Abstract
Background
Anxiety disorders first emerge during the critical developmental periods of childhood and adolescence. This review synthesizes recent findings on the prevalence, risk factors, and course of the anxiety disorders; and their neurobiology and treatment.
Methods
For this unstructured review, searches were conducted using PubMed, PsychInfo, and clinicaltrials.gov. Findings related to the epidemiology, neurobiology, risk factors and treatment of pediatric anxiety disorders were then summarized.
Findings
Anxiety disorders are high prevalence, and early onset conditions associated with multiple risk factors including early inhibited temperament, environment stress and structural and functional abnormalities in the prefrontal-amygdala circuitry as well as the default mode and salience networks. The anxiety disorders are effectively treated with cognitive behavioral therapy (CBT), selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs).
Conclusions
Anxiety disorders are high prevalence, early onset conditions associated with a distinct neurobiological fingerprint, and are consistently responsive to treatment. Questions remain regarding who is at risk for developing anxiety disorders as well as the way in which neurobiology predicts treatment response.
Keywords: generalized anxiety disorder; separation anxiety disorder; fMRI; pharmacogenomics; selective serotonin reuptake inhibitor (SSRI, SRI)
Introduction
Anxiety disorders begin in childhood and adolescence (Beesdo-Baum & Knappe, 2012; Beesdo, Pine, Lieb, & Wittchen, 2010) and with a lifetime prevalence close to 30% are the most common mental health conditions across the life span (Kathleen Ries Merikangas et al., 2010) Anxiety disorders are more prevalent, present earlier in development than depression (Beesdo et al., 2010) and if left untreated are associated with significant short and long term impairment (Kendall et al., 2010) (Ranøyen et al., 2018) and place children at high risk for subsequent mood disorders, substance misuse, disruptive behaviors, suicidal behavior, educational underachievement, and later adult economic disadvantage (Asselmann, Wittchen, Lieb, & Beesdo-Baum, 2018). Anxiety disorders as classified by the Diagnostic and Statistical Manual of Mental Disorders-Fifth Edition (DSM-5) include: generalized anxiety disorder, social anxiety disorder, separation anxiety disorder, specific phobia, panic disorder and agoraphobia. Although each of the anxiety disorders are defined by specific criteria generalized, separation and social anxiety disorders are often studied en block because of their common comorbidity, similar risk factors, shared neurobiology and similar responses to treatment, as discussed throughout this review.
Substantial progress in anxiety disorder treatment has occurred over the past decade and lays the groundwork for tailored interventions. Today, we have a better understanding of the neurobiology and psychological factors in youth with anxiety disorders as well as predictors and moderators of treatment outcome. At least a half dozen meta-analyses summarize treatment efficacy and tolerability outcome, and identify risk factors for developing pediatric anxiety disorders and predictors of pharmacological and psychotherapeutic treatment response, remission and tolerability. Randomized controlled trials including the large comparative efficacy trial, the Child/Adolescent Anxiety Multimodal Treatment of Anxiety (CAMS) (Walkup et al., 2008) shed light on the phenomenology and assessment of pediatric anxiety disorders and the efficacy of pharmacological and psychological treatment. Importantly, accumulated data from CAMS and other randomized controlled trials have identified parental and family factors that affect youth with anxiety and their response to treatment.
Given the significant progress that has occurred over the past decade, we aimed to summarize and discuss the current knowledge regarding pediatric anxiety disorders. Herein, we review (1) epidemiology and course, (2) risk factors, (3) neurobiology and (4) psychopharmacologic and psychotherapeutic treatment of pediatric anxiety disorders. Assessment of anxiety disorders has recently been the subject of a Practitioner Review in this journal. (see Creswell, Waite, & Hudson, 2020) and is not specifically covered in this review.
Methods
For this unstructured review, searches were conducted in PubMed, PsychINFO, clinicaltrials.gov and the abstracts from the last 5 years from the Annual Meeting of the American Academy of Child & Adolescent Psychiatry (AACAP). In terms of the time frame for this review, per the Journal of Child Psychology and Psychiatry practice for these reviews, we have focused on findings from the last decade, although the search of AACAP abstracts was limited to the prior 5 years secondary to availability of the AACAP Confex system. Search results were compiled and reviewed to provide an overview of the current knowledge regarding pediatric anxiety disorders including epidemiology, neurobiology and predictors, outcomes and tolerability of current treatments. For the PubMed search (inception through August 1, 2019), we used the following search strategy (adolescent* OR children OR pediatric OR youth) AND (anxiety OR social phobia OR social anxiety disorder OR SAD OR generalized anxiety disorder OR GAD OR separation anxiety disorder OR obsessive compulsive disorder*) AND (fMRI or functional magnetic resonance imaging OR voxel based morphometry OR VBM or functional connectivity OR amygdala OR prefrontal cortex OR spectroscopy OR psychotherapy OR cognitive behavioral therapy OR CBT OR selective serotonin reuptake inhibitor OR SSRI OR selective serotonin norepinephrine reuptake inhibitor OR SNRI OR selective serotonin norepinephrine reuptake inhibitor OR fluoxetine OR fluvoxamine OR citalopram OR escitalopram OR fluoxetine OR paroxetine OR venlafaxine OR desvenlafaxine OR duloxetine OR vortioxetine OR vilazodone). The results of the search were then manually limited to randomized, placebo-controlled trials. The references of all eligible trials and review articles were searched for additional clinical trials. For this unstructured review, we also attempted to highlight key findings and developments that have been published in the last decade.
Results
Epidemiology and course
With a median age of onset of 6 years of age, anxiety disorders are among the first psychiatric conditions to emerge and precede the onset of depression (median age of onset 13) as well as substance use disorders (median age of onset 15) (Merikangas et al., 2010). The onset and course of the pediatric anxiety disorders is interwoven with other psychiatric disorders in a complex developmental trajectory (Asselmann, Wittchen, Lieb, Höfler, & Beesdo-Baum, 2014; Beesdo-Baum & Knappe, 2012; Beesdo et al., 2007). Individuals with the greatest vulnerability move in and out of anxiety diagnoses overtime (homotypic comorbidity) and also morph overtime into other diagnoses (heterotypic comorbidity) (Caspi & Moffitt, 2018). Put another way, the adolescent with panic and generalized anxiety disorders was once a boy with separation anxiety disorder and was a toddler with extreme shyness or the young adult with major depression once suffered from social anxiety disorder as a teen.
Specific phobia, characterized by a circumscribed fear related to a common situation/focus, is among the first anxiety disorders to emerge in children and adolescents with a mean age of onset of around 6 years of age (Beesdo, Knappe, & Pine, 2009; Wehry et al., 2015). Similar to the other DSM-5 anxiety disorders, specific phobia has high heterotypic continuity with other anxiety disorders, including the fear-based anxiety disorders: separation, social and separation anxiety disorders.
With a mean age of onset of 8 years of age separation anxiety disorder (SAD) is typically the next anxiety disorder to emerge (Beesdo et al., 2010). As its name implies, separation anxiety disorder is characterized by distress if and when a child is separated from his or her close attachment figures. Separation anxiety disorder affects 6.7% of US youth and disproportionately affects females (9% vs. 6.3%) (Merikangas et al., 2010). Having separation anxiety disorder as a child increased the risk of developing later panic disorder (HR: 3.5, p=0.001) and strongly increased the risk of developing generalized anxiety disorder (HR: 7.7, p=0.02) (Beesdo et al., 2010).
Youth with social anxiety disorder often begin life with behavioral inhibition, and go on to experience full onset of anxiety symptoms near age 12. Children with social anxiety disorder are self-conscious and experience intense anxiety in social situations, worry about embarrassing themselves and fearing negative evaluation by peers or others. Social anxiety disorder, which occurs in approximately 9% of adolescents (11.2% of females and 7% of males) (Merikangas et al., 2010), has also been associated with an increased risk of developing other anxiety disorders and the avoidance observed in youth with social anxiety disorder may result in adolescent school refusal and overlap diagnostically with agoraphobia.
Generalized Anxiety Disorder (GAD) is characterized by excessive, difficult-to-control, diffuse anxiety that is accompanied by initial insomnia, difficulty with concentration, irritability, fatigue and muscle tension as well as numerous somatic symptoms (Crawley et al., 2014). Like most anxiety disorders, post-pubertal rates are greater in females compared to males (3% vs. 1.5%) and affect 2.2% of adolescents aged 13–18 (Merikangas et al., 2010).
Panic disorder, typically the ‘last’ anxiety disorder to emerge (Beesdo et al., 2010), is characterized by discrete, rapid onset and intense periods (i.e. attacks) of distressing somatic and cognitive symptoms (Wehry et al., 2015). Panic attacks occur “out of the blue” and in response to cues. When triggered panic attacks are observed in another psychiatric disorder they are recognized by the DSM-5 modifier, “with panic attacks”. Panic attacks as well as “fearful spells”—independent of panic disorder, quadruple the risk of developing any anxiety disorder, panic disorder, agoraphobia, GAD as well as depressive disorders (Asselmann et al., 2014). The prevalence of panic disorder like social anxiety disorder and GAD increases with age: 1.8% in 13–14-year-olds, 2.3% in 15–16-year-olds and 3.3% in 17–18-year-olds (Kessler, Chiu, Demler, Merikangas, & Walters, 2005).
The proportion of youth affected by severe anxiety (defined as having “a lot” or “extreme” impairment in daily activities or having “severe or very severe distress”) varies by diagnosis (Merikangas et al., 2010). Severe panic disorder, social anxiety disorder and GAD increase with age with severe anxiety affecting 8.3% of adolescents. Most adolescents with panic disorder or agoraphobia had “severe” anxiety, compared to about half of those with GAD. Adolescents with “severe” anxiety comprised a minority of those with social anxiety disorder (14.3%), separation anxiety disorder (7.9%) and relatively few with specific phobia (<5%) (Merikangas et al., 2010). Finally, agoraphobia has a prevalence of approximately 2.5% from age 13–17 (Merikangas et al., 2010). Finally, it is noteworthy that studies conducted using DSM-IV classification included posttraumatic stress disorder (PTSD) among the anxiety disorders and may not have included separation anxiety disorder which was, until 2014 not classified among the anxiety disorders.
Risk factors for pediatric anxiety disorders
Although, environmental, biological and developmental risk factors for anxiety disorders have been identified it is largely unclear how these forces interact to result in the development of an anxiety disorder. Only recently have studies linked neurobiology and temperament as well as specific psychological characteristics (e.g. fear learning) with the development of anxiety during certain developmental periods. For example, fear conditioning (i.e., the degree to which an individual associates a neutral stimulus with a threatening unconditioned stimulus) is nonlinear during development (Lau et al., 2011) and may be related to maturational changes in the connectivity of prefrontal cortical structures with the amygdala and other subcortical structures (Jarcho et al., 2015).
Cognitive risk factors
Bias towards threat-related stimuli (i.e., threat bias), intolerance of uncertainty (i.e., cognitive bias that determines how an individual perceives and reacts to uncertain situations (Yook, Kim, Suh, & Lee, 2010)) and learned behaviors (e.g., avoidance) are cognitive risk factors that have received considerable attention (Lau & Waters, 2017). Avoidance of situations in which a child or adolescent experienced anxiety is negatively reinforcing—anxiety is reduced by avoiding the situation. An additional risk factor—intolerance of uncertainty—represents “a dispositional characteristic that results from a set of negative beliefs about uncertainty and its implications and involves the tendency to react negatively on an emotional, cognitive, and behavioral level to uncertain situations and events” (Buhr & Dugas, 2009). Intolerance of uncertainty potentially relates to fear extinction and results in increased expectation of threat in ambiguous situations which produces anxiety-related responses to both “learned threat and safety cues” (Morriss, Christakou, & van Reekum, 2016). However, the degree to which intolerance of uncertainty represents a risk factor for developing anxiety disorders or an epiphenomenal process remains unclear. Some, but not all (Britton et al., 2013; Shechner et al., 2015) studies suggest that anxious children also exhibit decreased fear extinction compared to healthy youth (Craske et al., 2008). In this regard, Britton and colleagues suggest that, from a developmental perspective, “abnormal safety learning in childhood may establish threat-related appraisal biases early” which contributes to the development of anxiety disorders (Britton, Lissek, Grillon, Norcross, & Pine, 2011). In following up on the neurophysiology of this association, a cross-sectional study of anxious and non-anxious youth found decreased anterior cingulate cortex activation in anxious youth compared to those without anxiety during an fear extinction recall fMRI task (Britton et al., 2013) and a subsequent report found prefrontal cortex activation in response to a conditioned stimulus differs based on the age suggesting that maturation or developmental psychopathology is related to this enhanced negative association between age and prefrontal cortex activation (Haddad, Bilderbeck, James, & Lau, 2015).
Behavioral Inhibition as a risk factor for developing anxiety disorders
Behavioral inhibition, the tendency to feel overwhelmed and to withdraw from unfamiliar situations, individuals or settings (Svihra & Katzman, 2004), is present in about 15% of children increases the risk of developing anxiety disorders (Beesdo et al., 2010; Jacqueline A. Clauss & Blackford, 2012; Hudson, Dodd, Lyneham, & Bovopoulous, 2011; Shamir-Essakow, Ungerer, & Rapee, 2005). In the Early Developmental Stages of Psychopathology (EDSP) Study, behavioral inhibition predicted the development of generalized, separation and social anxiety disorders as well as panic disorder. However, some studies, including meta-analyses suggest that the link between behavioral inhibition and social anxiety disorders may be stronger than for other disorders. In this regard, in a meta-analysis of seven studies, behavioral inhibition increased the risk of developing social anxiety disorder by seven fold; this study suggested that almost half of inhibited children would develop social anxiety disorder. Interestingly, this risk is independent of differences in temperament and age (Clauss & Blackford, 2012). Further, specific aspects of behavioral inhibition (e.g., peer relationships, separation, fear of adults) may be uniquely associated with separation anxiety disorder (Pahl, Barrett, & Gullo, 2012). Other studies illustrate the multidimensionality of the relationship between behavioral inhibition and the development of anxiety disorders and suggest that certain parenting styles could “increase [the] risk for behavioral inhibition…which may then lead to the development of anxiety in later childhood” (Hudson et al., 2011).
Family environmental risk factors and attachment
In the EDSP study, having a parent with GAD as well as childhood separation events and “dysfunctional family functioning” (reflected by higher McMaster Family Assessment Device score, a 60-item self-report instrument that assesses problem solving, communication, roles, affective responsiveness, affective involvement and behavioral control) significantly increased the risk of developing GAD (Beesdo et al., 2010). Additionally, childhood separation-related events increased the risk of developing other anxiety disorders (Beesdo et al., 2010). Separation-related events also potentially interact with psychological factors, including attachment style (Lewis-Morrarty et al., 2015; Warren, Huston, Egeland, & Sroufe, 1997). Nearly 50 years ago, Bowlby (1973) described separation anxiety that develops in some healthy infants when they separate from their caregiver, although, he observed that healthy infants were calmed by the return of the caregiver and developed “confidence that the caregiver will help to protect him or her.” However, Bowlby noted that some children exhibit insecure attachment styles—a trait that increases their risk of developing an anxiety disorder. Subsequently, Warren and colleagues demonstrated in a prospective study that experimentally-determined attachment style, specifically anxious attachment, at 12 months of age predicted the development of anxiety disorders at 17½ years of age (Warren et al., 1997).
These early attachment relationships—and their disruption—have been examined in longitudinal studies of infants with early deprivation. These youth with early separation/institutionalization are at greater risk of developing anxiety disorders and this risk increases the longer they are institutionalized; however, they were also at risk of developing affective symptoms and physical growth delays (Ellis, Fisher, & Zaharie, 2004). In the Bucharest Early Intervention Project in which children were randomized to continued institutionalization or placement in foster care, earlier placement in foster care decreased the risk of internalizing symptoms (depressive and anxiety symptoms) and the “long-term stability of foster-care placements” predicted the development of anxiety/depressive symptoms during adolescence (Humphreys et al., 2015). In parallel a number of studies (discussed later) have examined frontolimbic circuitry in these youth with early deprivation who are at risk for developing anxiety disorders (Gee et al., 2013).
There are conflicting data on the influence of parental behavior on the development of anxiety disorders. Parental overprotection or “over controlling” parenting increases the risk of developing anxiety in some studies (Rapee & Melville, 1997; Turgeon, O’Connor, Marchand, & Freeston, 2002) while other studies suggest that this risk is associated primarily with the specific parental behaviors that potentiate avoidance and fear learning. However, in EDSP, parental overprotection was not consistently associated with an increased risk of developing an anxiety disorder (Beesdo et al., 2010). From a fear learning and threat bias standpoint, when parents model anxious behavior, children are reluctant to explore novel situations, have more avoidance and approach situations with caution. Finally, family accommodation, the degree to which a family changes/adapts behavior to decrease a child’s anxiety or avoid anxiety-provoking stimuli, is a risk factor for and potentiates the development of anxiety disorders in youth (Lebowitz et al., 2013). Accommodation, by families, is increased in youth with anxiety disorders and may increase distress (p<0.001), and anxiety severity (p=0.017) (Lebowitz, Scharfstein, & Jones, 2014).
Parental disorders
Having a parent with an anxiety disorder and certain personality disorder symptoms has been associated with an increased risk of their child offspring having anxiety disorders. In a longidutinal study of youth, parental anxiety disorders were associated with an increased risk of developing an anxiety disorder. Specifically, parental GAD increased the risk of child offspring developing GAD and other anxiety disorders, but not depressive disorders. However, the opposite was not true. Having a parent with an anxiety disorder and a depressive disorder increased the risk of a child developing an anxiety disorder without a depressive disorder (Beesdo et al., 2010). Steinsbekk and colleagues (2019) observed that in youth whose parents had cluster A and C personality disorder symptoms had more emergent anxiety symptoms when prospectively followed from age 4–8.
Substance use and the risk of developing anxiety disorders
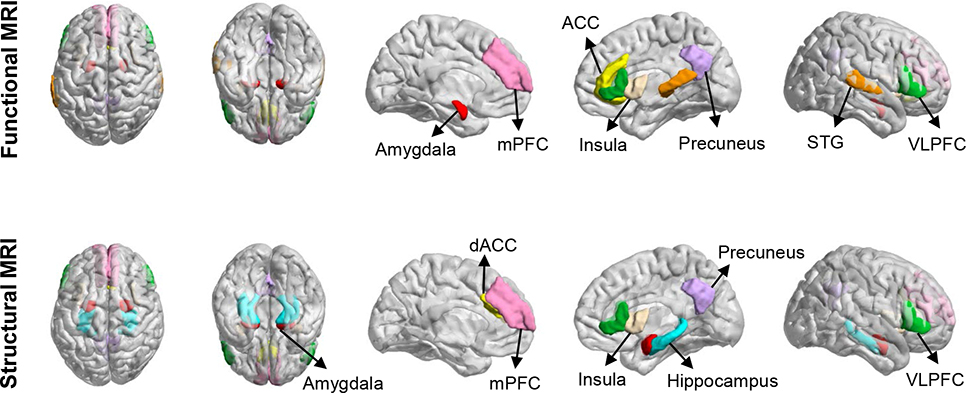
Alcohol, tobacco and cannabis use is more common in patients with anxiety disorders compared to the general population. However, like other risk factors for developing anxiety disorders, the relationship between exposure and pathogenesis is complex and often difficult to discern from cross sectional and prospective studies. Matthew and colleagues examined the relationship between alcohol use in adolescents and panic disorder—which typically emerges in late adolescence (Mathew, Norton, Zvolensky, Buckner, & Smits, 2011) and found that prior alcohol use among adolescents was associated with increased panic-related symptoms using a hyperventilation challenge paradigm. In this cohort, the history of panic attacks was not associated with a desire to consume alcohol suggesting that the “self-medication hypothesis” may not entirely explain this association (Blumenthal, Cloutier, Zamboanga, Bunaciu, & Knapp, 2015). The relationship between cannabis use and the development (and maintenance) of anxiety disorders has become clearer in recent years. Regular (i.e., daily) cannabis use during adolescence increases the risk of developing anxiety disorders, even in individuals who stopped using marijuana, suggesting that “early cannabis exposure causes enduring mental health risks in the general cannabis-using adolescent population” (Degenhardt et al., 2013). Finally, patients with high synthetic cannabinoid use have decreased gray matter volumes and altered functional activity in an ensemble of cortical structures that are implicated in the pathophysiology of pediatric anxiety disorders (e.g., cuneus, precuneus, insula, inferior frontal gyrus/ventrolateral prefrontal cortex and cingulate, Figure 1) (Livny et al., 2018). It is unknown whether this effect is present with other cannabinoids or which psychoactive components potentially contribute to these neurostructural and neurofunctional effects.
FIGURE 1: Neurofunctional (top) and neurofunctional (bottom) abnormalities in pediatric patients with generalized, separation and social anxiety disorders.
VLPFC, ventrolateral prefrontal cortex; STG, superior temporal gyrus; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex; dACC, dorsal anterior cingulate cortex
Environmental toxicants and risk factors for developing anxiety disorders
A relatively neglected area concerning the risk of developing anxiety disorders includes environmental exposures, including air pollution (Brokamp, Strawn, Beck, & Ryan, 2019). Mercury and other toxicants have also been associated with an increased risk of developing anxiety disorders, even when correcting for maternal age at delivery, fish intake, maternal ethnicity, household income, maternal education and marital status (Patel et al., 2019). A longitudinal study of nearly 400 mothers in whom environmental toxicants were measured during pregnancy in their homes and children, reveals that cord and maternal blood mercury concentrations at birth are associated with more anxiety disorders at age 8—the age when many anxiety disorders emerge (Patel et al., 2019). Traffic pollution is also associated with increases in generalized anxiety symptoms, and myo-inositol concentrations in the anterior cingulate cortex mediate this association between traffic-related air pollution and anxiety (Brunst et al., 2019). More recently, specific components of air pollution that induce inflammation and oxidative stress in the brain (e.g. particulate matter with an aerodynamic diameter <2.5 μm (PM2.5)) have been linked to exacerbations of anxiety disorders. Greater PM2.5 exposure is associated with an increased risk of psychiatric emergency department visits for anxiety disorders in children and adolescents and this association appeared to be mediated by community deprivation (Brokamp et al., 2019).
Neurobiology of anxiety disorders
Since the beginning of the century, nearly a dozen studies have examined the structural and functional neuroanatomy of pediatric anxiety disorders using magnetic resonance imaging (MRI) (Table 1, Figure 1). Collectively, these studies demonstrate neurostructural abnormalities in regions that regulate emotional processing, fear extinction (a process by which an individual develops new associations for a conditioned stimulus in which he or she now classifies this stimuli as ‘safe’ (Bouton, 2002)), appraisal of threat and mentalization—processes that are fundamentally disrupted in pediatric anxiety disorders (Nolte, Guiney, Fonagy, Mayes, & Luyten, 2011; Strawn, Wehry, Delbello, Rynn, & Strakowski, 2012). In the subsequent sections, we draw from three kinds of literature: (1) magnetic resonance spectroscopy studies which describe brain concentrations of neurotransmitters in patients with pediatric anxiety disorders, (2) neurostructural studies, which illustrate differences in gray matter volumes and cortical thickness, and (3) functional imaging studies that provide a glimpse into the activity of individual structures (both at rest and while performing anxiety-relevant tasks) and the ways in which their functional connectivity is altered in pediatric anxiety disorders.
TABLE 1.
Neurostructural findings in pediatric anxiety disorders.
| Study | Disorder | N | Age range | Amygdala | Hippocampus | Anterior cingulate cortex | Ventrolateral prefrontal cortex | Additional regions |
|---|---|---|---|---|---|---|---|---|
| De Bellis et al. 2000 | GAD | 12 | 8–16 | ↑GMV (total and L) | No significant difference in other regions between groups | |||
| De Bellis et al. 2002 | GAD | 13 | 8–16 | ↑ GMV and WMV of Superior temporal gyrus; Significant correlation between the Superior temporal gyrus white matter percent asymmetry index with SCARED score | ||||
| Milham et al. 2005 | Mixed AD | 17 | NR | ↓GMV (L, corrected) and (R, uncorrected) | No group differences | No group differences | ↓ GMV bilaterally (subthreshold, uncorrected) | ↓ GMV in bilateral precuneus (subthreshold, uncorrected) |
| Liao et al, 2013 | GAD | 26 | 16–18 | ↑ GMV in R putamen | ||||
| Liao et al, 2014 | GAD | 26 | 16–18 | Larger gray matter volume in R putamen | ||||
| Strawn et al, 2013 | GAD | 15 | 10–17 | ↑ GMV in precuneus and precentral gyrus; ↓GMV in orbital gyrus and posterior cingulate; ↑ WMV in L inferior temporal gyrus; ↓ WMV in medial and superior frontal gyri | ||||
| Mueller et al. 2013 | Mixed AD | 39 | NR | ↓GMV (R) | ↓GMV (R) | No group differences | ↑bilateral insula GMV | |
| Strawn et al, 2014 | Mixed AD | 13 | NR | ↓GMV bilaterally | ↑cortical thickness in the inferolateral and ventromedial PFC, inferior/middle temporal cortex and lateral occipital cortex | |||
| Strawn et al, 2015 | Mixed AD | 38 | ||||||
| Gold et al. 2016 | Mixed AD | 39 | 10–17 | ↑ DLPFC (L) GMV | ||||
| Gold et al. 2017 | Mixed AD | 75 | 8–18 | No group differences | ↓GMV (R), which was related to anxiety diagnosis and symptom severity | ↑cortical thickness in precentral gyrus and vmPFC. |
Neurobiology of risk
Altered structure and functional activity may precede the development of anxiety disorders. Adolescents who had behavioral inhibition during childhood, had thinner dorsal anterior cingulate cortices in late adolescence, although, more anxiety during adolescence was associated with thicker ventrolateral prefrontal cortex in adulthood among those with low behavioral inhibition as children (Sylvester et al., 2016). Youth with higher “trait anxiety” and behavioral inhibition have increased prefrontal activation (Fu, Taber-Thomas, & Perez-Edgar, 2017; Telzer et al., 2008). Additionally, in behaviorally inhibited children, anterior cingulate cortex activation to fearful faces correlates with the severity of anxiety that emerges over the subsequent 2 years (Clauss, Benningfield, Rao, & Blackford, 2016). Another line of evidence implicating this circuitry in the risk of developing anxiety disorders comes from children with early deprivation who were examined with fMRI and then followed prospectively. In pre-adolescents who received institutional care, Green and colleagues found attenuated amygdala responses to “social-affective cues of trustworthiness” that are typical of healthy subjects. Additionally, differences in amygdala response to trustworthy versus untrustworthy stimuli predicted separation anxiety severity over the subsequent 2 years (Green et al., 2016). Adolescents with behavioral inhibition in early childhood have increased amygdala-dorsolateral prefrontal cortex and amygdala-anterior insula connectivity (Hardee et al., 2013) in addition to increased amygdala reactivity. Interestingly, behavioral inhibition predicts distinct development of these amygdala-prefrontal circuits. In adolescents who had early-childhood behavioral inhibition, anxiety symptoms became negatively associated with right amygdala-left dorsolateral prefrontal cortex connectivity, as the children got older. However, those youth with low behavioral inhibition had a positive anxiety-connectivity association that increased as they grew older (Abend, Swetilitz et al, 2019). Additionally, “social reticence” assessed in toddlerhood (age 2–7) which predicts pre-adolescent social anxiety symptoms as well as social anxiety disorder in adolescence with pre-adolescents who had high social anxiety symptoms having increased bilateral insula engagement during a task in which children anticipated and then received positive or negative feedback from virtual peers (Clarkson et al., 2019).
At this juncture, structural and functional neuroimaging studies in youth who are at risk for developing anxiety disorders suggest abnormalities of networks that subserve cognitive control, attention and fear processing. Taken together, these findings raise the possibility of a vulnerability in the ability of these systems to manage an individual’s their internal and external environment. The networks in which ‘at risk’ youth have structural and functional differences compared to healthy youth dynamically interact. A failure of this interaction may ultimately result in the mis-interpreting of novel and even neutral stimuli as threatening, an inability to exert effective cognitive control under pressure, in the context of poor self-regulation (Walkup et al, 2020). Together this network dysfunction then sets the stage for a pattern of behavior that evolves and elaborates over time as anxiety disorders emerge.
Neuroanatomy of pediatric anxiety disorders
The amygdala which is intimately involved in fear processing across species and across development has been implicated in most neurostructural studies of pediatric anxiety disorders (Fox, Oler, Tromp, Fudge, & Kalin, 2015). The connectivity of the amygdala with subcortical and cortical structures as well as its anatomy and involvement in fear processing and anxiety have recently been reviewed in detail (Janak & Tye, 2015). Briefly, the amygdala receives input from the environment via the sensory thalamus and sensory cortices (primarily directed to the lateral amygdala). Within the amygdala, projections from the lateral amygdala send information to the basolateral and basomedial amygdala. The basolateral amygdala reciprocally connects with the prefrontal cortex and with regions discussed later in this review. Many of these target regions subserve fear processing and the processing of anxiety (Figure 1) (Janak & Tye, 2015). Early structural studies in children and adolescents with anxiety disorders demonstrated increased volumes in the amygdala (De Bellis et al., 2000). However, follow-up studies that specifically examined gray matter volumes in more detail found decreased amygdala gray matter volumes (Milham et al., 2005; Mueller et al., 2013; Strawn, Hamm, et al., 2015) in pediatric patients with generalized, separation and social anxiety disorders. Beyond the amygdala, pediatric patients with anxiety mixed anxiety disorders and GAD have decreased gray matter volume in posterior structures, including components of the default mode network (e.g., the posterior cingulate, cuneus and precuneus) (Milham et al., 2005; Mueller et al., 2013; Strawn et al., 2013) as well as the insula (Mueller et al., 2013), while two studies—derived from an overlapping sample—identified decreased hippocampal gray matter volumes (Gold et al., 2017; Mueller et al., 2013). The potentially conflicting results of volumetric studies of the amygdala, in the context of increased functional activity in the amygdala of youth with anxiety disorders (discussed below) has multiple potential mechanistic explanations. For example, these findings may result from loss of inhibitory GABAergic interneurons/neurons in the amygdala, which could explain both decreased volume and increased activity in the amygdala (Kalmar et al., 2009; Siegle, Konecky, Thase, & Carter, 2006). However, no direct evidence of decreased GABAergic neuronal populations exists in pediatric anxiety disorders. Future studies combing molecular cellular biology and neuroimaging are needed to verify this speculation.
In addition to differences in gray matter volumes between youth with anxiety and healthy subjects, several studies evaluated cortical thickness—a measure that has important developmental implications with regard to the pathophysiology of anxiety disorders in youth. Regarding this developmental significance, cortical thickness is, in part determined early (during the second trimester) and reflects the influence of multiple developmental processes at the cellular level, including alterations in synaptic density, changes in neuronal distributions and population shifts in cortical neurons.. As such proliferation of neurogenic progenitors, early in life, may alter cortical thickness (Pontious, Kowalczyk, Englund, & Hevner, 2007) and thus, changes in cortical thickness early in the course of illness raise the possibility that the pediatric anxiety disorders may be influenced by disruptions in cortical maturation that precede the development of the anxiety disorder. In pediatric anxiety disorders, cortical thickness is increased in inferior and middle temporal cortices and the medial prefrontal cortex (Strawn et al., 2014; Gold et al. 2017). In the ventromedial prefrontal cortex, cortical thickness and decreased gray matter volumes are associated with the severity of anxiety symptoms (Ducharme et al., 2013). Further, functional activity in this region is increased in adolescents with GAD (Strawn et al., 2012b; Roy et al., 2013) and in individuals with behavioral inhibition (Sechner et al., 2012)—a population who are at increased risk of developing anxiety disorders (Beesdo et al., 2010).
Taken together these findings suggest neurostructural findings implicate abnormalities within multiple networks, including the salience network which includes the amygdala and insula as well as the dorsal anterior cingulate, the frontoparietal/ventral attention network and the default mode network (including medial prefrontal cortex, precuneous/cuneus, and posterior cingulate). These networks subserve functions that are directly relevant to anxiety disorders. The salience network detects salient stimuli and recruits relevant networks to respond to them in an appropriate context-relevant manner. The frontoparietal and ventral attention networks subserve attentional processing, including shifts in attention and threat-oriented attention. Finally, the default mode network processes self-referential cognition (Domakonda, He, Lee, Cyr, & Marsh, 2019), generates mental representations of one’s self and future actions, thoughts and feelings in addition to assigning significance to thoughts about oneself (Li, Mai, & Liu, 2014; Xu, Lin, Han, He, & Bi, 2016).
Functional neuroimaging in pediatric anxiety disorders
Functional studies of pediatric anxiety disorders implicate many of the same structures as do structural studies (Figure 1) and commonly reveal amygdala hyperactivity (Blair et al., 2011; McClure, Monk, & Nelson, 2007; Monk, 2008; Thomas, Drevets, & Dahl, 2001). In general, the magnitude of amygdala activation correlates with anxiety severity (Killgore & Yurgelun-Todd, 2005); however, not all studies demonstrate increased amygdala activity in pediatric patients with anxiety disorders. One study that examined the specificity of amygdala hyperactivity in pediatric patients with anxiety disorders with and without co-occurring depression found that comorbid anxiety and depression was associated with greater amygdala reactivity compared to those with depression alone (Beesdo, Lau, et al., 2009), suggesting that amygdala reactivity is specific to anxiety. Of note, several studies failed to observe differences in amygdala activation between adolescents with and without diagnoses of GAD (Monk et al., 2006; Strawn, Bitter, et al., 2012).
In addition to the amygdala hyperactivity, many studies of youth with anxiety disorders have demonstrated increased activity in cortical regions (Figure 1) including the anterior cingulate cortex and ventrolateral prefrontal cortex (Monk et al., 2006, 2008; Strawn, Bitter, et al., 2012). Multiple studies in pediatric anxiety disorders demonstrate inverse relationships between the activity in the ventrolateral prefrontal cortex and anxiety severity consistent with the notion that activity in this region has a compensatory function. Also, compared to healthy youth, adolescents with social anxiety disorder exhibit increased dorsal anterior cingulate cortex activation (Blair et al., 2011) and activity within this region can be modified by context. Specifically, altered integration of anterior cingulate cortex activation and prefrontal activation has been observed in anxious youth in when shifting attention between threatening images and neutral images (Price et al., 2014), while among youth with anxiety disorders, those who struggle to tolerate uncertainty have more amygdala-anterior cingulate cortex activation (Krain et al., 2008).
Regarding connectivity within prefrontal circuits (Figure 1, bottom panel), considerable attention has focused on an ‘amygdalacentric’ model (Blackford & Pine, 2012; Guyer, Masten, & Pine, 2013; Monk & Pine, 2016; Strawn, Wehry, Delbello, Rynn, & Strakowski, 2012) given that multiple studies demonstrate altered connectivity between the amygdala and ventrolateral prefrontal cortex (Beesdo, Lau, et al., 2009; Strawn, Bitter, et al., 2012) as well as other structures within the prefrontal arousal networks. However, examining connectivity is nuanced with regard to the primary anxiety disorder. In adolescents with GAD, VLPFC-amygdala connectivity in response to viewing emotional faces is weaker compared to healthy youth (Monk, Telzer, & Mogg, 2008), although in adolescents with social anxiety disorder fronto-amygdala connectivity is increased when anticipating peer interaction (Guyer et al., 2008). Additionally, in adolescents with GAD, insula-amygdala connectivity is increased particularly among those with higher levels of anxiety (compared to those who are less anxious) (Roy et al., 2013) while in patients with mixed anxiety disorders, amygdala-insula and amygdala-posterior cingulate connectivity is increased (McClure et al., 2007; Strawn et al., 2012).
Imaging Studies Delineating the Neurocircuitry of Treatment Response in Pediatric Anxiety Disorders
As a general rule, increases in cortical activation which regulate anxiety within certain regions of the fear circuitry described above have been linked with symptomatic improvement—both with psychotherapy and SSRIs. One of the first fMRI studies to examine the neurophysiology of treatment response in pediatric GAD compared pre- and post-treatment activation in youth receiving fluoxetine and CBT. In this study, treatment-related increased in ventrolateral prefrontal cortex activity were observed following both CBT and fluoxetine (Maslowsky et al., 2010). Also, this group demonstrated that pre-treatment amygdala activation in youth with GAD correlated with the degree of anxiety improvement following treatment (CBT or fluoxetine) (McClure et al., 2007).
Neurochemistry of pediatric anxiety disorders
The relationship between function and neurochemistry—across multiple disorders—is complex with inhibitory and excitatory functions being interdependent (Lener et al., 2017; Tatti, Haley, Swanson, Tselha, & Maffei, 2017). Neurochemical studies are often difficult to interpret in that they either measure “whole brain” concentrations or concentrations within a specific region. Thus, it is difficult to determine whether findings relate to intracellular, extracellular/synaptic chemistry or potentially even altered cycling of these chemicals. One cross-sectional study examined glutamatergic tone in the anterior cingulate cortex in adolescents with GAD and found increased glutamate to creatine ratios are associated with more severe anxiety (Strawn et al., 2013). However, most evidence of the association between anxiety and either glutamatergic or GABAergic systems in youth has been indirect. Mutations in the glutamatergic gene, GRIK4, has been associated with sertraline response in children and adolescents with generalized, separation and social anxiety disorders (Sakolsky et al., 2010). Specifically, this mutation (rs1954787) is in the 3’ end of the first intron and may alter the expression of this kainic acid-type glutamate receptor that it encodes. However, the way in which this relates to function per se or the glutamatergic neurochemical milieu remains to be determined.
Children with separation anxiety disorders exhibit neuroendocrine dysregulation which includes increased separation-related secretion of cortisol compared to healthy youth (Brand, Wilhelm, Kossowsky, Holsboer-Trachsler, & Schneider, 2011). Children with separation anxiety also exhibit increase CO2 sensitivity (Roberson-Nay et al., 2010) as well as lower salivary oxytocin compared to pediatric patients with other anxiety disorders. Interestingly, lower salivary oxytocin levels were associated with decreased suicidality in children with anxiety disorders (Lebowitz, Blumberg, & Silverman, 2019) and salivary oxytocin concentrations correlate with cerebrospinal fluid oxytocin levels (R=0.657, p<0.001) (Martin et al., 2018).
Neurobiology of recovery
Several findings of the neurophysiology of treatment response in pediatric anxiety disorders emerge from studies conducted by Phan and colleagues in which children and adolescents (ages 9–19 years) with generalized, separation, and/or social anxiety disorder (as well as healthy controls) underwent functional magnetic resonance imaging scans approximately 12–13 weeks apart during which time they were treated with either CBT or sertraline (Burkhouse et al., 2018). In the most recent of these studies that leveraged an implicit threat task, anxious youth had reduced medial prefrontal cortex/anterior cingulate cortex activation, but effective treatment increased activation in this region with those patients who had greater improvement in social anxiety/avoidance symptoms displaying greater increase in anterior cingulate cortex activation (Burkhouse et al., 2018). In this sample, increase activation of the dorsolateral prefrontal cortex and ventrolateral prefrontal cortex, as well as precentral/postcentral gyri, prior to treatment, was associated with better improvement in anxiety symptoms regardless of whether youth received sertraline or CBT (Kujawa et al., 2016).
Finally, a in youth with generalized, social, and/or separation anxiety disorder who received 12 weeks of mindfulness-based cognitive therapy for children (MBCT-C), treatment-related increases in in activation of the bilateral insula and anterior cingulate cortex and treatment-related decrease in anxiety correlated with change in activation in these structures (p<0.005 corrected) (Strawn et al., 2016).
Psychopharmacologic treatment of pediatric anxiety disorders
Nearly two dozen randomized controlled trials have evaluated the efficacy of antidepressants, benzodiazepines, α2 agonists and other classes of medication for the treatment of pediatric anxiety disorders, and two studies directly compared more than one psychopharmacologic treatment (Bernstein, GARFINKEL, & BORCHARDT, 1990; da Costa et al., 2013). Among these, antidepressants are the most consistently studied and are efficacious compared to placebo (Locher et al., 2017; Strawn, Welge, Wehry, Keeshin, & Rynn, 2015; Zhen Wang et al., 2017). Indeed, antidepressants appear to be effective antianxiety medications and this underscores a recent shift in the field of psychopharmacology away from disease-focused terminology, often based on the first use of a medication, to neuroscience-based Nomenclature (NbN) (Caraci et al., 2017; Sultan, Correll, Zohar, Zalsman, & Veenstra-VanderWeele, 2018; Zohar & Kasper, 2016) which is based on mechanism of action and pharmacology. Thus, we will subsequently refer to psychopharmacologic interventions by the NbN terms.
SSRIs in pediatric anxiety disorders
Based on recent meta-analyses, selective serotonin reuptake inhibitors (SSRIs) are superior to other medication classes in pediatric anxiety disorders (Locher et al., 2017; Strawn, Mills, Sauley, & Welge, 2018), but patients are more like to discontinue SSRIs because of adverse events compared to SNRIs. Also, SSRIs are more likely to produce activation in children and adolescents with anxiety disorders compared to SNRIs (Mills and Strawn, 2020). Consistent with guidelines from the American Academy of Child & Adolescent Psychiatry that recommend SSRIs and SNRIs (Connolly & Bernstein, 2007), SSRIs are the most commonly prescribed initial medications for children and adolescents with anxiety disorders in the United States and approximately half of these youth continue treatment for at least 6 months (Bushnell et al., 2018). However, it is noteworthy that nearly on third of anxious who are treated with medication in the United States begin treatment with a non-SSRI medication (Bushnell et al., 2018). To date, sertraline (Rynn, Siqueland, & Rickels, 2001; Walkup et al., 2008), fluoxetine (Beidel et al., 2007; Birmaher et al., 2003; Clark et al., 2005), escitalopram (Strawn et al., 2019), fluvoxamine (The Research Unit on Pediatric Psychopharmacology Anxiety Study Group, 2001) and paroxetine (Wagner et al., 2004) have been evaluated in pediatric anxiety disorders. These individual clinical trials are reviewed in detail elsewhere and their primary findings are shown in Table 3. We will review the largest of these trials—the Child/Adolescent Anxiety Multimodal Anxiety Study (Walkup et al., 2008).
TABLE 3:
Randomized Placebo-Controlled Trials in Pediatric Anxiety Disorders
| Class | Reference | Disorder | N | Results |
|---|---|---|---|---|
| 5-HT1A modulator | Strawn et al, 2017 | GAD | 227 | Buspirone = placebo |
| Strawn et al, 2017 | GAD | 341 | Buspirone = placebo | |
| SSRI | RUPP, 2001 | Mixed anxiety disorders | 124 | Fluvoxamine > placebo |
| Strawn et al, 2019 | GAD | 51 | Escitalopram > placebo | |
| Rynn et al, 2001 | GAD | 22 | Sertraline > placebo | |
| Black and Uhde, 1994 | Selective mutism, SoAD, Avoidant Disorder | 15 | Fluoxetine > placebo | |
| Wagner et al, 2004 | SoAD | 322 | Paroxetine > placebo | |
| Beidel et al, 200* | SoAD | 122 | SET-C > fluoxetine > placebo | |
| Walkup et al, 2008 | Mixed anxiety disorders | 488 | CBT = sertraline > placebo CBT+sertraline > sertraline/ CBT CBT+sertraline > placebo |
|
| Birmaher et al, 2003 | Mixed anxiety disorders | 74 | Fluoxetine > placebo | |
| SNRI | Rynn et al, 2007 | GAD | 153 160 |
Venlafaxine ER > placebo |
| Strawn et al, 2015 | GAD | 272 | Duloxetine > placebo | |
| March et al, 200* | SoAD | 293 | Venlafaxine > placebo | |
| Geller et al, 2007 | Mixed anxiety disorders + ADHD | 176 | Atomoxetine > placebo | |
| TCA | Gittleman-Klein & Klein, 1971 | SoAD and mixed anxiety | 35 | Imipramine > placebo |
| Berney et al, 1981 | School refusal | 51 | Clomipramine = placebo | |
| Klein et al, 1992 | Separation anxiety with or without school avoidance | 21 | Imipramine = placebo | |
| BZD | Bernstein et al, 1990 | School refusal and Separation Anxiety Disorder | 24 | Alprazolam = imipramine = placebo |
| Simeon et al, 1992 | Overanxious Disorder and Avoidant Disorder | 30 | Alprazolam = placebo | |
| Grae et al, 1994 | Separation Anxiety Disorder | 15 | Clonazepam = placebo | |
| α2 agonist | Strawn et al, 2017 | Mixed anxiety disorders | 83 | Guanfacine = placebo for PARS Guanfacine > placebo for CGI-I. |
Abbreviations: SSRI- Selective Serotonin Reuptake Inhibitor; SNRI- Serotonin Norepinephrine Reuptake Inhibitor; BZD- Benzodiazepine; TCA, tricyclic antidepressant; PARS, pediatric anxiety rating scale, CGI-I, clinical global impression-improvement; SoAD, social anxiety disorder; SET-C- Social Effectiveness Therapy for Children; CBT- Cognitive Behavioral Therapy; CGI-S- Clinical Global Impression-Severity Scale; ADHD- Attention Deficit Hyperactive Disorder; GAD – Generalized Anxiety Disorder
The largest study of an SSRI in pediatric patients with anxiety, the Child/Adolescent Anxiety Multimodal Study (CAMS) (Walkup et al., 2008), compared sertraline (n=133), cognitive behavioral therapy (CBT) (n=139), combination therapy (CBT + sertraline) (n=140), and placebo (n=76). Patients were randomized 2:2:2:1 for treatment and placebo at six sites. CAMS included youth with separation, generalized and/or social anxiety disorders. This federally-funded trial included multiple measures of anxiety symptom severity (Caporino et al., 2013; Caporino et al., 2017) and safety (Rynn et al., 2015) as well as functional outcomes (Compton et al., 2010). CAMS facilitated the evaluation of treatment response as well as mediators and moderators of treatment response and its trajectory (Compton et al., 2014). In CAMS, sertraline was initiated at 25 mg/day and titrated to 200 mg/day with a mean dose of 134±60 mg per day in the combination-therapy group and 146± 61 mg per day in the sertraline group. More than half of sertraline patients (55%) exhibited improvement based on Clinical Global Impression- Improvement (CGI-I) scores while 81% of youth treated with cognitive based therapy (CBT) + sertraline met response criteria for (p<0.001) (compared to 60% in patients receiving CBT (p<0.001). All active treatments were statistically superior to placebo (Compton et al., 2010; Walkup et al., 2008) The most common adverse events for sertraline include headache, gastric distress, and insomnia. In terms of tolerability, there were no differences between the patients randomized to sertraline and placebo in terms of total physical and psychiatric adverse events or for any individual physical or psychiatric adverse events. Physical adverse events were more commonly reported in patients receiving sertraline compared to those receiving CBT (p<0.01) or CBT + sertraline (p<0.01), suggesting that CBT may ameliorate some adverse events, as has been observed in studies of adults with anxiety disorders. Additionally, the total psychiatric adverse event burden was higher in children (≤12 years) compared to adolescents across all treatment groups (Rynn et al., 2015). While this is the largest randomized controlled trial of any psychopharmacologic or psychotherapeutic combination (or their combination) in pediatric patients with anxiety disorders, there was no CBT+ placebo treatment. Some have suggested that the “absence of such a group prevented the investigators from determining whether the addition of sertraline to cognitive behavioral therapy resulted in more improvement than each treatment given separately because of an additive effect of two active treatments or because of the placebo effect of adding a pill to cognitive behavioral therapy” (Rifkin & Braga, 2009). In this regard, those receiving CBT in conjunction with a tablet knew they were receiving active medication, thus accentuating the expectation of treatment success in patients receiving sertraline + CBT (Rifkin & Braga, 2009).
In pediatric patients with anxiety disorders, the probability of SSRI-related improvement increases over time (Strawn, Dobson, et al., 2017) although response is, overall, logarithmic (Strawn, Mills, Sauley, & Welge, 2018)—70% of the improvement, observed at week 8, occurred in the first 4 weeks of treatment. This trajectory may not be unique SSRIs in pediatric anxiety disorders as SSRIs appear to produce similar, early improvement in youth with major depressive disorder and OCD (Varigonda, Jakubovski, & Bloch, 2016). Additionally, compared to SNRIs, SSRIs produce faster and greater improvement. Only 40% of the treatment response observed for SSRIs occurs in patients receiving SNRIs by the eighth week of treatment and the divergence in SSRI-SNRI trajectory emerges near the fourth week of treatment.
In general, SSRIs are well-tolerated in pediatric patients with generalized, separation and social anxiety disorders. However, only this year were adverse effects in these trials examined meta-analytically. In this met-analysis (κ=18, N=2,631) of 7 medications, SSRIs were associated with a greater likelihood of adverse event-related discontinuation (Relative Risk [RR]: 3.59, p=0.0003), activation (RR: 2.39, p=0.003), sedation (RR: 1.94, p=0.002), insomnia (RR: 1.93, p=0.001), abdominal pain (RR: 1.53, p=0.005) and headache (RR: 1.24, p=0.04). Activation was more common with SSRIs (vs. SNRIs, RR: 1.32, p=0.007). Neither SSRIs nor SNRIs were associated with treatment-emergent suicidality (Mills and Strawn, 2020). Among SSRIs, a meta-analysis found differences in suicidality, with sertraline potentially having lower rates of treatment-emergent suicidality while six treatments including paroxetine had higher rates of suicidality compared to placebo (Dobson, Bloch, & Strawn, 2019). That medications within the SSRI class are differentially associated with both higher and lower rates of suicidality compared to placebo with class-wise comparisons (e.g., SSRI vs placebo) finding no significant differences call into question the boxed warning applied to all antidepressants regardless of indication and specific medication (“US Food and Drug Administration. Relationship between psychotropic drugs and pediatric suicidality: review and evaluation of clinical data. Available at: http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004–4065b1–10-TAB08-Hammads-Review.pdf.,”). However, unlike recent network meta-analyses of SSRIs and SNRIs in youth (Cipriani et al., 2016; Dobson et al., 2019; Z Wang, SH, Sim, & al, 2017), the analyses that gave rise to the boxed warning on SSRIs and SNRIs as well as earlier analyses of suicidality utilized older meta-analytic techniques could not compare individual medications.
SNRIs in pediatric anxiety disorders
Several studies have examined venlafaxine in youth with generalized (κ=2, pooled analysis) ( Rynn, Riddle, Yeung, & Kunz, 2007) and social anxiety disorders (κ=1) (March, Entusah, Rynn, Albano, & Tourian, 2007) as well as duloxetine in generalized anxiety disorder (κ=1) (Strawn, Prakash, et al., 2015) and atomoxetine in pediatric patients with ADHD and co-morbid generalized, separation and/or social anxiety disorders (Geller et al., 2007).
In children and adolescents with social anxiety disorder, a 16-week randomized, placebo-controlled trial with venlafaxine ER randomized youth ages 8–17 (N=293) to venlafaxine ER or placebo. 56% of venlafaxine-treated patients exhibited improvement (CGI-I scale) compared to 37% of those receiving placebo. Two randomized, double-blind, placebo-controlled trials evaluated the efficacy and tolerability of venlafaxine ER in pediatric patients (aged 6 to 17 years) with generalized anxiety disorder (Rynn et al., 2007). Over the course of eight weeks, venlafaxine-treated patients (n=157) exhibited significant improvement in GAD symptom severity compared to those receiving placebo (n=163) for eight weeks. Overall, venlafaxine ER was well tolerated with the most common adverse events being: asthenia, anorexia, pain, and somnolence as well as increases in blood pressure and cholesterol levels (Rynn et al., 2007).
The SNRI duloxetine, is approved by the Food and Drug Administration for the treatment of GAD in children, adolescents and adults and has been evaluated in one double blind, placebo-controlled trial. In this study, pediatric patients with GAD (ages 7–17 years) were treated with flexibly-dosed duloxetine over a 10 week period (duloxetine: n=135; placebo: n=137) followed by 18 weeks of open-label duloxetine (30–120 mg daily) (Strawn et al., 2015). Compared to patients receiving with placebo, duloxetine-treated patients demonstrated reductions in anxiety symptom severity and had higher rates of remission. Additionally, CGI-S improved with 54% for patients treated with duloxetine attaining a score of ≤2 compared to 35% of patients receiving placebo (p<0.001) (Strawn, Prakash, et al., 2015).
One double-blind, placebo-controlled trial evaluated the potential efficacy of atomoxetine in patients with ADHD and co-occurring GAD, SAD and/or social anxiety disorder. Children and adolescents, aged 8–17 years, were randomized to atomoxetine (n=87) or placebo (n=89). Atomoxetine was titrated to 1.8 mg/kg and, over the course of 12 weeks of treatment, improvements were observed for the primary anxiety outcome measure, the Pediatric Anxiety Rating Scale (PARS, p=0.011). Atomoxetine was associated with an effect size (Cohen’s d) of 0.4 (Geller et al., 2007) which is lower that the effect size for SSRIs (Dobson et al., 2019; Locher et al., 2017), consistent with prior meta-analyses that suggest that more serotonergically selective antidepressants are associated with greater effect sizes (Strawn, Welge, et al., 2015) and that SSRIs are associated with greater and more rapid improvement compared to SNRIs (Strawn, Mills, Sauley, & Welge, 2018)
Tricyclics in the treatment of pediatric anxiety disorders
Two randomized, placebo-controlled trials evaluated imipramine in pediatric anxiety disorders. The first study focused on children and young adolescents with significant school avoidance, which often co-occurs with anxiety disorders and is seen by some as a behavioral symptom of anxiety and in particular separation anxiety disorder. This study evaluated youth who, during a two-week period, had significant school avoidance or “marked distress” at school (ages 6–14, N=35) (Gittelman-Klein & Klein, 1971). Imipramine was administered for six weeks (75 mg per day for two weeks, and flexibly titrated thereafter) and the final dose range was between 100 and 200 mg/day. Imipramine was superior to placebo in the study’s primary outcome measure, return to school (81% vs 47%, p<0.05). At six weeks, children treated with imipramine were also more likely to be rated as “much improved” by a psychiatrist when compared with placebo (73% vs 32%, p<0.025). Importantly, imipramine and placebo groups were not significantly different in terms of global improvement at three weeks. With the exception of dry mouth, no side effects were significantly more common compared to the rates observed in patients receiving placebo.
A second study evaluated the efficacy of imipramine in children with SAD (N=20, ages 6–15) (Klein, Koplewicz, & Kanner, 1992), but failed to replicate the earlier study’s findings. In this study, participants completed one month of an open-label behavioral intervention, and those who still met diagnostic criteria for SAD entered six weeks of double-blind, treatment with imipramine or placebo. Imipramine was initiated at 25 mg per day and titrated to a maximum dose of 5 mg/kg/day. Assessments of global improvement from treating psychiatrists, mothers, children’s self-report, and teachers ranged from 40 – 60% regardless of treatment and did not significantly differ between those treated with imipramine and those receiving placebo. Imipramine was associated with behavioral activation (e.g., “angry outbursts,” irritability, etc.) and, as with the first study of imipramine, dry mouth was commonly observed in the imipramine treated group (45% vs 11%).
Tricyclics (i.e., tricyclic antidepressants, TCAs), have fallen out of favor since the introduction of SSRIs and SNRIs due to comparatively poorer tolerability, including anticholinergic side effects, the need for electrocardiographic monitoring as well as their narrow therapeutic index that results in an increased risk of lethality in TCA overdoses (Woolf et al., 2007). Further, given the effects of TCAs on QTc (Corrected QT Interval) which may represent a risk factor for torsades de pointes (Leonard et al., 1995), baseline electrocardiograms (EKGs) are recommended to identify congenitally prolonged QTc or other arrhythmias and repeat EKGs should be obtained after dose titrations (Dodd et al., 2011).
5-HT1A modulators
Buspirone was evaluated in one flexibly-dosed (N=227) and one fixed-dose (N=341) trial in children and adolescents aged 6–17 years with a primary diagnosis of GAD (Strawn, Mills, et al., 2017). With regard to improvement in the sum of the Kiddie Schedule for Affective Disorders and Schizophrenia GAD items, buspirone did not separate from placebo in the fixed-dose trial at low (p=0.32,) or high dose (p=0.47) nor did it separate from placebo in the flexibly-dosed study (p=0.15). There were a number of factors that may have influenced these negative studies in terms of design and sample size (Strawn, Mills, et al., 2017).
In the analysis of pooled adverse events for the two efficacy studies, discontinuation related to an adverse event occurred more commonly in patients treated with buspirone relative to those treated with placebo (p<0.01). However, lightheadedness was the only adverse event that occurred more frequently, at a statistically significant level, in patients treated with buspirone relative to those receiving placebo (10% vs 2%, p<0.001). Finally, in the fixed-dose study the number of patients who dropped out as a result of an adverse event only trended towards being statically significant between the high-dose and low-dose groups (χ2=2.53, p=0.11). Finally, in two pharmacokinetic studies of buspirone in anxious youth, the maximum post-dose plasma concentrations (CMAX) for both buspirone and its metabolite were higher in children compared to adults (Salazar et al., 2001). This finding raises the possibility that in younger patients, dosing might need to be decreased and administered more frequently to approximate the pharmacokinetic profile of buspirone in older patients and adults.
Adrenergic receptor agonists in pediatric anxiety disorders
An extended-release preparation of the α2A agonist, guanfacine has been evaluated in youth aged 6 to 17 years with generalized, separation and/or social anxiety disorder. While this study was not designed or powered to detect efficacy and included an unbalanced randomization (3:1 guanfacine ER (GXR): placebo), exploratory efficacy measures were included (e.g., PARS; CGI). At endpoint, PARS scores improved in both groups with 32 GXR-treated patients having a CGI-I score ≤ 2 (54.2%) compared with only 6 patients who received placebo (31.6%). GXR was well tolerated with somnolence and fatigue (both p<0.03) occurring more frequently in GXR-treated patients compared to those receiving placebo. Additionally, decreases in heart rate, systolic and diastolic blood pressure were observed in GXR-treated patients. It is noteworthy that, in this study, nearly 50% of subjects had doses of 2 to 3 mg/day. This is important in light of fixed-dose studies of GXR that suggest that in pediatric patients with ADHD (Sallee, Lyne, Wigal, & McGough, 2009) greater weight-based doses are associated with increased improvement. Thus, to the extent that some anxiety symptoms may be adrenergically mediated, greater reductions in central noradrenergic tone could potentially yield greater improvement in anxiety symptoms.
Benzodiazepines in pediatric anxiety disorders
While there is considerable evidence for the use of benzodiazepines in the acute treatment of anxiety in youth (Kuang, Johnson, Mulqueen, & Bloch, 2017) and these medications appear to be well-tolerated in this setting, studies of chronic use of benzodiazepines in pediatric patients with generalized, separation and social anxiety disorders have generally failed to show benefit. In terms of treatment-emergent suicidality, benzodiazepines were, in our recent meta-analysis of psychopharmacologic treatments for pediatric anxiety disorders, associated with more suicidality-related adverse events than placebo (Dobson et al., 2019). However, this finding was driven by one small RCT (Graae, Milner, Rizzotto, & Klein, 1994) and additional studies are needed. Further, several recent studies in children and adolescents suggest adjunctive benzodiazepine use—at least in treatment resistant depression—are associated with significant tolerability concerns. For example, in the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) study, benzodiazepine use was associated with suicidal adverse events in 6 of 10 of patients who received benzodiazepines compared to 42 of 324 patients who did not receive benzodiazepines (Brent et al., 2009).
Combined psychotherapy and SSRIs in pediatric anxiety disorders
Expert reviews consistently recommend the combination of CBT+SSRIs in treating pediatric patients with anxiety disorders. However, relatively few studies have examined CBT in comparison to an SSRI or in combination with an SSRI in pediatric anxiety disorders. CAMS (N=488) found CBT + sertraline to be more effective than CBT or sertraline monotherapy. Remission rates for CBT + sertraline were superior to CBT (66% vs. 35%) after 12 weeks of treatment (Walkup et al., 2008). Yet, youth in CBT continued to improve relative to COMB such that by 24 weeks, the gap in remission rates narrowed (65% vs 45%) with no significant difference by week 36 (67% vs 58%) Piacetini. et al., 2014). Interpretation of the effects of CBT in CAMS has always been complicated by design decisions that may have diminished the effects of CBT: (1) CBT was shortened to conform to the ethically acceptable duration of pill placebo exposure; (2) sessions focused on exposure tasks; the key ingredient in CBT were reduced. (3) At the end of CAMS’ 12-week acute phase, regardless of remission status, CBT participants entered maintenance phase with a reduced visit schedule, and no initiation of any new CBT treatment potentially limiting restricting potential gains from week 12–24 and beyond. (3) CAMS CBT had no parent involvement until well into treatment, and even then, it was limited, an approach that is at odds with current practice. Despite these limitations, the differences between CBT and CBT+SSRI and SSRI treatment in CAMS warrant discussion. A recent study suggested that those youth with severe anxiety, require combined CBT+SSRI treatment (Taylor et al., 2018). Additionally, relative to either monotherapy, the probability of response for both CBT and CBT+ sertraline increases week-over week during the acute 12-week treatment period whereas the probability of achieving additional benefit plateaus by week 4 in patients receiving placebo and week 8 in patients receiving sertraline (Strawn et al., 2017). In youth with social anxiety disorder, aged 7–17 years, Beidel and colleagues examined Social Effectiveness Therapy for Children (SET-C) + fluoxetine and found that both fluoxetine and SET-C improved anxiety symptoms relative to placebo. Finally, adjunctive fluoxetine in adolescents with “school refusal” and at least one DSM-IV anxiety disorder (N = 62) who received CBT (15 sessions) over 22 weeks.(Melvin et al., 2017) Compared to CBT, CBT + fluoxetine and CBT + placebo. School attendance, the primary outcome measure, improved in all groups, as did anxiety and depressive symptoms and “clinician-rated global functioning,” dimensional measures of anxiety did not differ among treatment (Melvin et al., 2017).
Finally, regarding the relative improvement of cognitive and somatic symptoms in patients with anxiety disorders, prior studies suggested that both psychopharmacologic treatment and psychotherapy differentially improve these symptoms (Strawn, Geracioti, Rajdev, Clemenza, & Levine, 2018). However, relatively few studies have explored the differential effects of pharmacotherapy and psychotherapy on specific symptoms in pediatric anxiety disorders. Recently however, Hale and colleagues (2018) used causal mediation models to evaluate somatic symptoms in CAMS. Somatic symptom improvement was mediated by improvement in anxiety and global function in sertraline-treated patients, but a similar relationship was not observed in youth receiving CBT or CBT + sertraline (Hale et al., 2018).
Psychotherapy for Pediatric Anxiety Disorders
More than 20 randomized controlled trials (RCTs) document the benefits of cognitive behavior therapy (CBT) for pediatric anxiety disorders, with average between-group effect sizes ranging from 0.31 to 0.44 (see (Seligman & Ollendick, 2011; Silverman, Pina, & Viswesvaran, 2008, for reviews). However, several other psychotherapies have been examined in pediatric patients with anxiety disorders (e.g., mindfulness-based cognitive therapy (Cotton et al., 2016, 2019), psychodynamic psychotherapy (Abbass, Rabung, Leichsenring, Refseth, & Midgley, 2013; Göttken, White, Klein, & Von Klitzing, 2014), solution-focused brief therapy (Creswell et al., 2017)). However, among psychotherapies, CBT is considered a “well-established” treatment for pediatric anxiety as defined by stringent criteria (Chambless & Hollon, 1998; Southam-Gerow & Prinstein, 2014). It is recommended as a frontline intervention (Higa-McMillan, Francis, Rith-Najarian, & Chorpita, 2016; Mohatt, Bennett, & Walkup, 2014; Silverman et al., 2008) for pediatric anxiety given its efficacy, limited side effect burden, and patient and family preference for psychosocial treatment (Brown et al., 2007). It has established utility and child, family, and parent-only formats (Higa-McMillan et al., 2016).
CBT for pediatric anxiety involves a collection of therapeutic techniques, including psychoeducation, relaxation training, cognitive restructuring, and the practice of exposure tasks. Efforts to better understand its mechanisms of action and to isolate active ingredients have found that each of these components has therapeutic value for anxiety (Higa-McMillan et al., 2016) but that exposure practice is particularly important (Peris et al., 2015). The steepest clinical improvement emerges after its introduction in treatment (Peris et al., 2015) and more exposure—particularly more sessions in which it is practiced and more time spent on challenging tasks—is associated with better clinical response (Peris et al., 2017). Advances in inhibitory learning models of fear learning have shed light on the mechanisms underlying the practice of exposure and have in turn informed its application in treatment (Craske et al., 2008; Weisman & Rodebaugh, 2018). These models underscore the importance of eliciting variable levels of distress during practice and violating patient expectations about outcomes rather than focusing on habituation to distress.
Given that exposure practice requires youth to confront and tolerate distress, growing work has considered how therapeutic process variables shape clinical response to CBT for anxiety. Youth compliance and mastery of exposure are associated with better CBT response (Peris et al., 2017). In addition, those with more positive expectations about the value of exposure in therapy are more compliant with practice (Wu et al., 2019), underscoring the importance of psychoeducation. Indeed, compliance with exposure practice mediates the link between treatment expectations and anxiety symptom improvement (Wu et al., 2019). Other work has considered the role of therapeutic alliance and patient engagement, finding evidence for reciprocal relationships throughout the course of treatment (McLeod, Southam-Gerow, & Kendall, 2017). Importantly, youth report better relationships with their CBT therapists following the introduction of exposure (Cummings et al., 2014). At the same time, the gap between research and practice settings persists and among therapists, CBT adherence and competence—key components of treatment success-- are better in research versus community settings, with the latter group difference appearing particularly pronounced when exposures are introduced (McLeod et al., 2019).
These findings speak to the importance of expanding access to CBT interventions for anxiety. Accordingly, there has been growing emphasis on modular treatments that can be disseminated and implemented more easily and on alternate modes of delivery, including internet-based protocols. A recent meta-analysis of digital interventions examined 4 studies of internet-based CBT (iCBT) for pediatric anxiety disorders (Thabrew et al., 2018). Compared to treatment-as-usual or waitlist controls, iCBT improved anxiety symptoms with maintenance of gains at extended follow-up. iCBT platforms that included therapist support were more effective and had greater treatment engagement compared to self-guided internet interventions (Thabrew et al., 2018).
Predicting treatment response in pediatric anxiety disorders
In an examination of predictors and moderators of both improvement in anxiety (i.e., PARS) as well as response (at 12 weeks), in CAMS, more severe anxiety symptoms and parental caregiver strain at baseline predicted week 12 PARS scores regardless of treatment. Further, no demographic characteristics, parental /family psychopathology, expectation or other variables were associated with categorical response at week 12 (Compton et al., 2014). This study also examined moderators that “shed light on which treatment might confer the best outcomes for a child or adolescent with a certain baseline principal disorder.” In this analysis, patients with a primary diagnosis of separation anxiety disorder who received CBT + sertraline had the most “favorable outcomes” on the PARS, suggesting that for youth with a primary diagnosis of separation anxiety disorder, sertraline + CBT is superior to sertraline and CBT monotherapy and placebo. For patients with a primary diagnosis of SAD, treatments that included sertraline were associated with better outcomes compared to CBT monotherapy or placebo. CBT monotherapy was similar to placebo raising the possibility that for patients with a primary diagnosis of SAD, treatment should include an SSRI. Last, in patients with a primary diagnosis of GAD, treatments that included CBT were associated with better outcomes compared to sertraline monotherapy, although the latter was superior to placebo (Compton et al., 2014).
Studies of the pharmacogenomics and medication response in pediatric anxiety disorders are rare relative to studies in children and adolescents with major depressive disorder (MDD) and especially compared to adults (Ramsey, Bishop, & Strawn, 2019; Wehry, Ramsey, Dulemba, Mossman, & Strawn, 2018). Nonetheless, several studies have examined the impact of pharmacokinetically-relevant genes in pediatric patients with anxiety disorders and one double-blind, placebo-controlled trial examined pharmacokinetic and pharmacodynamic variants on the trajectory of SSRI response and SSRI tolerability. In sertraline treated pediatric patients with depressive and anxiety disorders, the maximum sertraline dose during the initial titration period is inversely associated with the number of CYP2C19 reduced functional alleles. Polymorphisms in the 5-HT2A receptor gene, HTR2A (rs6313) are associated with sertraline dose (p=0.011). In youth with anxiety and/or depressive disorders who were naturalistically treated with escitalopram or citalopram, slower CYP2C19 metabolizers experienced more untoward effects than faster metabolizers (p=0.015), including activation symptoms (p=0.029) and had more rapid weight gain (p=0.018) (Aldrich et al, 2017). In a prospective trial of adolescents with GAD who were treated with escitalopram (forced-flexible titration), slower metabolizers for CYP2C19 had greater and faster improvement (Strawn et al., 2019). In this sample, youth who were homozygous for the G allele of the HTR2A gene (consistent with lower expression) did not respond as well as those patients who had at least one A allele. Finally, adolescents who were homozygous short for the promotor region of the serotonin transporter polymorphism, SLC6A4, had reduced magnitude and trajectory of response compared to those who were had a long allele (Strawn et al., 2019). In this study, slower CYP2C19 metabolism was associated with greater escitalopram exposure and reduced clearance; the latter was also associated with escitalopram tolerability (Tulisiak et al., 2019).
Limitations
This review summarizes a broad and complex topic and our approach was intentionally unstructured so as to provide a general overview of selected and recent advances in the epidemiology, course, neurobiology and treatment of pediatric anxiety disorders. We reviewed and summarized a diverse body of the literature in each area of the review. However, this may over-represent heterogeneity among studies. Thus, we caution the reader not to indiscriminately take our conclusions but to review the studies that we included. Reviewing the primary studies, particularly the prospective treatment trials, is crucial to interpreting and contextualizing the data. Additionally, while we have summarized treatment findings, treating all psychotherapy or all CBT as one modality and all SSRIs as another and then comparing them risks losing the patient heterogeneity as well as the individual differences in psychotherapy (e.g., frequency of exposure, format of psychotherapy, developmental adaptation) and pharmacotherapy (e.g. dosing and exposure, pharmacogenetic factors, rate of titration, differences in tolerability). Ultimately, considering these factors is critical to the precision treatment for youth with anxiety disorders.
Conclusions
Anxiety disorders emerge during childhood and adolescence, with approximately 10% of youth receiving an anxiety disorder diagnosis prior to the age of 18 (Beesdo et al., 2010). Identifying the neurobiological mechanisms underlying pediatric anxiety disorders is critical to understand the emergence of anxiety during development, identify youth at elevated risk for anxiety, and optimize treatments for children and adolescents. Structural and functional alterations in frontolimbic circuitry have been consistently identified in pediatric patients with anxiety disorders and those at risk of developing anxiety disorders (Blackford & Pine, 2012; Guyer et al., 2013; Strawn, Bitter, et al., 2012; Strawn, Wehry, et al., 2013; Sylvester et al., 2016). However, we are only just beginning to understand the developmental aspects of this neurocircuitry and the way in which it can be leveraged to predict risk of developing anxiety and to predict treatment response. Collectively, recent research has significantly advanced the understanding of the epidemiology, risk, neurobiology and treatment of anxiety disorders in children and adolescents. Over the past decade, studies have refined our understanding of the trajectory, durability and heterogeneity of treatment response in children and adolescents with anxiety disorders. Increasingly, the questions asked by researchers in the field are more in line with the dilemmas encountered in the clinic. Taken together, these studies create many opportunities for researchers and clinicians to further expand our understanding in this field to areas such as:
Studies have identified risk factors for developing anxiety disorders. Now, we must develop interventions that that can halt the development of anxiety disorders.
We have developed a functional and structural neurobiologic scaffold for the pediatric anxiety disorders. Now, we must determine how neurobiology shifts in response to treatment or risk and how it can be leveraged to predict treatment response (or tolerability).
Dozens of studies demonstrate the efficacy of medications and psychotherapies. Now, we must determine how to select among these treatments and how to combine psychotherapy and medication and the longer-term outcomes of these treatments as well as what factors predict durability of response and remission.
Studies suggest that there are groups of patients who respond differently to broad classes of interventions. Now, we must identify multi-modal patient-level predictors of treatment response to identify, which patients will do best with what treatment. In other words, we must answer the question: Who gets better with what treatment? Should treatment differ based on family factors, patient characteristics (e.g., comorbidity, symptom severity)?
Table 2.
Functional neuroimaging findings in pediatric anxiety disorders
| Reference | Disorder | Patients (n) | Comparison subjects (n) | Mean Age | Medication | Task/Method | Findings |
|---|---|---|---|---|---|---|---|
| Thomas et al, 2001 | Mixed AD | 12 | 12 | 12 | NR | Passive viewing of fearful and neutral faces Whole-brain fMRI |
↑activation in the R amygdala for fearful faces than for neutral faces, which correlated with anxiety sx |
| Monk et al, 2006 | Mixed AD | 18 | 15 | 13 | Medication naïve | Probe detection task with emotional and neutral faces ROI-based fMRI |
↑ R VLPFC activation to angry faces which inversely correlates with the anxiety severity; no amygdala effects |
| McClure et al, 2007 | Mixed AD | 15 | 20 | 12 | Medication free | Face-emotion rating task with fearful, happy, neutral, and angry faces ROI-based fMRI and FC |
activation to fearful faces > happy faces in R amygdala, vPFC, and ACC; ↑FC between R amygdala and R insula, posterior cingulate cortex, L precuneus and right lingual gyrus (uncorrected) |
| Beesdo et al, 2009 | Mixed AD | 16 | 45 | 14 | Medication free | Face-emotion rating task with fearful, happy, neutral, and angry faces ROI-based fMRI |
↑ bilateral amygdala activation when viewing of fearful vs happy faces; ↑ L OFC activation during fearful-afraid vs fearful-passive contrast |
| Monk et al, 2008 | GAD | 17 | 12 | 14 | Medication naïve | Probe detection task with masked emotional (angry, happy) and neutral faces pair ROI-based fMRI and FC |
↑ R amygdala activation during viewing of angry faces, which correlates with anxiety sx severity. |
| Maslowsky et al, 2010 | Mixed AD | 14 | 10 | 14 | NR | Probe detection task with emotional (angry, happy) and neutral faces ROI-based fMRI |
↑bilateral activation during viewing of fearful faces following treatment with CBT. Treatment-associated ↑in R VLPFC activation during viewing of fearful faces, following CBT or fluoxetine. |
| McClure-Tone et al, 2011 | Mixed AD | 12 | 17 | 13 | Neural response to co-player defection during Prisoner's dilemma game ROI-based fMRI |
↑ activation in precuneus and right TPJ and ↓ activation in mPFC/ACC toward co-player defection, betrayal, and mutual defection | |
| Guyer et al. 2012 | SAD | 14 | 26 | 14 | Medication free | Monetary incentive delay task ROI-based fMRI |
↑ caudate and putamen activation as increasing magnitude of anticipating incentives; |
| Guyer et al. 2012 | GAD | 18 | 26 | 14 | Medication free | Monetary incentive delay task ROI-based fMRI |
the group effect on the putamen was modulated by valence, greater putamen activation during potential gain versus loss trials |
| Strawn et al. 2012a | GAD | 10 | 10 | 14 | NR | Continuous performance task with emotional and neutral distractors. Whole-brain fMRI and ROI-based FC | ↑ activation of the L mPFC and R VLPFC in response to emotional vs neutral images; ↑ Fc between R amygdala and L posterior cingulate, and R VLPFC and bilateral mPFC, ↓ Fc between L amygdala and ipsilateral precuneus |
| Fitzgerald et al. 2013 | Mixed AD | 23 | 25 | 14 | Medication free | Multisource interference task including conflict processing and error processing Whole-brain and ROI-based fMRI |
↓ dlPFC activation during error processing |
| Britton et al. 2013a | Mixed AD | 23 | 42 | 14 | Medication free | Fear conditioning and extinction ROI-based fMRI |
↑ vmPFC activation response to the morphed images at the extremes of the continuum relative to the blended images during threat appraisal; ↑ subgenual ACC activation during explicit memory |
| Britton et al. 2013b | Mixed AD | 17 | 16 | 15 | Medication free | dot-probe task ROI-based fMRI |
No difference between groups |
| Swartz et al. 2014a | Mixed AD | 34 | 35 | 15 | Medication free | Emotional faces shifting attention task ROI-based fMRI |
↑ activation in rACC during shape versus face matching; No difference after excluded participants older than 18 |
| Swartz et al. 2014b | Mixed AD | 34 | 19 | 14 | Medication free | Emotional face-matching task Dynamic fMRI and Fc |
↑ amygdala activation and significant ↓response over time; ↓FC of context-modulated PFC-amygdala during the beginning of scanning |
| Spielberg et al. 2014 | Mixed AD | 16 | 26 | 13 | NR | Anticipating social feedback from peers during chatroom task ROI-based fMRI and FC |
↑ L amygdala activation when anticipating feedback from rejected peers; ↓ NuAcc activation when anticipating feedback from selected peers; ↑ amygdala-ACC Fc when anticipating peer feedback |
| Williams et al. 2015 | Mixed AD | 20 | 20 | 10 | Medication free | Anticipation task including fear and neutral faces ROI-based and whole brain fMRI |
↑ L amygdala response to “uncertain” vs. “certain” cues; ↑ L amygdala activation compare faces preceded by an “uncertain” cue with “certain” cue; ↑ R lingual gyrus and L insula activation during “uncertain” cues |
| Haddad et al. 2015 | Mixed AD | 15 | 11 | 15 | Medication free | Fear conditioning paradigm, threat, safety and control cue ROI-based and whole brain fMRI |
↓ activation in medial PFC/paracingulate, bilateral amygdala, R hippocampus, and vmPFC for threat vs control cues |
| Jarcho et al. 2015 | Mixed AD | 15 | 24 | 13 | Medication free | Prediction errors of social feedback from peers during chatroom task Whole-brain fMRI and ROI-based FC | ↑ bilateral striatal activation and ↑ negative mPFC-striatal FC during unexpected relative to expected feedback |
| Carpenter et al. 2015 | Mixed AD | 22 | 23 | 7 | NR | Passive viewing of angry and fearful faces | ↓L dlPFC activity in response to angry faces |
| Kujawa et al. 2016 | Mixed AD | 57 | 61 | 17 | Medication free | Emotional face-matching task Whole-brain and ROI-based fMRI and FC |
No difference between groups |
| Gold et al. 2016 | Mixed AD | 14 | 25 | 14 | Medication free | Fear acquistion/extinction task and extinction recall task ROI-based FC |
↑ negative L amygdala–vmPFC connectivity in threat relative to nonthreat conditions |
| White et al. 2017 | Mixed AD | 54 | 51 | 12 | Medication free | dot-probe task (angry-neutral, neutral-neutral expressions) ROI-based FC |
No difference between groups on amygdala connectivity |
| Yin et al.2017 | GAD | 20 | 14 | 16 | Medication naïve | Emotional valence-evaluation task including positive, neutral and negative pictures Whole-brain fMRI |
↓ activation of the R inferior frontal gyrus in response to evaluation of negative versus neutral picture, which was negatively correlated with severity of anxiety symptoms |
| Carlisi et al. 2017 | Mixed AD | 14 | 19 | 14 | Medication free | Face-attention paradigm focused on negative versus neutral faces, across attention states Whole-brain fMRI |
↑activation to negative vs neutral faces in the bilateral cerebellum, and ↓ activation in vmPFC |
| Burkhouse et al. 2018 | Mixed AD | 25 | 29 | 16 | Medication free | Emotional faces shifting attention task Whole-brain fMRI |
↓ R ACC activation during implicit threat processing (pre-treatment) ↑ activation of rACC related to greater improvement in social anxiety/avoidance sx |
Abbreviations: GAD= Generalized Anxiety Disorder; SAD, Social Anxiety Disorder; SepAD, Separation Anxiety Disorder; Fc, Functional Connectivity; FCS, functional connectivity strength; SCARED-PC, Screen for Child Anxiety Related Emotional Disorders Parent and Child Composite Score; FCS, functional connectivity strength; NR, not report; GMV, gray matter volume; WMV, white matter volume; VBM, Voxel-based morphometry; SBM, Surface-Based Morphometry; L, left; R, right.
Key Points.
The risk of developing anxiety disorders varies substantially among children and adolescents and some risk factors can be identified early in life.
Structure, function and neurochemistry are altered in amygdala and prefrontal circuits in children and adolescents with anxiety disorders as well as those at risk for developing anxiety disorders.
Psychotherapeutically and psychopharmacologically, CBT and SSRIs represent the first line treatments for pediatric patients with anxiety disorders. However, the best outcomes are achieved when the treatments are combined.
Further research is needed to enhance outcomes and reduce the considerable public health burden of pediatric anxiety disorders in addition to the risk of developing secondary depressive disorders in youth with anxiety disorders.
Acknowledgments
This review was invited by the Editors of JCPP. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD) through Grant R01HD098757 and from the Yung Family Foundation. The authors appreciate the assistance from the Anxiety Disorders Research Program at the University of Cincinnati (Sarah Mossman, MA, Heidi Schroeder, BS, Ashely Spect, BS and Sara Varney, BS) for technical assistance with the preparation of this manuscript.
Disclosures: Dr. Strawn has received research support from the National Institutes of Health (NIMH/NIEHS/NICHD) as well as Allergan, Neuronetics and Otsuka. He has received material support from and provided consultation to Myriad Genetics and receives royalties from the publication of two texts (Springer) and serves as an author for UpToDate and an Associate Editor for Current Psychiatry. Dr. Strawn also receive research support from the Yung Family Foundation. Dr. Lu receives support from a Chinese Government Scholarship. Dr. Levine has received research support from the National Institutes of Health (NICHD). Dr. Peris has received research from the NIMH and receives royalties from Oxford Publishing. Dr. Walkup has received research support from the Tourette Syndrome Association of America and the Hartwell Foundation. He has received honoraria and travel expenses for speaking engagements and meetings sponsored by the Tourette Association of America. He has received royalties from Guilford Press and Oxford University Press for multi‐ author books published about Tourette’s syndrome and from Wolters Kluwer for CME activity on childhood anxiety. He has served as an unpaid advisor to the Anxiety Disorders Association of America and the Trichotillomania Learning Center. He has served as a paid speaker for the Tourette Syndrome—Center for Disease Control and Prevention outreach educational programs, the American Academy of Child and Adolescent Psychiatry, and the American Psychiatric Association.
References
- 1.Abbass AA, Rabung S, Leichsenring F, Refseth JS, & Midgley N (2013). Psychodynamic psychotherapy for children and adolescents: A meta-analysis of short-term psychodynamic models. Journal of the American Academy of Child and Adolescent Psychiatry 10.1016/j.jaac.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 2.Asselmann E, Wittchen H-U, Lieb R, & Beesdo-Baum K (2018). Sociodemographic, clinical, and functional long-term outcomes in adolescents and young adults with mental disorders. Acta Psychiatrica Scandinavica, 137(1), 6–17. 10.1111/acps.12792 [DOI] [PubMed] [Google Scholar]
- 3.Asselmann E, Wittchen H-U, Lieb R, Höfler M, & Beesdo-Baum K (2014). Associations of fearful spells and panic attacks with incident anxiety, depressive, and substance use disorders: A 10-year prospective-longitudinal community study of adolescents and young adults. Journal of Psychiatric Research, 55, 8–14. 10.1016/j.jpsychires.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 4.Beesdo-Baum K, & Knappe S (2012). Developmental Epidemiology of Anxiety Disorders. Child and Adolescent Psychiatric Clinics of North America. 10.1016/j.chc.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 5.Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, & Wittchen H-U (2007). Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry, 64(8), 903–12. 10.1001/archpsyc.64.8.903 [DOI] [PubMed] [Google Scholar]
- 6.Beesdo K, Knappe S, & Pine DS (2009). Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. The Psychiatric Clinics of North America, 32(3), 483–524. 10.1016/j.psc.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beesdo K, Lau JYF, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, … Pine DS (2009). Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry, 66(3), 275–85. 10.1001/archgenpsychiatry.2008.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beesdo K, Pine DS, Lieb R, & Wittchen H-U (2010). Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Archives of General Psychiatry, 67(1), 47–57. 10.1001/archgenpsychiatry.2009.177 [DOI] [PubMed] [Google Scholar]
- 9.Beidel DC, Turner SM, Sallee FR, Ammerman RT, Crosby LA, & Pathak S (2007). SET-C versus fluoxetine in the treatment of childhood social phobia. Journal of the American Academy of Child and Adolescent Psychiatry, 46(12), 1622–32. 10.1097/chi.0b013e318154bb57 [DOI] [PubMed] [Google Scholar]
- 10.BERNSTEIN GA, GARFINKEL BD, & BORCHARDT CM (1990). Comparative Studies of Pharmacotherapy for School Refusal. Journal of the American Academy of Child & Adolescent Psychiatry, 29(5), 773–781. 10.1097/00004583-199009000-00016 [DOI] [PubMed] [Google Scholar]
- 11.Birmaher B, Axelson D. a, Monk K, Kalas C, Clark DB, Ehmann M, … Brent D. a. (2003). Fluoxetine for the treatment of childhood anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 42(4), 415–23. 10.1097/01.CHI.0000037049.04952.9F [DOI] [PubMed] [Google Scholar]
- 12.Blackford JU, & Pine DS (2012). Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child Adolesc Psychiatr Clin N Am, 21(3), 501–525. 10.1016/j.chc.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumenthal H, Cloutier RM, Zamboanga BL, Bunaciu L, & Knapp AA (2015). A laboratory-based test of the relation between adolescent alcohol use and panic-relevant responding. Experimental and Clinical Psychopharmacology. 10.1037/pha0000022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouton ME (2002). Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry 10.1016/S0006-3223(02)01546-9 [DOI] [PubMed] [Google Scholar]
- 15.Brand S, Wilhelm FH, Kossowsky J, Holsboer-Trachsler E, & Schneider S (2011). Children suffering from separation anxiety disorder (SAD) show increased HPA axis activity compared to healthy controls. Journal of Psychiatric Research, 45(4), 452–459. 10.1016/j.jpsychires.2010.08.014 [DOI] [PubMed] [Google Scholar]
- 16.Brent DA, Emslie GJ, Clarke GN, Asarnow J, Spirito A, Ritz L, … Keller MB (2009). Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. American Journal of Psychiatry, 166(4), 418–426. 10.1176/appi.ajp.2008.08070976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Britton JC, Grillon C, Lissek S, Norcross MA, Szuhany KL, Chen G, … Pine DS (2013). Response to learned threat: An fMRI study in adolescent and adult anxiety. American Journal of Psychiatry. 10.1176/appi.ajp.2013.12050651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Britton JC, Lissek S, Grillon C, Norcross MA, & Pine DS (2011). Development of anxiety: The role of threat appraisal and fear learning. Depression and Anxiety 10.1002/da.20733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brokamp C, Strawn JR, Beck AF, & Ryan P (2019). Pediatric Psychiatric Emergency Department Utilization and Fine Particulate Matter : A Case-Crossover Study. Environmental Health Perspectives, 127(9), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunst KJ, Ryan PH, Altaye M, Yolton K, Maloney T, Beckwith T, … Cecil KM (2019). Myo-inositol mediates the effects of traffic-related air pollution on generalized anxiety symptoms at age 12 years. Environmental Research 10.1016/j.envres.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buhr K, & Dugas MJ (2009). The role of fear of anxiety and intolerance of uncertainty in worry: An experimental manipulation. Behaviour Research and Therapy 10.1016/j.brat.2008.12.004 [DOI] [PubMed] [Google Scholar]
- 22.Burkhouse KL, Kujawa A, Hosseini B, Klumpp H, Fitzgerald KD, Langenecker SA, … Phan KL (2018). Anterior cingulate activation to implicit threat before and after treatment for pediatric anxiety disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry 10.1016/j.pnpbp.2018.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bushnell GA, Compton SN, Dusetzina SB, Gaynes BN, Brookhart MA, Walkup JT, … Sturmer T (2018). Treating pediatric anxiety: Initial use of SSRIs and other antianxiety prescription medications. Journal of Clinical Psychiatry 10.4088/JCP.16m11415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caporino NE, Brodman DM, Kendall PC, Albano AM, Sherrill J, Piacentini J, … Walkup JT (2013). Defining treatment response and remission in child anxiety: signal detection analysis using the pediatric anxiety rating scale. J Am Acad Child Adolesc Psychiatry, 52(1), 57–67. 10.1016/j.jaac.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caporino NE, Sakolsky D, Brodman DM, McGuire JF, Piacentini J, Peris TS, … Birmaher B (2017). Establishing Clinical Cutoffs for Response and Remission on the Screen for Child Anxiety Related Emotional Disorders (SCARED). Journal of the American Academy of Child and Adolescent Psychiatry 10.1016/j.jaac.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caraci F, Enna SJ, Zohar J, Racagni G, Zalsman G, van den Brink W, … Drago F (2017). A new nomenclature for classifying psychotropic drugs. British Journal of Clinical Pharmacology. 10.1111/bcp.13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caspi A, & Moffitt TE (2018). All for one and one for all: Mental disorders in one dimension. American Journal of Psychiatry. 10.1176/appi.ajp.2018.17121383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambless DL, & Hollon SD (1998). Defining empirically supported therapies. Journal of Consulting and Clinical Psychology 10.1037/0022-006X.66.1.7 [DOI] [PubMed] [Google Scholar]
- 29.Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, … Xie P (2016). Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. The Lancet, 388(10047), 881–890. 10.1016/S0140-6736(16)30385-3 [DOI] [PubMed] [Google Scholar]
- 30.CLARK DB, BIRMAHER B, AXELSON D, MONK K, KALAS C, EHMANN M, … BRENT D (2005). Fluoxetine for the Treatment of Childhood Anxiety Disorders: Open-Label, Long-Term Extension to a Controlled Trial. Journal of the American Academy of Child & Adolescent Psychiatry, 44(12), 1263–1270. 10.1097/01.chi.0000183464.41777.c1 [DOI] [PubMed] [Google Scholar]
- 31.Clarkson T, Eaton NR, Nelson EE, Fox NA, Leibenluft E, Pine DS, … Jarcho JM (2019). Early childhood social reticence and neural response to peers in preadolescence predict social anxiety symptoms in midadolescence. Depression and Anxiety 10.1002/da.22910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clauss JA, Benningfield MM, Rao U, & Blackford JU (2016). Altered Prefrontal Cortex Function Marks Heightened Anxiety Risk in Children. Journal of the American Academy of Child and Adolescent Psychiatry 10.1016/j.jaac.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clauss JA, & Blackford JU (2012). Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry, 51(10), 1066–1075.e1. 10.1016/j.jaac.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Compton SN, Peris TS, Almirall D, Birmaher B, Sherrill J, Kendall PC, … Albano AM (2014). Predictors and moderators of treatment response in childhood anxiety disorders: results from the CAMS trial. Journal of Consulting and Clinical Psychology, 82(2), 212–24. 10.1037/a0035458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Compton SN, Walkup JT, Albano AM, Piacentini JC, Birmaher B, Sherrill JT, … March JS (2010). Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child Adolesc Psychiatry Ment Health, 4, 1 10.1186/1753-2000-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Compton SN, Walkup JT, Albano AM, Piacentini JC, Birmaher B, Sherrill JT, … March JS (2010). Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child and Adolescent Psychiatry and Mental Health, 4, 1 10.1186/1753-2000-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Connolly SD, & Bernstein GA (2007). Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 46(2), 267–83. 10.1097/01.chi.0000246070.23695.06 [DOI] [PubMed] [Google Scholar]
- 38.Cotton S, Kraemer KM, Sears RW, Strawn JR, Wasson RS, McCune N, … Delbello MP (2019). Mindfulness-based cognitive therapy for children and adolescents with anxiety disorders at-risk for bipolar disorder: A psychoeducation waitlist controlled pilot trial. Early Intervention in Psychiatry. 10.1111/eip.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cotton S, Luberto CM, Sears RW, Strawn JR, Stahl L, Wasson RS, … Delbello MP (2016). Mindfulness-based cognitive therapy for youth with anxiety disorders at risk for bipolar disorder: a pilot trial. Early Intervention in Psychiatry, 10(5), 426–434. 10.1111/eip.12216 [DOI] [PubMed] [Google Scholar]
- 40.Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, & Baker A (2008). Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy 10.1016/j.brat.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 41.Crawley SA, Caporino NE, Birmaher B, Ginsburg G, Piacentini J, Albano AM, … Kendall PC (2014). Somatic complaints in anxious youth. Child Psychiatry and Human Development 10.1007/s10578-013-0410-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Creswell C, Violato M, Fairbanks H, White E, Parkinson M, Abitabile G, … Cooper PJ (2017). Clinical outcomes and cost-effectiveness of brief guided parent-delivered cognitive behavioural therapy and solution-focused brief therapy for treatment of childhood anxiety disorders: a randomised controlled trial. The Lancet Psychiatry 10.1016/S2215-0366(17)30149-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Creswell C, Waite P, & Hudson J (2020). Practitioner Review: Anxiety disorders in children and young people – assessment and treatment. Journal of Child and Adolescent Psychopharmocology 10.1111/jcpp.13186 [DOI] [PubMed] [Google Scholar]
- 44.da Costa CZG, de Morais RMCB, Zanetta DMT, Turkiewicz G, Neto FL, Morikawa M, … Asbahr FR (2013). Comparison Among Clomipramine, Fluoxetine, and Placebo for the Treatment of Anxiety Disorders in Children and Adolescents. Journal of Child and Adolescent Psychopharmacology, 23(10), 687–692. 10.1089/cap.2012.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, … Ryan ND (2000). A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry, 48(1), 51–57. 10.1016/S0006-3223(00)00835-0 [DOI] [PubMed] [Google Scholar]
- 46.Degenhardt L, Coffey C, Romaniuk H, Swift W, Carlin JB, Hall WD, & Patton GC (2013). The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction 10.1111/j.1360-0443.2012.04015.x [DOI] [PubMed] [Google Scholar]
- 47.Dobson ET, Bloch MH, & Strawn JR (2019). Efficacy and tolerability of pharmacotherapy in pediatric anxiety disorders: a network meta-analysis. Journal of Clinical Psychiatry, 80(1), 17r12064. [DOI] [PubMed] [Google Scholar]
- 48.Dodd S, Malhi GS, Tiller J, Schweitzer I, Hickie I, Khoo JP, … Berk M (2011). A consensus statement for safety monitoring guidelines of treatments for major depressive disorder. The Australian and New Zealand Journal of Psychiatry, 45(9), 712–725. 10.3109/00048674.2011.595686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Domakonda MJJ, He X, Lee S, Cyr M, & Marsh R (2019). Increased Functional Connectivity Between Ventral Attention and Default Mode Networks in Adolescents With Bulimia Nervosa. Journal of the American Academy of Child and Adolescent Psychiatry 10.1016/j.jaac.2018.09.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellis HH, Fisher PA, & Zaharie S (2004). Predictors of disruptive behavior, developmental delays, anxiety, and affective symptomatology among institutionally reared Romanian children. Journal of the American Academy of Child and Adolescent Psychiatry 10.1097/01.chi.0000136562.24085.160 [DOI] [PubMed] [Google Scholar]
- 51.Fluvoxamine for the treatment of anxiety disorders in children and adolescents. The Research Unit on Pediatric Psychopharmacology Anxiety Study Group. (2001). N Engl J Med, 344(17), 1279–1285. 10.1056/nejm200104263441703 [DOI] [PubMed] [Google Scholar]
- 52.Fox AS, Oler JA, Tromp DPM, Fudge JL, & Kalin NH (2015). Extending the amygdala in theories of threat processing. Trends in Neurosciences. 10.1016/j.tins.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geller D, Donnelly C, Lopez F, Rubin R, Newcorn J, Sutton V, … Sumner C (2007). Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 46(9), 1119–1127. 10.1097/chi.0b013e3180ca8385 [DOI] [PubMed] [Google Scholar]
- 55.Gittelman-Klein R, & Klein DF (1971). School phobia: controlled imipramine treatment. California Medicine, 115(3), 42. [PMC free article] [PubMed] [Google Scholar]
- 56.Gold AL, Steuber ER, White LK, Pacheco J, Sachs JF, Pagliaccio D, … Pine DS (2017). Cortical thickness and subcortical gray matter volume in pediatric anxiety disorders. Neuropsychopharmacology 10.1038/npp.2017.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Göttken T, White LO, Klein AM, & Von Klitzing K (2014). Short-term psychoanalytic child therapy for anxious children: A pilot study. Psychotherapy 10.1037/a0036026 [DOI] [PubMed] [Google Scholar]
- 58.Graae F, Milner J, Rizzotto L, & Klein RG (1994). Clonazepam in childhood anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 33(3), 372–376. 10.1097/00004583-199403000-00011 [DOI] [PubMed] [Google Scholar]
- 59.Green SA, Goff B, Gee DG, Gabard-Durnam L, Flannery J, Telzer EH, … Tottenham N (2016). Discrimination of amygdala response predicts future separation anxiety in youth with early deprivation. Journal of Child Psychology and Psychiatry and Allied Disciplines 10.1111/jcpp.12578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guyer AE, Masten CL, & Pine DS (2013). Neurobiology of Pediatric Anxiety Disorders. Pediatric Anxiety Disorders, 23–46. 10.1007/978-1-4614-6599-7_2 [DOI] [Google Scholar]
- 61.Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler AD, … Ernst M (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20(9), 1565–1582. 10.1162/jocn.2008.20114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haddad ADM, Bilderbeck A, James AC, & Lau JYF (2015). Fear responses to safety cues in anxious adolescents: Preliminary evidence for atypical age-associated trajectories of functional neural circuits. Journal of Psychiatric Research. 10.1016/j.jpsychires.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 63.Hale AE, Ginsburg GS, Chan G, Kendall PC, McCracken JT, Sakolsky D, … Walkup JT (2018). Mediators of Treatment Outcomes for Anxious Children and Adolescents: The Role of Somatic Symptoms. Journal of Clinical Child and Adolescent Psychology 10.1080/15374416.2017.1280804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, … Pérez-Edgar K (2013). Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biological Psychiatry 10.1016/j.biopsych.2013.01.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higa-McMillan CK, Francis SE, Rith-Najarian L, & Chorpita BF (2016). Evidence Base Update: 50 Years of Research on Treatment for Child and Adolescent Anxiety. Journal of Clinical Child and Adolescent Psychology 10.1080/15374416.2015.1046177 [DOI] [PubMed] [Google Scholar]
- 66.Hudson JL, Dodd HF, Lyneham HJ, & Bovopoulous N (2011). Temperament and family environment in the development of anxiety disorder: Two-year follow-up. Journal of the American Academy of Child and Adolescent Psychiatry 10.1016/j.jaac.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 67.Humphreys KL, Gleason MM, Drury SS, Miron D, Nelson CA, Fox NA, & Zeanah CH (2015). Effects of institutional rearing and foster care on psychopathology at age 12 years in Romania: Follow-up of an open, randomised controlled trial. The Lancet Psychiatry 10.1016/S2215-0366(15)00095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.J., P., S., B., S.N., C., P.C., K., B., B., A.M., A., … Walkup J (2014). 24- and 36-week outcomes for the child/adolescent anxiety multimodal study (CAMS). Journal of the American Academy of Child and Adolescent Psychiatry 10.1016/j.jaac.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Janak PH, & Tye KM (2015). From circuits to behaviour in the amygdala. Nature 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jarcho JM, Romer AL, Shechner T, Galvan A, Guyer AE, Leibenluft E, … Nelson EE (2015). Forgetting the best when predicting the worst: Preliminary observations on neural circuit function in adolescent social anxiety. Developmental Cognitive Neuroscience 10.1016/j.dcn.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, … Blumberg HP (2009). Relation between amygdala structure and function in adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 10.1097/CHI.0b013e31819f6fbc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kendall PC, Compton SN, Walkup JT, Birmaher B, Albano AM, Sherrill J, … Piacentini J (2010). Clinical characteristics of anxiety disordered youth. Journal of Anxiety Disorders, 24(3), 360–365. 10.1016/j.janxdis.2010.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kessler RC, Chiu WT, Demler O, Merikangas KR, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 617–27. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Killgore WDS, & Yurgelun-Todd DA (2005). Social anxiety predicts amygdala activation in adolescents viewing fearful faces. NeuroReport 10.1097/01.wnr.0000180143.99267.bd [DOI] [PubMed] [Google Scholar]
- 75.Klein RG, Koplewicz HS, & Kanner A (1992). Imipramine treatment of children with separation anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 31(1), 21–28. 10.1097/00004583-199201000-00005 [DOI] [PubMed] [Google Scholar]
- 76.Kuang H, Johnson JA, Mulqueen JM, & Bloch MH (2017). The efficacy of benzodiazepines as acute anxiolytics in children: A meta-analysis. Depression and Anxiety 10.1002/da.22643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kujawa A, Swain JE, Hanna GL, Koschmann E, Simpson D, Connolly S, … Phan KL (2016). Prefrontal Reactivity to Social Signals of Threat as a Predictor of Treatment Response in Anxious Youth. Neuropsychopharmacology 10.1038/npp.2015.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lau JYF, & Waters AM (2017). Annual Research Review: An expanded account of information-processing mechanisms in risk for child and adolescent anxiety and depression. Journal of Child Psychology and Psychiatry and Allied Disciplines 10.1111/jcpp.12653 [DOI] [PubMed] [Google Scholar]
- 79.Lebowitz ER, Blumberg HP, & Silverman WK (2019). Negative peer social interactions and oxytocin levels linked to suicidal ideation in anxious youth. Journal of Affective Disorders, 245, 806–811. 10.1016/j.jad.2018.11.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lebowitz ER, Scharfstein LA, & Jones J (2014). Comparing family accommodation in pediatric obsessive-compulsive disorder, anxiety disorders, and nonanxious children. Depression and Anxiety. 10.1002/da.22251 [DOI] [PubMed] [Google Scholar]
- 81.Lebowitz ER, Woolston J, Bar-Haim Y, Calvocoressi L, Dauser C, Warnick E, … Leckman JF (2013). FAMILY ACCOMMODATION IN PEDIATRIC ANXIETY DISORDERS. Depression and Anxiety, 30(1), 47–54. 10.1002/da.21998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, & Zarate CA (2017). Glutamate and Gamma-Aminobutyric Acid Systems in the Pathophysiology of Major Depression and Antidepressant Response to Ketamine. Biological Psychiatry, 81(10), 886–897. 10.1016/j.biopsych.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leonard HL, Meyer MC, Swedo SE, Richter D, Hamburger SD, Allen AJ, … Tucker E (1995). Electrocardiographic changes during desipramine and clomipramine treatment in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 34(11), 1460–1468. 10.1097/00004583-199511000-00012 [DOI] [PubMed] [Google Scholar]
- 84.Lewis-Morrarty E, Degnan KA, Chronis-Tuscano A, Pine DS, Henderson HA, & Fox NA (2015). Infant Attachment Security and Early Childhood Behavioral Inhibition Interact to Predict Adolescent Social Anxiety Symptoms. Child Development 10.1111/cdev.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li W, Mai X, & Liu C (2014). The default mode network and social understanding of others: What do brain connectivity studies tell us. Frontiers in Human Neuroscience, 8(1 FEB). 10.3389/fnhum.2014.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Livny A, Cohen K, Tik N, Tsarfaty G, Rosca P, & Weinstein A (2018). The effects of synthetic cannabinoids (SCs) on brain structure and function. European Neuropsychopharmacology. 10.1016/j.euroneuro.2018.07.095 [DOI] [PubMed] [Google Scholar]
- 87.Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, … Kossowsky J (2017). Efficacy and Safety of Selective Serotonin Reuptake Inhibitors, Serotonin-Norepinephrine Reuptake Inhibitors, and Placebo in Common Psychiatric Disorders A Meta-analysis in Children and Adolescents. Jama Psychiatry, 74(10), 1011–1020. 10.1001/jamapsychiatry.2017.2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.March JS, Entusah AR, Rynn M, Albano AM, & Tourian KA (2007). A Randomized controlled trial of venlafaxine ER versus placebo in pediatric social anxiety disorder. Biological Psychiatry, 62(10), 1149–54. 10.1016/j.biopsych.2007.02.025 [DOI] [PubMed] [Google Scholar]
- 89.Martin J, Kagerbauer SM, Gempt J, Podtschaske A, Hapfelmeier A, & Schneider G (2018). Oxytocin levels in saliva correlate better than plasma levels with concentrations in the cerebrospinal fluid of patients in neurocritical care. Journal of Neuroendocrinology, 30(5). 10.1111/jne.12596 [DOI] [PubMed] [Google Scholar]
- 90.Maslowsky J, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS, & Monk CS (2010). A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. Journal of Child and Adolescent Psychopharmacology 10.1089/cap.2009.0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mathew AR, Norton PJ, Zvolensky MJ, Buckner JD, & Smits JAJ (2011). Smoking behavior and alcohol consumption in individuals with panic attacks. Journal of Cognitive Psychotherapy 10.1891/0889-8391.25.1.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, … Pine DS (2007). fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology 10.1007/s00213-006-0542-9 [DOI] [PubMed] [Google Scholar]
- 93.McLeod BD, Southam-Gerow MA, & Kendall PC (2017). Observer, youth, and therapist perspectives on the alliance in cognitive behavioral treatment for youth anxiety. Psychological Assessment. 10.1037/pas0000465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Melvin GA, Dudley AL, Gordon MS, Klimkeit E, Gullone E, Taffe J, & Tonge BJ (2017). Augmenting Cognitive Behavior Therapy for School Refusal with Fluoxetine: A Randomized Controlled Trial. Child Psychiatry and Human Development, 48(3), 485–497. 10.1007/s10578-016-0675-y [DOI] [PubMed] [Google Scholar]
- 95.Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry, 49(10), 980–9. 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Merikangas KR, He JP, Brody D, Fisher P, Bourdon K, & Koretz DS (2010). Prevalence and Treatment of Mental Disorders Among US Children in the 2001–2004 NHANES. Pediatrics, 125(1), 75–81. 10.1542/peds.2008-2598.Prevalence [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Milham MP, Nugent AC, Drevets WC, Dickstein DS, Leibenluft E, Ernst M, … Pine DS (2005). Selective reduction in amygdala volume in pediatric anxiety disorders: A voxel-based morphometry investigation. Biological Psychiatry, 57(9), 961–966. 10.1016/j.biopsych.2005.01.038 [DOI] [PubMed] [Google Scholar]
- 98.Mohatt J, Bennett SM, & Walkup JT (2014). Treatment of Separation, Generalized, and Social Anxiety Disorders in Youths. American Journal of Psychiatry, 171(7), 741–748. 10.1176/appi.ajp.2014.13101337 [DOI] [PubMed] [Google Scholar]
- 99.Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, … Pine DS (2006). Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. American Journal of Psychiatry. 10.1176/ajp.2006.163.6.1091 [DOI] [PubMed] [Google Scholar]
- 100.Monk CS, & Pine DS (2016). Implications for understanding amygdala function in mental disorders. Living without an Amygdala. [Google Scholar]
- 101.Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HMC, … Pine DS (2008). Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry, 65(5), 568–576. 10.1001/archpsyc.65.5.568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morriss J, Christakou A, & van Reekum CM (2016). Nothing is safe: Intolerance of uncertainty is associated with compromised fear extinction learning. Biological Psychology 10.1016/j.biopsycho.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mueller SC, Aouidad A, Gorodetsky E, Goldman D, Pine DS, & Ernst M (2013). Erratum: Grey matter volumein adolescent anxiety: An impact of the brain-derived neurotropic factor Val66Met polymorphism? (Journal of the American Academy of Child and Adolescent Psychiatry (2013) 52 (184–195)). Journal of the American Academy of Child and Adolescent Psychiatry. 10.1016/j.jaac.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nolte T, Guiney J, Fonagy P, Mayes LC, & Luyten P (2011). Interpersonal stress regulation and the development of anxiety disorders: An attachment-based developmental framework. Frontiers in Behavioral Neuroscience. 10.3389/fnbeh.2011.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pahl KM, Barrett PM, & Gullo MJ (2012). Examining potential risk factors for anxiety in early childhood. Journal of Anxiety Disorders, 26(2), 311–320. 10.1016/j.janxdis.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 106.Patel NB, Xu Y, McCandless LC, Chen A, Yolton K, Braun J, … Lanphear BP (2019). Very low-level prenatal mercury exposure and behaviors in children: The HOME Study. Environmental Health: A Global Access Science Source 10.1186/s12940-018-0443-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Peris TS, Caporino NE, O’Rourke S, Kendall PC, Walkup JT, Albano AM, … Compton SN (2017). Therapist-Reported Features of Exposure Tasks That Predict Differential Treatment Outcomes for Youth With Anxiety. Journal of the American Academy of Child and Adolescent Psychiatry 10.1016/j.jaac.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peris TS, Compton SN, Kendall PC, Birmaher B, Sherrill J, March J, … Piacentini J (2015). Trajectories of change in youth anxiety during cognitive-behavior therapy. Journal of Consulting and Clinical Psychology 10.1037/a0038402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pontious A, Kowalczyk T, Englund C, & Hevner RF (2007). Role of intermediate progenitor cells in cerebral cortex development. Developmental Neuroscience 10.1159/000109848 [DOI] [PubMed] [Google Scholar]
- 110.Ramsey LB, Bishop JR, & Strawn JR (2019). Pharmacogenetics of treating pediatric anxiety and depression. Pharmacogenomics, 20(12), 867–870. 10.2217/pgs-2019-0088 [DOI] [PubMed] [Google Scholar]
- 111.Ranøyen I, Lydersen S, Larose TL, Weidle B, Skokauskas N, Thomsen PH, … Indredavik MS (2018). Developmental course of anxiety and depression from adolescence to young adulthood in a prospective Norwegian clinical cohort. European Child and Adolescent Psychiatry, 27(11), 1413–1423. 10.1007/s00787-018-1139-7 [DOI] [PubMed] [Google Scholar]
- 112.Rapee RM, & Melville LF (1997). Recall of family factors in social phobia and panic disorder: Comparison of mother and offspring reports. Depression and Anxiety. [DOI] [PubMed] [Google Scholar]
- 113.Rifkin A, & Braga RJ (2009). Behavioral Therapy, Sertraline, or Both in Childhood Anxiety. New England Journal of Medicine, 360(23), 2475–2477. 10.1056/NEJMc090139 [DOI] [PubMed] [Google Scholar]
- 114.Roberson-Nay R, Klein DF, Klein RG, Mannuzza S, Moulton JL, Guardino M, & Pine DS (2010). Carbon Dioxide Hypersensitivity in Separation-Anxious Offspring of Parents with Panic Disorder. Biological Psychiatry, 67(12), 1171–1177. 10.1016/j.biopsych.2009.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roy AK, Fudge JL, Kelly C, Perry JSA, Daniele T, Carlisi C, … Ernst M (2013). Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 52(3), 290–299.e2. 10.1016/j.jaac.2012.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rynn MA, Riddle MA, Yeung PP, & Kunz NR Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: two placebo-controlled trials., 164 The American journal of psychiatry 290–300 (2007). 10.1176/appi.ajp.164.2.290 [DOI] [PubMed] [Google Scholar]
- 117.Rynn MA, Siqueland L, & Rickels K (2001). Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. The American Journal of Psychiatry, 158(12), 2008–14. [DOI] [PubMed] [Google Scholar]
- 118.Rynn MA, Walkup JT, Compton SN, Sakolsky DJ, Sherrill JT, Shen S, … Birmaher B (2015). Child/Adolescent anxiety multimodal study: evaluating safety. J Am Acad Child Adolesc Psychiatry, 54(3), 180–190. 10.1016/j.jaac.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sakolsky DJ, Nurmi EL, Birmaher B, March JS, Walkup JT, Piacentini JC, … Ginsburg GS (2010). Association of GRIK4 with Treatment Response in the Child/Adolescent Anxiety Multimodal Study (CAMS). In Annual Meeting of the American Academy of Child Adolescent Psychiatry (p. 4.3). [Google Scholar]
- 120.Salazar DE, Frackiewicz EJ, Dockens R, Kollia G, Fulmor IE, Tigel PD, … Cutler NR (2001). Pharmacokinetics and tolerability of buspirone during oral administration to children and adolescents with anxiety disorder and normal healthy adults. Journal of Clinical Pharmacology, 41(12), 1351–1358. 10.1177/00912700122012823 [DOI] [PubMed] [Google Scholar]
- 121.Sallee FR, Lyne A, Wigal T, & McGough JJ (2009). Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol, 19(3), 215–226. 10.1089/cap.2008.0080 [DOI] [PubMed] [Google Scholar]
- 122.Seligman LD, & Ollendick TH (2011). Cognitive-Behavioral Therapy for Anxiety Disorders in Youth. Child and Adolescent Psychiatric Clinics of North America. 10.1016/j.chc.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shamir-Essakow G, Ungerer JA, & Rapee RM (2005). Attachment, behavioral inhibition, and anxiety in preschool children. Journal of Abnormal Child Psychology, 33(2), 131–143. 10.1007/s10802-005-1822-2 [DOI] [PubMed] [Google Scholar]
- 124.Shechner T, Britton JC, Ronkin EG, Jarcho JM, Mash JA, Michalska KJ, … Pine DS (2015). Fear conditioning and extinction in anxious and nonanxious youth and adults: Examining a novel developmentally appropriate fear-conditioning task. Depression and Anxiety 10.1002/da.22318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.SIEGLE GJ, KONECKY RO, THASE ME, & CARTER CS (2006). Relationships between Amygdala Volume and Activity during Emotional Information Processing Tasks in Depressed and Never-Depressed Individuals. Annals of the New York Academy of Sciences. 10.1111/j.1749-6632.2003.tb07105.x [DOI] [PubMed] [Google Scholar]
- 126.Silverman WK, Pina AA, & Viswesvaran C (2008). Evidence-based psychosocial treatments for phobic and anxiety disorders in children and adolescents. Journal of Clinical Child and Adolescent Psychology 10.1080/15374410701817907 [DOI] [PubMed] [Google Scholar]
- 127.Southam-Gerow MA, & Prinstein MJ (2014). Evidence Base Updates: The Evolution of the Evaluation of Psychological Treatments for Children and Adolescents. Journal of Clinical Child and Adolescent Psychology 10.1080/15374416.2013.855128 [DOI] [PubMed] [Google Scholar]
- 128.Strawn JR, Bitter SM, Weber WA, Chu WJ, Whitsel RM, Adler C, … Delbello MP (2012). Neurocircuitry of generalized anxiety disorder in adolescents: A pilot functional neuroimaging and functional connectivity study. Depression and Anxiety, 29(11), 939–947. 10.1002/da.21961 [DOI] [PubMed] [Google Scholar]
- 129.Strawn JR, Chu WJ, Whitsel RM, Weber WA, Norris MM, Adler CM, … Delbello MP (2013). A pilot study of anterior cingulate cortex neurochemistry in adolescents with generalized anxiety disorder. Neuropsychobiology 10.1159/000347090 [DOI] [PubMed] [Google Scholar]
- 130.Strawn JR, Cotton S, Luberto CM, Patino LR, Stahl LA, Weber WA, … Delbello MP (2016). Neural Function before and after Mindfulness-Based Cognitive Therapy in Anxious Adolescents at Risk for Developing Bipolar Disorder. Journal of Child and Adolescent Psychopharmacology 10.1089/cap.2015.0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Strawn JR, Dobson ET, Mills JA, Cornwall GJ, Sakolsky D, Birmaher B, … Rynn MA (2017). Placebo Response in Pediatric Anxiety Disorders: Results from the Child/Adolescent Anxiety Multimodal Study. Journal of Child and Adolescent Psychopharmacology, 27(6), 501–508. 10.1089/cap.2016.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Strawn JR, Geracioti L, Rajdev N, Clemenza K, & Levine A (2018). Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: an evidence-based treatment review. Expert Opinion on Pharmacotherapy, 19(10), 1057–1070. 10.1080/14656566.2018.1491966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Strawn JR, Hamm L, Fitzgerald DA, Fitzgerald KD, Monk CS, & Phan KL (2015). Neurostructural abnormalities in pediatric anxiety disorders. Journal of Anxiety Disorders, 32, 81–88. 10.1016/j.janxdis.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Strawn JR, John Wegman C, Dominick KC, Swartz MS, Wehry AM, Patino LR, … DelBello MP (2014). Cortical surface anatomy in pediatric patients with generalized anxiety disorder. Journal of Anxiety Disorders, 28(7), 717–723. 10.1016/j.janxdis.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 135.Strawn JR, Mills JA, Cornwall GJ, Mossman SA, Varney ST, Keeshin BR, & Croarkin PE (2017). Buspirone in Children and Adolescents with Anxiety: A Review and Bayesian Analysis of Abandoned Randomized Controlled Trials. Journal of Child and Adolescent Psychopharmacology, 28(1), cap.2017.0060 10.1089/cap.2017.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Strawn JR, Mills JA, Sauley BA, & Welge JA (2018). The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 57(4), 235–244.e2. 10.1016/j.jaac.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Strawn JR, Mills JA, Sauley BA, & Welge JA (2018). The Impact of Antidepressant Dose and Class on Treatment Response in Pediatric Anxiety Disorders: A Meta-Analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 57(4). 10.1016/j.jaac.2018.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Strawn JR, Prakash A, Zhang Q, Pangallo BA, Stroud CE, Cai N, & Findling RL (2015). A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry, 54(4), 283–293. 10.1016/j.jaac.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 139.Strawn JR, Prakash A, Zhang Q, Pangallo BA, Stroud CE, Cai N, & Findling RL (2015). A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 54(4), 283–93. 10.1016/j.jaac.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 140.Strawn JR, Wehry AM, Chu WJ, Adler CM, Eliassen JC, Cerullo MA, … Delbello MP (2013). Neuroanatomic abnormalities in adolescents with generalized anxiety disorder: A voxel-based morphometry study. Depression and Anxiety, 30(9), 842–848. 10.1002/da.22089 [DOI] [PubMed] [Google Scholar]
- 141.Strawn JR, Wehry AM, Delbello MP, Rynn MA, & Strakowski S (2012). Establishing the neurobiologic basis of treatment in children and adolescents with generalized anxiety disorder. Depression and Anxiety, 29(4), 328–339. 10.1002/da.21913 [DOI] [PubMed] [Google Scholar]
- 142.Strawn JR, Welge JA, Wehry AM, Keeshin B, & Rynn MA (2015). Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depression and Anxiety, 32(3), 149–157. 10.1002/da.22329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sultan RS, Correll CU, Zohar J, Zalsman G, & Veenstra-VanderWeele J (2018). What’s in a Name? Moving to Neuroscience-Based Nomenclature in Pediatric Psychopharmacology. Journal of the American Academy of Child and Adolescent Psychiatry 10.1016/j.jaac.2018.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Svihra M, & Katzman MA (2004). Behavioural inhibition: A predictor of anxiety. Paediatrics and Child Health. 10.1093/pch/9.8.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sylvester CM, Barch DM, Harms MP, Belden AC, Oakberg TJ, Gold AL, … Pine DS (2016). Early Childhood Behavioral Inhibition Predicts Cortical Thickness in Adulthood. Journal of the American Academy of Child and Adolescent Psychiatry 10.1016/j.jaac.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tatti R, Haley MS, Swanson OK, Tselha T, & Maffei A (2017). Neurophysiology and Regulation of the Balance Between Excitation and Inhibition in Neocortical Circuits. Biological Psychiatry, 81(10), 821–831. 10.1016/j.biopsych.2016.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taylor JH, Lebowitz ER, Jakubovski E, Coughlin CG, Silverman WK, & Bloch MH (2018). Monotherapy Insufficient in Severe Anxiety? Predictors and Moderators in the Child/Adolescent Anxiety Multimodal Study. Journal of Clinical Child and Adolescent Psychology 10.1080/15374416.2017.1371028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thabrew H, Stasiak K, Hetrick SE, Wong S, Huss JH, & Merry SN (2018). E-Health interventions for anxiety and depression in children and adolescents with long-term physical conditions. Cochrane Database of Systematic Reviews. 10.1002/14651858.CD012489.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Turgeon L, O’Connor KP, Marchand A, & Freeston MH (2002). Recollections of parent-child relationships in patients with obsessive-compulsive disorder and panic disorder with agoraphobia. Acta Psychiatrica Scandinavica 10.1034/j.1600-0447.2002.1188.x [DOI] [PubMed] [Google Scholar]
- 150.US Food and Drug Administration. Relationship between psychotropic drugs and pediatric suicidality: review and evaluation of clinical data. : http://www.fda.gov/ohrms/dockets/ac/04/briefing/2004-4065b1-10-TAB08-Hammads-Review.pdf. (n.d.).
- 151.Varigonda AL, Jakubovski E, & Bloch MH (2016). Systematic review and meta-analysis: Early treatment responses of selective serotonin reuptake inhibitors and clomipramine in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 55(10), 851–859.e2. 10.1016/j.jaac.2016.07.768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wagner KD, Berard R, Stein MB, Wetherhold E, Carpenter DJ, Perera P, … Machin A (2004). A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Archives of General Psychiatry, 61(11), 1153–62. 10.1001/archpsyc.61.11.1153 [DOI] [PubMed] [Google Scholar]
- 153.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, … Kendall PC (2008). Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. The New England Journal of Medicine, 359(26), 2753–2766. 10.1056/NEJMoa0804633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang Z, SH W, Sim L, & al, et. (2017). Comparative effectiveness and safety of cognitive behavioral therapy and pharmacotherapy for childhood anxiety disorders: A systematic review and meta-analysis. JAMA Pediatrics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Z, Whiteside SPH, Sim L, Farah W, Morrow AS, Alsawas M, … Murad MH (2017). Comparative Effectiveness and Safety of Cognitive Behavioral Therapy and Pharmacotherapy for Childhood Anxiety Disorders: A Systematic Review and Meta-analysis. JAMA Pediatrics 10.1001/jamapediatrics.2017.3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Warren SL, Huston L, Egeland B, & Sroufe LA (1997). Child and adolescent anxiety disorders and early attachment. Journal of the American Academy of Child and Adolescent Psychiatry 10.1097/00004583-199705000-00014 [DOI] [PubMed] [Google Scholar]
- 157.Wehry AM, Beesdo-Baum K, Hennelly MM, Connolly SD, & Strawn JR, Wehry AMAM, … Strawn JRJR (2015). Assessment and treatment of anxiety disorders in children and adolescents. Current Psychiatry Reports, 17(7), 591 10.1007/s11920-015-0591-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wehry AM, Ramsey L, Dulemba SE, Mossman SA, & Strawn JR (2018). Pharmacogenomic Testing in Child and Adolescent Psychiatry: An Evidence-Based Review. Current Problems in Pediatric and Adolescent Health Care, 48(2), 40–49. 10.1016/j.cppeds.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weisman JS, & Rodebaugh TL (2018). Exposure therapy augmentation: A review and extension of techniques informed by an inhibitory learning approach. Clinical Psychology Review 10.1016/j.cpr.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 160.Woolf AD, Erdman AR, Nelson LS, Caravati EM, Cobaugh DJ, Booze LL, … Troutman WG (2007). Tricyclic antidepressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clinical Toxicology (Philadelphia, Pa.), 45(3), 203–233. 10.1080/15563650701226192 [DOI] [PubMed] [Google Scholar]
- 161.Wu MS, Caporino NE, Peris TS, Pérez J, Thamrin H, Albano AM, … Piacentini J (2019). The Impact of Treatment Expectations on Exposure Process and Treatment Outcome in Childhood Anxiety Disorders. Journal of Abnormal Child Psychology 10.1007/s10802-019-00574-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu Y, Lin Q, Han Z, He Y, & Bi Y (2016). Intrinsic functional network architecture of human semantic processing: Modules and hubs. NeuroImage, 132, 542–555. 10.1016/j.neuroimage.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 163.Yook K, Kim KH, Suh SY, & Lee KS (2010). Intolerance of uncertainty, worry, and rumination in major depressive disorder and generalized anxiety disorder. Journal of Anxiety Disorders, 24(6), 623–628. 10.1016/j.janxdis.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 164.Zohar J, & Kasper S (2016). Neuroscience-based Nomenclature (NbN): A call for action. World Journal of Biological Psychiatry 10.1080/15622975.2016.1193626 [DOI] [PMC free article] [PubMed] [Google Scholar]