Abstract
Neutrophils are key effector cells of the innate immune system, serving as a first line of defense in the response to injury and playing essential roles in the wound healing process. Following myocardial infarction (MI), neutrophils infiltrate into the infarct region to propagate inflammation and begin the initial phase of cardiac wound repair. Pro-inflammatory neutrophils release proteases to degrade extracellular matrix (ECM), a necessary step for the removal of necrotic myocytes as a prelude for scar formation. Neutrophils transition their phenotype over time to regulate MI inflammation resolution and stabilize scar formation. Neutrophils contribute to the evolution from inflammation to resolution and scar formation by serving anti-inflammatory and repair functions. As anti-inflammatory cells, neutrophils contribute ECM proteins during scar formation, in particular fibronectin, galectin-3, and vimentin. The diverse and polarizing functions that contribute to MI wound repair make this innate immune cell a viable target to improve MI outcomes. Thus, understanding the signaling involved in neutrophil physiology in the context of MI may help to identify novel therapeutic targets.
Keywords: neutrophil, myocardial infarction, signaling, leukocyte, inflammation, wound repair
Introduction
Myocardial infarction (MI) occurs when blood flow in the coronary arteries is reduced to the point where oxygen and nutrient demands of the heart cannot be met. In response to MI, neutrophils are early responders, infiltrating from the circulation into the myocardium to coordinate the initial pro-inflammatory response. Neutrophils are recruited from the bone marrow at the onset of MI and increase in numbers in the circulation. Within the circulation, neutrophils firmly adhere and crawl along the blood vessel wall using diapedesis. Once migrated into the tissue, neutrophils are chemotactically drawn to the infarct site to serve numerous functions including propagation of inflammation, degranulation and reactive oxygen species production, phagocytosis of cellular debris, and eventually inflammation resolution and scar formation.
In the mouse model of permanent occlusion MI, neutrophil numbers peak at MI days 1–3, and by one month after MI, there is little inflammation and few neutrophils in the infarct region. We have recently shown that neutrophils polarize after MI, exhibiting a pro-inflammatory signature at MI days 1–3 and transitioning to anti-inflammatory and then pro-repair phenotypes at days 3–7.[1] While global MI signaling has been investigated in the LV, the heterogeneity by which individual cell types signal in each phase of MI and temporally shift their phenotype is just now being understood. Table 1 summarizes current knowledge of neutrophil signaling following MI, concepts that are expanded on below.
Table 1.
Summary of current knowledge in neutrophil signaling during response to MI
| Neutrophil signaling during | Key pathways and effectors |
|---|---|
| Recruitment from the bone marrow | • G-CSF inhibition of CXCR4 • S100A8/A9 activation of TLR4 • CXCL1/2 activation of CXCR2 |
| Infiltration through the vessel wall | • Chemotaxis from circulating chemokines (IL-8, CXCL-1,2, C5a) • Integrin/ICAM interactions between neutrophil and vascular endothelial cells • Crawling via PLC-Ca2+ and the PI3K/Rho GTPase axes • JAM-A and PECAM-1 interactions at endothelial junctions |
| Migration into the infarct region | • GPCR induced PLC and PI3K signaling • Calcium induced contraction • Rho-GTPase induced f-actin protrusions and MLC phosphorylation • S100A8/A9 induced cytokine production |
| Degranulation and reactive oxygen species (ROS) production | • PLC induced calcium signaling • Calcium activation of of SNAREs and Pyk2 • Pyk2 promotes downstream Rac activity and actin polymerization • SNARE activity results in exocytosis • Proteases cleave ECM targets |
| Phagocytosis | • Integrin induced calcium release promotes calpain activation • Calpain cleaves p81, eliminating the link between f-actin and the cell membrane • Ezrin cleavage promotes cell spreading allowing for engulfment of debris |
| Resolution of inflammation | • Neutrophils stimulate anti-inflammatory macrophage polarization • Neutrophils produce anti-inflammatory proteins (IL-10) in the reparative phase of MI • Neutrophils produce ECM proteins and contribute to scar formation • Neutrophils undergo apoptosis through the classical extrinsic and intrinsic pathway • Apoptotic neutrophils bind and scavenge pro-inflammatory mediators |
Neutrophil signaling during recruitment from the bone marrow
MI induces pro-inflammatory cytokines that activate release of neutrophils from the bone marrow, the largest reservoir source in the body. Multiple pro-inflammatory stimuli may induce leukocytosis (an increase in white blood cells), including interleukin (IL)-1, complement component 5a (C5a), and granulocyte colony stimulating factor (G-CSF).[2] Mice lacking G-CSF display impaired neutrophil mobilization from the bone marrow,[3] which has a built-in system to regulate the release of neutrophils. In this system, CXCL12 binds C-X-C motif chemokine receptor 4 (CXCR4) on bone marrow stromal cells. CXCR4 is a major regulator of neutrophil trafficking to limit release, and Cxcr4−/− mice exhibit neutrophilia and persistent mobilization of neutrophils from bone marrow.[4] G-CSF induces cleavage of CXCR4 through the cell surface peptidase CD26 to inhibit the CXCL12-CXCR4 axis and stimulate release of neutrophils into the circulation.[5] CXCR4 inhibition in mice promotes cardiac wound healing after MI by stimulating release of neutrophils, lymphocytes, and monocytes into the circulation.[6] The mechanism for the beneficial effect seen was primarily attributed to an increase in the mobilization and activity of regulatory T cells rather than an effect on neutrophils.
An important component in neutrophil mobilization is the S100A8/S100A9 complex. S100A8 and S100A9 form a heterodimer stabilized by calcium binding of the EF-hand type domain.[7] S100A8/A9 dimers bind to toll-like receptor 4 and promote IL-1β secretion.[8–10] IL-1β interacts with the interleukin 1 receptor (IL-1R) on hematopoietic stem cells in bone marrow leading to granulopoiesis in a cell-autonomous manner.[11] S100A8/A9 is elevated and is an essential mechanism for neutrophil trafficking to the blood following MI.[11, 12] Blockade of S100A9 improves cardiac physiology following MI, in part by reducing granulopoiesis.[13] Consequently, S100A9 inhibition reduces but does not eliminate neutrophils in the LV infarct following MI.
Another central axis for neutrophil mobilization entails CXCL1/2-CXCR2 signaling. Acute administration of pro-inflammatory CXCL1 or CXCL2 rapidly releases neutrophils from the bone marrow.[14, 15] Conversely, 11β-hydroxysteroid dehydrogenase type 1 suppresses CXCL2-induced neutrophil recruitment to the injured myocardium.[15] CXCR2 is the only known receptor for CXCL1/2 and is expressed in bone marrow stromal cells.[16] Mice deficient in CXCR2 show retention of neutrophils in the bone marrow.[16] Once in the circulation, neutrophils respond to MI by homing to the heart and infiltrating into the infarct region.
Neutrophil signaling during infiltration
After MI, neutrophils infiltrate from the circulation into the infarct region. MI induces cardiomyocytes to undergo necrosis, apoptosis, and other forms of cell death.[17] Myocyte cell death releases damage associated molecular patterns (DAMPs) that serve as danger signals.[18, 19] Common DAMPs include high mobility group box 1 (HMGB-1), adenosine triphosphate, double stranded DNA, mitochondrial DNA (mtDNA), and IL-1α and IL-1β.[11, 20–24] DAMPs bind to pattern recognition receptors on neutrophils, to initiate the complement cascade and propagate inflammatory signaling by inducing cytokine and chemokine production. Common pattern recognition receptors include toll-like receptors (TLRs) and interleukin receptors.[25] As an example, HMGB1 forms a heterodimer with CXCL12 and engages with CXCR4 to induce neutrophil recruitment and migration.[26] MI activates endothelial cells, triggering vessels to lose integrity and increase permeability, allowing for increased neutrophil infiltration.
Upon reaching the vessels that supply the infarct, neutrophils adhere to endothelial cells through interaction between cell surface integrins and selectins, including CD11a and CD11b and L-selectin (CD62L).[27] Binding of chemoattractants by neutrophils causes a conformational change in neutrophil integrins, which mediates cell arrest on ICAM-1.[28] CD11a and CD11b on the neutrophils bind to intercellular adhesion molecule (ICAM)-1 and ICAM-2 on endothelial cells.[29] A rapid and reversible binding of integrins with ICAM-1 results in a rolling effect of the neutrophil against the vessel wall. L-selectin then binds to L-selectin ligands on the endothelial cell to induce firm adhesion.[27, 30]
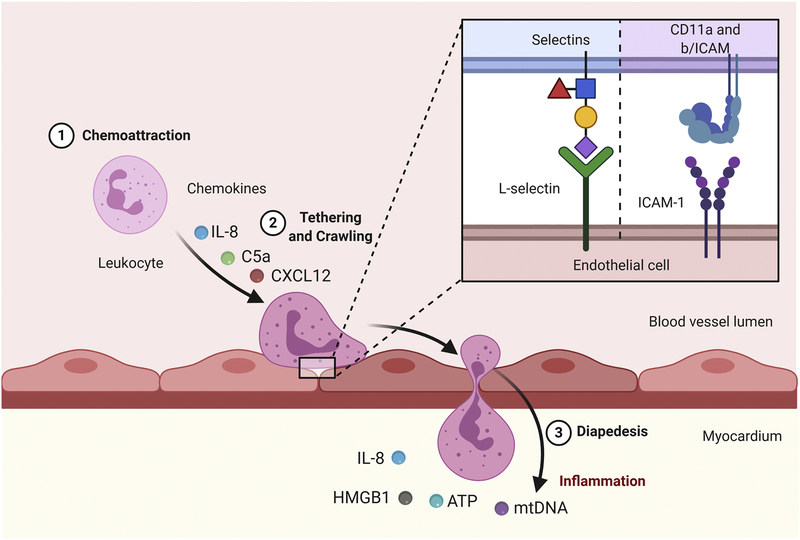
Following adhesion, the neutrophil crawls across the vessel wall following the chemotactic gradient, until it finds a suitable location for paracellular migration (Figure 1). The crawling process requires neutrophil polarity and involves a series of contractile interactions. The polarity creates an intracellular environment with distinct functions occurring on opposite sides of the neutrophil. The neutrophil creates F-actin protrusions known as lamellipodia on the side of the cell that faces and senses the chemotactic gradient. F-actin lamellipodia functions are dependent on non-muscle class II protein myosin heavy chain 9 (Myh9),[31] as loss of Myh9 reduces functional crawling and neutrophil diapedesis.[31] The neutrophil crawls along the endothelial layer, searching for a junction to migrate through. Junctions become leaky to facilitate diapedesis, and specific endothelial cell-neutrophil surface interactions allow the cell to pass between junctions. On the opposite side of the neutrophil, actin-myosin contractile interactions propel the neutrophil towards the gradient.[32, 33] Neutrophils maintain polarity (i.e., a side facing the chemotactic gradient and the opposite side propelling the cell along the vessel wall) through myosin-actin interactions.
Figure 1. Neutrophil signaling during infiltration.
Pro-inflammatory cytokines such as interleukin-8 (IL-8), C-X-C Ligand (CXCL)-12, and complement 5a (C5a) and DAMPs such as mitochondrial DNA (mtDNA) and high mobility group box 1 (HMGB1) recruit neutrophils to the vessel wall. Interactions between CD11 on the neutrophil and intercellular adhesion molecule 1, ICAM-1 on the endothelial cell surface as well as L-selectins found on both cells cause firm adhesion. Neutrophils follow the chemotactic gradient, crawling along the vessel wall. Neutrophils undergo diapedesis and continue to follow the chemotactic gradient to the site of injury. Created with BioRender.com
The phosphoinositide 3 kinase (PI3K) pathway is the major regulator of neutrophil polarity by initiating downstream signaling of three key Rho GTPases (Rac, RhoA, and Cdc42).[34] The second messengers, phosphatidylinositol bi-phosphate (PIP2) and phosphatidylinositol tri-phosphate (PIP3) activate Rac, RhoA, and Cdc42. GTP binding induces signaling to produce neutrophil polarity.[35] Rac is prominent in lamellipodia to promote F-actin polymerization by activating the Wiskott–Aldrich Syndrome protein (WASP) family.[36] RhoA is the key factor stimulating phosphorylation of myosin light chain (MLC) kinase through Rho associated protein kinase to promote contraction.[32] Activation of Rac is primarily dependent on PIP3, whereas RhoA is activated by PIP2, the downstream regulators of PI3K.[37] The split in this pathway allows the neutrophil to polarize. Cdc42 also plays a key role in maintaining a polarized neutrophil. Cdc42 induces guanine nucleotide exchange factors to keep Rac activated F-actin polymerization at the sight of the lamellipodia.[38] At the same time, Cdc42 improves RhoA signaling to enhance contraction on the opposite side of the cell. Cdc42 signals through WASP to increase MLC activity.[39, 40] Thus, Rac regulates function of the sensory side of the neutrophil, RhoA regulates contractile function propelling the cell forward, and Cdc42 regulates both roles.
ICAM-integrin interactions coordinate tight adhesion of the neutrophil at the endothelial tight junction. This interaction loosens the junction by inducing phosphorylation of vascular endothelial cadherin.[41] Once the junction becomes more permeable, junctional adhesion molecule (JAM), specifically JAM-A, and platelet endothelial cell adhesion molecule 1 (PECAM-1) are responsible for allowing the cell to pass through the junction.[42, 43] The function of these proteins is not completely clear, especially in vivo. In vitro, it appears that JAM-A binds to JAM-A on adjacent cells, and PECAM-1 works in a similar manner.[44, 45] The role of these proteins in endothelial cell-neutrophil interactions may be more complex. Loss of PECAM-1 in mice causes neutrophils to arrest before crossing the basement membrane into the extracellular space [45]. Similar to the processes of adhesion and crawling neutrophils require specific chemokines and chemokine receptor signaling specifically in diapedesis. Tumor necrosis factor alpha (TNF-α) induced CXCL2 is necessary for the neutrophil to pass through a junction but does not significantly hinder neutrophil adhesion and crawling.[46] These interactions between neutrophils and endothelial cells allow neutrophils to pass into the extracellular space and begin migrating to the infarct site.
Neutrophil signaling during migration into the infarct region
Migration to the infarct after diapedesis works through similar mechanisms as those described above for neutrophil crawling along the endothelium. The neutrophil must maintain polarity with sensors facing chemokines and a contractile mechanism pushing the cell towards the injury. A wide range of receptors on neutrophils activate contraction, including formyl-peptide receptors, leukotriene receptors, platelet activating factor receptor, CXCR1 and CXCR2, and complement receptors.[46, 47] In humans, C-X-C motif chemokines include CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8.[48] Our lab has investigated CXCL4 in the context of macrophages, in which CXCL4 overexpression increases LV dilation and reduces day 7 survival in mice. CXCL4 overexpression increases MMP-8 and MMP-9 levels at MI day 5, a point where MMP levels should be returning to baseline.[49]
Neutrophil signaling differs based on sex. In mice at MI day 1, males have a more robust inflammatory response in neutrophils showing elevated transcription of Cxcr3, IL6Rα, IL1r1, and IL13.[50] In addition, female mice have reduced IL6, IL12a, and TNFα mRNA and protein per cell in neutrophils isolated from the infarct at day 1. Overall, female mice show less pronounced inflammatory response to MI compared to their male counterparts. In line with a reduced inflammatory response in neutrophils, female outcomes were significantly better than males, showing survival of ~80% compared to ~60% for males and an LV rupture rate that is 4-fold lower than males.[50] One potential mechanism for the blunted inflammatory response in female mice is the desensitization to apoprotein F (Apo F) in females but not males. ApoF induces the liver X receptors/retinoid X receptor pathway through CD36 and peroxisome proliferator-activated receptor gamma (PPARγ) to activate NF-κB and produce pro-inflammatory cytokines such as IL-6.[50]
Sex steroids may also play an important role in the wound healing process and may account for some of the sex differences seen in MI. Androgen receptors drive neutrophil differentiation and thus regulate neutrophil counts. Wound healing is also accelerated following castration, deletion of androgen receptors, or androgen receptor blockade.[51] Therefore, there are a number of sex-mediated mechanisms of MI repair with neutrophil mechanisms.
The concentration of chemotactic factors directs the neutrophil to the injury site. DAMP receptors and cytokine receptors on the surface of the neutrophil lead to downstream activation of the PI3K pathway and the PLC-PKC pathway.[52] Thus, the higher concentration of signals transduced, the greater amount of cell contraction that occurs. Figure 2 describes the signaling involved in migration of the polarized neutrophil.
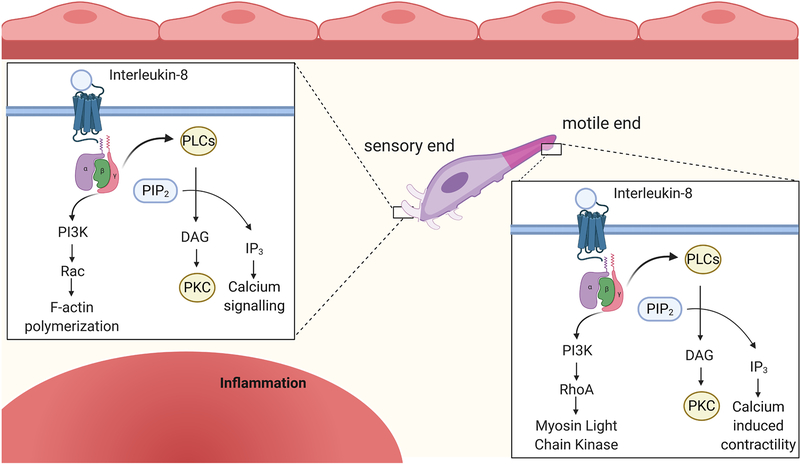
Figure 2. Neutrophil signaling during migration into the infarct region.
Following diapedesis, neutrophils continue to follow chemotactic gradients to the site of injury. Neutrophils bind IL-8, (CXCL)-1,2, and other stimuli that activate G protein coupled receptors (GPCRs). G protein subunits activate the phospholipase C (PLC) and the phosphoinositide 3-kinase (PI3K) pathway. At the sensory end (purple) of the polarized neutrophil the PLC pathway activates downstream calcium signaling and protein kinase C. The PI3K pathway activates Rac which permits F-actin polymerization and aids in production of protrusions that improve the neutrophil ability to respond to sensory information. On the other end of the polarized neutrophil, the motile end (pink), PLC induces calcium signaling that promotes contractility and motility. PI3K activates RhoA leading to downstream phosphorylation of myosin light chain (MLC) and aids in contraction. Together these mechanisms allow the neutrophil to navigate to the infarct area. Created with BioRender.com
G protein coupled receptor (GPCR) signaling induces both the phospholipase C (PLC) pathway and the PI3K pathway to induce neutrophil migration. Generally, ligands to GPCRs (e.g., C5a, IL-8, and CXCL-1 or 2) trigger a conformational change in the intracellular loop of the receptor resulting in dissociation of the heterotrimeric G-protein into its Gα and Gβγ subunits. Gβγ acts as the primary signal transducer by activating PLC following binding of formyl-peptide receptors, leukotriene receptors, platelet activating factor receptor, CXCR1 and CXCR2, and complement receptors. PLC cleaves PIP2, producing diacylglycerol (DAG) and inositol triphosphate (IP3). IP3 production activates the release of Ca2+ from the endoplasmic reticulum. Ca2+ regulates many aspects of neutrophil behavior, including migration, degranulation, and phagocytosis.[53] During neutrophil migration, Ca2+ undergoes complex interactions with integrins to induce both adhesion and reversal of adhesion to aid in migration.[54] When PLCβ isoforms are deleted, mouse neutrophils can still properly migrate to chemotactic signals ex vivo, indicating PI3K signaling is sufficient for chemotaxis or that compensatory mechanisms are activated.[53] The PI3K signaling pathway becomes activated by GPCR signaling in a similar manner to the PLC pathway through GPCR agonists. The Gβγ subunit activates PI3K and subsequently PIP2 and PIP3.[37, 53, 55] Production of PIPs activates ERK family proteins.[56] PI3K deletion in neutrophils results in reduced PIP3 production and defective neutrophil migration in response to C5a, IL-8, or CXCL-1,2. Neutrophil migration, therefore, is functionally dependent on activation of the PI3K pathway.[53, 55]
In addition to GPCR signaling to induce neutrophil migration, β-arrestin signaling also plays a role in neutrophil migration and activation following MI. Activation of multiple GPCRs on neutrophils, including CXCR1, CXCR 2, and CXCR4 bind GPCRs and the intracellular loop of the receptors catalytically activates β-arrestins. β-arrestin regulates actin reorganization by interacting with Ral GDP dissociation stimulator. This interaction results in activation of Rac1/RhoA activation and f-actin accumulation to promote neutrophil migration.[55] In addition, β-arrestins activate p38 mitogen activated kinase to further promote migration.[56] These signaling pathways are common across multiple inflammatory processes and are shared with the MI response.
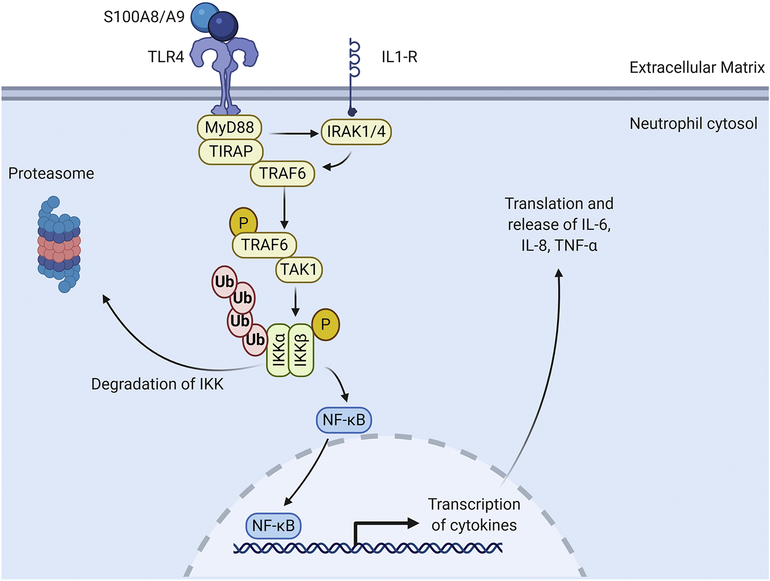
Neutrophil signaling involves the calcium binding S100 family of proteins, primarily S100A8 and S100A9 that induce cytokine generation in the neutrophil.[8, 10] The S100A8/A9 heterodimer binds TLR4 and the receptor for advanced glycation end products (RAGE).[8–10] S100A8 and S100A9 are secreted by neutrophils, monocytes, and endothelial cells and can function in an autocrine manner on neutrophils.[57] TLR4 activates myeloid differentiation factor 88 (MyD88) and its adapter Toll/IL-1R domain containing adapter protein (TIRAP).[24] The TIRAP/MyD88 complex recruits IL-1R associated kinases and (IRAKs) and TNF-α receptor associated factor 6 (TRAF6) to the receptor and undergoes a series of phosphorylation events.[58, 59] IRAK1 and TRAF6 dissociate from the receptor and complex with transforming growth factor activated kinase 1 (TAK1) to activate TAK1. Activated TAK1 phosphorylates the inhibitory κB kinases (IKKs).[60, 61] The IKKs phosphorylate IκB, which then dissociate from NF-κB to undergo ubiquitination and degradation (Figure 3). Upon release from IκB, NF-κB translocates to the nucleus and induces transcription of pro-inflammatory cytokines (e.g., IL-6, IL-8, and TNF-α). Treatment of monocytes with S100A8/A9 induces mediators downstream of TLR4, including MyD88 and IRAK-1.[62] Interestingly, S100A9 null mice show improved cardiomyocyte survival and improved LV physiology following ischemia/reperfusion.[63] S100A9 deletion attenuates granulopoiesis and improves cardiac physiology after MI.[11] Together, PLC, PI3K, and S100A8/A9 signaling promote migration of the neutrophil to the infarct area.
Figure 3. S100A8/A9 signaling in the neutrophil during migration to the infarct region.
The S100A8/S100A9 heterodimer binds to TLR4 on the neutrophil[7]. Toll like receptor 4 (TLR4) activates myeloid differentiation primary response 88 (MyD88) and TIR domain containing adaptor protein (TIRAP) which form a complex in the cytosol. The TIRAP/MyD88 complex recruits interleukin-1 receptor-associated kinase 1 (IRAKs) and tumor necrosis factor receptor (TNFR)-associated factor 6 (TRAF6). IRAK and TRAF6 dissociate from the receptor and complex with transforming growth factor beta-activated kinase 1 (TAK1) causing TAK1 to become activated. Activated TAK1 phosphorylate inhibitor of nuclear factor-κB (IκB) kinase (IKKs). The IKKs phosphorylate IκB which causes it to dissociate from nuclear factor kappa-light-chain-enhancer of activated B cells NF-κB, to undergo ubiquitination and degradation. NF-κB translocates to the nucleus and induces transcription of many pro-inflammatory cytokines including IL-6, IL-8, and TNF-α. Created with BioRender.com
Neutrophil signaling during degranulation and reactive oxygen species (ROS) production
Neutrophils are classified as granulocytes based upon their ability to generate granules. Neutrophil granules contain pro-inflammatory molecules such as complement proteins, gelatinases (e.g., matrix metalloproteinase (MMP)-9), and cysteine proteases (e.g., myeloperoxidase (MPO) and neutrophil elastase (NE)) that collectively contribute to MI-induced proteolytic break down of necrotic myocytes and existing ECM. In conjunction with degranulation, neutrophils produce and release ROS. While ROS generation is an efficient mechanism for killing and removing foreign pathogens, this mechanism is indiscriminate and can lead to injury of healthy tissue as well.[64] In the context of MI, ROS can damage healthy myocardium.[65, 66] Understanding the signaling mechanisms in the production and release of proteases and ROS from neutrophils may provide targets for intervention after MI.
There are four types of neutrophil granules: azurophilic (primary), specific (secondary), gelatinase (tertiary), and secretory granules. Azurophilic granules, the largest, are first formed in the neutrophil and contain myeloperoxidase (MPO), serine proteases, azurocidin, and α-defensins. Specific granules are smaller than azurophilic granules in diameter and contain lactoferrin, neutrophil gelatinase-associated lipocalin (NGAL), cathelicidin, and lysozyme. Gelatinase granules are smaller than specific granules and contain MMPs, particularly MMP-8 and −9. Secretory granules consist primarily of complement receptor 1, plasma protein albumin, CD13 (aminopeptidase N), CD14 (monocyte differentiation antigen), and CD16 (Fc gamma receptor III). In addition to having specialized protease components for each granule type, the excitation threshold required to induce degranulation differs.[67] Secretory vesicles require the lowest threshold, followed by gelatinase granules, then specific granules, and finally azurophillic granules.[68] Treatment with pro-inflammatory agonists such as N-formyl-met-leu-phe peptides at nanomolar concentrations in vitro robustly releases secretory vesicles with minimal release of other granule types.[69, 70] More potent agonists, such as phorbol myristate acetate, release gelatinase granules and not azurophillic granules.[71] This is an important quality for neutrophils to mitigate degranulation during the migration phase. Many of the important mediators for chemotaxis are the same signals that induce degranulation, such as CXCL-1 or 2, IL-1β, and IL-8.[69] As degranulation agonists become more potent at the site of injury, neutrophil degranulation is more readily stimulated.
The role of each granule type differs in the context of MI based on the granule components. Secretory vesicles are the first granule type to be released. Secretory vesicles contain numerous membrane bound receptors to improve the ability of neutrophils to respond to inflammatory stimuli when the granule fuses with the membrane, such as (CD-14) and Fc fragment of IgG receptor IIIa (CD-16). Inflammatory receptors found in secretory vesicles include complement receptor 1 and toll-like receptors.[79, 80] Secretory vesicles also contain and release albumin, although the role of neutrophil derived albumin in the context of MI has not been examined.
Gelatinase granules contain proteases such as MMP-8 and MMP-9. While removal of existing ECM by MMPs is needed to make space for replacement scar, excess MMP activity can worsen LV physiology after MI by increasing wall thinning.[76] While excess MMP activity is detrimental, early inhibition of MMP-9 also worsens cardiac dysfunction after MI by delaying resolution of pro-inflammatory neutrophils.[72] Global MMP-9 deletion promotes M2 macrophage polarization and inflammation resolution in aging and after MI.[73] Thus, shifting the timing and extent of inhibition can result in opposite results. In addition, MMPs contribute a wide-range of roles in addition to break down of ECM, including regulation of the inflammatory process by proteolytically activating or de-activating cytokines, chemokines, and growth factors, all of which are applicable to MI. Specific granules contain neutrophil gelatinase associated lipocalin (NGAL). In humans, NGAL complexes with MMP-9 and prolongs MMP-9 function and inflammation following MI.[[74],77] Mice are missing the cysteine at amino acid 87 of NGAL that allows proper binding of NGAL to MMP-9. NGAL is considered a biomarker for cardiovascular disease as it is upregulated in coronary artery disease, heart failure, and stroke, although limited data in human patients restricts NGAL as a prognostic marker in humans. [78]
Specific granules contain overlap with gelatinase granules in protein content, including MMP-9, macrophage 1 antigen, and NGAL. Although there is overlap in granule protein composition, concentrations differ as does granule susceptibility to degranulation.[75] Specific granules are well known for antimicrobial activity through release of lactoferrin, which is primarily found in specific granules. Lactoferrin is also known for regulating neutrophil recruitment by attenuating cytokine release.[76]
Azurophillic granules contain MPO and NE. MPO is primarily known as an antimicrobial agent, however, MPO stimulates oxidative stress in response to MI.[77] MPO catalyzes the formation of hypochlorous acid (HOCl), a powerful oxidant derived from chloride ions and hydrogen peroxide (H2O2). HOCl interacts with other small molecules, including NH3, to form monochloramines (NH2Cl) or with other ROS to yield peroxynitrite (ONOO−) and hydroxyl radical (·OH).[78] MPO itself induces feed forward degranulation.[79] MPO is a biomarker in ischemic heart disease, associating with abnormal LV remodeling.[80] NE can activate other proteases and cleaves multiple ECM targets, including fibronectin and collagens. NE acts as a major activator of MMP-9, further inducing ECM degradation following MI.[81]
NE even has the capacity to propagate chemotaxis by inducing IL-8 release from endothelial cells.[82] Proteinase 3 activates TNF-α, IL-1β, and IL-18, and degrades ECM components including fibronectin and collagen IV.[83] Proteinase 3 plasma levels are elevated in chronic MI patients who have adverse outcomes compared to event-free survivors.[83] Some proteases found in azurophillic granules have not been assessed in the context of MI, such as cathepsin G. However a new granule subtype (ficolin-1 rich granules), have been described in neutrophils and contain a several cathepsins, including Cathepsin B and D which have been shown to be upregulated in neutrophils following MI, warranting investigation of these in the context of MI. [1, 84]
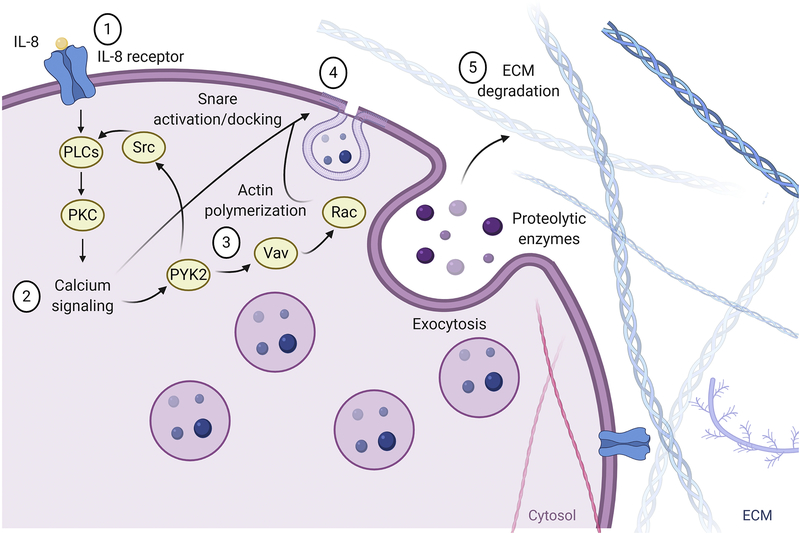
Figure 4 describes the steps that lead to neutrophil granule exocytosis. Exocytosis is tightly regulated by calcium signaling and soluble N-ethylmaleimide sensitive factor attaching proteins (SNAPs) and SNAP receptors (SNAREs) that regulate granule docking to the cell membrane. A key step for neutrophil degranulation is induction of calcium signaling, and stepwise increases in intracellular calcium correlate with the degree in which neutrophils degranulate. Upon reaching the site of injury, larger amplifications of Ca2+ induce neutrophil degranulation, causing the exocytosis of proteases to regulate inflammation and break down ECM and other debris in the infarcted area. This process involves docking of neutrophil granules to the membrane through the activity of SNAREs, followed by granule exocytosis. PKC-mediated calcium release from the ER regulates common exocytosis machinery and is important for granule release.
Figure 4. Neutrophil signaling during degranulation and reactive oxygen species (ROS) production.
1) Pro-inflammatory cytokines bind neutrophil GPCRs and 2) activate downstream calcium signaling through PLC. 3) Calcium activates proline rich kinase 2 (PYK2) which promotes SRC kinase activity and feeds forward to induce further calcium release. 4) Calcium also binds soluble NSF attachment proteins (SNAP) receptors (SNAREs) and activates them for proper granule docking to the neutrophil membrane. PYK2 activates Vav which, in turn, activates Rac. Rac promotes F-actin polymerization at the membrane of the neutrophil and aids in exocytosis of granules. 5) Granules containing proteases cleave ECM targets creating space for the new ECM scar. Created with BioRender.com
SNAPs and SNAREs regulate attachment of vesicles to the cell membrane.[85] V-SNAREs on vesicles attach to t-SNAREs found on the cell membrane. SNAPs are required for v-SNARE/t-SNARE attachment, and SNAPs themselves are calcium-binding proteins. Vesicle associated membrane protein-2 (VAMP-2) and secretory carrier membrane protein (SCAMP) have been identified in neutrophils, in the membranes of secretory vesicles as well as in gelatinase and specific granules.[86] Some of the characterized SNARE complexes include syntaxin 4/SNAP-23/VAMP-1 and syntaxin 4/SNAP-23/VAMP-2, which are involved in the exocytosis of specific and tertiary granules, whereas interactions between syntaxin 4 and VAMP-1/VAMP-7 are involved in the exocytosis of azurophillic granules.[87] Adding complexity, syntaxin-6 and −7 are also found in the cell membrane and regulate granule docking to the cell membrane. Blockade of syntaxin-6 activity with antibody treatment primarily inhibits release of azurophil and specific granules.[88] These findings provide further evidence for the specificity by which neutrophil granules are exocytosed. Proline rich kinase 2 (Pyk2) is another earlier mediator of neutrophil granule exocytosis that is calcium dependent. Increases in intracellular calcium induce autophosphorylation of Pyk2.[89] Pyk2 phosphorylates Vav.[89] Vav directly upregulates Rac signaling that induces actin remodeling to prepare granules for docking and fusion to the cell membrane via SNAREs.[90] In addition to Vav upregulation, Pyk2 activate Src family kinases that aid in activation of the PLC signaling cascade.[91]
Neutrophil signaling during phagocytosis
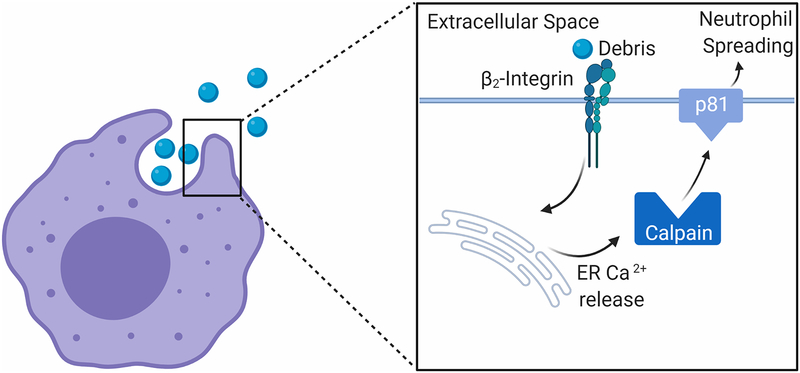
While neutrophil activity is far more diverse than originally thought, phagocytosis is a classic function of neutrophils (Figure 5). Phagocytosis in both neutrophils and macrophages is critical for removal of debris in the infarct area to prepare for adequate scar formation. A key step in neutrophil phagocytosis is increasing the size of the cell membrane, a process termed neutrophil spreading. This process is dependent on increasing intracellular calcium.[92] The increase of calcium is likely induced by neutrophil adhesion to the particle being engulfed through β2-integrin signaling.[93, 94] The calcium dependent pathways responsible for membrane spreading are not entirely clear.
Figure 5. Neutrophil signaling during phagocytosis.
Neutrophil phagocytosis is initiated by adhesion of integrins to debris particles in the extracellular space. β2-integrin signaling leads to calcium release from the endoplasmic reticulum. The rise in intracellular calcium activates calpain. Calpain proteolytically cleaves p81, a protein that binds the lipid bilayer to the f-actin protrusions found at the neutrophil membrane. This cleavage induces neutrophil spreading, allowing the neutrophil to engulf the debris particle. Created with BioRender.com
Just before particles are engulfed, there is a large spike in neutrophil intracellular calcium to induce neutrophil spreading.[95] Calpain expressed in neutrophils cleaves multiple structural proteins and could be a possible mechanism to induce neutrophil spreading, allowing the neutrophil to engulf debris found in the ECM.[96] Calpain requires a calcium concentration of 20 μM in vitro to become active, which is much higher than resting calcium concentrations found in neutrophils, suggesting calpain may only be active in the neutrophil upon increases in intracellular calcium.[97] Activation of calpain causes the protease to translocate from the cytosol to the cell membrane and aid in formation of the phagocytic cup.[98] Calpain cleaves p81, a protein that links f-actin projections to the phospholipid bilayer of the plasma membrane.[99–101] Cleavage of p81 creates space between the f-actin protrusions and the f-actin membrane allowing for the neutrophil membrane to increase in size. The neutrophil adheres to particles in the ECM and through calcium signaling undergoes neutrophil spreading. The increase in the size of the plasma membrane allows the neutrophil to engulf the particle and remove it from the extracellular space.
Neutrophil signaling during resolution of inflammation
While neutrophils are traditionally viewed as a pro-inflammatory cell type, neutrophils can also play an important role in inflammation resolution. In the past, neutrophils were deemed detrimental in MI due to expression of pro-inflammatory cytokines and production of reactive oxygen species that, when in excess, induce damage to the LV. Although high neutrophil counts are often used as a clinical predictor of poor outcomes following coronary events,[102] deletion of neutrophils in mice worsens cardiac physiology after MI.[103] Hence, neutrophils play an important role in the shift from the inflammatory phase to the reparative phases of cardiac wounding healing following MI, using both direct and indirect mechanisms.
Neutrophils, similar to macrophages, undergo polarization from a pro-inflammatory N1 phenotype during the inflammatory phase of remodeling to an N2 anti-inflammatory phenotype during the reparative phase and the maturation phase of remodeling.[104, 105] At day 1 after MI, less than 3% of neutrophils stain positive for CD206, a marker for N2 neutrophils. At day 7, this value increases more than 6-fold to nearly 20% of neutrophils staining positive for CD206.[106] N2 neutrophils express anti-inflammatory molecules, such as Arg1 and IL10 in the later stages of cardiac remodeling.[106]
Proteomic analysis of neutrophils over the MI time continuum supports these findings. D1 MI neutrophils release proteases such as MMP-8 and MMP-9 and express high levels of S100A9 indicating infiltration. At MI day 3, neutrophils begin to undergo apoptosis and reduce the inflammatory signaling while beginning to aid in ECM reorganization. At MI days 5 and 7 neutrophils produce ECM proteins necessary for scar formation, including fibronectin, vimentin, and fibrinogen.[1] Thus, neutrophils directly contribute to the reparative phase.
The Steffens lab reported that neutrophils interact with macrophages during the reparative phase to promote M2 polarization of macrophages.[103] They showed that NGAL secreted by the neutrophils induced M2 polarization. M2 macrophages produce higher levels of myeloid-epithelial-reproductive tyrosine kinase (MerTK), a marker of improved neutrophil phagocytosis. MerTK has been well characterized in phagocytosis and specifically in the context of MI for clearance of dead cardiomyocytes.[107, 108] Treatment of macrophages in vitro with neutrophil supernatant induces MerTK activation and improves macrophage phagocytosis.[103] In addition, depletion of neutrophils blocks M2 polarization and M2 polarization was shown to be necessary for clearance of apoptotic neutrophils, a finding confirmed by our lab.[109] We showed that treatment with IL-4 induces M2 polarization of macrophages and increased MerTK expression and increased phagocytosis of neutrophils at day 3 after MI. Thus, neutrophils indirectly contribute to the reparative phase.
In addition to polarizing from a pro-inflammatory to an anti-inflammatory state and expressing anti-inflammatory cytokines, neutrophils respond to anti-inflammatory cytokines as well. Exogenous administration of interleukin-4 (IL-4) at the time of MI reduces infarct size and decreases expression of pro-inflammatory cytokines in neutrophils, including Ccl3, IL12α, and TNFα at day 3 after MI.[109, 110] Neutrophils increase expression of interleukin 4 receptor (IL4r), which transduces IL-4 signaling.[109, 110] Neutrophils ability to respond to anti-inflammatory signaling may play an important role in the shift from inflammatory to anti-inflammatory signaling in the context of MI. There are also sex differences involved in the resolution of neutrophil mediated inflammation after MI. Females produce higher levels of N2 anti-inflammatory markers such as Ym1 and CD206.[50] Production of anti-inflammatory markers in neutrophils by females suggest neutrophils are polarized to a phenotype that aids inflammation resolution.
Neutrophils are designed to be a short-lived cell as a conserved mechanism to prohibit prolonged inflammation. At the end of their lifespan, neutrophils undergo apoptosis. Apoptosis, in comparison to necrosis, minimizes inflammation. The packaging process of apoptosis reduces further inflammation by limiting DAMP exposure to the extracellular space. The functional capacity of the M2 macrophage to phagocytose neutrophils provides a safe route of neutrophil clearance from the infarct area without prolonging inflammation. Neutrophil apoptosis also causes pro-inflammatory cytokine scavenging. While apoptotic neutrophils may not be functional, the cell membranes remain intact.[111] Thus, apoptotic neutrophils can bind and scavenge pro-inflammatory signals without signaling further inflammation.
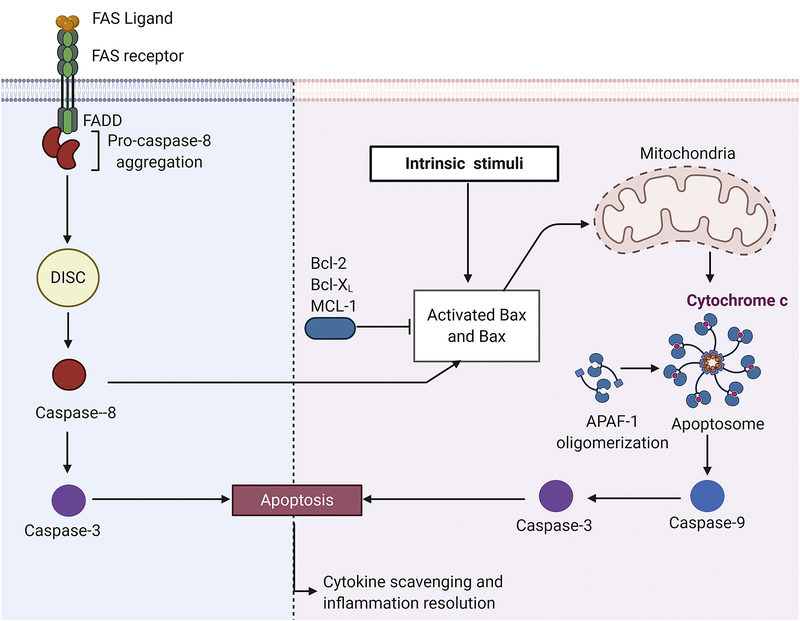
Neutrophil apoptosis occurs primarily through the classical caspase dependent pathways, both the intrinsic and extrinsic pathways (Figure 6). The intrinsic pathway relies primarily on the Bcl-2 family of proteins. Most prominent of these are pro-apoptotic Bax and anti-apoptotic Bcl-2. Following activation of the intrinsic pathway, Bax, normally bound and inhibited by Bcl-2, becomes active and travels to the mitochondria to promote release of cytochrome c into the cytosol.[112, 113] Cytochrome c binds with apoptotic protease activating factor-1 and forms a protein complex called the apoptosome which cleaves procaspase-9.[114, 115] There are multiple receptors that can induce the extrinsic pathway, including TNFR and Fas receptor.[116] Activation of these receptors promotes binding of procaspase-8 to adaptor proteins associated with the receptor.[117] These receptors are found in close proximity to lipid rafts. Additional procaspase-8 binds to the lipid-receptor complex, creating the death inducing signaling complex (DISC) causing autocatalysis of procaspases-8 to caspase-8.[117] Both caspase-8 and caspase-9 cleave procaspase-3 to induce apoptosis. In summary, neutrophils undergo apoptosis which is critical in attenuating prolonged inflammatory signaling, promoting recruitment and polarization of inflammation-resolving macrophages, and binding and scavenging of pro-inflammatory cytokines further resolve inflammation.[118]
Figure 6. Neutrophil signaling during resolution of inflammation.
Neutrophil apoptosis occurs through and intrinsic and extrinsic pathways. Intrinsic stimuli cause Bax to dissociate from anti-apoptotic Bcl-2, Bcl-x, or Mcl-1. Activated Bax stimulates cytochrome c release from mitochondria. Cytochrome c forms a complex with apoptotic protease activating factor 1 (APAF1) forming the apoptosome. The apoptosome activates caspase-9 which in turn activates caspase-3, the effector of neutrophil apoptosis. The extrinsic pathway is dependent on binding of external signals such as FAS ligand. Neutrophils bind FAS ligand through the FAS receptor. FAS receptor activation leads to procaspase-8 aggregation at the site of the receptor. Aggregation of procaspase-8 around lipid rafts in the membrane form (death-inducing signaling complex) DISC. DISC leads to autocrine cleavage of caspase-8. Caspase-8 activates caspase-3 inducing apoptosis. Created with BioRender.com
Conclusions
Neutrophils are highly complex granulocytes that perform a variety of functions in the LV after MI. The plasticity and broad range of functions that neutrophils produce makes them an exciting target for future study in the context of scar formation after MI. Targeting early pro-inflammatory migration to temper neutrophil infiltration into the infarct could be promising. The signaling involved in diapedesis and migration of neutrophils could be targeted to reduce neutrophil counts during the inflammatory phase. Neutrophil degranulation is another target. Limiting excess neutrophil degranulation could limit damage to healthy heart tissue and limit inflammatory signaling. Shifting the neutrophil phenotype from pro- to anti-inflammatory signaling has had some success in mouse models. Targeting neutrophil apoptosis, particularly pro-inflammatory neutrophils, may reduce inflammatory status in the early stages of MI. Initiating apoptosis could promote earlier induction of inflammation resolution. As our understanding of neutrophil physiology continues to evolve, options for therapeutic interventions targeting neutrophils to improve outcomes following MI continue to grow.
Highlights.
Neutrophils play key roles during repair after myocardial infarction (MI)
Neutrophils transition from pro-inflammatory to anti-inflammatory to reparative over the MI time continuum
Targeting neutrophil signaling is an unexplored avenue for therapeutic intervention
Acknowledgements
Dr. Lindsey is a Stokes-Shackleford Professor at UNMC. We acknowledge funding from the National Institutes of Health under award numbers HL123471, HL129823, and HL137319, the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development under award number 5I01BX000505, the American Cancer Society Research Scholar Grant under award number RSG-19-127-01-CSM, and the Swedish Society for Medical Research under award number P19-0144. The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding agencies. All authors have reviewed and approved the article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Daseke MJ 2nd, Valerio FM, Kalusche WJ, Ma Y, DeLeon-Pennell KY, Lindsey ML, Neutrophil proteome shifts over the myocardial infarction time continuum, Basic Res Cardiol 114(5) (2019) 37 10.1007/s00395-019-0746-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Opdenakker G, Fibbe WE, Van Damme J, The molecular basis of leukocytosis, Immunology Today 19(4) (1998) 182–189. 10.1016/S0167-5699(97)01243-7 [DOI] [PubMed] [Google Scholar]
- [3].Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, Fowler KJ, Basu S, Zhan YF, Dunn AR, Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization, Blood 84(6) (1994) 1737–46. [PubMed] [Google Scholar]
- [4].Eash KJ, Means JM, White DW, Link DC, CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions, Blood 113(19) (2009) 4711–4719. 10.1182/blood-2008-09-177287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Christopherson KW II, Cooper S, Broxmeyer HE, Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells, Blood 101(12) (2003) 4680–4686. 10.1182/blood-2002-12-3893 [DOI] [PubMed] [Google Scholar]
- [6].Wang Y, Dembowsky K, Chevalier E, Stüve P, Korf-Klingebiel M, Lochner M, Napp LC, Frank H, Brinkmann E, Kanwischer A, Bauersachs J, Gyöngyösi M, Sparwasser T, Wollert KC, C-X-C Motif Chemokine Receptor 4 Blockade Promotes Tissue Repair After Myocardial Infarction by Enhancing Regulatory T Cell Mobilization and Immune-Regulatory Function, Circulation 139(15) (2019) 1798–1812. 10.1161/circulationaha.118.036053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vogl T, Roth J, Sorg C, Hillenkamp F, Strupat K, Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 detected by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry, Journal of the American Society for Mass Spectrometry 10(11) (1999) 1124–1130. 10.1016/S1044-0305(99)00085-9 [DOI] [PubMed] [Google Scholar]
- [8].Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM, RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides, Cell 97(7) (1999) 889–901. 10.1016/s0092-8674(00)80801-6 [DOI] [PubMed] [Google Scholar]
- [9].Volz HC, Laohachewin D, Seidel C, Lasitschka F, Keilbach K, Wienbrandt AR, Andrassy J, Bierhaus A, Kaya Z, Katus HA, Andrassy M, S100A8/A9 aggravates post-ischemic heart failure through activation of RAGE-dependent NF-κB signaling, Basic Res Cardiol 107(2) (2012) 250 10.1007/s00395-012-0250-z [DOI] [PubMed] [Google Scholar]
- [10].Roth J, Burwinkel F, van den Bos C, Goebeler M, Vollmer E, Sorg C, MRP8 and MRP14, S-100-like proteins associated with myeloid differentiation, are translocated to plasma membrane and intermediate filaments in a calcium-dependent manner, Blood 82(6) (1993) 1875–83. [PubMed] [Google Scholar]
- [11].Sreejit G, Abdel-Latif A, Athmanathan B, Annabathula R, Dhyani A, Noothi SK, Quaife-Ryan GA, Al-Sharea A, Pernes G, Dragoljevic D, Lal H, Schroder K, Hanaoka BY, Raman C, Grant MB, Hudson JE, Smyth SS, Porrello ER, Murphy AJ, Nagareddy PR, Neutrophil-Derived S100A8/A9 Amplify Granulopoiesis After Myocardial Infarction, Circulation 141(13) (2020) 1080–1094. 10.1161/circulationaha.119.043833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marinković G, Koenis DS, de Camp L, Jablonowski R, Graber N, de Waard V, de Vries CJ, Goncalves I, Nilsson J, Jovinge S, Schiopu A, S100A9 Links Inflammation and Repair in Myocardial Infarction, Circ Res 127(5) (2020) 664–676. 10.1161/circresaha.120.315865 [DOI] [PubMed] [Google Scholar]
- [13].Marinković G, Grauen Larsen H, Yndigegn T, Szabo IA, Mares RG, de Camp L, Weiland M, Tomas L, Goncalves I, Nilsson J, Jovinge S, Schiopu A, Inhibition of pro-inflammatory myeloid cell responses by short-term S100A9 blockade improves cardiac function after myocardial infarction, Eur Heart J 40(32) (2019) 2713–2723. 10.1093/eurheartj/ehz461 [DOI] [PubMed] [Google Scholar]
- [14].Burdon PC, Martin C, Rankin SM, The CXC chemokine MIP-2 stimulates neutrophil mobilization from the rat bone marrow in a CD49d-dependent manner, Blood 105(6) (2005) 2543–8. 10.1182/blood-2004-08-3193 [DOI] [PubMed] [Google Scholar]
- [15].Wengner AM, Pitchford SC, Furze RC, Rankin SM, The coordinated action of G-CSF and ELR + CXC chemokines in neutrophil mobilization during acute inflammation, Blood 111(1) (2008) 42–49. 10.1182/blood-2007-07-099648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Eash KJ, Greenbaum AM, Gopalan PK, Link DC, CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow, J Clin Invest 120(7) (2010) 2423–2431. 10.1172/JCI41649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mishra PK, Adameova A, Hill JA, Baines CP, Kang PM, Downey JM, Narula J, Takahashi M, Abbate A, Piristine HC, Kar S, Su S, Higa JK, Kawasaki NK, Matsui T, Guidelines for evaluating myocardial cell death, Am J Physiol Heart Circ Physiol 317(5) (2019) H891–h922. 10.1152/ajpheart.00259.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Arslan F, de Kleijn DP, Pasterkamp G, Innate immune signaling in cardiac ischemia, Nature Reviews Cardiology 8(5) (2011) 292–300. 10.1038/nrcardio.2011.38 [DOI] [PubMed] [Google Scholar]
- [19].Ogawa S, Koga S, Kuwabara K, Brett J, Morrow B, Morris SA, Bilezikian JP, Silverstein SC, Stern D, Hypoxia-induced increased permeability of endothelial monolayers occurs through lowering of cellular cAMP levels, Am J Physiol 262(3 Pt 1) (1992) C546–54. 10.1152/ajpcell.1992.262.3.C546 [DOI] [PubMed] [Google Scholar]
- [20].Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, Hofmann MA, Kislinger T, Ingram M, Lu A, Tanaka H, Hori O, Ogawa S, Stern DM, Schmidt AM, Blockade of RAGE–amphoterin signalling suppresses tumour growth and metastases, Nature 405(6784) (2000) 354–360. 10.1038/35012626 [DOI] [PubMed] [Google Scholar]
- [21].Li J, Kokkola R, Tabibzadeh S, Yang R, Ochani M, Qiang X, Harris HE, Czura CJ, Wang H, Ulloa L, Wang H, Warren HS, Moldawer LL, Fink MP, Andersson U, Tracey KJ, Yang H, Structural Basis for the Proinflammatory Cytokine Activity of High Mobility Group Box 1, Molecular Medicine 9(1) (2003) 37–45. 10.1007/BF03402105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pisetsky DS, The origin and properties of extracellular DNA: From PAMP to DAMP, Clinical Immunology 144(1) (2012) 32–40. 10.1016/j.clim.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Prince LR, Allen L, Jones EC, Hellewell PG, Dower SK, Whyte MKB, Sabroe I, The role of interleukin-1beta in direct and toll-like receptor 4-mediated neutrophil activation and survival, Am J Pathol 165(5) (2004) 1819–1826. 10.1016/s0002-9440(10)63437-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lugrin J, Parapanov R, Rosenblatt-Velin N, Rignault-Clerc S, Feihl F, Waeber B, Müller O, Vergely C, Zeller M, Tardivel A, Schneider P, Pacher P, Liaudet L, Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction, J Immunol 194(2) (2015) 499–503. 10.4049/jimmunol.1401948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG, Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling, Am J Pathol 173(1) (2008) 57–67. 10.2353/ajpath.2008.070974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, Apuzzo T, De Marchis F, Pedotti M, Bachi A, Thelen M, Varani L, Mellado M, Proudfoot A, Bianchi ME, Uguccioni M, HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4, J Exp Med 209(3) (2012) 551–563. 10.1084/jem.20111739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Martinez M, Joffraud M, Giraud S, Baïsse B, Bernimoulin MP, Schapira M, Spertini O, Regulation of PSGL-1 interactions with L-selectin, P-selectin, and E-selectin: role of human fucosyltransferase-IV and -VII, J Biol Chem 280(7) (2005) 5378–90. 10.1074/jbc.M410899200 [DOI] [PubMed] [Google Scholar]
- [28].Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K, Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo, J Clin Invest 102(8) (1998) 1526–1533. 10.1172/JCI119893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Abram CL, Lowell CA, The Ins and Outs of Leukocyte Integrin Signaling, Annual Review of Immunology 27(1) (2009) 339–362. 10.1146/annurev.immunol.021908.132554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P, Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade, Journal of Experimental Medicine 203(12) (2006) 2569–2575. 10.1084/jem.20060925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zehrer A, Pick R, Salvermoser M, Boda A, Miller M, Stark K, Weckbach LT, Walzog B, Begandt D, A Fundamental Role of Myh9 for Neutrophil Migration in Innate Immunity, J Immunol 201(6) (2018) 1748–1764. 10.4049/jimmunol.1701400 [DOI] [PubMed] [Google Scholar]
- [32].Xu J, Wang F, Van Keymeulen A, Herzmark P, Straight A, Kelly K, Takuwa Y, Sugimoto N, Mitchison T, Bourne HR, Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils, Cell 114(2) (2003) 201–14. 10.1016/s0092-8674(03)00555-5 [DOI] [PubMed] [Google Scholar]
- [33].Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ, Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions, J Immunol 170(11) (2003) 5652–7. 10.4049/jimmunol.170.11.5652 [DOI] [PubMed] [Google Scholar]
- [34].Gambardella L, Vermeren S, Molecular players in neutrophil chemotaxis--focus on PI3K and small GTPases, J Leukoc Biol 94(4) (2013) 603–12. 10.1189/jlb.1112564 [DOI] [PubMed] [Google Scholar]
- [35].Lawson CD, Ridley AJ, Rho GTPase signaling complexes in cell migration and invasion, J Cell Biol 217(2) (2018) 447–457. 10.1083/jcb.201612069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Weiner OD, Servant G, Welch MD, Mitchison TJ, Sedat JW, Bourne HR, Spatial control of actin polymerization during neutrophil chemotaxis, Nat Cell Biol 1(2) (1999) 75–81. 10.1038/10042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, Crabbe T, Finan P, Jones G, Jackson S, Camps M, Rommel C, Wymann M, Hirsch E, Hawkins P, Stephens L, PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis, Nat Cell Biol 9(1) (2007) 86–91. 10.1038/ncb1517 [DOI] [PubMed] [Google Scholar]
- [38].Cau J, Hall A, Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways, J Cell Sci 118(Pt 12) (2005) 2579–87. 10.1242/jcs.02385 [DOI] [PubMed] [Google Scholar]
- [39].Van Keymeulen A, Wong K, Knight ZA, Govaerts C, Hahn KM, Shokat KM, Bourne HR, To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front, The Journal of cell biology 174(3) (2006) 437–445. 10.1083/jcb.200604113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kumar S, Xu J, Perkins C, Guo F, Snapper S, Finkelman FD, Zheng Y, Filippi M-D, Cdc42 regulates neutrophil migration via crosstalk between WASp, CD11b, and microtubules, Blood 120(17) (2012) 3563–3574. 10.1182/blood-2012-04-426981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Allingham MJ, van Buul JD, Burridge K, ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration, J Immunol 179(6) (2007) 4053–64. 10.4049/jimmunol.179.6.4053 [DOI] [PubMed] [Google Scholar]
- [42].Lou O, Alcaide P, Luscinskas FW, Muller WA, CD99 is a key mediator of the transendothelial migration of neutrophils, J Immunol 178(2) (2007) 1136–43. 10.4049/jimmunol.178.2.1136 [DOI] [PubMed] [Google Scholar]
- [43].Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA, CD99 plays a major role in the migration of monocytes through endothelial junctions, Nat Immunol 3(2) (2002) 143–50. 10.1038/ni749 [DOI] [PubMed] [Google Scholar]
- [44].Woodfin A, Reichel CA, Khandoga A, Corada M, Voisin M-B, Scheiermann C, Haskard DO, Dejana E, Krombach F, Nourshargh S, JAM-A mediates neutrophil transmigration in a stimulus-specific manner in vivo: evidence for sequential roles for JAM-A and PECAM-1 in neutrophil transmigration, Blood 110(6) (2007) 1848–1856. 10.1182/blood-2006-09-047431 [DOI] [PubMed] [Google Scholar]
- [45].Woodfin A, Voisin M-B, Imhof BA, Dejana E, Engelhardt B, Nourshargh S, Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1, Blood 113(24) (2009) 6246–6257. 10.1182/blood-2008-11-188375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Girbl T, Lenn T, Perez L, Rolas L, Barkaway A, Thiriot A, Del Fresno C, Lynam E, Hub E, Thelen M, Graham G, Alon R, Sancho D, von Andrian UH, Voisin MB, Rot A, Nourshargh S, Distinct Compartmentalization of the Chemokines CXCL1 and CXCL2 and the Atypical Receptor ACKR1 Determine Discrete Stages of Neutrophil Diapedesis, Immunity 49(6) (2018) 1062–1076.e6. 10.1016/j.immuni.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Edwards LJ, Constantinescu CS, Platelet activating factor/platelet activating factor receptor pathway as a potential therapeutic target in autoimmune diseases, Inflamm Allergy Drug Targets 8(3) (2009) 182–90. 10.2174/187152809788681010 [DOI] [PubMed] [Google Scholar]
- [48].Rajarathnam K, Schnoor M, Richardson RM, Rajagopal S, How do chemokines navigate neutrophils to the target site: Dissecting the structural mechanisms and signaling pathways, Cell Signal 54 (2019) 69–80. 10.1016/j.cellsig.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lindsey ML, Jung M, Yabluchanskiy A, Cannon PL, Iyer RP, Flynn ER, DeLeon-Pennell KY, Valerio FM, Harrison CL, Ripplinger CM, Hall ME, Ma Y, Exogenous CXCL4 infusion inhibits macrophage phagocytosis by limiting CD36 signalling to enhance post-myocardial infarction cardiac dilation and mortality, Cardiovascular research 115(2) (2019) 395–408. 10.1093/cvr/cvy211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].DeLeon-Pennell KY, Mouton AJ, Ero OK, Ma Y, Padmanabhan Iyer R, Flynn ER, Espinoza I, Musani SK, Vasan RS, Hall ME, Fox ER, Lindsey ML, LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling, Basic research in cardiology 113(5) (2018) 40–40. 10.1007/s00395-018-0699-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lai J-J, Lai K-P, Zeng W, Chuang K-H, Altuwaijri S, Chang C, Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: lessons from conditional AR knockout mice, Am J Pathol 181(5) (2012) 1504–1512. 10.1016/j.ajpath.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].de Haan JJ, Smeets MB, Pasterkamp G, Arslan F, Danger Signals in the Initiation of the Inflammatory Response after Myocardial Infarction, Mediators of Inflammation 2013 (2013) 206039 10.1155/2013/206039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D, Roles of PLC-β2 and -β3 and PI3Kγ in Chemoattractant-Mediated Signal Transduction, Science 287 (2000) 1046–1049. 10.1126/science.287.5455.1046 [DOI] [PubMed] [Google Scholar]
- [54].Gong Y, Zhang Y, Feng S, Liu X, Lü S, Long M, Dynamic contributions of P- and E-selectins to β2-integrin-induced neutrophil transmigration, The FASEB Journal 31(1) (2017) 212–223. 10.1096/fj.201600398rrr [DOI] [PubMed] [Google Scholar]
- [55].Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP, Central Role for G Protein-Coupled Phosphoinositide 3-Kinase γ in Inflammation, Science 287(5455) (2000) 1049–1053. 10.1126/science.287.5455.1049 [DOI] [PubMed] [Google Scholar]
- [56].Mócsai A, Jakus Z, Vántus T, Berton G, Lowell CA, Ligeti E, Kinase Pathways in Chemoattractant-Induced Degranulation of Neutrophils: The Role of p38 Mitogen-Activated Protein Kinase Activated by Src Family Kinases, The Journal of Immunology 164(8) (2000) 4321–4331. 10.4049/jimmunol.164.8.4321 [DOI] [PubMed] [Google Scholar]
- [57].Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J, S100A8/A9 in Inflammation, Front Immunol 9 (2018) 1298 10.3389/fimmu.2018.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Prabhu SD, Frangogiannis NG, The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis, Circ Res 119(1) (2016) 91–112. 10.1161/CIRCRESAHA.116.303577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lin S-C, Lo Y-C, Wu H, Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling, Nature 465(7300) (2010) 885–890. 10.1038/nature09121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S, Essential function for the kinase TAK1 in innate and adaptive immune responses, Nat Immunol 6(11) (2005) 1087–95. 10.1038/ni1255 [DOI] [PubMed] [Google Scholar]
- [61].Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S, TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo, Genes Dev 19(22) (2005) 2668–81. 10.1101/gad.1360605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MAD, Nacken W, Foell D, van der Poll T, Sorg C, Roth J, Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock, Nature Medicine 13(9) (2007) 1042–1049. 10.1038/nm1638 [DOI] [PubMed] [Google Scholar]
- [63].Li Y, Chen B, Yang X, Zhang C, Jiao Y, Li P, Liu Y, Li Z, Qiao B, Bond Lau W, Ma XL, Du J, S100a8/a9 Signaling Causes Mitochondrial Dysfunction and Cardiomyocyte Death in Response to Ischemic/Reperfusion Injury, Circulation 140(9) (2019) 751–764. 10.1161/circulationaha.118.039262 [DOI] [PubMed] [Google Scholar]
- [64].Reactive Oxygen Species in Inflammation and Tissue Injury, Antioxidants & Redox Signaling 20(7) (2014) 1126–1167. 10.1089/ars.2012.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Frangogiannis NG, Regulation of the inflammatory response in cardiac repair, Circ Res 110(1) (2012) 159–173. 10.1161/CIRCRESAHA.111.243162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hori M, Nishida K, Oxidative stress and left ventricular remodelling after myocardial infarction, Cardiovasc Res 81(3) (2009) 457–64. 10.1093/cvr/cvn335 [DOI] [PubMed] [Google Scholar]
- [67].Ma Y, Yabluchanskiy A, Lindsey ML, Neutrophil roles in left ventricular remodeling following myocardial infarction, Fibrogenesis Tissue Repair 6(1) (2013) 11. 10.1186/1755-1536-6-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sengeløv H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N, Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils, The Journal of Immunology 154(8) (1995) 4157–4165. [PubMed] [Google Scholar]
- [69].Bradford PG, Rubin RP, Quantitative changes in inositol 1,4,5-trisphosphate in chemoattractant-stimulated neutrophils, J Biol Chem 261(33) (1986) 15644–7. [PubMed] [Google Scholar]
- [70].Sengeløv H, Kjeldsen L, Diamond MS, Springer TA, Borregaard N, Subcellular localization and dynamics of Mac-1 (alpha m beta 2) in human neutrophils, J Clin Invest 92(3) (1993) 1467–1476. 10.1172/JCI116724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kjeldsen L, Bjerrum OW, Askaa J, Borregaard N, Subcellular localization and release of human neutrophil gelatinase, confirming the existence of separate gelatinase-containing granules, Biochem J 287 (Pt 2)(Pt 2) (1992) 603–610. 10.1042/bj2870603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Iyer RP, de Castro Brás LE, Patterson NL, Bhowmick M, Flynn ER, Asher M, Cannon PL, Deleon-Pennell KY, Fields GB, Lindsey ML, Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution, J Mol Cell Cardiol 100 (2016) 109–117. 10.1016/j.yjmcc.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, Altara R, Halade GV, Voorhees AP, Nguyen NT, Jin YF, Winniford MD, Hall ME, Han HC, Lindsey ML, Myocardial Infarction Superimposed on Aging: MMP-9 Deletion Promotes M2 Macrophage Polarization, J Gerontol A Biol Sci Med Sci 71(4) (2016) 475–83. 10.1093/gerona/glv034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sivalingam Z, Larsen SB, Grove EL, Hvas AM, Kristensen SD, Magnusson NE, Neutrophil gelatinase-associated lipocalin as a risk marker in cardiovascular disease, Clin Chem Lab Med 56(1) (2017) 5–18. 10.1515/cclm-2017-0120 [DOI] [PubMed] [Google Scholar]
- [75].Cowland JB, Borregaard N, Granulopoiesis and granules of human neutrophils, Immunological Reviews 273(1) (2016) 11–28. 10.1111/imr.12440 [DOI] [PubMed] [Google Scholar]
- [76].Crouch SP, Slater KJ, Fletcher J, Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin, Blood 80(1) (1992) 235–40. [PubMed] [Google Scholar]
- [77].Haghi Aminjan H, Abtahi SR, Hazrati E, Chamanara M, Jalili M, Paknejad B, Targeting of oxidative stress and inflammation through ROS/NF-kappaB pathway in phosphine-induced hepatotoxicity mitigation, Life Sciences 232 (2019) 116607 10.1016/j.lfs.2019.116607 [DOI] [PubMed] [Google Scholar]
- [78].Hampton MB, Kettle AJ, Winterbourn CC, Inside the Neutrophil Phagosome: Oxidants, Myeloperoxidase, and Bacterial Killing, Blood 92(9) (1998) 3007–3017. 10.1182/blood.V92.9.3007 [DOI] [PubMed] [Google Scholar]
- [79].Grigorieva DV, Gorudko IV, Sokolov AV, Kostevich VA, Vasilyev VB, Cherenkevich SN, Panasenko OM, Myeloperoxidase Stimulates Neutrophil Degranulation, Bull Exp Biol Med 161(4) (2016) 495–500. 10.1007/s10517-016-3446-7 [DOI] [PubMed] [Google Scholar]
- [80].Ndrepepa G, Myeloperoxidase - A bridge linking inflammation and oxidative stress with cardiovascular disease, Clin Chim Acta 493 (2019) 36–51. 10.1016/j.cca.2019.02.022 [DOI] [PubMed] [Google Scholar]
- [81].Garratt LW, Sutanto EN, Ling K-M, Looi K, Iosifidis T, Martinovich KM, Shaw NC, Kicic-Starcevich E, Knight DA, Ranganathan S, Stick SM, Kicic A, Matrix metalloproteinase activation by free neutrophil elastase contributes to bronchiectasis progression in early cystic fibrosis, European Respiratory Journal 46(2) (2015) 384–394. 10.1183/09031936.00212114 [DOI] [PubMed] [Google Scholar]
- [82].Chen HC, Lin HC, Liu CY, Wang CH, Hwang T, Huang TT, Lin CH, Kuo HP, Neutrophil elastase induces IL-8 synthesis by lung epithelial cells via the mitogen-activated protein kinase pathway, J Biomed Sci 11(1) (2004) 49–58. 10.1007/bf02256548 [DOI] [PubMed] [Google Scholar]
- [83].Ng LL, Khan SQ, Narayan H, Quinn P, Squire IB, Davies JE, Proteinase 3 and prognosis of patients with acute myocardial infarction, Clin Sci (Lond) 120(6) (2011) 231–238. 10.1042/CS20100366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rørvig S, Østergaard O, Heegaard NHH, Borregaard N, Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors, Journal of Leukocyte Biology 94(4) (2013) 711–721. 10.1189/jlb.1212619 [DOI] [PubMed] [Google Scholar]
- [85].Lacy P, Eitzen G, Control of granule exocytosis in neutrophils, Front Biosci 13 (2008) 5559–70. 10.2741/3099 [DOI] [PubMed] [Google Scholar]
- [86].Mollinedo F, Calafat J, Janssen H, Martín-Martín B, Canchado J, Nabokina SM, Gajate C, Combinatorial SNARE Complexes Modulate the Secretion of Cytoplasmic Granules in Human Neutrophils, The Journal of Immunology 177(5) (2006) 2831–2841. 10.4049/jimmunol.177.5.2831 [DOI] [PubMed] [Google Scholar]
- [87].Xie L.-x., Calafat J, Janssen H, de la Iglesia-Vicente J, Mollinedo F, Intracellular location of syntaxin 7 in human neutrophils, Immunology Letters 129(2) (2010) 72–77. 10.1016/j.imlet.2010.02.003 [DOI] [PubMed] [Google Scholar]
- [88].Martín-Martín B, Nabokina SM, Blasi J, Lazo PA, Mollinedo F, Involvement of SNAP-23 and syntaxin 6 in human neutrophil exocytosis, Blood 96(7) (2000) 2574–83. [PubMed] [Google Scholar]
- [89].Kamen LA, Schlessinger J, Lowell CA, Pyk2 Is Required for Neutrophil Degranulation and Host Defense Responses to Bacterial Infection, The Journal of Immunology 186(3) (2011) 1656–1665. 10.4049/jimmunol.1002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kim C, Marchal CC, Penninger J, Dinauer MC, The Hemopoietic Rho/Rac Guanine Nucleotide Exchange Factor Vav1 Regulates N-Formyl-Methionyl-Leucyl-Phenylalanine-Activated Neutrophil Functions, The Journal of Immunology 171(8) (2003) 4425–4430. 10.4049/jimmunol.171.8.4425 [DOI] [PubMed] [Google Scholar]
- [91].Sheshachalam A, Srivastava N, Mitchell T, Lacy P, Eitzen G, Granule Protein Processing and Regulated Secretion in Neutrophils, Frontiers in Immunology 5(448) (2014). 10.3389/fimmu.2014.00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Kruskal BA, Shak S, Maxfield FR, Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium, Proceedings of the National Academy of Sciences 83(9) (1986) 2919–2923. 10.1073/pnas.83.9.2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Xu W, Wang P, Petri B, Zhang Y, Tang W, Sun L, Kress H, Mann T, Shi Y, Kubes P, Wu D, Integrin-induced PIP5K1C kinase polarization regulates neutrophil polarization, directionality, and in vivo infiltration, Immunity 33(3) (2010) 340–350. 10.1016/j.immuni.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Pettit EJ, Hallett MB, Release of ‘caged’ cytosolic Ca2+ triggers rapid spreading of human neutrophils adherent via integrin engagement, Journal of Cell Science 111(15) (1998) 2209–2215. [DOI] [PubMed] [Google Scholar]
- [95].Heinrich V, Controlled One-on-One Encounters between Immune Cells and Microbes Reveal Mechanisms of Phagocytosis, Biophys J 109(3) (2015) 469–76. 10.1016/j.bpj.2015.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Dewitt S, Hallett MB, Cytosolic free Ca2+ changes and calpain activation are required for β integrin–accelerated phagocytosis by human neutrophils, Journal of Cell Biology 159(1) (2002) 181–189. 10.1083/jcb.200206089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Imajoh S, Aoki K, Ohno S, Emori Y, Kawasaki H, Sugihara H, Suzuki K, Molecular cloning of the cDNA for the large subunit of the high-Ca2+-requiring form of human Ca2+-activated neutral protease, Biochemistry 27(21) (1988) 8122–8. 10.1021/bi00421a022 [DOI] [PubMed] [Google Scholar]
- [98].Dewitt S, Tian W, Hallett MB, Localised PtdIns(3,4,5)P3 or PtdIns(3,4)P2 at the phagocytic cup is required for both phagosome closure and Ca2+ signalling in HL60 neutrophils, J Cell Sci 119(Pt 3) (2006) 443–51. 10.1242/jcs.02756 [DOI] [PubMed] [Google Scholar]
- [99].Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M, Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker, Journal of Cell Biology 120(1) (1993) 129–139. 10.1083/jcb.120.1.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Shcherbina A, Bretscher A, Kenney DM, Remold-O’Donnell E, Moesin, the major ERM protein of lymphocytes and platelets, differs from ezrin in its insensitivity to calpain, FEBS Letters 443(1) (1999) 31–36. 10.1016/S0014-5793(98)01674-3 [DOI] [PubMed] [Google Scholar]
- [101].Dewitt S, Francis RJ, Hallett MB, Ca2+ and calpain control membrane expansion during the rapid cell spreading of neutrophils, Journal of Cell Science 126(20) (2013) 4627–4635. 10.1242/jcs.124917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Guasti L, Dentali F, Castiglioni L, Maroni L, Marino F, Squizzato A, Ageno W, Gianni M, Gaudio G, Grandi AM, Cosentino M, Venco A, Neutrophils and clinical outcomes in patients with acute coronary syndromes and/or cardiac revascularisation. A systematic review on more than 34,000 subjects, Thromb Haemost 106(4) (2011) 591–9. 10.1160/th11-02-0096 [DOI] [PubMed] [Google Scholar]
- [103].Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S, Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype, Eur Heart J 38(3) (2017) 187–197. 10.1093/eurheartj/ehw002 [DOI] [PubMed] [Google Scholar]
- [104].Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, Richmond A, Delayed wound healing in CXCR2 knockout mice, J Invest Dermatol 115(2) (2000) 234–244. 10.1046/j.1523-1747.2000.00034.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Vafadarnejad E, Rizzo G, Krampert L, Arampatzi P, Arias-Loza PA, Nazzal Y, Rizakou A, Knochenhauer T, Reddy Bandi S, Nugroho VA, Schulz DJ, Roesch M, Alayrac P, Vilar J, Silvestre JS, Zernecke A, Saliba AE, Cochain C, Dynamics of Cardiac Neutrophil Diversity in Murine Myocardial Infarction, Circ Res (2020). 10.1161/circresaha.120.317200 [DOI] [PubMed] [Google Scholar]
- [106].Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, Henry J, Cates CA, Deleon-Pennell KY, Lindsey ML, Temporal neutrophil polarization following myocardial infarction, Cardiovasc Res 110(1) (2016) 51–61. 10.1093/cvr/cvw024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK, Phagocytosis and clearance of apoptotic cells is mediated by MER, Nature 411(6834) (2001) 207–211. 10.1038/35075603 [DOI] [PubMed] [Google Scholar]
- [108].Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB, Enhanced Efferocytosis of Apoptotic Cardiomyocytes Through Myeloid-Epithelial-Reproductive Tyrosine Kinase Links Acute Inflammation Resolution to Cardiac Repair After Infarction, Circ Res 113(8) (2013) 1004–1012. doi: 10.1161/CIRCRESAHA.113.301198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Daseke MJ 2nd, Tenkorang-Impraim MAA, Ma Y, Chalise U, Konfrst SR, Garrett MR, DeLeon-Pennell KY, Lindsey ML, Exogenous IL-4 shuts off pro-inflammation in neutrophils while stimulating anti-inflammation in macrophages to induce neutrophil phagocytosis following myocardial infarction, J Mol Cell Cardiol 145 (2020) 112–121. 10.1016/j.yjmcc.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Shintani Y, Ito T, Fields L, Shiraishi M, Ichihara Y, Sato N, Podaru M, Kainuma S, Tanaka H, Suzuki K, IL-4 as a Repurposed Biological Drug for Myocardial Infarction through Augmentation of Reparative Cardiac Macrophages: Proof-of-Concept Data in Mice, Scientific Reports 7(1) (2017) 6877 10.1038/s41598-017-07328-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bonecchi R, Garlanda C, Mantovani A, Riva F, Cytokine decoy and scavenger receptors as key regulators of immunity and inflammation, Cytokine 87 (2016) 37–45. 10.1016/j.cyto.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Weinmann P, Gaehtgens P, Walzog B, Bcl-Xl- and Bax-alpha-mediated regulation of apoptosis of human neutrophils via caspase-3, Blood 93(9) (1999) 3106–15. [PubMed] [Google Scholar]
- [113].Liu X, Kim CN, Yang J, Jemmerson R, Wang X, Induction of Apoptotic Program in Cell-Free Extracts: Requirement for dATP and Cytochrome c, Cell 86(1) (1996) 147–157. 10.1016/S0092-8674(00)80085-9 [DOI] [PubMed] [Google Scholar]
- [114].Kim H-E, Du F, Fang M, Wang X, Formation of apoptosome is initiated by cytochrome c-induced dATP hydrolysis and subsequent nucleotide exchange on Apaf-1, Proceedings of the National Academy of Sciences of the United States of America 102(49) (2005) 17545–17550. 10.1073/pnas.0507900102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Li Y, Zhou M, Hu Q, Bai X.-c., Huang W, Scheres SHW, Shi Y, Mechanistic insights into caspase-9 activation by the structure of the apoptosome holoenzyme, Proceedings of the National Academy of Sciences 114(7) (2017) 1542–1547. 10.1073/pnas.1620626114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Trauth BC, Klas C, Peters AM, Matzku S, Möller P, Falk W, Debatin KM, Krammer PH, Monoclonal antibody-mediated tumor regression by induction of apoptosis, Science 245(4915) (1989) 301–5. 10.1126/science.2787530 [DOI] [PubMed] [Google Scholar]
- [117].Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME, Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor, EMBO J 14(22) (1995) 5579–5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bourke E, Cassetti A, Villa A, Fadlon E, Colotta F, Mantovani A, IL-1β Scavenging by the Type II IL-1 Decoy Receptor in Human Neutrophils, The Journal of Immunology 170(12) (2003) 5999–6005. 10.4049/jimmunol.170.12.5999 [DOI] [PubMed] [Google Scholar]