Abstract
Triple-negative breast cancer (TNBC) has the worst prognosis of all breast cancers, and lacks effective targeted treatment strategies. Previously, we identified 33 transcription factors highly expressed in TNBC. Here, we focused on six SOX transcription factors (SOX4, 6, 8, 9, 10 and 11) highly expressed in TNBCs. Our siRNA screening assay demonstrated that SOX9 knock-down suppressed TNBC cell growth and invasion in vitro. Thus, we hypothesized that SOX9 is an important regulator of breast cancer survival and metastasis, and demonstrated that knockout of SOX9 reduced breast tumor growth and lung metastasis in vivo. In addition, we found that loss of SOX9 induced profound apoptosis, with only a slight impairment of G1 to S progression within the cell cycle, and that SOX9 directly regulates genes controlling apoptosis. Based on published CHIP-seq data, we demonstrated that SOX9 binds to the promoter of apoptosis-regulating genes (tnfrsf1b, fadd, tnfrsf10a, tnfrsf10b, and ripk1), and represses their expression. SOX9 knock-down upregulates these genes, consistent with the induction of apoptosis. Analysis of available CHIP-seq data showed that SOX9 binds to the promoters of several EMT- and metastasis-regulating genes. Using CHIP assays, we demonstrated that SOX9 directly binds the promoters of genes involved in EMT (vim, cldn1, ctnnb1, and zeb1) and that SOX9 knock-down suppresses the expression of these genes.
Keywords: SOX9, triple-negative breast cancer, cell growth, metastasis, apoptosis
Introduction
Breast cancer is the most frequently diagnosed cancer in women and the second leading cause of cancer-related death of women in the United States (1). Breast cancer can be divided into clinically defined subtypes including estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-positive, and ‘triple-negative’ breast cancers (TNBC), which lack of ER, progesterone receptor (PR), and the HER2 protein expression. TNBCs represent approximately 10–15% of all breast cancers. Patients with TNBC have a poor outcome compared to the other subtypes of breast cancer (2), and there are few effective targeted therapies available for TNBC patients (2,3). A better understanding of the critical molecular regulators of TNBC is necessary to develop effective targeted therapies for this aggressive type of breast cancer.
Transcription factors (TFs) are critical molecules that regulate gene expression, ultimately controlling biologic processes such as cell growth, survival, and metastasis. To identify transcription factors that control TNBC survival and metastasis, we previously used a genomic screen to identify 33 transcription factors that are highly expressed and activated in TNBCs (as compared to non-TNBCs) (4). We hypothesized that some of these highly expressed transcription factors regulate the aggressive behavior of TNBCs, such as enhanced survival and metastasis. Of these 33 transcription factors, six are members of the SOX (Sex Determining Region Y-related HMG-box) family of transcription factors. Therefore, in this study we investigated the role of the SOX transcription factors in regulating breast cancer growth, survival, and metastasis.
We investigated the ability of six SOX transcription factors (SOX4, 6, 8, 9, 10 and 11) that are highly expressed in TNBCs to regulate TNBC growth and invasion. While we found that SOX transcription factors are essential for TNBC growth, SOX9 was required for both growth and invasion. SOX9 has been previously shown to be highly expressed in aggressive cancers (5,6), and has also been identified as a negative prognostic factor in lung cancer (7,8). Therefore, we focused on SOX9 and determined its role in regulating TNBC growth, cancer survival, and metastasis. We used SOX9-overexpressing, or SOX9 inhibited (by knock-down and knock-out strategies) breast cancer cell lines to determine whether SOX9 is necessary and/or sufficient to regulate TNBC cellular survival and metastasis. Our results demonstrated that SOX9 is critical for TNBC growth and metastasis in vitro and in vivo. Loss of SOX9 induced profound apoptosis of TNBC cells, with only a slight impairment of the G1 to S cell cycle progression, demonstrating that SOX9 is required for cancer survival. We then demonstrate that SOX9 directly binds to the promoter of several extrinsic apoptosis-inducing factors (such as fadd, tnfrsf10a, tnfrsf10b, and ripk1) and showed that loss of SOX9 caused up-regulation of these genes, consistent with the induction of apoptosis. Loss of SOX9 induced down regulation of many EMT factors. SOX9 directly binds the promoters of vim (Vimentin), cldn1 (Claudin-1), ctnnb1 (β-Catenin) and zeb1 (Zinc finger E-box-binding homeobox 1) genes and increased expression of SOX9 increased the expression of vim and ctnnb1 genes demonstrating that SOX9 regulates EMT in TNBCs. Overall, our data demonstrate that SOX9 is a critical regulator of TNBC survival and EMT that ultimately causes TNBC tumors to be highly aggressive. Our studies provide a strong basic science rational to develop SOX9 inhibitors for the treatment of TNBC, the most aggressive form of breast cancer.
Materials and Methods
Cell line culture
Breast cancer cell lines were purchased from American Type Culture Collection (ATCC, Manassas, VA), and were maintained according to ATCC recommendations. MCF7, MDA-MB-231, and MDA-MB-468 cell lines were cultured in DMEM medium; the ZR75-1, T47D, HCC1937, HCC1143, and HCC70 cell lines were cultured in RPMI-1640 medium, MCF10A and MCF12A cell lines were cultured in DMEM/F12 medium (Cellgro by Mediatech, Inc., Manassas, VA). Growth media for all cell lines was supplemented with 10% FBS, penicillin (100 mg/ml), and streptomycin (100 mg/ml). For cell authentication, STR profiles were compared to: (1) known ATCC fingerprints (ATCC.org); (2) the Cell Line Integrated Molecular Authentication database (CLIMA) version 0.1.200808 (http://bioinformatics.istge.it/clima/); and (3) the MD Anderson fingerprint database.
Plasmids and lentiviral vectors
The cDNA representing the complete open reading frame of human SOX9 (pOTB7-hSOX9) was obtained from Dharmacon (USA) and cloned into the pInducer20 vector (Addgene, USA) using Gateway™ cloning technology (Invitrogen™, ThermoFisher Scientific, USA) to generate the doxycycline (DOX) inducible expression plasmid. Lentiviral vectors were produced in HEK 293T cells by co-tranfection of a lentiviral expression plasmid, a packaging plasmid VPR, and a pseudotyping plasmid encoding the G protein of the Vesicular Stomatitis Virus (VSV-G) envelope gene using X-treme-Gene9 transfection reagent (XTG9-RO, Roche), following the manufacturer’s instructions. Lenti-virus medium was used to transduce the target cells. CRISPR/Cas9 was used to knockout target gene expression in breast cancer cells (9). Cells were infected with DOX-inducible Lenti-Cas9 (Tet-on pCW-Cas9, Addgene) followed by antibiotic selection to isolate stable cell clones. Stable cells were then infected with Lenti-sgSOX9 (HCP217635-LvSG03–3, Genecopoeia) or sgSCramble control (LPPCCPCTR01L03–025, Genecopoeia). After the integration of the viral RNA, cells were treated with or without DOX (2 μg/ml, unless stated otherwise) for indicated days, then cells were harvested to detect the expression of target factors. Mycoplasma testing was performed with the Lonza mycoplasma detection kit (Catalog # LT07–418), using luminescence reading after incubation of the cell culture media with the reagent.
siRNA transfection
The siRNA oligos targeting specific SOXs used in the screening assay were purchased from Sigma-Aldrich (St. Louis, MO). Transfection of cells was performed using DharmaFECT1 (T-2001–03, Dharmacon) with a pool of three independent siRNA duplexes at a total concentration of 20 nM siRNA for SOXs or non-specific siRNA (SIC001, Sigma), following the manufacturer’s instructions. Cells will be harvest or reseeded for next analysis after 36–48 hours of siRNA transfection.
Cell number counting
After treatment for indicated time, cells were plated in triplicate in 48-well plates. Cell proliferation was measured by counting cell numbers at indicated time points using the Countess Automated Cell counter (Invitrogen, Life Technologies, Grand Island, NY).
Cell cycle analysis and apoptosis assay
Cell cycle analysis was performed as previous described (10). Cells were treated with siRNA for 48 hours. The cell cycle was synchronized using lovastatin (30 μM) treatment for 24 hours, followed by mevalonate (3mM) treatment to release cell cycle. At indicated time point, cells were harvested and fixed in 4% formaldehyde, then treated with Triton X-100 (0.1%) and stained in 0.5 mL of PI/RNase staining buffer (550825, BD Biosciences). Samples were analyzed using a BD Accuri C6 flow cytometer (BD Biosciences, San Jose, CA) and conducted in triplicate. Apoptosis assays were performed using Annexin V staining kit (88–8007-72, eBioscience) following the manufacturer’s instructions. After treatment with siRNA for 48 hours, cells were harvested, stained, and analyzed using a BD Accuri C6 flow cytometer (BD Biosciences, San Jose, CA) and conducted in triplicate.
RNA isolation and mRNA expression analysis
Total RNA was isolated from cells using the RNeasy Mini Kit (74104, QIAGEN). After being reverse transcribed, real-time quantitative PCR was performed using an ABI 7500 System (Applied Biosystems, Foster City, CA). The relative gene expression was determined using the comparative Ct method and normalized to cyclophilin (2−ΔΔCt method). The qRT-PCR assays were performed in triplicate. Primer and probe sequences of SOX9 for qRT-PCR analysis are listed in Supplementary Table 1.
CHIP assay
Cells were treated with formaldehyde to cross-link proteins with DNA, followed by treatment with lysis buffer. Lysed samples were sonicated 6–12 times to get DNA fragment size between 200–500 bps. DNA-protein complexes were incubated with specific antibody (anti-SOX9: AB5535, Millipore; anti-H3K27AC: ab4729, abcam; anti-H3K27me3: mAbcam 6002, abcam) or normal IgG (Rabbit: 2729, CST; Mouse: 12–371, Sigma) overnight at 4ºC, followed by incubation with protein-A/G agarose beads (20421, Thermo Scientific) for 1 hour at 4ºC to pull down targeted binding DNA fragments. After reverse of cross-linking, the DNA template was analyzed by qRT-PCR with specific primers of interested target genes using a SYBR green assay (1725274, BioRad). The recruitment of targeted protein was presented as an enrichment fold change of PCR amplification in specific antibody pull down samples, compared with that of IgG samples. CHIP assay primers for EMT pathway and apoptosis pathway are listed in Supplementary Tables 2–4.
Western blot analysis
Cells were harvested and lysed in RIPA buffer (R0278, Sigma Aldrich) with protease inhibitors (11697498001, Sigma). Protein samples were separated on a 10% gradient SDS-gel, and transferred onto nitrocellulose membrane. Membrane was incubated with primary antibody overnight at 4°C, then washed with 0.1% TBS-T and incubated with secondary antibodies for 1 hour at room temperature. Bands were detected using the enhanced chemiluminescence western blotting substrate (32106, Thermo Scientific) method. Experiments were performed in triplicate. The primary antibodies used were SOX9 (D8G8H, CST), β-catenin (92G2, CST), PARP (9542, CST), cleaved PARP (9541, CST), caspase-3 (9662, CST), cleaved Caspase-3 (9661, CST), FoxC1 (D8A6, CST), TCF3/TCF7L1 (D15G11, CST), TRAIL (C92B9, CST), FasL (4273S, CST), Death Receptor Antibody Sampler Kit (8356, CST), Epithelial-Mesenchymal Transition (EMT) Antibody Sampler Kit (9782, CST), GAPDH (D4C6R, CST), Vinculin (4650S, CST), β-Actin (A5441, Sigma).
Migration and invasion assays
Cells in serum-free media were seeded in the top chamber of 24-well cell culture insert with 8-μm pores (353097, Corning), with full growth media in bottom chamber. For invasion studies, the top chamber was coated with 20 μl of Matrigel (354230, Corning). Cells were allowed to migrate for 16–18 hours, then material was removed from top chamber, and passed cells were fixed with 4% paraformaldehyde at room temperature for 30 min and stained with 0.05% crystal violet for 30 min. Cell images were captured with a Nikon microscope and counted in 5 fields at 20×magnification.
Xenograft growth and metastasis in vivo
This study was conducted using a M. D. Anderson Institutional Animal Care and Use Committee (IACUC) approved animal protocol. Female nude mice (4–6 weeks old) were obtained from Charles River Laboratories (Wilmington, MA). For the xenograft growth analysis, MDA MB-231-iCas9/sgSOX9 or sgScramble cells were injected subcutaneously into the right 2nd inguinal mammary fat pad (MFP) of nude mice. When xenograft tumor size reached 50–100mm3, mice were divided randomly into 2 groups and treated with sucrose (50 mg/mL) or DOX (0.2 mg/mL) water. Xenograft tumor sizes were measured every 3–7 days, and growth rates were compared between sucrose and DOX water groups. Mice were sacrificed when tumor size reached ≥2000mm3. For the in vivo metastasis analysis, MDA MB-231-LM2 (LM2) breast cancer cells (a highly metastatic variant of MDA MB-231 cells which metastasize to the lungs (11)) were stably transfected with the DOX-inducible Cas9/sgSOX9 or sgScramble. Cells were then treated with or without DOX (2 μg/mL) in vitro for 5 days. After that, cells were injected into nude mice via the tail vain. Firefly D-luciferin postassium salt (150 mg/kg, Cat. #122799, Perkin Elmer, MA, USA) was injected intraperitoneally (IP) just before in vivo luminescent imaging assay (IVIS 100 imaging system, Xenogen, MA, USA).
Cell death detection by enzyme-linked immunosorbent assay (ELISA)
The cell death ELISA assay was performed following manufacturer’s instructions (12). Briefly, cells were treated with or without siRNA targeting SOX9 for 48 hours. The cell lysate samples (100 μL) were prepared in lysis buffer and placed into wells of anti-histone antibody-coated microplate, followed by addition of conjugated solution (containing anti-DNA-peroxidase (POD) antibody). After this, 100 μL of substrate solution was added and incubated for the color development. The apoptotic DNA fragments in the cytoplasmic fraction of cell lysates form immunocomplex with anti-DNA-POD antibody, and the amount of peroxidase was detected using a Synergy™ HT microplate reader (BioTek Instruments, Inc. Vermont, USA) at a wavelength 405 nm and reference wavelength of 490 nm to indicate cell death signaling.
Computational biology analysis and CHIP-Enrich analysis
Expression data of SOX transcription factors were obtained from the Oncomine platform (13) using publicly available datasets. SOX9 expression in the TCGA (14,15), Curtis (16,17), Esserman (18) breast datasets and Neve cell lines (19) is reported as Log2 median-centered intensity between TNBC and non-TNBC tissues, or among different stages. The Boersma (20), Van deVijver (21), Sorlie (22), Sotirious (23), Curtis (16), Desmedt (24), Hatzis (25), Minn (26), Schmidt (27), and Symmans (28) breast datasets were used to analyze survival according to SOX9 expression using Kaplan-Meier survival curves and statistical significance was determined using the log-rank test. CHIP-Enrich analysis was used to test gene set enrichment for available CHIP-seq data as descripted (29,30).
Results
Expression of SOX transcription factors in TNBC and non-TNBC tissues and cell lines
There are approximately 20 SOX transcription factors (TFs) that have been identified in human tissue. Expression levels of each SOX transcription factor were compared among different breast cancer subtypes according to ER, HER2, and TNBC status in the Curtis breast cancer dataset (16). Consistent with our previous report (4), SOX4, 6, 8, 9, 10 and 11 are more highly expressed in TNBCs than in non-TNBC (TCGA (14,15); Curtis (16,17)) (Figure 1A, 1B, and Supplementary Figure 1). The level of SOXs mRNA were also compared in basal breast cancers (which typically consists of 80–90% TNBC) to Normal like, Luminal A, Luminal B, or Her2-enriched breast cancers using the Esserman dataset (18). SOX9 and SOX6, especially SOX9, show highest expression level in basal breast cancers (Supplementary Figure 1B). Expression levels of SOX4, 6, 8, 9, 10, and 11 in the Neve breast cancer cell line dataset were analyzed and are shown in Supplementary Figure 1C (SOX6 and SOX8 expressions are not included in the Neve dataset) (19). In two non-TNBC cell lines (MCF7 and ZR75-1) and two TNBC cell lines (MDA MB-231 and MDA MB-468), expression of SOX6 was not detectable; expression of SOX4 was high in both MDA MB-468 and ZR75-1 cell lines; however, expression of SOX8, 9 and 11 was higher in the TNBC cells than that in the non-TNBC cells, (Supplementary Figure 2A).
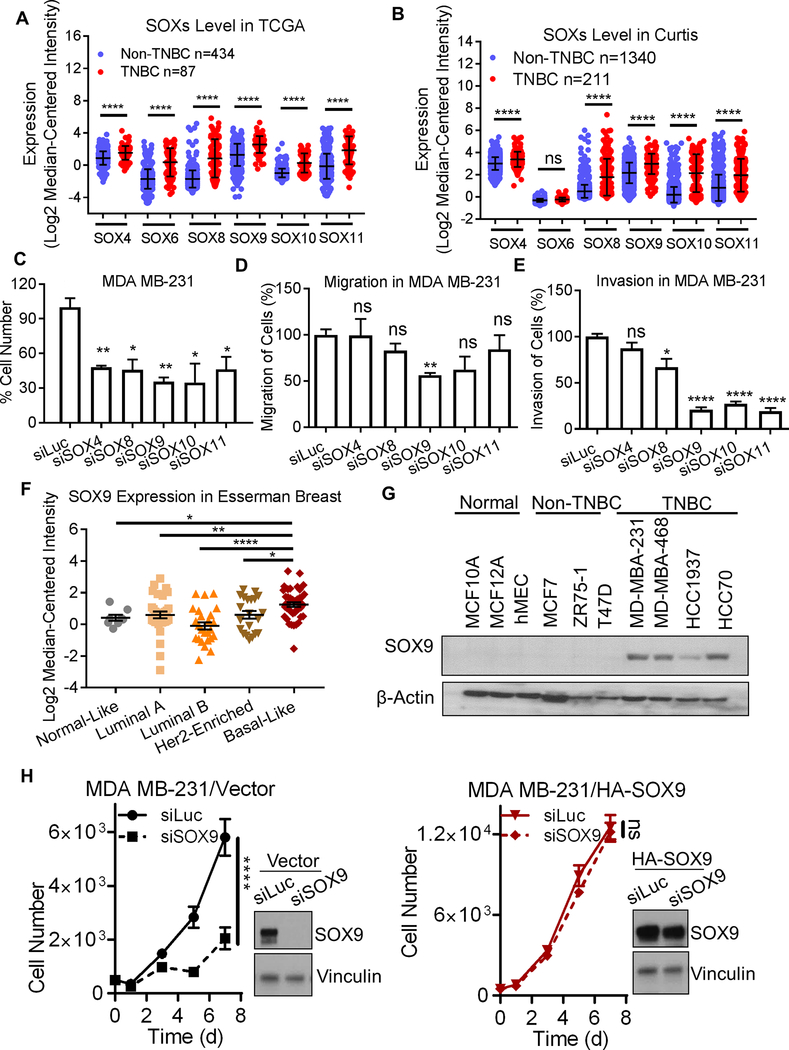
Figure 1. SOX transcription factors are important regulators in TNBCs.
A and B. Expression of SOX Transcription Factors in Clinical Subtypes of Breast Cancer from TCGA and Curtis Datasets (14–16). Expression level of each SOX transcription factor was compared between TNBC and non-TNBC subtypes of breast cancer. The mRNA expression is shown as log2 median centered, and compared with one-way ANOVA with Bonferroni’s multiple comparisons test. C-E. Cell growth, migration, and invasion were assessed in MDA MB-231 cells in vitro. The suppression percent (%) of growth, migration, and invasion was calculated by comparing to the siLuc control group. F. Relative mRNA expression level of SOX9 in different breast cancer subtypes were compared in Esserman breast cancer data set (18). Dot plots demonstrate the expression of SOX9 in different subtypes. Significance was determined with Student’s t-test. G. SOX9 protein expression was determined by western blot analysis. Data shown as representative results of three independent experiments. H. The association between MDA MB-231 cell growth and SOX9 protein level. SOX9 protein level after siRNA treatment was determined by western blot analysis in control or SOX9-ORF overexpression cell lines. Data shown as Mean ± SD of three independent experiments, in triplicate. Significance was determined using Student’s t-test (ns: not significant; * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001).
SOXs regulates TNBC growth and invasion in vitro
To investigate the role of SOX transcription factors in regulating breast cancer cell growth and invasion, MDA MB-231 and MDA MB-468 cells were transfected with siLuc or siRNA-targeting SOX4, SOX8, SOX9, SOX10 or SOX11 for 48 hours (SOX6 was undetectable in tested cells). Treatment with siRNA for SOX factors decreased mRNA expression of each of the SOX transcription factors in MDA MB-231 and MDA MB-468 cells (Supplementary Figure 2B). Cell numbers were counted at day 6 after siRNA treatment. SOX4, 8, 9, 10, and SOX11-knockdown decreased cell growth by approximately 50% in MDA MB-231 cells (Figure 1C). Knockdown of SOX4, 9, 10 and 11 suppressed cell growths by 40% in MDA MB-468 cells but not in MCF7 cells (Supplementary Figure 3A).We next assessed the effect of SOX-knockdown on cell migration and invasion in vitro. Knockdown of SOX8, 9, 10, and 11 reduced cell invasion of MDA MB-231 cells, but only knockdown of SOX9 significantly decreases both migration and invasion of MDA MB-231 and MDA MB-468 cells (Figure 1D and 1E, and Supplementary Figure 3B). To study the effect of SOX transcription factors on metastasis, we focused on SOX9 instead of other SOX family members.
SOX9 expression in human breast cancer
Given the biologic importance of SOX9 in regulating growth and invasion, we examined the expression of SOX9 in breast cancer subtypes. The level of SOX9 mRNA is highest in basal breast cancers when compared to Normal like, Luminal A, Luminal B, and Her2-enriched breast cancers (using the Esserman dataset) (18) (Figure 1F). In the Curtis breast dataset (16), SOX9 mRNA expression is higher in stage IV breast cancers than in stage III or stage II breast cancers (Supplementary Figure 4A), and higher in TNBC breast cancers as compared to non-TNBC breast cancers (Supplementary Figure 4B). We next measured protein expression of SOX9 in breast cell lines including normal human breast epithelial cell lines (MCF10A, MCF12A, and HMEC) and human breast cancer cell lines (non-TNBC cell lines: MCF7, ZR75-1, and T47D; and TNBC cell lines: MDA MB-231, MDA MB-468, HCC1937 and HCC70). These studies demonstrated that the level of SOX9 protein is highest in TNBC cells (Figure 1G). Expression levels of SOX9 in the Neve breast cancer cell line dataset (19) were analyzed and are shown in Supplementary Figure S4C. Using publicly-available breast cancer datasets, we sought to determine whether SOX9 expression is associated with overall survival in breast cancer. We queried 5 publicly available datasets, and found that high expression of SOX9 (as defined by being above the median value) is associated with worse metastasis-free survival only in Schmidt dataset (p=0.0378) (28). We also examined overall survival in 6 databases (only one of which was used for both overall survival and for metastasis-free survival). We found that high SOX9 expression was associated with worse overall survival in only one of these datasets (the Boersma Breast dataset (21) (p=0.0338)) (see Supplementary Figure 5 for examples). Our observation shows SOX9 expression did not generally correlate with metastasis free survival or overall survival and suggests that high SOX9 expression is not the only molecular that promotes metastasis and leads to death from breast cancer.
SOX9 regulates breast cancer cells growth in vitro and in vitro
As shown above, knockdown of SOX9 by siRNA suppressed TNBC cells growth. To investigate whether this cell growth suppression was induced by off-target effect of siRNA treatment, we conducted TNBC cell growth experiments using a TNBC cell line that was stably transfected with a SOX9 cDNA (ORF: open reading frame) lacking the 3’ untranslated region (3’UTR). We then transfected siRNAs targeting 3’UTR of SOX9 in SOX9 ORF-overexpressed MDA MB-231 cells and vector control cells. SOX9-knockdown suppressed cell growth in vector control MDA MB-231 cells (Figure 1H). In SOX9 ORF-overexpressed cells, siRNAs targeting 3’UTR of SOX9 did not reduce SOX9 expression level, and did not suppress cell growth of MDA MB-231 cells (Figure 1H). Our data suggest SOX9 expression positively regulates TNBC cell growth.
To further confirm the effect of SOX9 expression on breast cancer cell growth in vitro, SOX9 was overexpressed in non-TNBC cell lines or knocked-out in several TNBC breast cancer cell lines. Overexpression of SOX9 in MCF7 cells (Luminal, ER-positive breast cancer cells) stimulated cell growth (Figure 2A). On the other hand, knockout of SOX9 in MDA MB-231 cells (triple negative breast cancer cells) resulted in the suppression of cell growth (Figure 2A). In addition, increased SOX9 expression level in MCF7 was associated with increased cell growth rate (Figure 2B and 2C). SOX9 protein expression was much higher in MDA MB-231 than in MCF7 cells, and that high expression was associated with higher cell growth rate (Figure 2B and 2C). SOX9 expression may play a role in the difference of TNBC and non-TNBC, which need to be investigated further in the future.
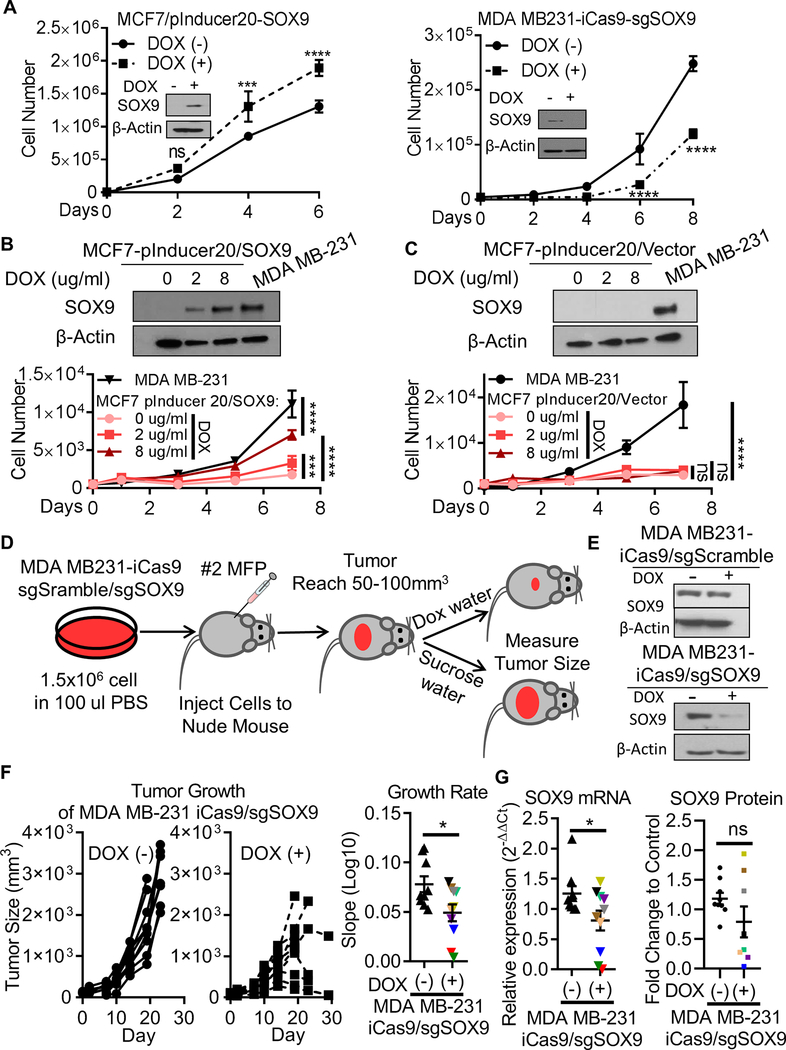
Figure 2. SOX9 regulates breast cancer cells growth in vitro and in vivo.
A. Effect of SOX9 expression on breast cancer cell growth in vitro. SOX9 was overexpressed in non-TNBC cell lines (MCF7) or knocked out in TNBC cell lines (MDA MB-231) through 2 days of DOX treatment (2ug/mL). SOX9 protein expression was determined by western blotting analysis. Cell number was determined by cell counting. B-C. SOX9 dependent breast cancer cell growth in vitro. SOX9 expression was induced by DOX treatment (0, 2, 8 ug/mL) in non-TNBC cell lines (MCF7), and compared with MDA MB-231 cells by western blotting analysis. Cell growth was examined by cell counting. D. Scheme of xenograft experiment. To study the effect of SOX9 on tumor growth, MDA MB-231 cells were stably transfected with a Doxycycline (DOX)-inducible Cas9, and then infected with sgSOX9 or sgScramble guide RNA. These cells were then injected subcutaneously into the right 2nd inguinal mammary fat pad (MFP) of nude mice. When xenograft tumor size reached 50–100 mm3, the mice were divided randomly into 2 groups, and feed with 50 mg/mL sucrose water or 0.2 mg/mL DOX in sucrose water to induce in vivo knockout of SOX9. The size of the xenograft tumors was measured and the tumor growth rates were compared between sucrose and DOX water treated groups. E. SOX9 expression in DOX-inducible Cas9/sgRNA transfected MDA MB-231 cells in vitro was determined by western blotting. F. Xenograft growth of MDA MB-231/DOX-inducible Cas9 cells. Tumor size was measured. G. SOX9 mRNA and protein level in MDA MB-231 xenograft tumor tissues. Total RNA was extracted from tissue fragments of all MDA MB-231 xenograft tumors, and SOX9 mRNA expression levels were evaluated with qRT-PCR. SOX9 protein level was examined by western blotting assay. Two xenograft tumors with small size in DOX water treatment group were not available for western blotting assay. Data shown as Mean ± SD of three independent experiments in triplicate. Significance was determined using Student’s t-test (ns: not significant; * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001)
To study the effect of SOX9 on tumor growth in vivo, MDA MB-231 and HCC1937 cells were stably transfected with a doxycycline-inducible Cas9, and then infected with sgSOX9 or sgScramble guide RNA. These cells were then injected subcutaneously into the right 2nd inguinal mammary fat pad (MFP) of nude mice to examine xenograft growth as shown in Figure 2D and Supplementary Figure 6C. DOX treatment in vitro induced knockout of SOX9 protein in the sgSOX9 guide RNA transfected cells, but not in the sgScramble guide RNA transfected cells (Figure 2E and Supplementary Figure 6D). In the sgScramble guide RNA group, DOX water feed did not affect tumor growth rates (Supplementary Figure 6A and E). However, in the sgSOX9 guide RNA group, DOX treatment decreased MDA MB-231 xenograft growth (Figure 2F and Supplementary Figure 6A and E). To assess SOX9 expression ex vivo, total RNAs were extracted from MDA MB-231 xenograft tumors, and mRNA expression levels were evaluated by qRT-PCR. SOX9 mRNA expression was detected in both DOX and sucrose water-feed mice tumor tissues. In MDA MB-231/iCas9/sgScramble xenograft tumors, SOX9 mRNA expression was similar in DOX and sucrose water treated groups (Supplementary Figure 6B). However, lower SOX9 mRNA expression level was observed in DOX water feed group compared with sucrose water feed group in MDA MB-231/iCas9/sgSOX9 xenograft tumors (Figure 2G). SOX9 protein expression in available xenograft tumor tissues was examined (two xenograft tumors in DOX water treatment group were not available for protein expression assay, but SOX9 expression in these two xenograft tumors was determined by qRT-PCR assay). There was variable SOX9 expression in both control and DOX water treatment groups.
SOX9 regulates breast cancer cells metastasis in vitro and in vivo
To study the effect of SOX9 on cell migration and invasion, MCF7 and ZR75-1 cells were infected with pInducer20-hSOX9 lentivirus. Doxycycline (DOX) (2ug/ml) induced overexpression of SOX9 in these cells and increased cell migration and invasion by at least 2-fold change (Figure 3A, and Supplementary Figure 7A). On the other hand, MDA MB-231 and MDA MB-468 cells were transfected with DOX inducible CRISPR/Cas9/sgRNA. Doxycycline (DOX) (2ug/ml) induced SOX9 knockout and suppressed cell migration and invasion by nearly 50% in these two TNBC cell lines (Figure 3B, and Supplementary Figure 7B).
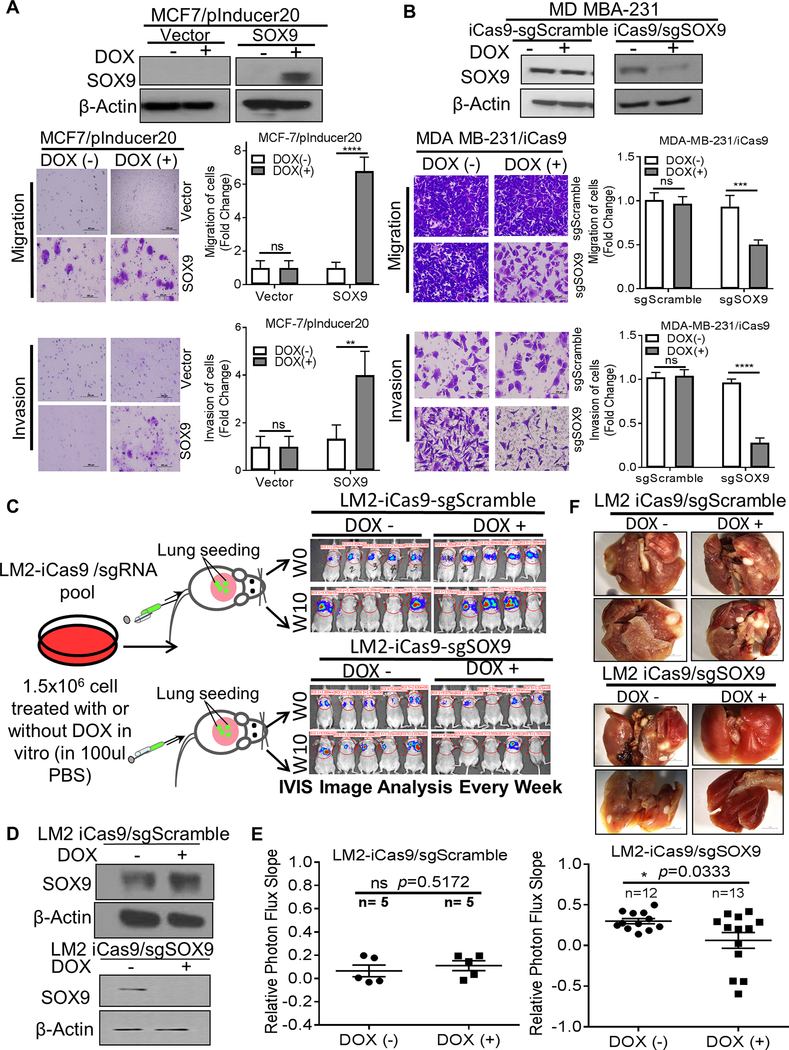
Figure 3. SOX9 regulates breast cancer cells metastasis in vitro and in vivo.
A and B. Effect of SOX9 expression on breast cancer cell migration and invasion in vitro. With or without DOX (2 μg/ml) treatment, SOX9 protein expression was determined by Western blot in A) MCF7/pInducer20-SOX9 or vector cells, and in B) MD MBA-231/ iCas9/sgSOX9 or sgScramble cells. Cell migration and invasion in cells were assessed. The suppression percent (%) of migration and invasion was calculated compared. C. Scheme of breast cancer metastasis experiment. To determine whether breast cancer cell metastasis was affected by loss of SOX9 expression in vivo, MDA MB-231-LM2 (LM2) breast cancer cells were stably transfected with the DOX-inducible Cas9/sgSOX9 or sgScramble. Cells were then treated with or without DOX (2 μg/ml) in vitro for 5 days. After that, cells were injected into nude mice via the tail vain, and cell metastases in lung were evaluated by an in vivo bioluminescent assay (IVIS assay). Metastatic lung tumors were detected by D-luciferin signaling in lung and were examined every week. Representative IVIS images are shown. D. After treated with or without DOX in vitro. SOX9 expression in LM2/DOX-inducible Cas9 cells was determined by western blotting assay. E. Relative photon flux was used to measure the lung metastasis signal of LM2 cells. The slope of signal increase in lung was compared between SOX9-knockout and control group. F. Metastatic tumor nodules on the surface of lung. Data shown as Mean ± SD of three independent experiments in triplicate. Student’s t-test was used (ns: not significant; * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001).
To determine whether breast cancer cell metastasis was affected by loss of SOX9 expression in vivo, LM2-iCas9/sgRNA cells were treated with or without DOX (2ug/ml) for 5 days and were injected into nude mice via the tail vain, and lung metastasis of LM2 cells was detected as shown in Figure 3C. DOX treatment in vitro induced knockout of SOX9 protein in the sgSOX9 guide RNA transfected cells, but not in the sgScramble guide RNA transfected cells (Figure 3D). Mice were imaged weekly to detect D-luciferin signaling. SOX9 knockout reduced growth of lung metastatic tumors (Figure 3E) and decreased the number of metastatic nodules on the lung surface (Figure 3F), suggesting that high SOX9 expression plays a critical role in promoting the growth and survival of metastatic TNBC. The process of metastasis includes extravasation after tail vain injection, cell survival, and growth in the lungs. Data from this experiment reflects the final outcome of these processes. The reduction in the size of lung tumors suggests that loss of SOX9 caused reduced growth of metastatic tumors.
SOX9 knockdown induces apoptosis and delay of G1-to-S phase transition
To investigate the mechanism by which SOX9 loss inhibits tumor growth, cell apoptosis was analyzed after knock-down expression of SOX9. SOX9-knockdown induced apoptosis in MDA MB-231 (increasing apoptosis by nearly 3-fold), and in MDA MB-468 cells (increasing apoptosis by nearly 2.5-fold change) (Figure 4A). SOX9 protein level was determined by western blot analysis as shown in Figure 4B. SOX9 knockdown-induced cell apoptosis was also examined by cell death ELISA analysis in TNBC cells (MDA MB-231, MDA MB-468 and HCC1937 cells) and non-TNBC cells (MCF7 and ZR-75-1 cells). Cells treated with siSOX9 or staurosporine (STP, used as a positive control to induce apoptosis) presented increased apoptotic DNA fragments in TNBC cells comparing to siLuc or wild type (no treatment control) cells (Supplementary Figure 8A).
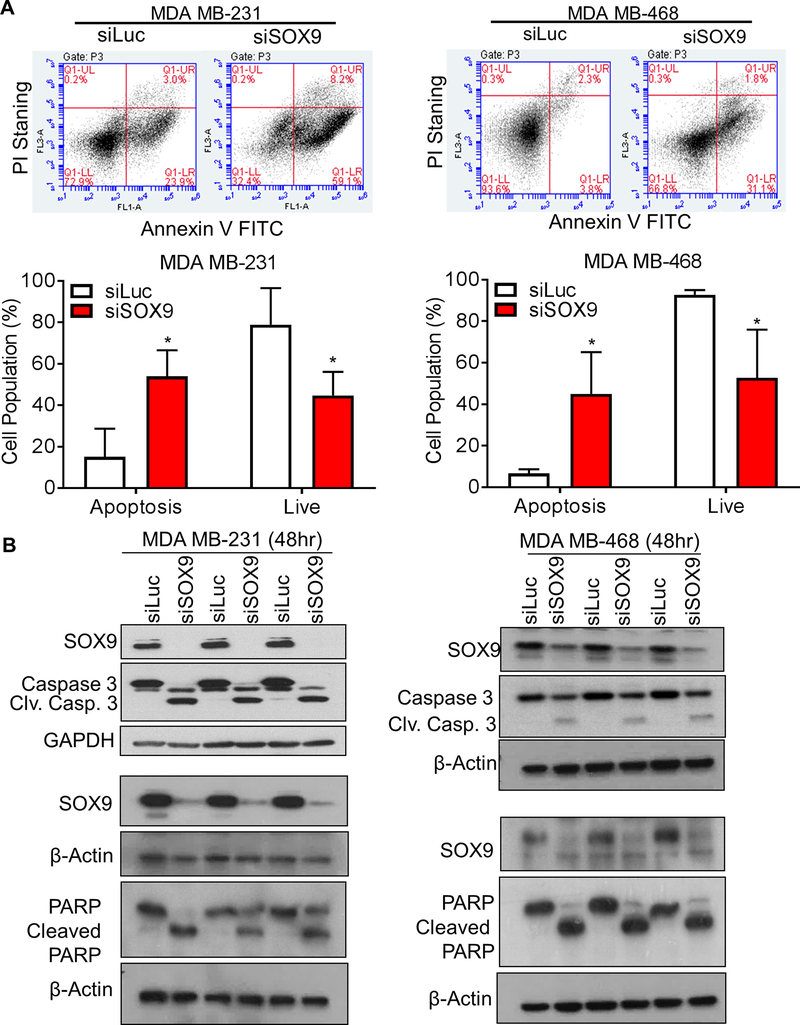
Figure 4. Loss of SOX9 induces TNBC cell apoptosis.
A. Knockdown of SOX9 by siRNA treatment induces TNBC cells apoptosis. Cells were treated with siLuc or siSOX9 for 48 hours. Population of apoptotic cells and live cells were compared between siLuc and siSOX9 treatment groups. Data shown as Mean ± SD of three independent experiments in triplicate. Significance was determines using Student’s t-test (ns: not significant; * p<0.05). B. Apoptotic pathway is induced by SOX9 knockdown in MDA MB-231 cells and MDA MB-468 cells.
To investigate the mechanism by which SOX9 knockdown induces apoptosis, we assessed expression of proteins involved in the apoptotic cascade effector, including caspase-3/cleaved caspase-3 and PARP/cleaved PARP. In MDA MB-231 and MDA MB-468 cells, with decreased SOX9 expression the levels of cleaved PARP and cleaved caspase-3 were increased, along with a corresponding decrease in procaspase-3 and PARP level (Figure 4B). On the other hand, siSOX9 treatment in MCF7 and ZR75-1 cells did not induce caspase 3 activity (Supplementary Figure 8B), or induce apoptosis as measured by flow cytometry analysis or cell death ELISA assay (Supplementary Figure 8C and D).
To study the effect of SOX9 on cell cycle regulation, MDA MB-231 cells were treated and analyzed as shown in Supplementary Figure 9A. A parallel panel of MDA MB-231 cells were used to confirm SOX9 protein knockdown by western blot. SOX9 knockdown was present at the 0 hour time point and maintained at 60 hours (Supplementary Figure 9B). MDA MB-231 cells treated with siSOX9 had slightly fewer cells in S-phase compared to the siRNA control group 39–48 hours after the cell cycle block was released. Conversely, knockdown of SOX9 caused a slight increase in the proportion of cells in the G0/G1 phase 42–48 hours after the cell cycle block was released (Supplementary Figure 9C).
Mechanism by which SOX9 regulates cell survival, cell death, and EMT
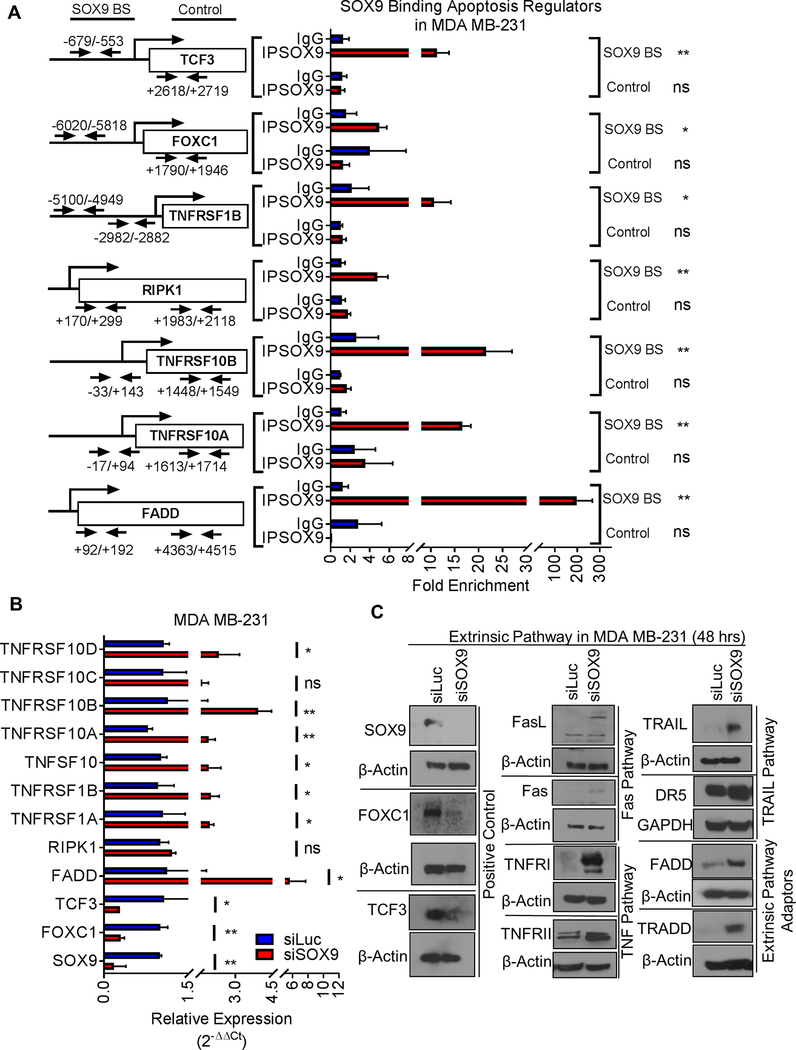
To investigate whether SOX9 regulates genes controlling apoptosis and invasion, we examined whether SOX9 binds to the promoters of apoptosis and EMT regulatory genes. We performed a CHIP-Enrich analysis to test gene set enrichments using available CHIP-seq data (31,32). As shown in Supplementary Figure 10, signaling pathways of death receptors and ligands, cell death, caspase activation via extrinsic apoptosis, as well as EMT signaling in cancer are on the top list of SOX9-CHIP gene enrichment analysis. We confirmed SOX9 binding in the promoter regions of genes regulating apoptosis and EMT using publicly available SOX9-CHIP seq datasets (Supplementary Figures 11–15) (31–33). Our CHIP assay data further confirmed that SOX9 directly binds to promoter of the following apoptosis-regulating genes: foxc1, tcf3, tnfrsf1b, fadd, tnfrsf10a, tnfrsf10b, and ripk1 (Figure 5A and Supplementary Figure 11A). We next investigated whether knockdown of SOX9 affected the expression of these genes in TNBC and non-TNBC cells. Expression of fadd, tnfrsf1a (TNFRI), tnfsf10 (TRAIL), and tnfrsf10b (Death receptor 5) was increased by SOX9 knockdown in TNBC cells (Figure 5B and Supplementary Figure 11B), and expression of fadd, ripk1, tnfsf10, tnfrsf10a, tnfrsf10c, fas, fas ligand, and tnfrsf1a was suppressed by SOX9-overexpression in non-TNBC cells (Figure 11B and 11C). Protein expression of TNFR1, TRAIL, Death receptor 5 (DR5), TRADD and FADD was increased by SOX9 knockdown in TNBC cells, while expressions of FOXC1 and TCF3 were decreased by SOX9 knockdown (Figure 5C).
Figure 5. Mechanism of SOX9 regulating TNBC survival.
A. SOX9 binding in promoter area of genes involved in cell death and survival was examined by CHIP assay. BS: Binding Site. B. SOX9 regulates cell death and survival gene expression. Upon SOX9 knockdown, expression of genes involved in cell death was examined by qRT-PCR assay. C. SOX9 regulates protein expressions of factors involved in cell death pathways. The representative image of one of the three independent experiments with similar outcomes were shown. Data shown as Mean ± SD of three independent experiments in triplicate. Significance determined by Student’s t-test (ns: not significant; * p<0.05; ** p<0.01)
Analysis of available CHIP-seq data also showed that SOX9 binds to the promoters of several EMT- and metastasis-regulating genes. Using CHIP assays, we demonstrated that SOX9 directly binds the promoters of vim (Vimentin), cldn1 (Claudin-1), ctnnb1 (β-Catenin) and zeb1 (Zinc finger E-box-binding homeobox 1) genes to regulate the expression of these genes (Figure 6A and Supplementary Figure 12A). Analysis of RNA expression using an EMT PCR array demonstrated that SOX9 knockdown in MDA MB-231 cells decreased the expression of many EMT-regulatory genes (Supplementary Figure 12B). Knockdown of SOX9 suppresses the mRNA expression of vim (Vimentin) and ctnnb1 (β-Catenin) while increasing cdh1 (E-Cadherin) gene expression (Figure 6B). Conversely, overexpression of SOX9 in MCF7 cells increased the expression of vim (Vimentin) and ctnnb1 (β-Catenin) but decreased cdh1 (E-Cadherin) gene expression (Supplementary Figure 12C). Protein expression of Vimentin and β-Catenin was reduced by SOX9 knockdown, but protein level of E-cadherin was increased by SOX9 knockdown in TNBC cells (MDA MB-468) (Figure 6C). On the other hand, protein expression of β-Catenin was enhanced by SOX9-overexpression in non-TNBC (MCF7) and non-tumorigenic (MCF10A) cells (Figure 6C).
Figure 6. Mechanism of SOX9 controlling EMT pathway in TNBCs.
A. SOX9 binding in promoter area of genes involved in EMT signaling was determined by CHIP assays. B. SOX9 regulates EMT gene mRNA expression. Upon SOX9 knockdown, expression of genes involved in EMT was examined by qRT-PCR assay. C. SOX9 regulates EMT protein signature level. A representative image of one of three independent experiments with similar outcome was shown. D-E. The status of histone modification (H3K27AC/Me3) in promoter area of potential SOX9 regulating genes upon SOX9 knockdown. Data shown as Mean ± SD of three independent experiments in triplicate. Significance determined with Student’s t-test (ns: not significant; * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001). BS: Binding Site.
Based on published CHIP-Seq data (Supplementary Figure 16), histone modification was evaluated by CHIP assay. SOX9 expression was first knocked down by siRNA treatment (Figure 6D). CHIP assays were then done to investigate promoter acetylation (for the SOX9 up-regulated genes, FOXC1 and VIM) using the H3K27Ac tag, or promoter methylation (for SOX9 down-regulated gene, FADD) using the H3K27me3 tab. The results demonstrated that the H3K27Ac levels present in the promoters of the FOXC1 and VIM genes (both of which were down-regulated upon SOX9 knockdown) was reduced upon SOX9 knockdown in MDA MB-231 cells (Figure 6D–E). Conversely, the H2K27me3 level present in the promoter of the FADD gene (which was found to be up-regulated upon SOX9 knockdown) was decreased upon SOX9 knockdown (Figure 6E).
Discussion
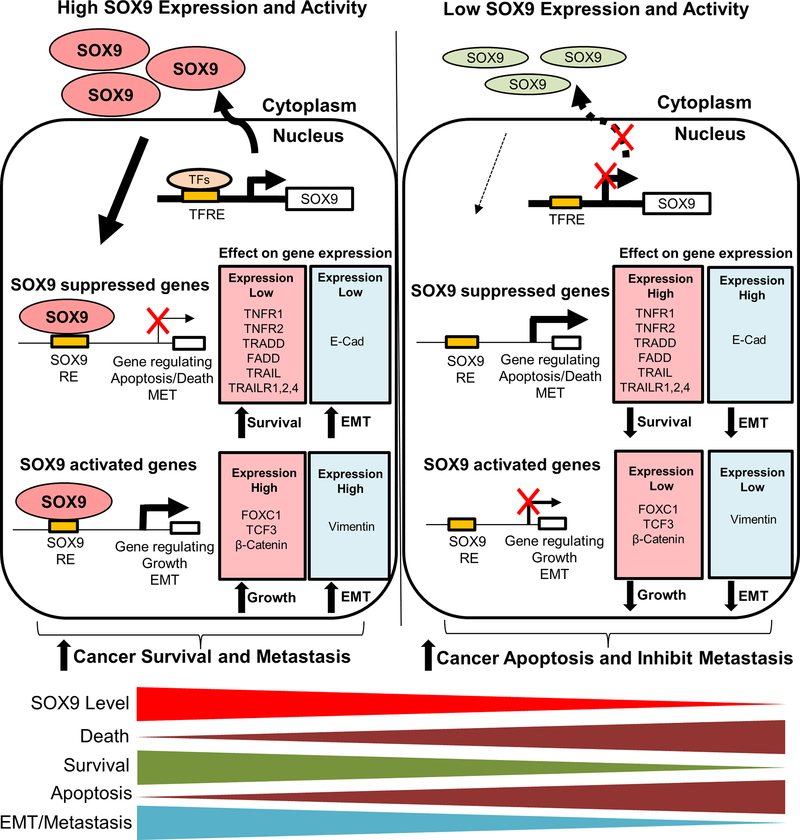
In this study, we investigated the role of the SOX transcription factors in regulating TNBC growth, survival, and metastasis. We discovered that, while many of the SOX transcription factors regulate breast cancer growth, SOX9 is a critical regulator of TNBC growth, survival, and metastasis. Inhibition of SOX9 induced TNBC cell death and reduced invasion in vitro and reduced tumor growth and metastasis in vivo. We also investigated the mechanism by which SOX9 affects these biologic processes as shown in Figure 7. In breast cancers that have high SOX9 expression, SOX9 directly binds to the promoter area of death-inducing genes, and subsequently suppresses the expression of these genes. Additionally, SOX9 directly binds to the promoters of EMT and metastasis-promoting genes causing up-regulation of genes. High SOX9 expression and activity promotes cancer cell survival and metastasis, and suppresses cell apoptosis and death contributing to the aggressive development of TNBC. Conversely, in breast cancers that have low SOX9 expression, the absence of SOX9 releases the suppressive effect of SOX9 on death-inducing gene expression and reduces the expression of EMT genes which may lead to reduced cell growth, decreased cell survival, EMT, and metastasis.
Figure 7. Summary of the mechanism that SOX9 promotes breast cancer survival and metastasis.
In TNBCs that highly express SOX9, SOX9 directly binds to the promoter region of death-inducing genes and suppresses the expression of these genes, while also directly binds the promoters of EMT and metastasis-promoting genes causing up-regulation of these genes. High expression and activity of SOX9 promotes cancer cell survival, metastasis, and suppresses cell apoptosis and death contributing to the aggressive development of TNBC. Conversely, in breast cancers that have low SOX9 expression, the absence of SOX9 releases the suppressive effect of SOX9 on death-inducing gene expression and reduces the expression of EMT genes leading to reduced cell growth, decreased cell survival, EMT and metastasis. TFRE: transcription factor response elements; RE: response elements.
Tumor growth is the integrated result of increased proliferation and reduced apoptosis (34). Induction of apoptosis is one of the most important mechanisms for chemotherapy and radiotherapy in cancer treatment. The extrinsic apoptosis pathway is mediated by death receptors, including Fas receptors, tumor necrosis factor (TNF) receptor superfamily (TNFRSF), and TNF-related apoptosis-inducing ligand (TRAIL or TNFRSF10) receptors. These receptors will interact with the ligands to induce the recruitment of adaptor proteins, eventually resulting in the activation of Caspase-3, −6, and −7, leading to cell apoptosis (35). For the first time, our study revealed that SOX9 knockdown induces cell apoptosis in TNBC cells through activation of extrinsic apoptotic pathways. Interestingly, we did not observe apoptosis induction after SOX9-knockdown in MCF7 and ZR75-1 non-TNBC cell lines. The low SOX9 level in MCF7 and ZR75-1 may indicate that these cells do not require SOX9 for their survival. Our results suggest that only cells with high expression of SOX9 require SOX9 for survival. In breast cancer, the expression of estrogen receptor (ER) represses SOX9 expression (36). In ER-positive non-TNBCs, up-regulation of SOX9 causes endocrine resistance (37), and SOX9 high expression is required for maintenance of ER-positive breast cancer stem cells (38). These additional studies show that the SOX9 transcription factor plays an important, but distinct role in the different forms of breast cancer. In ER-positive breast cancers SOX9 serves to maintain stemness and may be involved in hormonal therapy resistance, while in TNBC, SOX9 appears to be predominantly involved in regulating proliferation, invasion and metastasis.
Previous studies have also demonstrated the role of SOX9 in regulating cell apoptosis (39,40), however our results provide a novel mechanism by which the absence of SOX9 in TNBC cells, but not in SOX9-low non-TNBC cells, decreased cell survival. Additionally, SOX9 is also an important regulator of cell cycle. Previous studies have reported that SOX9 overexpression causes a G1 cell cycle block, while SOX9-knockdown causes delayed S-phase progression (41–44). However, our results demonstrate that SOX9-knockdown in TNBC cells decreased cell cycle progression only slightly. In TNBCs, SOX9 functions predominantly to promote survival (and prevent apoptosis) rather than as a cell cycle regulator.
Epithelial-to-mesenchymal transition (EMT) is a major hallmark for tumorigenic progression. The EMT program endows cancer cells with increased invasion and migratory abilities to become metastatic (45). Studies of transcription factors regulating the expression of genes involved in EMT program are critical for understanding tumorigenic mechanisms and development of novel therapeutic strategies to treat metastatic cancers. SOX9 is one of the transcription factors involved in the EMT process of cancer. SOX9 transduces Wnt/β-catenin signals and induces EMT, and contributes to cancer cell invasion and metastasis (5,6,32,46–49). Our data indicates that SOX9 protein is expressed at higher levels in the majority of TNBC tumors than in non-TNBC tumors. Our results further demonstrate that SOX9 regulates breast cancer growth and metastasis by directly regulating the expression of apoptosis and EMT genes in breast cancer cells. We show that SOX9 directly binds to the promoters of apoptosis and EMT-regulating genes (such as FADD and VIM)), and regulates the H3K27Ac/Me3 status of these target genes to control gene expression. These results, taken together, show that SOX9 is an essential transcription factor regulating apoptosis and EMT, contributing to breast cancer metastasis.
As shown in our SOX9 CHIP enrichment analysis, several important pathways involved in cancer development are revealed as SOX9 regulated pathways. These include: 1) The PIP2 phospholipid signaling pathway controls cell growth, and cytokine and growth factor signal transduction; 2) The Rho GTPase signaling pathway that controls cell apoptosis, abnormal tumor growth and metastasis (50); 3) The YAP1- and WWTR1 (TAZ)-stimulated gene expression pathway regulates gene expression, control cell proliferation and apoptosis (51); 4) The SIgnal Regulatory Protein (SIRP) family of proteins that are membrane proteins that transduce signals in immune cells to regulate immune cell interaction with other cells (52); and 5) Rap1 signaling GTPase that control cell proliferation and cell-cell adhesion. Because we observed an effect on EMT in our SOX9 knockdown studies, we focused on the EMT and cell survival pathways in this study,. However, SOX9 is indeed involved in controlling other cellular processes including the response to extracellular signals that stimulate growth. This is consistent with our finding that SOX9 knockdown inhibited growth of TNBC cells. These data also serve to reinforce our proposal that SOX9 is a master regulator of proliferation, apoptosis, and metastasis. In the future, we will study the role of SOX9 in controlling these other pathways.
Although our study focused on SOX9, the most effective SOX factor that regulating cell growth and metastasis in TNBC based on our 2D culture screening, other SOX factors (such as SOX2, SOX4, SOX6, SOX10 and SOX11) play important roles in cancer, and are needed to cooperate with SOX9 to control cancer progression. For example, SOX9 cooperate with SOX10 to control a tumorigenic program in melanoma (8); SOX9 acts as a downstream target of SOX2, and the SOX2-SOX9 signaling axis is required for maintaining cancer stem cells (36,53). Furthermore, SOX2 controls proliferation and metastasis of breast cancer cells (54,55). In triple negative breast cancer, SOX2 has been reported to be a tumor promoter and could be a potential therapeutic target (55). SOX4, also abnormally overexpressed in TNBC, is a critical transcription factor in activating EMT program in immortalized human mammary epithelial cells and is required for TGF-β-induced EMT (56). Sox6 overexpression suppressed pancreatic cancer cell proliferation and migration in vitro and tumor growth and liver metastasis in vivo (57). In addition, SOX11 has been shown to increase TNBC cell growth and invasion in vitro (4), and thus may be able to compensate for SOX9 in cells with low expression of SOX9. Our future studies are focused on investigating the cooperation between SOX9 and other SOX factors that highly expressed in TNBC.
SOX9 has been shown by us (in this report) and others (58,59) to induce the expression of many genes. However, our results also suggest that SOX9 can repress the expression of other genes (such as FADD). These observation may be supported by the results published by Kadaja et al. of SOX9 RNA-Seq data, which suggest that SOX9 suppresses the expression of many genes (58). Their studies show that 17% of genes found to be up-regulated after SOX9 knock-out had SOX9 binding elements within their promoters. These results suggest that such genes are repressed by SOX9 (58). Ma et al. using SOX9 ChIP sequencing analysis and transcriptome profiling of prostate cancer cells, showed that siRNA to SOX9 induces the expression of many genes (consistent with the hypothesis that SOX9 represses the expression of these genes) (59). The same effect has also been observed when other transcription factors are inhibited or knocked down. Previous studies have shown that the estrogen receptor when activated by estrogen represses the expression of more genes than it induces (60).
To develop novel therapeutic strategy for TNBC, it will be important to discover ways to target this “master regulator”. Inhibition of the SOX9 transcription factor may effectively prevent the growth of metastatic cells and may induce death of existing metastatic lesions. However, as a transcription factor, targeting SOX9 directly for cancer treatment may be difficult. SOX9 is a ubiquitously expressed factor in normal cells, and the complete loss of SOX9 is embryonically lethal (61). However, in adult tissues, SOX9 may not be essential. Conditional SOX9 deletion in the normal mammary gland did not affect development of mammary gland (62). These observations imply SOX9 would be a promising target in treatment of metastatic cancer diseases. Our current studies are focused on identifying critical up-stream activators of SOX9 that are potentially more amenable to drug targeting. Ultimately, it will be important to develop inhibitors of SOX9 that have minimal toxicity. The results from these studies provide the basic science rationale to more effectively treat triple-negative breast cancer, the most aggressive and lethal form of breast cancer.
Supplementary Material
Implications.
Our studies identified the SOX9 protein as a “master regulator” of breast cancer cell survival and metastasis, and provide preclinical rationale to develop SOX9 inhibitors for the treatment of women with metastatic triple-negative breast cancer.
Acknowledgments
We would like to thank Michelle Savage for editing the manuscript, and Sam Short for assisting in the submission.
Financial Support:
This work was supported by a NCI Cancer Center Support Grant (P30CA016672, PHB), a Susan G. Komen Scientific Advisory Board Grant, SAB1600006 (PHB), and a grant from the Breast Cancer Research Foundation 2015–2016 BCRF grant (PHB), and by the John Charles Cain Endowment (PHB).
Footnotes
Conflict of interest:
P. Brown is a minor stock-holder (<1%) of the GeneTex Biotechnology Company. No reagents from this company were used in this study. Other remaining authors declare no actual, potential, or perceived conflict of interest that would prejudice the impartiality of this article.
References
- 1.Van Swearingen AE, Siegel MB, Anders CK. Breast cancer brain metastases: evidence for neuronal-like adaptation in a ‘breast-to-brain’ transition? Breast Cancer Res 2014;16:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders CK, Carey LA. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin Breast Cancer 2009;9 Suppl 2:S73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepherd JH, Uray IP, Mazumdar A, Tsimelzon A, Savage M, Hilsenbeck SG, et al. The SOX11 transcription factor is a critical regulator of basal-like breast cancer growth, invasion, and basal-like gene expression. Oncotarget 2016;7:13106–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling S, Chang X, Schultz L, Lee TK, Chaux A, Marchionni L, et al. An EGFR-ERK-SOX9 signaling cascade links urothelial development and regeneration to cancer. Cancer Res 2011;71:3812–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrasco-Garcia E, Lopez L, Aldaz P, Arevalo S, Aldaregia J, Egana L, et al. SOX9-regulated cell plasticity in colorectal metastasis is attenuated by rapamycin. Sci Rep 2016;6:32350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao B, Peng R, Li Z, Ding J. Effects of spreading areas and aspect ratios of single cells on dedifferentiation of chondrocytes. Biomaterials 2014;35:6871–81 [DOI] [PubMed] [Google Scholar]
- 8.Shakhova O, Cheng P, Mishra PJ, Zingg D, Schaefer SM, Debbache J, et al. Antagonistic cross-regulation between Sox9 and Sox10 controls an anti-tumorigenic program in melanoma. PLoS Genet 2015;11:e1004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science 2014;343:80–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamali T, Kavoosi G, Safavi M, Ardestani SK. In-vitro evaluation of apoptotic effect of OEO and thymol in 2D and 3D cell cultures and the study of their interaction mode with DNA. Sci Rep 2018;8:15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest 2005;115:44–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balijepalli MK, Tandra S, Pichika MR. Antiproliferative activity and induction of apoptosis in estrogen receptor-positive and negative human breast carcinoma cell lines by Gmelina asiatica roots. Pharmacognosy Res 2010;2:113–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes DR, Chinnaiyan AM. Bioinformatics strategies for translating genome-wide expression analyses into clinically useful cancer markers. Ann N Y Acad Sci 2004;1020:32–40 [DOI] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas N Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciriello G, Gatza ML, Beck AH, Wilkerson MD, Rhie SK, Pastore A, et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 2015;163:506–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012;486:346–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira B, Chin SF, Rueda OM, Vollan HK, Provenzano E, Bardwell HA, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun 2016;7:11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esserman LJ, Berry DA, Cheang MC, Yau C, Perou CM, Carey L, et al. Chemotherapy response and recurrence-free survival in neoadjuvant breast cancer depends on biomarker profiles: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Breast Cancer Res Treat 2012;132:1049–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006;10:515–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boersma BJ, Reimers M, Yi M, Ludwig JA, Luke BT, Stephens RM, et al. A stromal gene signature associated with inflammatory breast cancer. Int J Cancer 2008;122:1324–32 [DOI] [PubMed] [Google Scholar]
- 21.van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347:1999–2009 [DOI] [PubMed] [Google Scholar]
- 22.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A 2003;100:10393–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desmedt C, Piette F, Loi S, Wang Y, Lallemand F, Haibe-Kains B, et al. Strong time dependence of the 76-gene prognostic signature for node-negative breast cancer patients in the TRANSBIG multicenter independent validation series. Clin Cancer Res 2007;13:3207–14 [DOI] [PubMed] [Google Scholar]
- 25.Hatzis C, Pusztai L, Valero V, Booser DJ, Esserman L, Lluch A, et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA 2011;305:1873–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature 2005;436:518–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt M, Bohm D, von Torne C, Steiner E, Puhl A, Pilch H, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res 2008;68:5405–13 [DOI] [PubMed] [Google Scholar]
- 28.Symmans WF, Hatzis C, Sotiriou C, Andre F, Peintinger F, Regitnig P, et al. Genomic index of sensitivity to endocrine therapy for breast cancer. J Clin Oncol 2010;28:4111–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavalcante RG, Lee C, Welch RP, Patil S, Weymouth T, Scott LJ, et al. Broad-Enrich: functional interpretation of large sets of broad genomic regions. Bioinformatics 2014;30:i393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato T, Kaneda A, Tsuji S, Isagawa T, Yamamoto S, Fujita T, et al. PRC2 overexpression and PRC2-target gene repression relating to poorer prognosis in small cell lung cancer. Sci Rep 2013;3:1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawai T, Yasuchika K, Ishii T, Miyauchi Y, Kojima H, Yamaoka R, et al. SOX9 is a novel cancer stem cell marker surrogated by osteopontin in human hepatocellular carcinoma. Sci Rep 2016;6:30489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsimont JC, Youssef KK, Sanchez-Danes A, Sukumaran V, Defrance M, Delatte B, et al. Sox9 Controls Self-Renewal of Oncogene Targeted Cells and Links Tumor Initiation and Invasion. Cell Stem Cell 2015;17:60–73 [DOI] [PubMed] [Google Scholar]
- 33.Shi Z, Chiang CI, Labhart P, Zhao Y, Yang J, Mistretta TA, et al. Context-specific role of SOX9 in NF-Y mediated gene regulation in colorectal cancer cells. Nucleic Acids Res 2015;43:6257–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamm I, Schriever F, Dorken B. Apoptosis: implications of basic research for clinical oncology. Lancet Oncol 2001;2:33–42 [DOI] [PubMed] [Google Scholar]
- 35.Elmore S Apoptosis: a review of programmed cell death. Toxicol Pathol 2007;35:495–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domenici G, Aurrekoetxea-Rodriguez I, Simoes BM, Rabano M, Lee SY, Millan JS, et al. A Sox2-Sox9 signalling axis maintains human breast luminal progenitor and breast cancer stem cells. Oncogene 2019;38:3151–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeselsohn R, Cornwell M, Pun M, Buchwalter G, Nguyen M, Bango C, et al. Embryonic transcription factor SOX9 drives breast cancer endocrine resistance. Proc Natl Acad Sci U S A 2017;114:E4482–E91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue Y, Lai L, Lian W, Tu X, Zhou J, Dong P, et al. SOX9/FXYD3/Src Axis Is Critical for ER(+) Breast Cancer Stem Cell Function. Mol Cancer Res 2019;17:238–49 [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Garbutt CC, Spentzos D, Choy E, Hornicek FJ, Duan Z. Expression and Therapeutic Potential of SOX9 in Chordoma. Clin Cancer Res 2017;23:5176–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xue Y, Yang G, Wang C, Li X, Du G. Effects of shRNA-Mediated SOX9 Inhibition on Cell Proliferation and Apoptosis in Human HCC Cell Line Hep3B Mediated by Ultrasound-Targeted Microbubble Destruction (UTMD). Cell Biochem Biophys 2015;73:553–8 [DOI] [PubMed] [Google Scholar]
- 41.Stockl S, Gottl C, Grifka J, Grassel S. Sox9 Modulates proliferation and expression of osteogenic markers of adipose-derived stem cells (ASC). Cell Physiol Biochem 2013;31:703–17 [DOI] [PubMed] [Google Scholar]
- 42.Shi G, Wang TT, Quan JH, Li SJ, Zhang MF, Liao PY, et al. Sox9 facilitates proliferation, differentiation and lipogenesis in primary cultured human sebocytes. J Dermatol Sci 2017;85:44–50 [DOI] [PubMed] [Google Scholar]
- 43.Liu C, Liu L, Chen X, Cheng J, Zhang H, Shen J, et al. Sox9 regulates self-renewal and tumorigenicity by promoting symmetrical cell division of cancer stem cells in hepatocellular carcinoma. Hepatology 2016;64:117–29 [DOI] [PubMed] [Google Scholar]
- 44.Stockl S, Bauer RJ, Bosserhoff AK, Gottl C, Grifka J, Grassel S. Sox9 modulates cell survival and adipogenic differentiation of multipotent adult rat mesenchymal stem cells. J Cell Sci 2013;126:2890–902 [DOI] [PubMed] [Google Scholar]
- 45.Ye X, Weinberg RA. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol 2015;25:675–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol 2004;166:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dupasquier S, Abdel-Samad R, Glazer RI, Bastide P, Jay P, Joubert D, et al. A new mechanism of SOX9 action to regulate PKCalpha expression in the intestine epithelium. J Cell Sci 2009;122:2191–6 [DOI] [PubMed] [Google Scholar]
- 48.Santos JC, Carrasco-Garcia E, Garcia-Puga M, Aldaz P, Montes M, Fernandez-Reyes M, et al. SOX9 Elevation Acts with Canonical WNT Signaling to Drive Gastric Cancer Progression. Cancer Res 2016;76:6735–46 [DOI] [PubMed] [Google Scholar]
- 49.Capaccione KM, Hong X, Morgan KM, Liu W, Bishop JM, Liu L, et al. Sox9 mediates Notch1-induced mesenchymal features in lung adenocarcinoma. Oncotarget 2014;5:3636–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clin Exp Metastasis 2007;24:657–72 [DOI] [PubMed] [Google Scholar]
- 51.Long F Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 2011;13:27–38 [DOI] [PubMed] [Google Scholar]
- 52.Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, et al. Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res 2011;71:1374–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garros-Regulez L, Aldaz P, Arrizabalaga O, Moncho-Amor V, Carrasco-Garcia E, Manterola L, et al. mTOR inhibition decreases SOX2-SOX9 mediated glioma stem cell activity and temozolomide resistance. Expert Opin Ther Targets 2016;20:393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu K, Xie F, Gao A, Zhang R, Zhang L, Xiao Z, et al. SOX2 regulates multiple malignant processes of breast cancer development through the SOX2/miR-181a-5p, miR-30e-5p/TUSC3 axis. Mol Cancer 2017;16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu P, Tang H, Song C, Wang J, Chen B, Huang X, et al. SOX2 Promotes Cell Proliferation and Metastasis in Triple Negative Breast Cancer. Front Pharmacol 2018;9:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang J, Liang Q, Lei Y, Yao M, Li L, Gao X, et al. SOX4 induces epithelial-mesenchymal transition and contributes to breast cancer progression. Cancer Res 2012;72:4597–608 [DOI] [PubMed] [Google Scholar]
- 57.Jiang W, Yuan Q, Jiang Y, Huang L, Chen C, Hu G, et al. Identification of Sox6 as a regulator of pancreatic cancer development. J Cell Mol Med 2018;22:1864–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kadaja M, Keyes BE, Lin M, Pasolli HA, Genander M, Polak L, et al. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev 2014;28:328–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma F, Ye H, He HH, Gerrin SJ, Chen S, Tanenbaum BA, et al. SOX9 drives WNT pathway activation in prostate cancer. J Clin Invest 2016;126:1745–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 2003;144:4562–74 [DOI] [PubMed] [Google Scholar]
- 61.Koopman P Sex determination: a tale of two Sox genes. Trends Genet 2005;21:367–70 [DOI] [PubMed] [Google Scholar]
- 62.Malhotra GK, Zhao X, Edwards E, Kopp JL, Naramura M, Sander M, et al. The role of Sox9 in mouse mammary gland development and maintenance of mammary stem and luminal progenitor cells. BMC Dev Biol 2014;14:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.