Abstract
Introduction:
Markers of monocyte/macrophage activation and vascular inflammation are associated with HIV-related cardiovascular diseases (CVD) and mortality. We compared these markers among African people living with HIV (PLWH) and HIV-negative adults, and examined risk factors associated with elevated biomarkers(>75% percentile) in PLWH on antiretroviral therapy (ART).
Design:
Cross-sectional study.
Methods:
We measured serum concentrations of a gut integrity biomarker (I-FABP), monocyte/macrophage activation biomarkers (soluble CD14 and CD163), and vascular inflammation biomarkers (soluble/circulating intercellular[sICAM-1] and vascular[sVCAM-1] cell adhesion molecules-1). We assessed the relationship of these inflammatory parameters with HIV, using logistic regression adjusting for traditional CVD risk factors.
Results:
Among the 541 participants, median age was 43 years and half were female. Among 275 PLWH, median CD4+T-cell count and duration of ART use was 509 cells/mm3 and 8 years respectively. PLWH had significantly higher prevalence of elevated inflammatory biomarkers compared to HIV-negative individuals even after adjustment for traditional CVD risk factors. Compared to subjects without HIV-infection, the prevalence of elevated biomarkers was highest among persons with detectable viral load and CD4+T-cell counts≤200 cells/mm3. In a sub-analysis among PLWH, nadir CD4+T-cell count≤200 cells/mm3 was associated with elevated sCD14; dyslipidemia with elevated sCD14, sICAM-1, and sVCAM-1; and overweight/obesity with reduced sCD14. Longer ART exposure (>4 years) was associated with reduced sVCAM-1 and sICAM-1.
Conclusion:
HIV and not traditional CVD risk factors is a primary contributor of monocyte/macrophage activation and inflammation despite ART. Anti-inflammatory therapies in addition to ART may be necessary to reduce these immune dysregulations and improve health outcomes of African PLWH.
Keywords: Inflammation, HIV, Africa, monocyte activation, CVD
Introduction
Antiretroviral therapy (ART) has greatly reduced mortality associated with human immunodeficiency virus (HIV), however this success has been tempered by increased rates of cardiovascular diseases (CVD)[1–7]. Despite sub-Saharan Africa (SSA) carrying the highest burden of HIV, data on potential drivers of HIV-associated risk of CVD remain scant[2, 8]. HIV has been associated with persistent immune activation and biomarkers of monocyte activation (soluble CD14[sCD14] and sCD163) and vascular inflammation (soluble/circulating intercellular[sICAM-1] and vascular [sVCAM-1] cell adhesion molecules-1)[6, 9–19], have been associated with increased risk for premature CVD and mortality in people living with HIV (PLWH) in studies conducted in resource-rich settings [6, 13, 19–24]. The mechanisms underlying this immune dysregulation are not clear and may include ART toxicity, traditional CVD risk factors, and co-infections. The effect of HIV independent of these factors is also likely to be substantial[6, 25]. Although few studies in the SSA have reported an increase in markers of immune activation and inflammation among PLWH, the results may have been confounded by the lack of data on co-morbid diseases, lack of thorough consideration for traditional CVD risk factors and HIV specific risk factors, and the lack of HIV-negative reference group for comparison[26–29]. This is particularly important because there may be unique factors in SSA that promote inflammation (poor sanitation and nutrition) regardless of the HIV status[30–32].
We examined an independent association of HIV with markers of monocyte/macrophages activation and vascular inflammation after adjustment for all traditional CVD risk factors among PLWH and behaviorally similar (i.e. similar prevalence of tobacco use) HIV-negative adults. We also identified clinical and HIV-specific risk factors associated with residual immune activation during ART.
Methods
We analyzed samples from a cross-sectional study of 275 PLWH on stable ART for >6 months and 266 HIV-negative adults enrolled between 2017-2018 from the Kisumu District Hospital HIV clinic and voluntary HIV testing centers in western Kenya. Recruitment and study procedures have been described elsewhere[33]. Briefly, data were collected through a questionnaire; including anthropometric measurements; laboratory examination; and medical chart abstraction. The study sample was a convenience sample of adults ≥30 years old. Individuals with a recorded history of CVD, neoplasia, active infection, and those who at the time of recruitment were on medications that could influence their immune status were excluded. The Ethics and Research Committee of Kenyatta National Hospital and the Institutional Review Board at University of Washington approved the study. All participants provided written informed consent.
Dependent Variable
Serum levels of intestinal-fatty acid binding protein (I-FABP), sCD14, and sCD163 were quantified using ELISA (Quantikine ELISA kit, R&D systems) while sICAM-1 and sVCAM-1 were measured using multiplex ELISA-based assay (Meso-Scale Discovery). Assays were performed in the same UW laboratory in duplicate. The inter-assay coefficients of variation were <11%.
Independent Variables
The main independent variable of interest was HIV status. Data on covariates such as age, alcohol use, smoking status, ART duration, type of ART regimen, and nadir CD4+T-cell count (CD4) were obtained from the participants and confirmed using medical records. Body mass index (BMI) was calculated from weight and height measurements. Fasting blood samples were used to assess for blood glucose (FBG), lipids, current CD4+T-cell count, and HIV RNA viral load (VL). Elevated BP was defined when systolic BP≥140 mmHg or diastolic BP≥90 mmHg or if the participant was on antihypertensives. Diabetes mellitus was defined as FBG ≥126 mg/dl or the use of medications for diabetes. Dyslipidemia was defined as highdensity (HDL) <40 mg/dl or low density lipoprotein (LDL) ≥130 mg/dl, or triglycerides(TG) ≥150 mg/dl. Undetectable viremia was defined as VL<50 copies/mL and viral suppression as VL<1000 copies/mL per Kenyan guidelines[34].
Statistical Analyses
Comparison between groups were analyzed using X2 test, t test or Wilcoxon rank-sum test. We used multivariate logistic regression to estimate the association between HIV-infection and prevalence of elevated (highest quartile [>75th percentile] vs. lower three quartiles) biomarkers. Fully adjusted model included covariates such as age, hypertension, diabetes, smoking status, alcohol consumption status, TG, HDL, and BMI. Secondary analyses stratified HIV status by CD4, ART duration, and VL were done.
Additional analysis was done to determine factors associated with elevated biomarkers in PLWH with additional adjustment for nadir and current CD4, ART regimen, and ART duration. Significance was set at a p value <0.05. Analyses were performed using STATA® version 13 (San Antonio, Texas).
Results
Study participants characteristics are presented in Supplementary Table 1. Compared with HIV-negative participants, PLWH were older and less likely to be hypertensive, diabetic or obese (p<0.05 for all). Almost all PLWH (97%) were virally suppressed. The median current CD4 was 509 cells/mm3. Only 13% were on protease inhibitors.
HIV-infection is associated with elevated biomarkers of monocyte activation and inflammation.
Age-adjusted levels of sCD14, sCD163, I-FABP, sICAM-1, and sVCAM-1 were higher among PLWH compared to the HIV-negative participants (Figure 1). HIV-infection was associated with elevated I-FABP (Odds ratio (OR),3.59; p<0.001), sCD14 (OR,8.58; p <0.001), sCD163 (OR,2.03; p=0.007), sICAM-1 (OR,3.17; p<0.001), and sVCAM-1 (OR,1.92; p=0.001) in unadjusted analysis. The association remained unchanged after adjustment for CVD risk factors (Supplementary Table 2).
Figure 1. Serum levels of biomarkers by HIV serostatus, a) CD4+T-cell count and b) viral load.
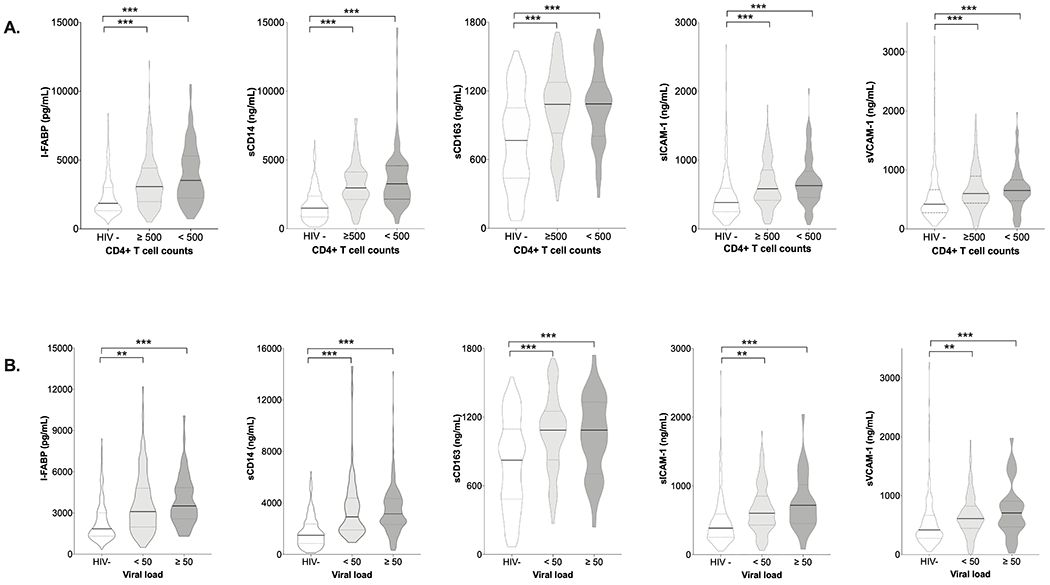
For all biomarkers, 266 HIV-negative participants (HIV-), 220 PWH with viral load<50 copies/mL (<50), 55 PWH with viral load ≥50 copies/mL (≥50), 146 PLWH with CD4+ T-cell count ≥500 cells/mm3 (≥500), and 129 PLWH with CD4+ T-cell count <500 cells/mm3 (<500). Pairwise comparisons were adjusted for age.***P<0.001. ***P<0.001.
When we further stratified PLWH by HIV-specific factors, those with CD4≤200 cells/mm3, detectable viremia, and those on ART<4 years had the highest prevalence of elevated biomarkers when compared to the HIV-negative persons (Table 1). However, the odds of having elevated biomarkers remained high for PLWH with undetectable VL and CD4≥500 cells/mm3 compared to the HIV-negative persons. When we stratified by duration of ART use (<4 years, 4-10 years, >10 years), we observed a trend that the odds of elevated biomarkers decreased with longer ART exposure. In fact, only PLWH on ART for <4 years had statistically significant elevated sVCAM-1 and sICAM-1 compared to HIV-negative participants.
Table 1:
Association of HIV Specific Characteristics and Elevated Biomarkers.
| sCD14† OR (95%CI) |
sCD163† OR (95%CI) |
I-FABP† OR (95%CI) |
sVCAM-1† OR (95%CI) |
sICAM-1†
OR (95%CI) |
|
|---|---|---|---|---|---|
| Stratified by HIV status and current CD4 count (cells/mm3) | |||||
| HIV- | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| HIV+, CD4 ≥500 | 7.87 (4.36 - 12.22) | 7.70 (1.56 - 5.38) | 2.21 (1.31 - 3.77) | 2.34 (1.24 - 3.14) | 3.86 (2.25 - 6.63) |
| HIV+, 200> CD4 <500 | 8.79 (4.66 - 15.59) | 2.39 (1.10 - 5.21) | 3.43 (1.95 - 6.02) | 1.73 (1.17 - 3.08) | 3.54 (1.94 - 6.45) |
| HIV+, CD4 ≤200 | 9.32 (3.49 - 22.85) | 2.49 (1.11 - 5.88) | 4.59 (1.80 - 11.72) | 2.36 (1.05 - 6.10) | 3.61 (1.33 - 9.74) |
| Stratified by HIV status and viral load (copies/mL) | |||||
| HIV- | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| HIV+,VL <50 | 7.61 (3.06 - 15.95) | 2.40 (1.28 - 4.55) | 2.71 (1.76 - 4.35) | 1.91 (1.21 - 3.02) | 3.65 (2.22 - 5.99) |
| HIV+, VL ≥50 | 8.61 (4.94 - 14.99) | 4.88 (1.71 - 9.97) | 3.26 (1.38 - 7.69) | 3.97 (1.71 - 9.19) | 5.29 (2.20 - 12.67) |
| Stratified by HIV status and ART duration (years) | |||||
| HIV- | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| HIV+, ART <4 | 8.35 (4.77 - 18.33) | 3.53 (1.73 - 5.88) | 3.74 (2.04 - 6.87) | 3.10 (1.71 - 5.62) | 4.42 (2.36- 8.28) |
| HIV+, ART 4-10 | 7.83 (4.23- 13.65) | 2.83 (1.42 - 4.93) | 2.91 (1.67- 5.06) | 1.05 (0.88 - 2.70) | 1.01 (0.78- 3.26) |
| HIV+, ART >10 | 7.29 (3.92 - 12.52) | 1.81 (1.33 - 4.01) | 2.61 (1.48 - 4.59) | 1.02 (0.82 - 2.56) | 1.08 (0.79- 3.48) |
OR, Odds Ratio; CI, confidence interval. ART, antiretroviral therapy; and VL, viral load. Other abbreviations as in Table 1
Model are adjusted for age, dyslipidemia, BMI, hypertension, diabetes, smoking status.
Factors associated with elevated biomarkers among PLWH on ART.
When we restricted our analysis to PLWH, low nadir CD4≤200 cells/m3 was associated with elevated sCD14 while ART use >4 years was associated with r educed sICAM-1 and sVCAM-1 (p<0.05 for all; Supplementary Table 3). Other covariates associated with elevated biomarkers included: triglycerides>150 mg/dL with elevated sCD14, sICAM-1, and sVCAM-1; and BMI<18 kg/m2 with I-FABP.
Compared with a nevirapine based regimen, PI-based regimens were associated with elevated sCD163 (OR, 2.83, 95%CI;1.98-5.47) and efavirenz-based regimens with elevated I-FABP (OR, 2.34, 95%CI;1.31-4.18).
Discussion
In this well characterized African cohort, we observed a significantly higher prevalence of elevated monocyte/macrophages activation, and vascular inflammation in PLWH compared to HIV-negative individuals. This was evident among the virally suppressed and those with CD4+T-cell count≥500 cells/m3. Most importantly, we identified clinical and HIV-specific factors associated with this immune dysregulation. To our knowledge, this investigation is the largest and first of its kind to thoroughly investigate the relationship between HIV, traditional CVD risk factors, and markers of vascular inflammation, gut permeability and macrophage/monocyte activation among African PLWH in the ART era.
Our observation of persistent elevation of sCD14 and sCD163 while taking ART is of relevance since both markers have been linked to CVD risk and mortality among PLWH[18, 19, 35–37]. These findings concur with limited studies conducted in SSA that found minimal effect of ART on reducing these markers of monocyte/macrophage activation[23, 24, 27, 29, 38, 39]. In a large African HIV cohort, sCD14 and sCD163 were significantly elevated in untreated adults and decreased during ART-mediated viral suppression compared with an HIV-negative group; notably, pre-ART levels of each of the biomarkers were the strongest predictors of residual immune activation during ART[27]. In a community-based, longitudinal, cohort study comprised of PLWH on ART and HIV-negative individuals in Uganda, there was persistent elevation of sCD14 and I-FABP among PLWH but not sCD163 and discordant to our study they found evidence of sex disparities where women tended to have significantly higher levels of these markers compared to men[24]. Our study also found that HIV and not CVD risk factors was a strong predictor for the residual monocyte/macrophage activation. In addition, we observed a negative association between nadir CD4+T-cell count and sCD14 supporting the concept that early ART may be important for the prevention of future complications related to HIV-associated immune activation.
Our study results contrast three large US based studies, the Multicenter AIDS Cohort Study[40] and the Women’s Interagency Health Study[41] that showed normalization of inflammatory markers after ART initiation, and the Veterans Aging Cohort Study that reported a high prevalence of elevated sCD14 only in veteran with detectable viremia and those with low CD4+T-cell count [38]. The low prevalence of CVD risk factors among our HIV-negative cohort compared to the HIV-negative participants in the aforementioned cohorts, differences in the type of ART drugs, and other unmeasured confounders unique and prevalent to the African setting (i.e. co-infections known to modulate host immunity) could account for the differences between our study and other studies.
Another interesting finding of this study is that PLWH with undetectable viremia had elevated vascular inflammation markers, independent of traditional risk factors. This is important as sICAM-1 and sVCAM-1 have been found to predict risk of future CVD events[9, 10]. We also showed for the first time that long-term use of ART yielded a significant reduction of sICAM-1 and sVCAM-1 among African PLWH, suggesting that host factors and not ART toxicity may be directly involved in promoting vascular inflammation in this setting. Our findings are in keeping with a report from US based study that showed significant reduction of levels of sICAM-1 and sVCAM-1 after a year of ART[42]. Our observation of elevated vascular inflammation markers among virally suppressed African PLWH is noteworthy because the majority had very low prevalence of CVD risk factors compared to PLWH in previous studies[43–45]. Thus, given that sICAM-1 and sVCAM-1 are expressed early in atherosclerosis, prospective studies correlating these markers with future cardiovascular events may offer insight into their prognostic value in African PLWH[46, 47].
The drivers of this immune dysregulation in SSA are less clear and could be multifactorial[48],[49],[50],[51]. The fact that long-term use of ART was associated with reduced levels of all biomarkers suggests features unique to PLWH per se and not ART as the likely contributors. Traditional CVD risk factors have been associated with HIV-associated inflammation[52–54]. In this study, only hypertriglyceridemia was associated with elevated sCD14, sICAM-1, and sVCAM-1 consistent with previous reports[6, 13]. We found no influence of any other traditional CVD risk factors on the biomarkers. This finding, together with the fact that our PLWH did not have a higher burden of CVD risk factors than the HIV-negative participants further suggests that features unique in HIV may have provided the stimulus. More studies from Africa are warranted to confirm these findings.
The main strength of our study is the ability to assess the effect of HIV on a wide range of biomarkers and the thorough assessment of CVD risk factors that allowed us to control for confounders that were not accounted for in previous studies from Africa. The relatively matched HIV-negative persons allowed for direct comparison between groups and identification of factors that may be particularly unique for PLWH in SSA. Inclusion of equal number of women in this study also makes our results more generalizable. We acknowledge certain limitations to our study. We did not adequately assess the effect of individual ART drugs. We did not include data on other known CVD inflammatory risk factor (i.e. high sensitivity-C-reactive protein[hsCRP]). However, based on previous reports demonstrating positive correlation between hsCRP and monocyte/macrophage activation markers, we hypothesize that hsCRP will be elevated among PLWH[21]. Future studies are needed to confirm this. As with any cross-sectional study, we cannot infer causation or control for unmeasured confounders.
In summary, monocyte/macrophage activation and vascular inflammation persist despite viral suppression and restoration of CD4+T-cell count in African PLWH. Long-term receipt of ART resulted in significant reduction of vascular inflammation. Traditional risk factors such as hypertension and diabetes were not strong predictors of the immunological dysregulation observed during ART in this setting. Our findings have important implications for African PLWH and health care professionals. In addition to focusing on guideline-recommended HIV treatment, anti-inflammatory therapies may be necessary to prevent immune activation and vascular inflammation associated with chronic HIV-infection in this setting. Future studies are urgently needed to test whether anti-inflammatory therapies are able to prevent this immune dysregulation and improve health outcomes of African PLWH on long-term ART
Supplementary Material
Acknowledgments
We thank Paul Macharia, Geoffrey Omondi, Victor Omodi, and Ana Gervassi, for their contribution. This project was supported by National Institutes of Health (NIH) grant R21TW010459, R21TW010459-02S1 from the Fogarty International Center (FIC), EDCTP2 programme supported by the European Union grant TMA-2016-1598-Kenya CVHIV, and Washington/Fred Hutch Center for AIDS Research, an NIH-funded program under award number AI027757. TT is supported by grant 3R01AI134130-03S1 from the National Institute of Allergy and Infectious Diseases. The funders did not participate in data collection or any activity that is directly related to the execution of the research. TT, CF, SP, SP contributed to the conception and design of the study, the supervision, data acquisition, analysis and interpretation, and the critical revision of the manuscript. JZ, CW, JK, GB, JN, SM contributed to the data analysis, the data interpretation, the manuscript drafting, and the critical revision of the manuscript.
This project was supported by National Institutes of Health (NIH) grant R21TW010459, AI027757, and R21TW010459-02S1; EDCTP2 programme grant TMA-2016-1598-Kenya CVHIV. The funders did not participate in data collection or any activity that is directly related to the execution of the research.
Footnotes
Author Disclosure Statement: All authors declare no financial conflicts of interest.
Reference
- 1.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah ASV, Stelzle D, Lee KK, Beck EJ, Alam S, Clifford S, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation 2018; 138(11):1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow FC, Price RW, Hsue PY, Kim AS. Greater Risk of Stroke of Undetermined Etiology in a Contemporary HIV-Infected Cohort Compared with Uninfected Individuals. J Stroke Cerebrovasc Dis 2017; 26(5):1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS 2010; 24(10):1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr 2009; 51(3):268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos AG, Hulzebosch A, Grobbee DE, Barth RE, Klipstein-Grobusch K. Association between Immune Markers and Surrogate Markers of Cardiovascular Disease in HIV Positive Patients: A Systematic Review. PLoS One 2017; 12(1):e0169986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis 2012; 205 Suppl 3:S375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osoti A, Temu TM, Kirui N, Ngetich EK, Kamano JH, Page S, et al. Metabolic Syndrome Among Antiretroviral Therapy-Naive Versus Experienced HIV-Infected Patients Without Preexisting Cardiometabolic Disorders in Western Kenya. AIDS Patient Care STDS 2018; 32(6):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 1998; 351(9096):88–92. [DOI] [PubMed] [Google Scholar]
- 10.de Lemos JA, Hennekens CH, Ridker PM. Plasma concentration of soluble vascular cell adhesion molecule-1 and subsequent cardiovascular risk. J Am Coll Cardiol 2000; 36(2):423–426. [DOI] [PubMed] [Google Scholar]
- 11.Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS 2009; 23(9):1059–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamtchum-Tatuene J, Mwandumba H, Al-Bayati Z, Flatley J, Griffiths M, Solomon T, et al. HIV is associated with endothelial activation despite ART, in a sub-Saharan African setting. Neurol Neuroimmunol Neuroinflamm 2019; 6(2):e531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-Inflammatory Markers in Relation to Cardiovascular Disease in HIV Infection. A Systematic Review. PLoS One 2016; 11(1):e0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204(8):1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis 2013; 208(11):1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanna DB, Lin J, Post WS, Hodis HN, Xue X, Anastos K, et al. Association of Macrophage Inflammation Biomarkers With Progression of Subclinical Carotid Artery Atherosclerosis in HIV-Infected Women and Men. J Infect Dis 2017; 215(9):1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caillon A, Schiffrin EL. Role of Inflammation and Immunity in Hypertension: Recent Epidemiological, Laboratory, and Clinical Evidence. Curr Hypertens Rep 2016; 18(3):21. [DOI] [PubMed] [Google Scholar]
- 18.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis 2015; 211(8):1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203(6):780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Younas M, Psomas C, Reynes J, Corbeau P. Immune activation in the course of HIV-1 infection: Causes, phenotypes and persistence under therapy. HIV Med 2016; 17(2):89–105. [DOI] [PubMed] [Google Scholar]
- 21.Neuhaus J, Jacobs DR, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis 2010; 201(12):1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rezer JFP, Adefegha SA, Ecker A, Passos DF, Saccol RSP, Bertoldo TMD, et al. Changes in inflammatory/cardiac markers of HIV positive patients. Microb Pathog 2018; 114:264–268. [DOI] [PubMed] [Google Scholar]
- 23.Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis 2010; 202(5):723–733. [DOI] [PubMed] [Google Scholar]
- 24.Siedner MJ, Zanni M, Tracy RP, Kwon DS, Tsai AC, Kakuhire B, et al. Increased Systemic Inflammation and Gut Permeability Among Women With Treated HIV Infection in Rural Uganda. J Infect Dis 2018; 218(6):922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006; 12(12):1365–1371. [DOI] [PubMed] [Google Scholar]
- 26.Siedner MJ, Bwana MB, Asiimwe S, Amanyire G, Musinguzi N, Castillo-Mancilla J, et al. Timing of Antiretroviral Therapy and Systemic Inflammation in Sub-Saharan Africa: Results From the META Longitudinal Cohort Study. J Infect Dis 2019; 220(7):1172–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroeze S, Wit FW, Rossouw TM, Steel HC, Kityo CM, Siwale M, et al. Plasma Biomarkers of Human Immunodeficiency Virus-Related Systemic Inflammation and Immune Activation in Sub-Saharan Africa Before and During Suppressive Antiretroviral Therapy. J Infect Dis 2019; 220(6):1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siedner MJ, Kim JH, Nakku RS, Bibangambah P, Hemphill L, Triant VA, et al. Persistent Immune Activation and Carotid Atherosclerosis in HIV-Infected Ugandans Receiving Antiretroviral Therapy. J Infect Dis 2016; 213(3):370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabatanzi R, Bayigga L, Cose S, Rowland Jones S, Joloba M, Canderan G, et al. Monocyte Dysfunction, Activation, and Inflammation After Long-Term Antiretroviral Therapy in an African Cohort. J Infect Dis 2019; 220(9):1414–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koethe JR, Blevins M, Nyirenda C, Kabagambe EK, Shepherd BE, Wester CW, et al. Nutrition and inflammation serum biomarkers are associated with 12-week mortality among malnourished adults initiating antiretroviral therapy in Zambia. J Int AIDS Soc 2011; 14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramendra R, Isnard S, Lin J, Fombuena B, Ouyang J, Mehraj V, et al. CMV seropositivity is associated with increased microbial translocation in people living with HIV and uninfected controls. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sullivan ZA, Wong EB, Ndung’u T, Kasprowicz VO, Bishai WR. Latent and Active Tuberculosis Infection Increase Immune Activation in Individuals Co-Infected with HIV. EBioMedicine 2015; 2(4):334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masyuko S, Page Stephanie, Kinuthia John et al. Metabolic syndrome and 10-year cardiovascular risk among HIV-positive and HIV-negative adults: A cross-sectional study. In. Medicine; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ministry of Health NASCP. Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection in Kenya 2018. In: NASCOP; 2018. [Google Scholar]
- 35.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28(7):969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis 2012; 206(10):1558–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsue PY, Scherzer R, Hunt PW, Schnell A, Bolger AF, Kalapus SC, et al. Carotid Intima-Media Thickness Progression in HIV-Infected Adults Occurs Preferentially at the Carotid Bifurcation and Is Predicted by Inflammation. J Am Heart Assoc 2012; 1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis 2012; 55(1):126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Méndez-Lagares G, Romero-Sánchez MC, Ruiz-Mateos E, Genebat M, Ferrando-Martínez S, Muñoz-Fernández M, et al. Long-term suppressive combined antiretroviral treatment does not normalize the serum level of soluble CD14. J Infect Dis 2013; 207(8):1221–1225. [DOI] [PubMed] [Google Scholar]
- 40.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29(4):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, et al. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr 2012; 60(4):359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francisci D, Giannini S, Baldelli F, Leone M, Belfiori B, Guglielmini G, et al. HIV type 1 infection, and not short-term HAART, induces endothelial dysfunction. AIDS 2009; 23(5):589–596. [DOI] [PubMed] [Google Scholar]
- 43.de Gaetano Donati K, Rabagliati R, Iacoviello L, Cauda R. HIV infection, HAART, and endothelial adhesion molecules: current perspectives. Lancet Infect Dis 2004; 4(4):213–222. [DOI] [PubMed] [Google Scholar]
- 44.Mayne ES, Louw S. Good Fences Make Good Neighbors: Human Immunodeficiency Virus and Vascular Disease. Open Forum Infect Dis 2019; 6(11):ofz303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marincowitz C, Genis A, Goswami N, De Boever P, Nawrot TS, Strijdom H. Vascular endothelial dysfunction in the wake of HIV and ART. FEBS J 2019; 286(7):1256–1270. [DOI] [PubMed] [Google Scholar]
- 46.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 2007; 27(11):2292–2301. [DOI] [PubMed] [Google Scholar]
- 47.Ridker PM. Intercellular adhesion molecule (ICAM-1) and the risks of developing atherosclerotic disease. Eur Heart J 1998; 19(8):1119–1121. [DOI] [PubMed] [Google Scholar]
- 48.Hatano H, Delwart EL, Norris PJ, Lee TH, Neilands TB, Kelley CF, et al. Evidence of persistent low-level viremia in long-term HAART-suppressed, HIV-infected individuals. AIDS 2010; 24(16):2535–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis 2011; 203(10):1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piconi S, Parisotto S, Rizzardini G, Passerini S, Meraviglia P, Schiavini M, et al. Atherosclerosis is associated with multiple pathogenic mechanisms in HIV-infected antiretroviral-naive or treated individuals. AIDS 2013; 27(3):381–389. [DOI] [PubMed] [Google Scholar]
- 51.Zevin AS, McKinnon L, Burgener A, Klatt NR. Microbial translocation and microbiome dysbiosis in HIV-associated immune activation. Curr Opin HIV AIDS 2016; 11(2):182–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baker J, Ayenew W, Quick H, Hullsiek KH, Tracy R, Henry K, et al. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis 2010; 201(2):285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Kort S, Keszthelyi D, Masclee AA. Leaky gut and diabetes mellitus: what is the link? Obes Rev 2011; 12(6):449–458. [DOI] [PubMed] [Google Scholar]
- 54.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57(6):1470–1481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


