Abstract
Lynch syndrome (LS) is a prevalent hereditary cancer predisposition syndrome. While colorectal cancer is the most common gastrointestinal (GI) cancer in LS, there is also increased risk of gastric and small intestinal cancers. Recommendations for upper GI cancer surveillance in LS vary widely with limited data supporting effectiveness. Herein, we collected data on individuals with a diagnosis of Lynch syndrome seen at our tertiary care referral center. We identified individuals who underwent upper endoscopy and those with upper GI cancers, and associated demographics, genetic testing results, and endoscopic information. Standard statistical analyses were performed. Among 295 individuals with LS seen at our center, 217 (73.6%) underwent 660 total upper endoscopies. Of these 217, pre-cancerous upper endoscopy findings included Barrett’s esophagus (7, 3.2%), gastric intestinal metaplasia (18, 8.3%), and duodenal adenomas (4, 1.8%), and Helicobacter pylori was identified in 6 (2.8%). Upper GI cancers were diagnosed in eleven individuals (3.7%), including esophageal in 1, gastric in 6, and duodenal in 4. Five (1.7%) of these upper GI cancers were identified on surveillance. Individuals with upper GI cancers identified on surveillance were older at first surveillance endoscopy, with median age 63.3 vs 44.9 years, P<0.001. Of the upper GI cancers detected on surveillance, 80% (4/5) occurred within 2 years of last upper endoscopy and 80% were stage I. In conclusion, upper endoscopy surveillance in LS identifies upper GI cancers. For individuals with LS who undergo upper GI surveillance, a short surveillance interval may be warranted.
Keywords: Lynch syndrome, endoscopic surveillance, gastric cancer, duodenal cancer
INTRODUCTION
Lynch syndrome (LS) is among the most prevalent hereditary cancer predisposition syndromes with a population incidence estimated at 1:279 or greater.[1] This autosomal dominant syndrome results from germline disease-causing variants in DNA mismatch repair genes including MLH1, MSH2, MSH6, PMS2 or EPCAM. Individuals with LS have increased lifetime risk of multiple cancers, with the highest gastrointestinal (GI) cancer risk being for colorectal cancer (CRC).[2,3] LS-related CRCs develop at an accelerated rate with polyp to CRC progression within a few years, as opposed to 10 or more years in individuals without Lynch syndrome.[4] Based on increased neoplastic progression and significant CRC risk, frequent colonoscopic surveillance has proven to prevent death from CRC and is widely accepted as standard of care.[5–12] Accordingly, colonoscopic surveillance for LS in the United States is recommended at least every 1–2 years.[6–8,11]
Apart from CRC risk, LS is also associated with increased risk of other GI cancers, including gastric and small intestinal adenocarcinoma. However, unlike colonoscopy surveillance guidelines, the role for upper GI tract surveillance in LS is far less clear. Currently, there is a lack of consensus among guidelines from different groups about the age at which to begin surveillance, the frequency of surveillance, and even the individuals with LS for which upper GI surveillance is recommended.[5–11] This leads to widely variable practice patterns and can result in confusion for both patients and providers.[3,6,13] Lastly, as more individuals are now pursuing genetic testing due to improved access and decreasing costs, it is inevitable that LS will be increasingly identified, further emphasizing the need to define the most effective surveillance strategies.[14]
Recent studies have begun to identify factors associated with upper GI cancers in LS, suggesting that surveillance could be recommended for individuals at high risk. Among individuals testing positive for LS at a commercial laboratory, factors associated with gastric cancer included male sex, increasing age, family history, and a disease-causing variant in MLH1 or MSH2.[15] The international, multicenter Prospective Lynch Syndrome Database also identified age and disease-causing variants in MLH1 or MSH2 as factors independently associated with upper GI cancers in LS.[16] Additionally, studies from European cancer registries have identified MLH1 or MSH2 disease-causing variants in particular as being associated with upper GI cancer.[17–19] While these studies suggest which particular subgroups of LS should be surveilled, there continue to be knowledge gaps in the efficacy of and optimal intervals for surveillance. Therefore, we present our experience with upper endoscopic surveillance in a LS cohort from a tertiary care referral center, highlighting the discovery of early stage upper GI cancers in patients with LS undergoing surveillance.
MATERIALS AND METHODS
This is a retrospective analysis of individuals with LS seen at our tertiary care referral center at the University of Pennsylvania (Philadelphia, Pennsylvania), approved by the University of Pennsylvania Institutional Review Board. Individuals had a medical record with at least one visit to the University of Pennsylvania Health System as well as a documented pathogenic or likely pathogenic variant in MLH1, MSH2, MSH6, PMS2, or EPCAM, or were a confirmed obligate carrier of a known familial pathogenic/likely pathogenic variant. Information obtained from this cohort included demographics, personal history, family history, and upper endoscopy records. We identified individuals with upper GI cancers detected on surveillance as well as those detected outside of surveillance. Records for data abstraction included medical encounters and endoscopy reports from our center, as well as all available data from outside our institution.
At our institution, we routinely offer upper GI cancer surveillance in LS starting at age 30, and recommend 1–2 year intervals between upper endoscopies, consistent with recommendations from the American Society of Clinical Oncology, the European Society for Medical Oncology, and the European Society of Digestive Oncology, though patients receive care outside of our center as well.[5,7,9] Patients underwent upper endoscopy with a standard adult endoscope, and biopsy for Helicobacter pylori was performed by some providers, but was not performed universally. Upper GI cancers were defined as esophageal, gastric, or duodenal cancers, but did not include non-duodenal small intestinal cancers (as these would not be expected to be detected on standard endoscopic surveillance). Patients with surveillance detected upper GI cancers were asymptomatic, and apart from LS had no other known significant risk factors for upper GI cancers. Comparative statistical analyses were performed using Stata/IC 15.1 (College Park, TX).
RESULTS
At our tertiary care referral center, 295 individuals with LS were identified as described in Table 1. Of this cohort, 174 (59.0%) were female, 255 (86.4%) were of reported White race, 5 (1.7%) were of reported Hispanic / Latino ethnicity, and 28 (9.5%) reported an Ashkenazi Jewish ancestry. A pathogenic or likely pathogenic variant was noted in MLH1 in 22.7%, MSH2 in 32.9%, MSH6 in 21.0%, PMS2 in 20.3%, and EPCAM in 3.1%. A personal history of CRC was present in 71 (24.1%). A family history of CRC was reported in 223 (75.6%), while a family history of gastric cancer was reported in 55 (18.6%), and of small intestinal cancer in 13 (4.4%).
Table 1:
Lynch syndrome cohort characteristics (N = 295)
| N (%) | |
|---|---|
| Female | 174 (59.0%) |
| Hispanic / Latino | 5 (1.7%) |
| Race | |
| White | 255 (86.4%) |
| Black | 10 (3.4%) |
| Asian | 15 (5.1%) |
| Other/unknown | 15 (5.1%) |
| Ashkenazi Jewish ancestry | 28 (9.5%) |
| Lynch syndrome gene | |
| MLH1 | 67 (22.7%) |
| MSH2 | 97 (32.9%) |
| MSH6 | 62 (21.0%) |
| PMS2 | 60 (20.3%) |
| EPCAM | 9 (3.1%) |
| Personal history of upper GI cancer* | 16 (5.4%) |
| Esophageal | 1 (0.3%) |
| Gastric | 6 (2.0%) |
| Duodenal | 4 (1.3%) |
| Non-duodenal small intestinal | 5 (1.7%) |
| Personal history of colorectal cancer | 71 (24.1%) |
| Family history gastric cancer | 55 (18.6%) |
| Family history small intestinal cancer | 13 (4.4%) |
| Family history colorectal cancer | 223 (75.6%) |
| Individuals who underwent upper endoscopy | 217 (73.6%) |
| Number of upper endoscopies performed, median (IQR) | 2.0 (1.0, 4.0) |
| Total upper GI cancers diagnosed | 11 (5.1%) |
| UGI cancer detected on surveillance endoscopy | 5 (2.3%) |
These cancers were detected during the study period, however the non-duodenal small intestinal cancers were not detected by upper endoscopy given their location distal to the duodenum.
Of the 295 with Lynch syndrome, 217 (73.6%) underwent at least 1 upper endoscopy. There were a total of 660 upper endoscopies performed with a median of 2 (IQR 1–4) upper endoscopies performed per person. In total, 11 (3.7%) of our entire cohort were diagnosed with an upper GI cancer including 1 with esophageal squamous cell carcinoma, 6 with gastric adenocarcinoma, and 4 with duodenal adenocarcinoma. Table 2 compares the characteristics of those who developed an upper GI cancer (including outside of surveillance) to those who had no upper GI cancer and had undergone at least one upper GI endoscopy. There were no significant differences in terms of gender, race, ethnicity, Ashkenazi Jewish ancestry, LS gene, or family histories between the groups. Individuals who developed cancer were overall older, with median age at first endoscopy of 63.3 years (IQR 54.8–67.9) versus 44.9 years (IQR 34.7–55.7), P<0.001.
Table 2:
Characteristics of individuals with Lynch syndrome who underwent upper endoscopy.
| No upper GI cancer (n=206) | Upper GI cancer (n=11) | p-value | |
|---|---|---|---|
| Female | 121 (58.7%) | 6 (54.5%) | 0.78 |
| Hispanic/Latino ethnicity | 5 (2.4%) | 0 (0.0%) | 0.23 |
| Race | 0.80 | ||
| White | 179 (86.9%) | 11 (100.0%) | |
| Black | 7 (3.4%) | 0 (0.0%) | |
| Asian | 9 (4.4%) | 0 (0.0%) | |
| Other/Unknown | 11 (11.2%) | 0 (0.0%) | |
| Ashkenazi Jewish ancestry | 24 (11.8%) | 1 (9.1%) | 0.12 |
| Lynch syndrome gene | 0.31 | ||
| MLH1 | 48 (23.3%) | 2 (18.2%) | |
| MSH2 | 70 (34.0%) | 4 (36.4%) | |
| MSH6 | 40 (19.4%) | 0 (0.0%) | |
| PMS2 | 42 (20.4%) | 4 (36.4%) | |
| EPCAM | 6 (2.9%) | 1 (9.1%) | |
| Personal history of colon cancer | 52 (25.2%) | 4 (36.3%) | 0.47 |
| Family history gastric cancer | 44 (22.0%) | 3 (27.3%) | 0.68 |
| Family history small intestinal cancer | 9 (4.4%) | 0 (0.0%) | 0.47 |
| Number of EGDs, median (IQR) | 2.0 (1.0, 4.0) | 3.0 (1.0, 8.0) | 0.22 |
| Age at first EGD, median (IQR) | 44.9 (34.7, 55.7) | 63.3 (54.8, 67.9) | <0.001 |
Table 3 describes non-cancerous endoscopic findings among individuals with LS who underwent upper endoscopy. Among the 217 individuals who underwent upper endoscopy, pre-cancerous findings were observed, including Barrett’s esophagus (7, 3.2%), gastric intestinal metaplasia (18, 8.3%), and duodenal adenomas (4, 1.8%); Helicobacter pylori was identified in 6 (2.8%) individuals. Those with upper GI cancer were more likely to have duodenal adenomas, 18.2% vs 1.0%, P<0.001, though this should be interpreted with caution given the small numbers. There were no significant differences regarding esophageal or gastric endoscopic findings in those with versus those without upper GI cancers, and overall, Helicobacter pylori infection was rare among this LS cohort.
Table 3:
Upper endoscopy findings in Lynch syndrome.
| Total (n=217) | No upper GI cancer (n=206) | Upper GI cancer (n=11) | p-value | |
|---|---|---|---|---|
| Esophagitis | 37 (17.1%) | 36 (17.5%) | 1 (9.1%) | 0.46 |
| Barrett’s esophagus | 7 (3.2%) | 7 (3.4%) | 0 (0.0%) | 0.53 |
| Fundic gland polyp | 53 (24.4%) | 48 (23.3%) | 5 (45.5%) | 0.11 |
| Gastritis | 55 (25.3%) | 51 (24.8%) | 4 (36.4%) | 0.41 |
| Gastric intestinal metaplasia | 18 (8.3%) | 17 (8.3%) | 1 (9.1%) | 0.94 |
| Helicobacter pylori | 6 (2.8%) | 6 (2.9%) | 0 (0.0%) | 0.56 |
| Duodenal adenoma | 4 (1.8%) | 2 (1.0%) | 2 (18.2%) | <0.001 |
p-value compares persons with known upper GI cancer versus those without Findings were per person, categorized as ever present / never present
Of the 11 individuals who developed an upper GI cancer, 5 of these cancers were detected on surveillance. Individuals detected outside of surveillance underwent endoscopy for clinical indications, including anemia, abnormal imaging, and dysphagia. Table 4 describes demographics, personal and family history, and endoscopic details of all of the detected cancers, while Figure 1 illustrates the chronology of upper GI cancer diagnosis and previous surveillance endoscopies (if they were performed). The endoscopy interval before detection of the upper GI cancer was short: 4 of the 5 individuals (80%) with cancer detected on surveillance had a prior negative upper endoscopy within 2 years of their diagnosis with an upper GI cancer. Additionally, 4 of the 5 surveillance-detected upper GI cancers (80%) were detected at stage I, whereas only 2 of the 6 (33.3%) detected outside of surveillance were stage I.
Table 4:
Upper GI cancers identified in the Lynch syndrome cohort
| Individual | Age/sex/variant | Upper GI cancer | Personal history colorectal cancer (age) | Personal history other cancer (age) | Tobacco use | Family history gastric cancer | Family history small intestinal cancer | Family history colorectal cancer | Number of endoscopies prior to cancer | Interval between last surveillance endoscopy and cancer (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| LS1 | 79/F/MSH2 | Gastric (2cm polyp in body) | No | No | Former | No | No | Yes | 1 | 5 |
| LS2 | 71/F/MSH2 | Gastric (2cm polyp in body) | Yes (53) | Uterine (52), Breast (66), Cutaneous T-cell lymphoma (71) | Never | No | No | Yes | 5 | 1 |
| LS3 | 69/M/EPCAM | Gastric (5cm mass in body) | No | Urinary tract (58), Melanoma (64) | Former | No | No | Yes | 3 | 2 |
| LS4 | 65/M/MSH2 | Duodenal | No | Prostate (65), Urinary tract (66) | Never | No | No | Yes | 1 | 1 |
| LS5 | 62/F/MSH2 | Duodenal | Yes (38) | Urinary tract (53) | Never | No | No | No | 5 | 1 |
| LS6 | 78/M/PMS2 | Esophageal | No | No | Never | No | No | Yes | - | - |
| LS7* | 68/M/MLH1 | Gastric (4cm mass in fundus) | Yes (49) | No | Never | Yes | No | Yes | - | - |
| LS8 | 62/F/PMS2 | Gastric (5cm mass in body) | No | Uterine (50), Pancreatic (65) | Never | No | No | Yes | - | - |
| LS9 | 54/M/MLH1 | Duodenal | Yes (35) | Lung (54) | Current | Yes | No | Yes | - | - |
| LS10 | 48/F/PMS2 | Duodenal | No | Breast (55) | Former | No | No | Yes | - | - |
| LS11 | 41/F/PMS2 | Gastric (1cm polyp in antrum) | No | No | Never | Yes | No | Yes | - | - |
All patients were White, no known Ashkenazi Jewish history or Hispanic / Latino ethnicity
Deceased, 3 years after cancer diagnosis
Figure 1:
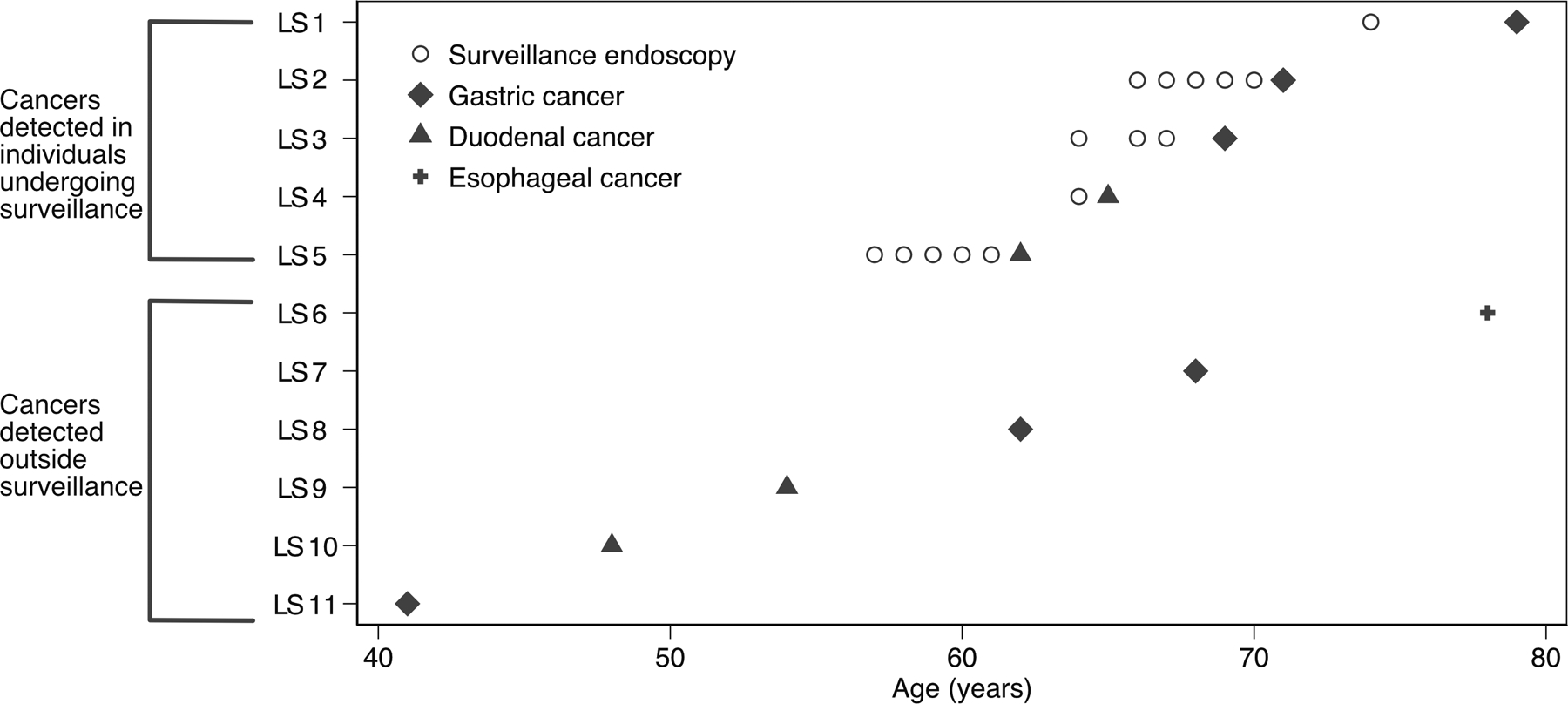
Plot of upper GI cancers identified in the Lynch syndrome cohort. Individual subjects are shown along the y-axis. Circles represent endoscopies performed for surveillance prior to cancer, if any, at individual’s age at the time of upper endoscopy (x-axis). Cancer diagnoses are represented by diamond (gastric), triangle (duodenal), and plus sign (esophageal).
DISCUSSION
In this retrospective analysis of individuals with LS, we find that among 217 subjects who underwent upper endoscopy, 5 (2.3%) had surveillance-detected gastric or duodenal cancer, and 80% of detected cancers were stage I. To our knowledge, this is the largest study to describe outcomes of endoscopic surveillance in Lynch syndrome in a granular manner. These results support that upper endoscopy surveillance in LS detects upper GI cancers, with the majority being detected at an early stage, and also provides evidence to support consideration of upper endoscopy surveillance in the management of Lynch syndrome. Since upper endoscopy is an overall safe procedure and individuals with LS already undergo sedation for colonoscopies at frequent intervals, the addition of upper endoscopy to LS surveillance would not significantly add to patient or procedural burden, though future studies should evaluate the cost-effectiveness of screening for all LS individuals versus those with particular risk factors.[11,20,21] As recent studies have sought to identify which LS subgroups should be surveilled, knowledge gaps and a paucity of literature regarding outcomes of upper GI surveillance remain. Thus, our study helps to begin filling an important knowledge gap that will directly impact surveillance strategies in LS.
Previous studies evaluating the impact of upper GI cancer surveillance in LS have been markedly smaller. A 2002 Finnish study of 73 subjects with LS identified only 1 duodenal cancer.[22] A 2017 Turkish study that surveilled 21 LS individuals found no cancers.[23] These studies both concluded that upper GI surveillance was not beneficial. A 2018 German study of 44 subjects, however, found surveillance was associated with earlier stage of detection and suggested benefit to endoscopic surveillance for upper GI cancers.[24] Early detection of upper GI cancers improves survival overall; though the data for this is not Lynch-specific, there is no suggestion that it would be different in LS.[25] Although 80% of individuals with surveillance-detected cancers in our cohort had stage I disease, future studies are needed in multi-center cohorts to investigate the mortality benefit of early detection of upper GI cancers in LS.
Another stated knowledge gap relates to the intervals between endoscopies, and recommendations differ widely among societies.[3] Individuals in our cohort had 1–2 year intervals between upper endoscopies, consistent with recommendations from the American Society of Clinical Oncology, the European Society for Medical Oncology, and the European Society of Digestive Oncology.[5,7,9] This is more aggressive than American College of Gastroenterology and National Comprehensive Cancer Network recommendations, which recommend longer intervals.[8,11] When it comes to CRC, it is thought that adenomas progress to adenocarcinoma at a more rapid rate in LS than in non-LS CRC; thus, a 5–10 year surveillance interval between colonoscopies in LS is not sufficient.[26] It is unclear if LS-associated upper GI cancers have a similar rapid progression to adenocarcinoma. While our sample size is not robust enough to allow for definitive recommendations on interval timing, it does appear from our data that upper GI cancers develop relatively rapidly in LS as observed in Figure 1. In their recently published study identifying clinical factors associated with gastric cancer, Kim et al. propose 3-year intervals for endoscopy and suggest more frequent surveillance if a patient has multiple risk factors for gastric cancer (sex, age, pathogenic variant of LS gene, and family history).[15] We argue this proposed interval should be further studied, as even without multiple risk factors, individuals in our study with cancer detected outside surveillance may have benefited from more frequent endoscopy to facilitate earlier detection. While older age has been considered a risk factor, we also demonstrate that in LS, upper GI cancers can occur early, as seen in our Figure 1, emphasizing that early upper GI surveillance should be considered, as some society guidelines also suggest.[5–9,11] Lastly, given that many of the patients who developed upper GI cancers were older, future studies should also evaluate if upper endoscopy surveillance intervals should shorten with increasing age.
Among our LS cohort, upper endoscopy did not find a high prevalence of intestinal metaplasia. Moreover, there was no difference in intestinal metaplasia rates between those who developed cancer and those who did not. This is contrary to another study of a LS cohort, where those who developed gastric cancer had high rates (70+%) of concomitant chronic immune gastritis upon histological examination of the cancer.[27] In that study, individuals who developed gastric cancer were not clearly undergoing endoscopic surveillance. Interestingly, while consensus guidelines for LS are relatively consistent regarding support for Helicobacter pylori infection testing and eradication when upper endoscopy surveillance is performed, in our cohort Helicobacter pylori infection was quite rare. This may be related to the lack of racial diversity of the cohort, as it is known that Helicobacter pylori infection is more prevalent in racial and ethnic minorities.[28,29] This finding, though, is consistent with a study from the Dutch Hereditary Cancer Registry, where Helicobacter pylori was not associated with increased gastric cancer risk among Lynch patients, with similar findings in other studies.[17,30]
Our study has several limitations, including that it is a single institution, retrospective study at a referral center which limits generalizability. However, our cohort includes good representation among all known LS-causing genes, suggesting our results are applicable to LS in general. Our relatively small sample size limits the ability to detect differences among patients who developed and did not develop upper GI cancer and to identify with accuracy optimal surveillance intervals. Our small sample size also limits the ability to stratify by pathogenic variant. We further did not have MMR status available on the majority of the cancers, limiting our ability to determine if the tumors were LS related. There is also selection bias in that those who elect to undergo endoscopic surveillance may be more motivated due to personal/family history or other factors. For example, Figure 1 demonstrates that those who underwent upper GI surveillance and developed cancer were generally older than those who developed an upper GI cancer but did not undergo surveillance. Thus age, or some other factor not captured in our data, may help explain differences in decisions to undergo surveillance. However, our overall rates of upper GI cancer are generally similar to another cohort describing Lynch patients, providing support regarding the external validity of our results.[27] Lastly, our subset of patients with cancers within 1–2 years of a prior endoscopy raises the question of endoscopy quality. It is possible that patients had incomplete endoscopies or missed lesions, leading to an apparent short interval between prior surveillance endoscopy and cancer diagnosis. However, it is also possible that, similar to CRC, LS-associated upper GI cancers may develop quickly with an accelerated progression, which may be the case as 80% of the upper GI cancers discovered were stage I despite recent normal upper endoscopy. However, at this time, it remains unclear if there is an accelerated carcinoma sequence in LS-related upper GI cancers, similar to CRC, or if these cancers develop via other mechanisms. Our study provides evidence in an area with a marked paucity of literature, which has been highlighted as an area of critical need to improve LS medical management. As such, despite the aforementioned limitations and our small sample size (of both LS individuals and detected upper GI cancers), this study begins to lay the groundwork to establish concrete upper endoscopic surveillance guidelines for LS, with future studies needed to focus on validating these results in larger and ethnically diverse cohorts.
The utility of endoscopic surveillance for upper GI cancers in LS continues to be an area of uncertainty. Using the largest cohort of surveilled LS subjects to date, we find that among 217 individuals who underwent upper endoscopy, 5 (2.3%) were identified to have a surveillance-detected gastric or duodenal cancer. These surveillance-detected upper GI cancers were primarily detected at an early stage, and were discovered with a median surveillance interval of 1 year, underscoring that, similar to LS-associated CRCs, LS-associated UGI cancers may develop quickly. Taken together, this study supports upper GI surveillance as part of a LS management program, and that if upper GI surveillance is performed, providers should consider using a shorter surveillance interval of 1–2 years, though this interval should be further studied. Future prospective studies should aim to identify persons with LS most likely to benefit from surveillance, and to identify the optimal surveillance modalities and intervals for these individuals.
Acknowledgements / grant support:
NIH/NIDDK grants T32DK774022 (SK), K08DK106489 (BWK), and R03DK120946 (BWK); The Jason and Julie Borrelli Lynch Syndrome Research Fund (BWK).
We would like to acknowledge Dr. E. John Wherry at the University of Pennsylvania Perelman School of Medicine for his help, guidance, and advice related to this project.
Footnotes
Disclosures:
SK: Travel (Boston Scientific Corporation, Olympus Corporation)
CMD: None
MR: None
JML: None
KJW: None
BWK: Consulting (Exact Sciences), Travel (Janssen)
The authors declare no other personal, professional, or financial conflicts of interests.
REFERENCES
- 1.Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, et al. Prevalence and Penetrance of Major Genes and Polygenes for Colorectal Cancer. Cancer Epidemiol Biomarkers Prev. 2017. March;26(3):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015. March;15(3):181–94. [DOI] [PubMed] [Google Scholar]
- 3.Mankaney G, Macaron C, Burke CA. Refining Risk Factors for Gastric Cancer in Patients With Lynch Syndrome to Optimize Surveillance Esophagogastroduodenoscopy. Clin Gastroenterol Hepatol. 2020. April;18(4):780–782. [DOI] [PubMed] [Google Scholar]
- 4.Lynch HT, Smyrk T, Jass JR. Hereditary nonpolyposis colorectal cancer and colonic adenomas: aggressive adenomas? Semin Surg Oncol. 1995. Nov-Dec;11(6):406–10. [DOI] [PubMed] [Google Scholar]
- 5.Balmana J, Balaguer F, Cervantes A, Arnold D, Group EGW. Familial risk-colorectal cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2013. October;24 Suppl 6:vi73–80. [DOI] [PubMed] [Google Scholar]
- 6.Giardiello FM, Allen JI, Axilbund JE, Boland CR, Burke CA, Burt RW, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology. 2014. August;147(2):502–26. [DOI] [PubMed] [Google Scholar]
- 7.Stoffel EM, Mangu PB, Gruber SB, Hamilton SR, Kalady MF, Lau MW, et al. Hereditary colorectal cancer syndromes: American Society of Clinical Oncology Clinical Practice Guideline endorsement of the familial risk-colorectal cancer: European Society for Medical Oncology Clinical Practice Guidelines. J Clin Oncol. 2015. January 10;33(2):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015. February;110(2):223–62; quiz 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vangala DB, Cauchin E, Balmana J, Wyrwicz L, van Cutsem E, Guller U, et al. Screening and surveillance in hereditary gastrointestinal cancers: Recommendations from the European Society of Digestive Oncology (ESDO) expert discussion at the 20th European Society for Medical Oncology (ESMO)/World Congress on Gastrointestinal Cancer, Barcelona, June 2018. Eur J Cancer. 2018. November;104:91–103. [DOI] [PubMed] [Google Scholar]
- 10.Vasen HF, Blanco I, Aktan-Collan K, Gopie JP, Alonso A, Aretz S, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut. 2013. June;62(6):812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oncology NCPGi. Genetic/Familial high-risk assessment: colorectal. Version 3.2019 Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf
- 12.de Jong AE, Hendriks YM, Kleibeuker JH, de Boer SY, Cats A, Griffioen G, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology. 2006. March;130(3):665–71. [DOI] [PubMed] [Google Scholar]
- 13.Vasen HF, Velthuizen ME, Kleibeuker JH, Menko FH, Nagengast FM, Cats A, et al. Hereditary cancer registries improve the care of patients with a genetic predisposition to cancer: contributions from the Dutch Lynch syndrome registry. Fam Cancer. 2016. July;15(3):429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S, Long JM, Ginsberg GG, Katona BW. The role of endoscopy in the management of hereditary diffuse gastric cancer syndrome. World J Gastroenterol. 2019. June 21;25(23):2878–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Braun D, Ukaegbu C, Dhingra TG, Kastrinos F, Parmigiani G, et al. Clinical Factors Associated With Gastric Cancer in Individuals With Lynch Syndrome. Clin Gastroenterol Hepatol. 2020. April;18(4):830–837 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moller P, Seppala TT, Bernstein I, Holinski-Feder E, Sala P, Gareth Evans D, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 2018. July;67(7):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capelle LG, Van Grieken NC, Lingsma HF, Steyerberg EW, Klokman WJ, Bruno MJ, et al. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology. 2010. February;138(2):487–92. [DOI] [PubMed] [Google Scholar]
- 18.Engel C, Loeffler M, Steinke V, Rahner N, Holinski-Feder E, Dietmaier W, et al. Risks of less common cancers in proven mutation carriers with lynch syndrome. J Clin Oncol. 2012. December 10;30(35):4409–15. [DOI] [PubMed] [Google Scholar]
- 19.Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011. June 8;305(22):2304–10. [DOI] [PubMed] [Google Scholar]
- 20.Richter JM, Kelsey PB, Campbell EJ. Adverse Event and Complication Management in Gastrointestinal Endoscopy. Am J Gastroenterol. 2016. March;111(3):348–52. [DOI] [PubMed] [Google Scholar]
- 21.Levy I, Gralnek IM. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract Res Clin Gastroenterol. 2016. October;30(5):705–718. [DOI] [PubMed] [Google Scholar]
- 22.Renkonen-Sinisalo L, Sipponen P, Aarnio M, Julkunen R, Aaltonen LA, Sarna S, et al. No support for endoscopic surveillance for gastric cancer in hereditary non-polyposis colorectal cancer. Scand J Gastroenterol. 2002. May;37(5):574–7. [DOI] [PubMed] [Google Scholar]
- 23.Galiatsatos P, Labos C, Jeanjean M, Miller K, Foulkes WD. Low yield of gastroscopy in patients with Lynch syndrome. Turk J Gastroenterol. 2017. November;28(6):434–438. [DOI] [PubMed] [Google Scholar]
- 24.Ladigan S, Vangala DB, Kuhlkamp J, Pox C, Engel C, Hueneburg R, et al. Value of EGD for gastric cancer surveillance in patients with hereditary non-polyposis colorectal cancer (HNPCC) or Lynch syndrome (LS). Journal of Clinical Oncology. 2018;36(15_suppl):1522–1522. [Google Scholar]
- 25.Jim MA, Pinheiro PS, Carreira H, Espey DK, Wiggins CL, Weir HK. Stomach cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer. 2017. December 15;123 Suppl 24:4994–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boland CR. Speed kills. Clin Gastroenterol Hepatol. 2011. April;9(4):290–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adar T, Friedman M, Rodgers LH, Shannon KM, Zukerberg LR, Chung DC. Gastric cancer in Lynch syndrome is associated with underlying immune gastritis. J Med Genet. 2019. December;56(12):844–845. [DOI] [PubMed] [Google Scholar]
- 28.Crowe SE. Helicobacter pylori Infection. N Engl J Med. 2019. March 21;380(12):1158–1165. [DOI] [PubMed] [Google Scholar]
- 29.Kumar SM DC, Ellenberg S, Kaplan DE, Goldberg DS Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology. 2019. (Accepted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soer EC, Leicher LW, Langers AM, van de Meeberg PC, van der Wouden EJ, Koornstra JJ, et al. Equivalent Helicobacter pylori infection rates in Lynch syndrome mutation carriers with and without a first-degree relative with gastric cancer. Int J Colorectal Dis. 2016. March;31(3):693–7. [DOI] [PubMed] [Google Scholar]


