Abstract
Objectives.
HIV-exposed uninfected (HEU) infants exhibit altered vaccine responses and an increased mortality compared to HIV-unexposed (HU) infants. Here, vaccine responses in HEU and HU cord blood monocytes (CBMs) were assessed following Bacillus Calmette-Guerín (BCG) treatment.
Design.
Innate responses to in vitro BCG treatment were assessed through transcriptional profiling using CBMs obtained from a Nigerian cohort of HIV-infected and uninfected women.
Methods.
HU (n=9) and HEU (n=10) infant CBMs were treated with BCG and transcriptionally profiled with the Nanostring nCounter platform. Differential expression and pathway enrichment analyses were performed, and transcripts were identified with enhanced or dampened BCG responses.
Results.
Following BCG stimulation, several pathways associated with inflammatory gene expression were upregulated irrespective of HIV exposure status. Both HU and HEU monocytes increased expression of several cytokines characteristic of innate BCG responses, including IL1β, TNFα, and IL6. Using differential expression analysis, we identified genes significantly upregulated in HEU compared to HU monocytes including monocyte chemokine CCL7 and anti-inflammatory cytokine TNFAIP6. In contrast, genes significantly upregulated in HU compared to HEU monocytes include chemokine CCL3 and cytokine IL23A, both of which influence anti-mycobacterial T cell responses. Finally, two genes which regulate prostaglandin production, CSF2 and PTGS2, were also more significantly upregulated in the HU cord blood indicating that inflammatory mediators are suppressed in the HEU infants.
Conclusions.
HEU monocytes exhibit altered induction of several key innate immune responses, providing mechanistic insights into dysregulated innate response pathways that can be therapeutically targeted to improve vaccine responses in HEU infants.
Keywords: BCG, HIV, HIV-exposed, infants, tuberculosis, monocytes
INTRODUCTION
Currently, an estimated 1.6 million infants are born to HIV infected mothers but are not themselves infected by HIV. These HIV-exposed uninfected (HEU) infants exhibit an elevated risk of contracting infectious diseases as well as increased rates of morbidity and mortality [1–6]. In addition, exposure to both maternal HIV and ART in utero has been linked to altered physical and immunological development, including reduced birth weight and altered composition of myeloid and T cells [7–11]. Indeed, responses to postnatal antigenic challenges such as infections and vaccines, including cellular responses to vaccination, appear to be selectively altered and impaired in HEU infants [12–14]. These differential cellular responses may be the result of an altered composition and function of innate cells in HEU infants, particularly in monocytes and dendritic cells, resulting in insufficient T cell recruitment and activation [7–9, 15].
The effectiveness of vaccine-induced newborn immunity is of particular concern in regions where tuberculosis is endemic and the Bacillus Calmette-Guerin (BCG) vaccine is administered at the time of birth. While correlates of protection are poorly defined, several reports support the essential roles Th1-polarized CD4 responses, with expression of IFNγ, TNFα and IL-2 widely considered a marker for quality BCG responses in infants [16, 17]. Additionally, several studies suggest early IL-23 driven Th17 responses are necessary for driving BCG-specific Th1 responses [18–20]. Indeed, HEU infants show altered immune responses to BCG, including reduced IFNγ secretion and reduced cytokine polyfunctionality [21–23].
We hypothesize that altered innate immune responses associated with in utero HIV exposure have the potential to influence responses to infant vaccinations such as BCG. To address this hypothesis, we investigated transcriptomic changes in monocyte immune response to BCG treatment using cord blood obtained from a Nigerian cohort of HU and HEU infants to address this. We observed differential expression of immune genes within the HEU monocyte response to BCG, revealing insights into innate immune responses and vaccine responses that support, and provide mechanistic insights into, previous reports of impaired BCG-specific T cell responses in HEU infants [21–23].
METHODS
Study design/Participants
Samples used in this study were obtained with consent from Nigerian women enrolled in the Microbiome Affects Risk of Development and Growth in HIV-exposed and Uninfected Infants in Nigeria (MARGIN) Study carried out by the Institute of Human Virology (IHV) at the University of Maryland (Baltimore, MD). This is a five-year NIH funded longitudinal study enrolling 150 HIV-infected and 150 HIV-uninfected women from Benin City, Nigeria and followed from 18 weeks of gestation to 18 months post-partum. All sample collection described was carried out using procedures approved by the University of Maryland Baltimore. Cord blood was collected at the time of delivery from infants born to either HIV-negative (n=12) or HIV-positive (n=12) women at clinical sites in rural Nigeria. Samples were collected in heparinized vacutainer tubes and shipped overnight to IHV where cord blood mononuclear cells were isolated and cryopreserved within 24 h of collection. All HIV positive mothers were reported at stage 1, receiving cART (zidovudine/nevirapine/lamivudine, tenofovir disoproxil fumarate/lamivudine/efavirenz, *azidothymidine/nevirapine/nevirapine, or **lopinavir/ritonavir), and having mean antenatal CD4+ counts of 387.5 cells/mm3 of (range 138 – 694 cells/mm3)(Table S1).
Monocyte isolation
CBMC from both unexposed and HIV-exposed human infant samples were thawed for positive selection of CD14+ monocytes. RPMI supplemented with 10% fetal bovine serum, without antibiotics (R10) was added to vials of frozen CBMCs. Thawed cells and media were transferred into tubes containing R10 and centrifuged for 10 minutes at 250 RCF. Media was removed and cells were resuspended in DPBS followed by a second centrifugation as described previously. CBMCs were resuspended in 4 ml of DNase solution (100μg/ml) and incubated for 15 minutes at room temperature. R10 was added to stop digestion (6ml). Cell suspension was filtered through a 40 μm filter to remove aggregates and cell counts were obtained.
CD14+ Cell Purification
CBMCs were pelleted and resuspended in DPBS containing 2% FBS with 1mM EDTA (filter sterilized; Buffer ES) at 1×108 cells/ml in 0.1 to 2 ml. Immunomagnetic positive selection of CD14+ cells from CBMC samples was achieved using EasySepTM Human CD14 Positive Selection Kit II (STEMCELL Technologies Inc.) using the manufacturer’s instructions. Purified samples contained >1.0 × 10E6 CD14+ cells and were subsequently transferred to polypropylene conical tubes for further experiments.
BCG Stimulation
Twenty-four hours prior to stimulation, actively replicating Mycobacterium bovis BCG Russia strain (obtained from the laboratory of David Sherman at University of Washington, Dept of Microbiology) was used to inoculate 7H9 media. Upon reaching log phase growth (OD600 = 1.0), BCG cultures were pelleted, washed in DPBS and resuspended in 1.3 ml of R10 for a final concentration of 2.75 × 107 cfu/ml. Monocytes were spun and resuspended in R10 to a final concentration of 5.5 × 105 cells/500 μl. Monocytes from each donor (HEU and HU) were distributed into two wells (5.5 × 105 cells per well), one well treated with BCG, the other remaining untreated. BCG treatment consisted of 100 μl Tissue culture wells containing monocytes from either HU and HEU infants (1 × 106 cells were assessed at each condition) received either 200 μl of BCG suspension (MOI 10) or R10 (untreated controls). Plates were incubated for 6 hours at 37°C and 5% CO2. Cells were harvested by centrifugation at 1200 RPM for 10 minutes, removal of supernatants, and lysis in 300μl of buffer RA1 with 1% β-mercapto-ethanol. Cell lysates were stored at −80° C. Purified CD14+ cell lysates were thawed and immediately followed by RNA isolation using an RNA Nucleospin kit (Takara Bio).
Immune Gene Expression Profiling
Experiments and plate layouts were designed to control for batch effects between plate runs and technical replicates were used to test for variability between samples. Nanostring nCounter technology uses fluorescently barcoded single-stranded oligonucleotide probes to hybridize and immobilize purified mRNA, thus allowing for direct identification and quantitation of hundreds of target genes without the need for preamplification and library preparation [24]. Transcriptomic profiles using 581 immune genes were acquired from 10 HIV-exposed BCG treated, 9 HIV-exposed untreated, 8 Unexposed BCG treated, and 9 unexposed untreated samples were run using an nCounterTM MAX platform (Nanostring Technologies Inc.) with the Human Immunology v2.0 chipset. RNA (100 ng) was directly hybridized for 18 hours and images were acquired from 280 fields of view for each sample and data were tabulated. Subsequent quality control and normalization analyses were carried out with nSolver software (v 4.0). After filters were applied, 7 unexposed BCG treated, 9 unexposed untreated 10 HIV-exposed BCG treated, and 8 HIV-exposed untreated remained.
Statistical Analysis
All statistical analyses were performed using either R version 3.5.1 or nCounter Advanced Analysis 2.0 software (Nanostring). Two-proportion z-tests were used to determine if binary data (eg. sex, delivery, etc) were different between HU and HEU donor groups, while t tests were used to test for differences in continuous data. Pathway scores were calculated using nCounter Pathway scoring module, which uses the first principle component of a set of genes’ (within a given pathway) normalized expression, with BCG treatment set as a covariate in HU and HEU groups. For differential gene expression data, infant ID was set as a confounder for analysis of matched samples. Differences in gene expression between BCG treated and untreated conditions were calculated using nSolver analysis software (Nanostring). For these data, either mixed or simplified negative binomial models or loglinear models were used depending on the data collected for each gene. Adjusted p values were calculated using the Benjamini-Yekutieli correction [25]. To evaluate differences in BCG-induced gene expression, the ratio was calculated for linear fold change Exposed:Unexposed (FC HEU:HU) for each gene with significantly altered expression following stimulation. Z-scores were then calculated from FC HEU:HU values, and genes where z < −1 or z > 1 were designated differentially altered in HEU infants following BCG, compared to HU infants. Linear fold changes of differentially altered genes were then compared in HU and HEU infants by multiple t-tests with the Holm-Sidak correction for multiple comparisons to confirm significant differences between HU and HEU groups in degree of differential gene upregulation.
RESULTS
BCG stimulation induces robust expression of proinflammatory gene signatures in HU and HEU infants.
Cord blood mononuclear cells (CBMC) obtained from Nigerian infants were classified as either in utero HIV exposed-uninfected (HEU) (n=10) or HIV-unexposed (HU) (n=9). Monocytes were isolated from cryopreserved cells and transcriptionally evaluated following in vitro stimulation with live BCG. In addition to HIV exposure status, data from infants and mothers was also evaluated (Table S1). No significant differences were identified among maternal age, infant birth weight, infant sex, and gestational age (days) between HU and HEU infants. All HIV positive mothers were reported at stage 1, receiving cART (zidovudine/nevirapine/lamivudine, tenofovir disoproxil fumarate/lamivudine/efavirenz, *azidothymidine/nevirapine/nevirapine, or **lopinavir/ritonavir), and having mean antenatal CD4+ counts of 387.5 (range 138 – 694).
Transcriptional evaluation was undertaken via Nanostring analysis utilizing purified cord blood monocytes following 6-hour exposure to BCG (MOI 10). Comparisons between BCG treated and untreated cells were generated using unsupervised clustering of normalized expression data, revealing that BCG influences expression profiles across multiple functional gene sets in both HU and HEU monocytes (Figure S1). Expression levels within a subset of 22 genes involved in anti-mycobacterial responses reveals that both HU and HEU monocytes upregulate key cytokines, chemokines and transcription factors (Fig. 1A–B). Specifically, increased levels are consistently observed for TNF(TNFα), IL1B(IL-1β), IL6, CCL2(MCP-1), CCL3(MIP-1α), CCL4(MIP-1β), SOCS3 and NFKB1 in BCG treated monocytes compared to untreated controls for HU and HEU groups. Somewhat surprisingly, increases were not observed for IL12A, IL-15 and IL18. However, previous studies using BCG-treated adult blood suggest that these genes exhibit delayed kinetics, with protein being detected no earlier than 10 hours following treatment [26]. Additionally, these results indicate that cord blood monocytes transcriptionally respond to BCG in a manner similar to both peripheral blood monocytes and monocyte-derived macrophages [26, 27].
Figure 1: Cord Blood Monocytes derived from HIV-Exposed and HIV-Unexposed infants increase inflammatory gene expression in response to BCG treatment.

Heatmaps depict nonhierarchical clustering of normalized mRNA counts from untreated and BCG untreated monocytes from HU (A) and HEU (B) cord blood. Genes are selected based on previously reported associations with innate mycobacterial responses.
BCG treatment alters gene expression pathways in HU and HEU monocytes
Pathway module analysis was performed to evaluate altered immunological pathway usage between HU and HEU monocytes (Fig. 2A–B). Using expression of genes within predefined pathways in relation to BCG treatment, principle component pathway scores were generated, with higher scores corresponding to expression of the majority of genes in a given pathway. Scores were calculated for 182 pathways containing overlapping gene sets for HU and HEU monocyte responses and trends were assessed using non-hierarchical clustering. Eighteen pathways relevant to innate anti-BCG responses were selected for further evaluation, with pathway scores calculated for untreated and BCG treated samples.
Figure 2. BCG treatment alters gene alters gene expression pathways in HU and HEU monocytes.
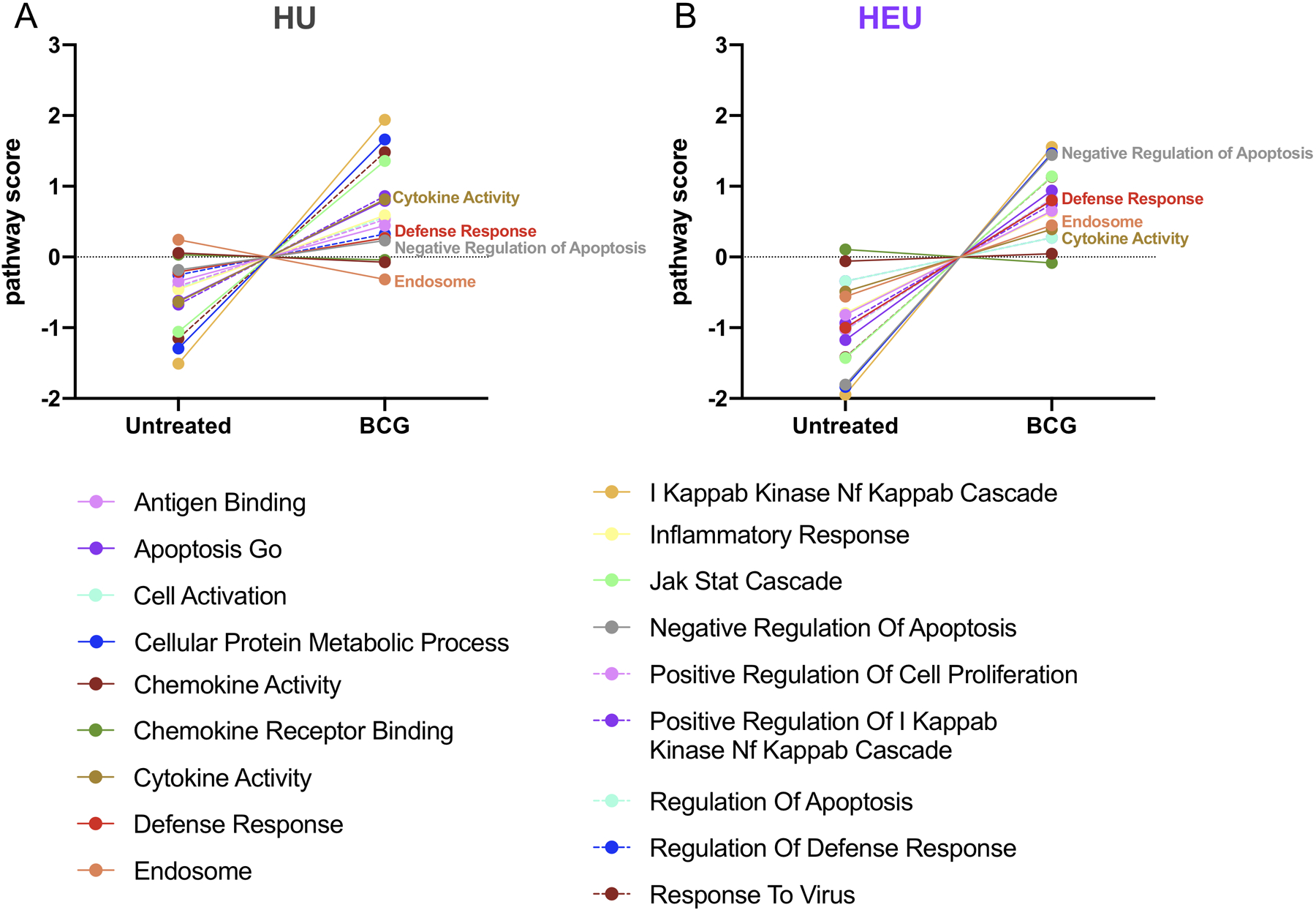
Pathway scores were generated by comparing differential genes expression for gene modules potentially implicated in BCG responses in untreated and BCG treated monocytes from HU (A) and HEU (B) cord blood. Pathways that are differentially altered following BCG in HU and HEU are labeled in the graphs.
Following BCG treatment, a number of pathways were upregulated in monocytes from both the HU and HEU derived cord blood monocytes, with the highest change observed in the IκKB NκFB Cascade pathway (Fig. 2A and B). This is perhaps unsurprising, given the central role of this pathway, particularly as TLR2/4 and NFκB signaling are associated with the earliest responses to BCG [26, 28]. While utilization of pathways was generally conserved between HU and HEU infants, greater increases in pathway scores were observed in HEU monocytes for Negative Regulation of Apoptosis and Defense Response. Conversely, the pathway score increase for Cytokine Activity was more pronounced in HU monocytes when compared to HEU. Genes involved in the Endosome pathway were moderately downregulated and upregulated with BCG treatment in HU and HEU, respectively. These data identify differential responses between monocytes from HU and HEU infants, revealing clues as to how the HIV infection of the mother influences monocyte function within the infants.
Following BCG stimulation HU and HEU cord blood monocytes differentially increase expression of genes associated with innate BCG responses
Differential expression analysis was carried out on normalized mRNA data from HU (Fig. 3A) and HEU infants (Fig 3B) and the data presented as volcano plots. A number of genes were significantly upregulated following BCG treatment; 86 genes in HU and 89 in HEU monocytes. In addition, many gene transcripts were significantly reduced following BCG treatment; 31 in HU and 28 in HEU monocytes. The highest linear fold changes in both HU and HEU were for genes generally associated with inflammation, including IL1B (252-HU / 286-HEU), TNF (38.4-HU / 29.2-HEU), IL6 (224-HU / 210-HEU), IL1A (183-HU / 173-HEU), and IL8 (22.1-HU / 19.3-HEU). In addition to responses following BCG treatment, we also compared baseline gene expression across HEU and HU monocytes and found no significant differences between the two groups (Figure S2). To clearly convey differences in BCG-induced changes in gene expression between HU and HEU, fold change for the top 50 upregulated genes was compared (Fig. 3C).
Figure 3: HU and HEU cord blood monocytes increase expression of genes associated with innate responses to BCG in vitro.
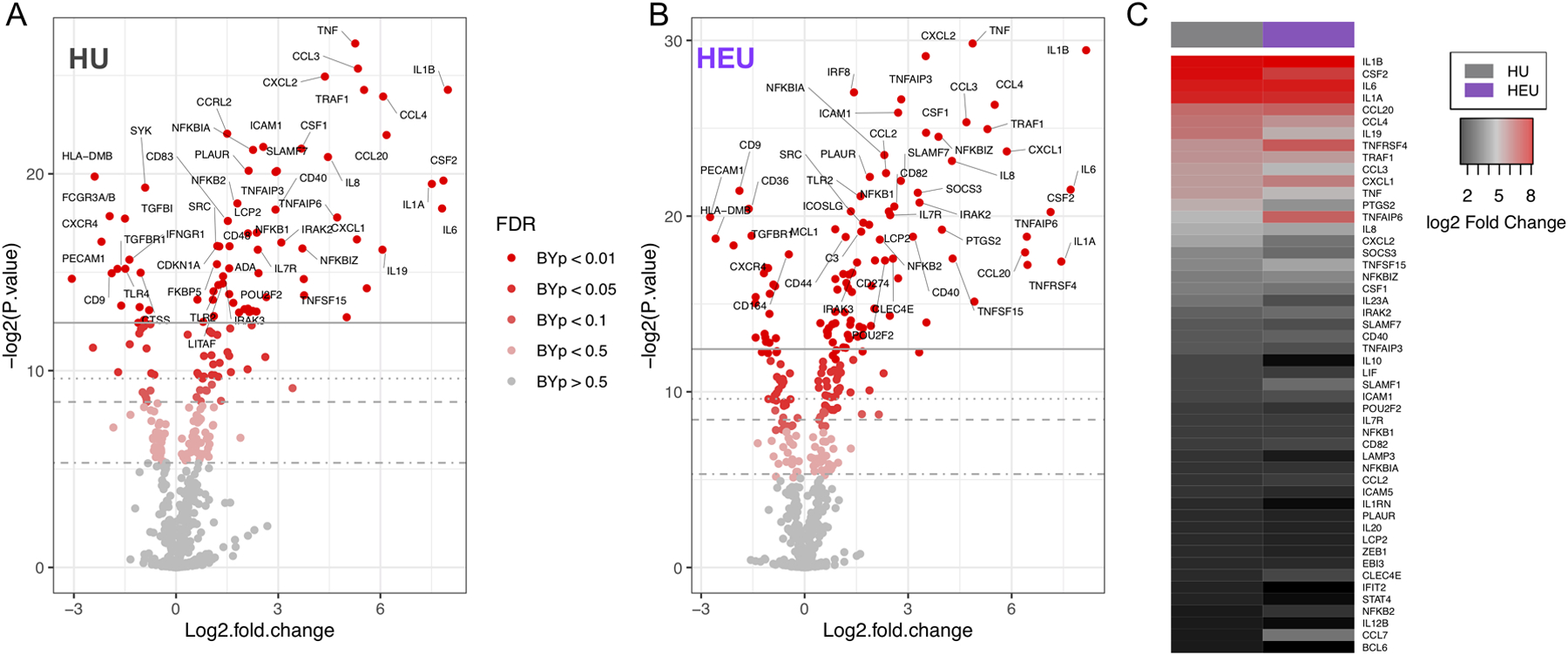
Fold change in gene expression following BCG treatment in monocytes from HU(A) and HEU (B) cord blood samples. Differences in gene upregulation between HU and HEU monocytes after BCG treatment were compared using the top 50 upregulated genes in HU infants (C). Heatmap depicts log2 fold change from untreated conditions.
Eleven genes that were significantly upregulated after BCG treatment exhibited significant differences in linear fold change between HU and HEU monocytes (Fig. 4). Six of the upregulated genes exhibited significantly higher fold induction in the HU compared to HEU monocytes: IL23A(10.7-HU / 6.5-HEU), IL19(66.8-HU / 30.3-HEU), CCL3(40.5-HU / 25.7-HEU), CXCL2(20.6-HU / 11.4-HEU), CSF2(230-HU /140-HEU) and PTGS2(32.3-HU / 15.8-HEU) (Fig 4A–F). Conversely, 5 genes were found to exhibit elevated fold changes with BCG treatment in HEU compared to HU monocytes: CCL7 (3.3-HU / 11.6-HEU), CXCL1 (39.6-HU / 58-HEU), TNFRSF4 (48.4-HU / 87.8-HEU), TNFSF15 (13.4-HU / 19.5-HEU) and TNFAIP6 (26.4-HU / 86.6-HEU) (Fig. 4G–K). Differential upregulation of these genes has the potential to influence multiple pathways involved in recruitment and activation of T cells.
Figure 4: BCG responses are differentially modulated in several genes in HEU cord blood monocytes.
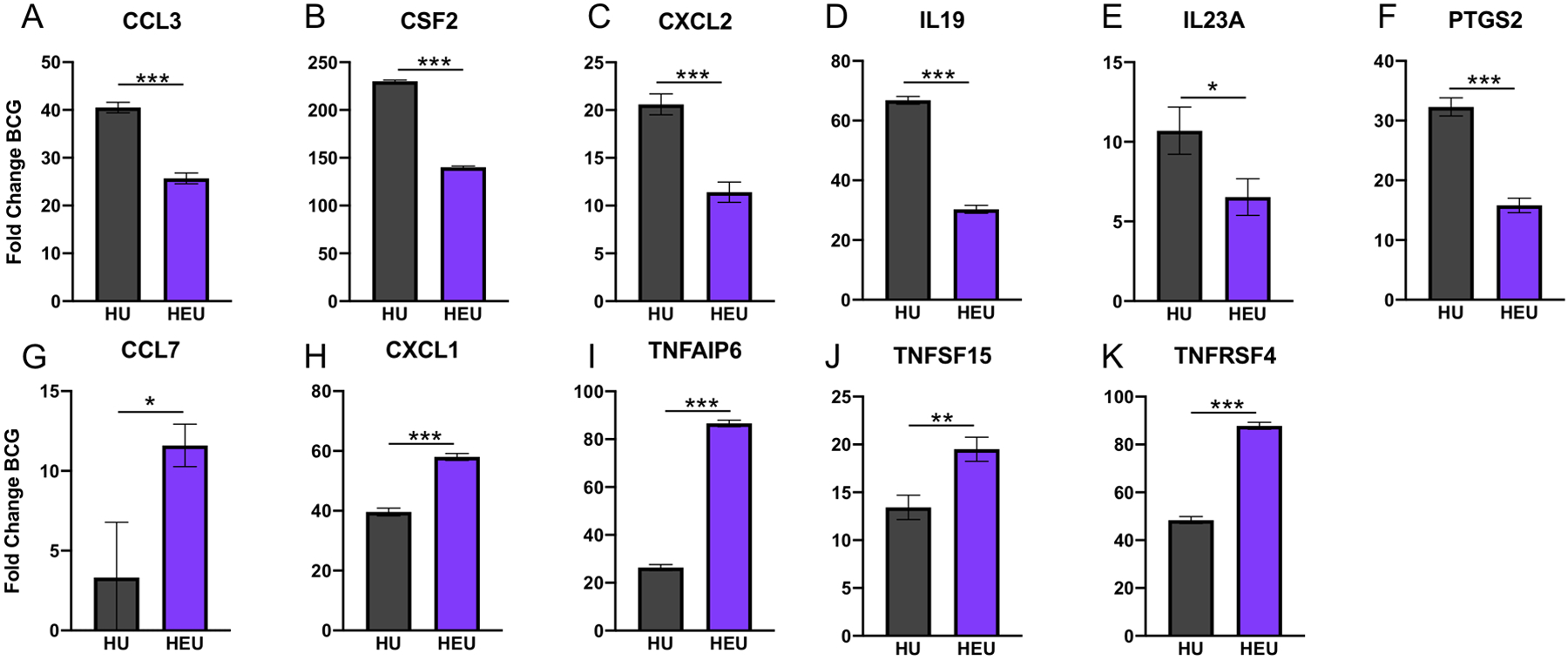
Following BCG treatment, monocytes from HEU infant cord blood exhibited either significantly dampened (A-F) or enhanced (G-K) elevation of genes involved in regulation of inflammatory responses. Results are based on thresholding of differentially expressed genes by fold increase and adjusted p-values. Genes depicted in (A-K) code for the respective proteins: CCL3/MIP-1α, GM-CSF, CXCL2/MIP-2, IL-19, IL-23p19, COX-2, CCL7/MCP-3, CXCL1/GROα, TSG-6, TNF-SF15, TNF-SF4/OX40. Average linear fold change with BCG treatment is depicted with error bars representing standard error of the mean. Comparisons were made using the Holm-Sidak method with correction for multiple comparisons (see methods). * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
The 11 genes identified as differentially upregulated in HEU monocytes are involved with a number of key pathways with potential to influence monocyte response to the BCG vaccination (Fig. 5). In this model, initial BCG sensing upstream of the MyD88/NFκB pathway results in BCG-associated gene transcription. Differentially upregulated genes are identified and subdivided according to pathway specific effects, including pro- and anti-inflammatory activity. In addition, two genes associated with prostaglandin production, CSF2 and PTGS2, were upregulated to a lesser extent in the HEU cord blood monocytes compared to HU. This model indicates that the differential induction of gene transcripts has the potential to play important roles in the immune response mounted by monocytes of HU and HEU infants.
Figure 5: Simplified diagram illustrating altered innate signaling following BCG treatment of HU and HEU monocytes.
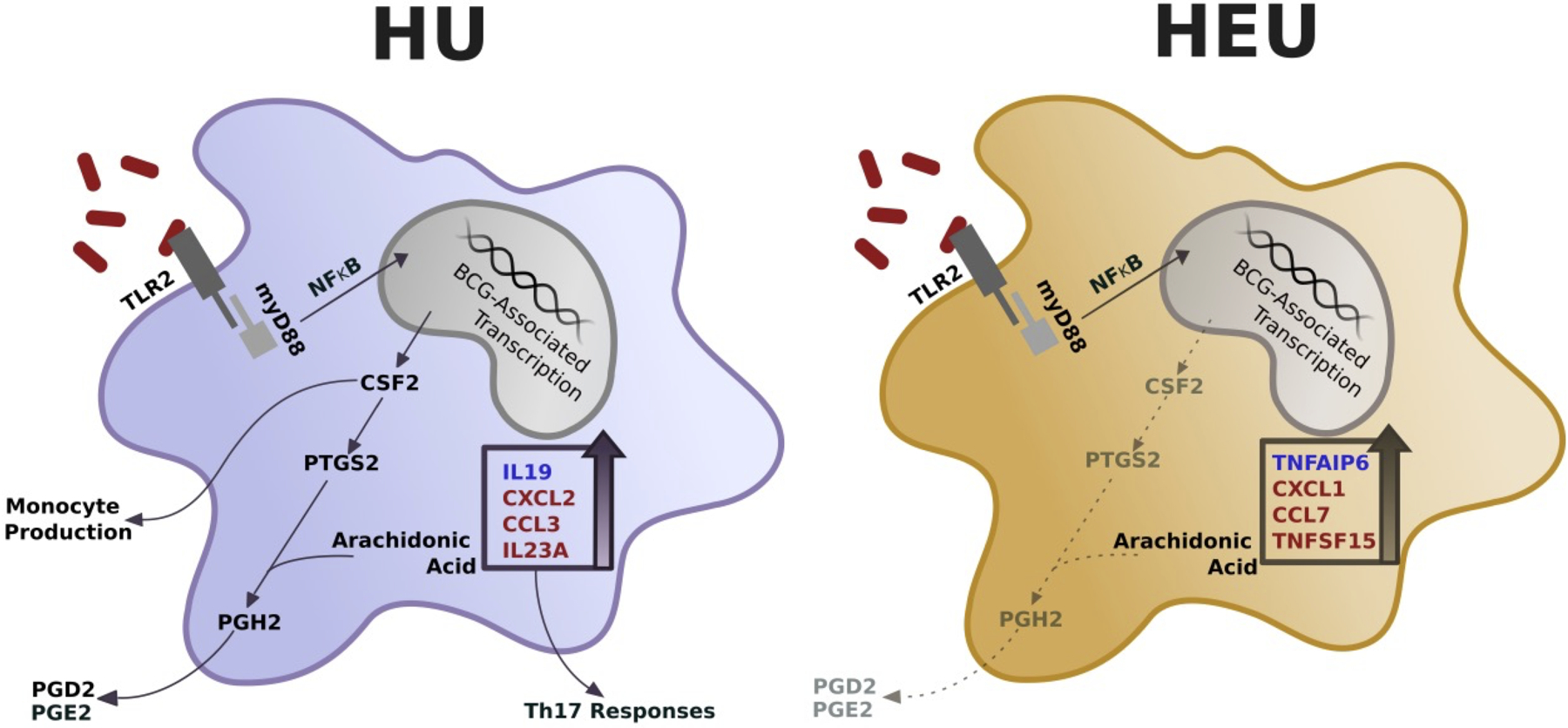
Eleven differentially upregulated genes between HU and HEU infants were submitted into the KEGG pathway finder to determine pathway specific effects. Genes with higher upregulation involved in anti- (blue) and pro-inflammatory (red) responses are shown for the corresponding HU or HEU condition. Effect of dampened CSF2 and PTGS2 are shown for HEU monocytes (gray text, dashed lines)
DISCUSSION
Previous reports have identified an increase in morbidity and mortality rates in uninfected infants born to HIV infected mothers [2, 29]. To date, the literature contains conflicting reports regarding monocyte responses in HIV-exposed uninfected infants with both enhanced and dampened pro-inflammatory signaling being observed depending on both time from birth and the bacterial or viral ligands utilized [8, 30]. The goal of this work was to evaluate BCG responses in cord blood monocytes from HU infants and compare these to HEU infants. BCG is generally administered at birth and T cell responses to BCG have been demonstrated to be impaired in HEU [12–14]. Since T cell responses to BCG are largely shaped by innate responses, including responses from monocytes, it is critical to evaluate the monocyte response in HEU infants in the context of BCG vaccination. We determined that monocytes from both HU and HEU infants responded to BCG with gene upregulation characteristic of canonical anti-mycobacterial innate responses, including those for IL-6, TNFα, IL-1β, GM-CSF, and IL-23. Furthermore, pathway analysis suggests that expression of these inflammatory mediators is likely occurring through similar pathways involving NFκB in both HU and HEU infants. However, upregulation of multiple genes varied greatly between the HEU and HU monocytes, with several of these genes potentially playing central roles in regulating downstream inflammatory and adaptive responses.
Interestingly, the pro-inflammatory genes, CXCL1, CCL7 and TNFSF15 were expressed at a heightened level in monocytes from HEU compared to HU infants (Fig. 5). The chemokines CXCL1 and CCL7 mediate migration of neutrophils and monocytes during innate immune responses through engagement of CXCR2 and CCR2, respectively [31, 32]. There have been few reports on CCL7, previously called MCP-3, in regard to BCG responses. However, in a study from 1997, mycobacterial lipoarabinomannan (LAM) was shown to induce CCL7 expression in peripheral blood-derived monocytes, with whole BCG inducing similarly strong expression [33]. Several reports have also demonstrated that CCL7 plays a key role in recruitment of tumor-associated monocytes (TAMs) and macrophages [34]. It is worth noting that these TAMs suppress T cell function through expression of tolerogenic cytokines and surface proteins, including TGF-β, IL-10 and PD-L1 [35]. In contrast, the levels of the pro-inflammatory cytokines CXCL2, CCL3 and IL23A, were suppressed following BCG stimulation in HEU infants (Fig. 5). CXCL2 and CCL3 (MIP-2α and MIP-1α) are secreted by macrophages and mediate recruitment of multiple effector cells, including neutrophils, to sites of inflammation [32]. CCL3/MIP-1α in particular has been shown to be among the earliest cytokines produced following ex vivo stimulation of whole blood with BCG, suggesting its overall importance in shaping downstream responses [26].
Following exposure to inflammatory cytokines and microbial products, TNFAIP6 expression is rapidly upregulated in monocytes resulting in production of the anti-inflammatory protein TNFα stimulated gene 6 (TSG-6) [36, 37]. We have identified TNFAIP6/TSG-6 to be significantly upregulated following BCG treatment in both HU and HEU infants. Furthermore, we observed a remarkable increase in production of TNFAIP6 in HEU monocytes (26-fold increase in HU compared to an 86-fold increase in HEU). Little is known regarding the role of TSG-6 in bacterial responses, however evidence suggests TSG-6 exerts its anti-inflammatory effects following zymosan stimulation through binding to CD44 on macrophages and inhibiting TLR2-induced nuclear translocation of NFκB [38–40]. The role of TLR2 signaling and subsequent NFκB-induced inflammatory cytokine production is of particular importance in innate responses to mycobacteria, and therefore increased TSG-6 expression could result in less effective innate responses [28, 41, 42]. Taken together, the dramatic elevation of TNFAIP6/TSG-6 observed in HEU infants is potentially linked to dampening of downstream innate responses through negative feedback, which may impair the development of anti-BCG or other vaccine associated T cell responses.
While we did not observe differences in expression of IL12A, possibly due to the 6-hour time point chosen for analysis, we did observe increased induction of IL12B and IL23A in both HU and HEU infants, suggesting increased production of cytokine IL-23 [26]. In addition, the induction of IL23A was significantly dampened in the HEU compared to HU monocytes following BCG treatment. IL23A promotes the production of pro-inflammatory cytokines as well as the maintenance and expansion of Th17 cells [43, 44]. Interestingly, M. bovis BCG as well as several other intracellular bacteria have been shown to require IL-23 driven Th17 responses to drive protective Th1 responses [20, 45–47].
Prenatal exposure to HIV has been associated with reduced levels of monocytes and their associated cytokines during the first 6 months of life [2, 7, 8]. One factor potentially driving reported deficiencies in monocyte production in HEU infants is a dampened GM-CSF (CSF2) response, as was observed here following BCG treatment. GM-CSF is a key stimulator of monocyte production from bone marrow stem cells, plays a central role as an inflammatory mediator and induces expression of PTGS2 (COX-2), the key inducible enzyme in the biosynthesis of prostaglandins (i.e. PGD2, PGE2) (Fig. 5). Interestingly, Gopal et al. demonstrated that in mice PGE2 suppresses IL-12 in monocytes and dendritic cells while inducing expression of IL-23, thus promoting Th17 cell differentiation [20, 48]. This was shown to result in downregulation of IL-10, increased IL-12, and increased BCG-specific Th1 cell responses. Our observations of significantly higher expression of prostaglandin associated genes CSF2 and PTGS2, as well as IL-23A/IL-23 in HU monocytes, compared to HEU, may therefore be linked to the observed impairment of BCG-specific Th1/IFNγ responses reported in HEU infants [21–23].
Exposure to HIV antigens, anti-retroviral treatment, and the altered immunological environment of HIV infected mothers each have the potential to impact the developing neonate immune system. Insights into how these changes may shape responses to vaccines and pathogens are necessary as more infants are born HIV-exposed. Our findings provide mechanistic insights with regard to the impaired T cell responses previously identified in lymphocytes from HEU infants. The transcriptional analysis described here identifies pathways that could be therapeutically modulated to reduce the impact of in utero HIV exposure and improve vaccine responsiveness in HEU infants.
Supplementary Material
Heatmaps depict nonhierarchical clustering of normalized mRNA counts from untreated and BCG untreated monocytes from HU (A) and HEU (B) cord blood. Genes were combined from 5 predefined gene sets which exhibited clustering of BCG treated and untreated samples: programmed cell death, Jak-Stat signaling, cell proliferation, inflammatory responses, and NFκB cascade. Clusters of genes with increased mRNA levels with BCG treatment are highlighted.
Differential gene expression analysis was used to assess differences in immune gene expression in HEU monocytes compared to HU in untreated conditions.
Acknowledgements
We thank the members of the Sodora Laboratory including Cole Fisher and Katie Fancher for their critical assessment of data and assisting in preparation of the manuscript. Gallya Gannot and Isaac Rodriguez-Chavez for support in the undertaking of these experiments.
Footnotes
Conflicts of Interest and Source of Funding
This work was supported by a grant from the NIH-NIDCR R01 DE023047. The authors declare no conflicts of interest.
References
- 1.Wedi CO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV 2016; 3(1):e33–48. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Raya B, Kollmann TR, Marchant A, MacGillivray DM. The Immune System of HIV-Exposed Uninfected Infants. Front Immunol 2016; 7:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuhn L, Thea DM, Aldrovandi GM. Bystander effects: children who escape infection but not harm. J Acquir Immune Defic Syndr 2007; 46(5):517–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mussi-Pinhata MM, Motta F, Freimanis-Hance L, de Souza R, Szyld E, Succi RC, et al. Lower respiratory tract infections among human immunodeficiency virus-exposed, uninfected infants. Int J Infect Dis 2010; 14 Suppl 3:e176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet 2007; 369(9571):1440–1451. [DOI] [PubMed] [Google Scholar]
- 6.Slogrove A, Reikie B, Naidoo S, De Beer C, Ho K, Cotton M, et al. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. J Trop Pediatr 2012; 58(6):505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunders MJ, Bekker V, Scherpbier HJ, Boer K, Godfried M, Kuijpers TW. Haematological parameters of HIV-1-uninfected infants born to HIV-1-infected mothers. Acta Paediatr 2005; 94(11):1571–1577. [DOI] [PubMed] [Google Scholar]
- 8.Reikie BA, Adams RCM, Leligdowicz A, Ho K, Naidoo S, Rusk CE, et al. Altered innate immune development in HIV-exposed uninfected infants. J Acquir Immune Defic Syndr 2014; 66(3):245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velilla PA, Montoya CJ, Hoyos A, Moreno ME, Chougnet C, Rugeles MT. Effect of intrauterine HIV-1 exposure on the frequency and function of uninfected newborns’ dendritic cells. Clin Immunol 2008; 126(3):243–250. [DOI] [PubMed] [Google Scholar]
- 10.Clerici M, Saresella M, Colombo F, Fossati S, Sala N, Bricalli D, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood 2000; 96(12):3866–3871. [PubMed] [Google Scholar]
- 11.Pacheco SE, McIntosh K, Lu M, Mofenson LM, Diaz C, Foca M, et al. Effect of perinatal antiretroviral drug exposure on hematologic values in HIV-uninfected children: An analysis of the women and infants transmission study. J Infect Dis 2006; 194(8):1089–1097. [DOI] [PubMed] [Google Scholar]
- 12.Hygino J, Lima PG, Filho RG, Silva AA, Saramago CS, Andrade RM, et al. Altered immunological reactivity in HIV-1-exposed uninfected neonates. Clin Immunol 2008; 127(3):340–347. [DOI] [PubMed] [Google Scholar]
- 13.Chougnet C, Kovacs A, Baker R, Mueller BU, Luban NL, Liewehr DJ, et al. Influence of human immunodeficiency virus-infected maternal environment on development of infant interleukin-12 production. J Infect Dis 2000; 181(5):1590–1597. [DOI] [PubMed] [Google Scholar]
- 14.Rich KC, Siegel JN, Jennings C, Rydman RJ, Landay AL. Function and phenotype of immature CD4+ lymphocytes in healthy infants and early lymphocyte activation in uninfected infants of human immunodeficiency virus-infected mothers. Clin Diagn Lab Immunol 1997; 4(3):358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetghebuer T, Smolen KK, Adler C, Das J, McBride T, Smits G, et al. Initiation of Antiretroviral Therapy Before Pregnancy Reduces the Risk of Infection-related Hospitalization in Human Immunodeficiency Virus-exposed Uninfected Infants Born in a High-income Country. Clin Infect Dis 2019; 68(7):1193–1203. [DOI] [PubMed] [Google Scholar]
- 16.Watkins ML, Semple PL, Abel B, Hanekom WA, Kaplan G, Ress SR. Exposure of cord blood to Mycobacterium bovis BCG induces an innate response but not a T-cell cytokine response. Clin Vaccine Immunol 2008; 15(11):1666–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansoor N, Scriba TJ, de Kock M, Tameris M, Abel B, Keyser A, et al. HIV-1 infection in infants severely impairs the immune response induced by Bacille Calmette-Guerin vaccine. J Infect Dis 2009; 199(7):982–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khader SA, Pearl JE, Sakamoto K, Gilmartin L, Bell GK, Jelley-Gibbs DM, et al. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-gamma responses if IL-12p70 is available. J Immunol 2005; 175(2):788–795. [DOI] [PubMed] [Google Scholar]
- 19.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007; 8(4):369–377. [DOI] [PubMed] [Google Scholar]
- 20.Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M, et al. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur J Immunol 2012; 42(2):364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazzola TN, da Silva MT, Abramczuk BM, Moreno YM, Lima SC, Zorzeto TQ, et al. Impaired Bacillus Calmette-Guerin cellular immune response in HIV-exposed, uninfected infants. AIDS 2011; 25(17):2079–2087. [DOI] [PubMed] [Google Scholar]
- 22.Kidzeru EB, Hesseling AC, Passmore JA, Myer L, Gamieldien H, Tchakoute CT, et al. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS 2014; 28(10):1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hesseling AC, Jaspan HB, Black GF, Nene N, Walzl G. Immunogenicity of BCG in HIV-exposed and non-exposed infants following routine birth or delayed vaccination. Int J Tuberc Lung Dis 2015; 19(4):454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, Dunaway DL, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 2008; 26(3):317–325. [DOI] [PubMed] [Google Scholar]
- 25.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat 2001; 29(4):1165–1188. [Google Scholar]
- 26.Bisiaux A, Boussier J, Duffy D, Quintana-Murci L, Fontes M, Albert ML, et al. Deconvolution of the Response to Bacillus Calmette-Guerin Reveals NF-kappa B-Induced Cytokines As Autocrine Mediators of Innate Immunity. Frontiers in Immunology 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasper MA, Biswas SP, Fisher BS, Ehnert SC, Sherman DR, Sodora DL. Nonpathogenic SIV and Pathogenic HIV Infections Associate with Disparate Innate Cytokine Signatures in Response to Mycobacterium bovis BCG. PLoS One 2016; 11(8):e0158149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci U S A 1999; 96(25):14459–14463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J 2007; 26(6):519–526. [DOI] [PubMed] [Google Scholar]
- 30.Simani OE, Adrian PV, Violari A, Kuwanda L, Otwombe K, Nunes MC, et al. Effect of in-utero HIV exposure and antiretroviral treatment strategies on measles susceptibility and immunogenicity of measles vaccine. AIDS 2013; 27(10):1583–1591. [DOI] [PubMed] [Google Scholar]
- 31.Widmer U, Manogue KR, Cerami A, Sherry B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J Immunol 1993; 150(11):4996–5012. [PubMed] [Google Scholar]
- 32.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013; 121(24):4930–4937. [DOI] [PubMed] [Google Scholar]
- 33.Vouret-Craviari V, Cenzuales S, Poli G, Mantovani A. Expression of monocyte chemotactic protein-3 in human monocytes exposed to the mycobacterial cell wall component lipoarabinomannan. Cytokine 1997; 9(12):992–998. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Cai Y, Liu L, Wu Y, Xiong X. Crucial biological functions of CCL7 in cancer. PeerJ 2018; 6:e4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009; 206(6):1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe R, Sato Y, Ozawa N, Takahashi Y, Koba S, Watanabe T. Emerging Roles of Tumor Necrosis Factor-Stimulated Gene-6 in the Pathophysiology and Treatment of Atherosclerosis. Int J Mol Sci 2018; 19(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wisniewski HG, Maier R, Lotz M, Lee S, Klampfer L, Lee TH, et al. TSG-6: a TNF-, IL-1-, and LPS-inducible secreted glycoprotein associated with arthritis. J Immunol 1993; 151(11):6593–6601. [PubMed] [Google Scholar]
- 38.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 2011; 118(2):330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang H, Tian W, Wang S, Liu X, Wang Z, Hou L, et al. TSG-6 secreted by bone marrow mesenchymal stem cells attenuates intervertebral disc degeneration by inhibiting the TLR2/NF-kappaB signaling pathway. Lab Invest 2018; 98(6):755–772. [DOI] [PubMed] [Google Scholar]
- 40.Wisniewski HG, Vilcek J. TSG-6: an IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev 1997; 8(2):143–156. [DOI] [PubMed] [Google Scholar]
- 41.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2001; 2(8):675–680. [DOI] [PubMed] [Google Scholar]
- 42.Heldwein KA, Liang MD, Andresen TK, Thomas KE, Marty AM, Cuesta N, et al. TLR2 and TLR4 serve distinct roles in the host immune response against Mycobacterium bovis BCG. J Leukoc Biol 2003; 74(2):277–286. [DOI] [PubMed] [Google Scholar]
- 43.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol 2008; 181(9):5948–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005; 6(11):1123–1132. [DOI] [PubMed] [Google Scholar]
- 45.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 2009; 31(5):799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, et al. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol 2009; 183(9):5886–5895. [DOI] [PubMed] [Google Scholar]
- 47.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol 2007; 178(6):3786–3796. [DOI] [PubMed] [Google Scholar]
- 48.Kalinski P Regulation of immune responses by prostaglandin E2. J Immunol 2012; 188(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Heatmaps depict nonhierarchical clustering of normalized mRNA counts from untreated and BCG untreated monocytes from HU (A) and HEU (B) cord blood. Genes were combined from 5 predefined gene sets which exhibited clustering of BCG treated and untreated samples: programmed cell death, Jak-Stat signaling, cell proliferation, inflammatory responses, and NFκB cascade. Clusters of genes with increased mRNA levels with BCG treatment are highlighted.
Differential gene expression analysis was used to assess differences in immune gene expression in HEU monocytes compared to HU in untreated conditions.


