2018; 32(4):513–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12(9):1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998; 9(2):117–125. [PubMed] [Google Scholar]
- 13.Kaslow R, Ostrow D, Detels R, Phair J. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–318. [DOI] [PubMed] [Google Scholar]
- 14.Hofferkamp J Standards for Cancer Registries Volume III: Standards for Completeness, Quality, Analysis, Management, Security and Confidentiality of Data. In. Springfield, Ill: North American Association of Central Cancer Registries; August 2008. [Google Scholar]
- 15.Surveillance E, and End Results (SEER) Program. Surveillance Research Program, Cancer Statistics Branch In. SEER 9 Regs Public-Use (1973–2001) ed: National Cancer Institute, Division of Cancer Control and Population Science; 2004. [Google Scholar]
- 16.Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018; 68(1):31–54. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 18.Moore TO, Moore AY, Carrasco D, Vander Straten M, Arany I, Au W, et al. Human papillomavirus, smoking, and cancer. J Cutan Med Surg 2001; 5(4):323–328. [DOI] [PubMed] [Google Scholar]
- 19.Peto J That the effects of smoking should be measured in pack-years: misconceptions 4. Br J Cancer 2012; 107(3):406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freedman ND, Abnet CC, Caporaso NE, Fraumeni JF Jr., Murphy G, Hartge P, et al. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int J Epidemiol 2016; 45(3):846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laaksonen MA, Virtala E, Knekt P, Oja H, Harkanen T. SAS Macros for Calculation of Population Attributable Fraction in a Cohort Study Design. J Stat Softw 2011; 43(7):1–25. [Google Scholar]
- 22.SAS Institute Inc. SAS/STAT User’s Guide, Version 9.4. In. Cary, North Carolina: SAS Institute Inc.; 2014. [Google Scholar]
- 23.Kirk GD, Merlo C, P OD, Mehta SH, Galai N, Vlahov D, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis 2007; 45(1):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiels MS, Cole SR, Mehta SH, Kirk GD. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr 2010; 55(4):510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigel K, Wisnivesky J, Gordon K, Dubrow R, Justice A, Brown ST, et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012; 26(8):1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004; 54(2):78–93. [DOI] [PubMed] [Google Scholar]
- 27.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and racial differences in the smoking-related risk of lung cancer. N Engl J Med 2006; 354(4):333–342. [DOI] [PubMed] [Google Scholar]
- 28.Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest 2005; 128(1):370–381. [DOI] [PubMed] [Google Scholar]
- 29.Henschke CI, Yip R, Miettinen OS. Women’s susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA 2006; 296(2):180–184. [DOI] [PubMed] [Google Scholar]
- 30.Pakkala S, Chen Z, Rimland D, Owonikoko TK, Gunthel C, Brandes JR, et al. Human immunodeficiency virus-associated lung cancer in the era of highly active antiretroviral therapy. Cancer 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marquez-Garban DC, Pietras RJ. Estrogen-signaling pathways in lung cancer. Adv Exp Med Biol 2008; 617:281–289. [DOI] [PubMed] [Google Scholar]
- 32.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 1996; 88(3–4):183–192. [DOI] [PubMed] [Google Scholar]
- 33.Althoff KN, Gebo KA, Moore RD, Boyd CM, Justice AC, Wong C, et al. Contributions of traditional and HIV-related risk factors on non-AIDS-defining cancer, myocardial infarction, and end-stage liver and renal diseases in adults with HIV in the USA and Canada: a collaboration of cohort studies. Lancet HIV 2019; 6(2):e93–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TR. Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am J Public Health 2010; 100(10):1896–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Gao L, Li H, Gao J, Yang Y, Zhou F, et al. Human papillomavirus infection and laryngeal cancer risk: a systematic review and meta-analysis. J Infect Dis 2013; 207(3):479–488. [DOI] [PubMed] [Google Scholar]
- 36.Reddy KP, Kong CY, Hyle EP, Baggett TP, Huang M, Parker RA, et al. Lung Cancer Mortality Associated With Smoking and Smoking Cessation Among People Living With HIV in the United States. JAMA Intern Med 2017; 177(11):1613–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shepherd L, Ryom L, Law M, Petoumenos K, Hatleberg CI, d’Arminio Monforte A, et al. Cessation of Cigarette Smoking and the Impact on Cancer Incidence in Human Immunodeficiency Virus-infected Persons: The Data Collection on Adverse Events of Anti-HIV Drugs Study. Clin Infect Dis 2019; 68(4):650–657. [DOI] [PubMed] [Google Scholar]
- 38.Bigna JJ, Kenne AM, Asangbeh SL, Sibetcheu AT. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: a systematic review and meta-analysis. Lancet Glob Health 2018; 6(2):e193–e202. [DOI] [PubMed] [Google Scholar]
- 39.Benard A, Mercie P, Alioum A, Bonnet F, Lazaro E, Dupon M, et al. Bacterial pneumonia among HIV-infected patients: decreased risk after tobacco smoking cessation. ANRS CO3 Aquitaine Cohort, 2000–2007. PLoS One 2010; 5(1):e8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitzpatrick ME, Kunisaki KM, Morris A. Pulmonary disease in HIV-infected adults in the era of antiretroviral therapy. AIDS 2018; 32(3):277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Revised surveillance case definition for HIV infection--United States, 2014. MMWR Recomm Rep. 2014;63(RR-03):1–10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


