Abstract
Green synthesis of nanoparticles is an important area in the field of nanotechnology, which has cost effective and environment friendly benefit over physical and chemical methods. The present study aims at preparation of silver nanoparticles through green route using leaves of Ocimum canum Sims, a widely distributed medicinal herb. The synthesized silver nanoparticles were characterized by SEM and XRD. The spherical and rod like morphological shapes were proven by SEM techniques. Crystallographic structure was confirmed by XRD and average particle size of synthesized silver nanoparticles was calculated which was found to be of 15.72 nm. The antibacterial activity of these prepared silver nanoparticles against pathogenic bacterium Escherichia coli (E. coli) has shown the highest ZOI of 2.45 cm at 30 ppm.
Keywords: Antibacterial, Silver, Nanoparticles, SEM, XRD, ZOI
Highlights
-
•
Synthesis of Silver nanoparticles by green and ecofriendly methods.
-
•
Structural studies of silver nanoparticles. Morphological study of the silver nanoparticles.
-
•
Αaverage particle size also determined by Scherrer formula.
-
•
Determination of the antimicrobial activity of the synthesize nanoparticles.
1. Introduction
Metal incorporated nanoparticles have received wide interest in the area of industrial and medicinal applications. Among them, Nobel metals gold, silver, platinum and palladium based nanoparticles have received much attention due to their unique electrical, optical and electronic as well as catalytic properties. Nanoparticles reveal atom like behaviours due to high surface to volume area and wide gap between valance band and conduction band [1,2]. Silver Nanoparticles have initiated useful interest not only fundamental development in research but also the industrial level owing to their versatile properties [[3], [4], [5], [6], [7]]. This feature has attracted many researchers to find new methods for their synthesis [8]. A number of physical and chemical methods like reduction in solutions, thermal decomposition of silver compound, microwave assisted and photochemical reactions have been reported by several workers. The most common method for the synthesis of silver nanoparticles is a chemical reduction using inorganic and organic reducing agents such as hydrazine [9], N-dimethylformamide [10], Sodium borohydride [11], poly(ethyleneglycol) [12] and surfactant template approach [10,13]. The synthesis of nanoparticles through green routes using microorganisms [[14], [15], [16]] enzymes [17] and plant extracts [[18], [19], [20], [21], [22], [23]]were suggested as possible environmental friendly alternatives to chemical methods. Additionally, these methods were reported to be of cost effective and synthesized particles were found more stable. Several medicinal plants such as Acorus calamus [24], Alternanthera dentata [25], Ocimumsanctum [26], Azadirachta indica [27], Brassica rapa [28], Coccinia indica [29], Vitex negundo [30], Melia dubia [31]have already been used to synthesize and stabilize metallic NPs, very particularly biogenic AgNPs.
A thorough survey of literature indicated that not much work has been done on the use of jungli tulsi an aromatic herb (Ocimum canum Sims.) in nanotechnology. Ocimum canum Sims. (hoary basil) of family Lamiaceae locally known as Jungli tulsi, Bapchi, Naked bapchi, Ban tulsi etc. and is widely occurring in India, China, Indonesia, Malaysia, Myanmar, Philippines, Srilanka, Africa and south west Asia. It's strong aromatic smell makes the plant conspicuous [32,33]. The plant is medicinally used in the treatment of headaches, cough, cold, fever, urinary tract infections and sexual debility in traditional health care systems of tribals of Rajasthan [34,35]. The essential oil from the leaves of this herb has been reported to have insecticidal and antiplasmodial properties and is effective in Malaria. The main compound in the essential oil of this plant is 1,8-Cineole [36] which is also used as insect-repellent [32].
The present study aims at first time synthesis and characterization of silver nanoparticles using aqueous leaf extract of Ocimum canum Sims., (JungliTulsi). We also attempted to study the antibacterial activity of the nanoparticle synthesized.
2. Experimental
2.1. Plant collection and identification
Ocimum canum Sims., is an aromatic much branched erect herb with 4 angled stems, bearded nodes and lanceolate or ovate lanceolate leaves. It is a common weed of open waste lands. For the present study fresh plants were collected from Chittorgarh fort locality which lies in between 74038’57.9″E and 24053’47.06″N at an elevation of 544 m in district Chittorgarh of Rajasthan in the month of September and brought to laboratory in air tight polythene bags for further processing.
2.2. Preparation of leaf extract
For the preparation of leaf extract, fresh leaves were collected in a beaker and washed several times with water to remove the dust and finally with double distilled water. 10 g washed leaves were cut into fine pieces and crushed with the help of mortar and pestle in 100 ml double distilled water. After grinding the aqueous extract was taken in 250 ml beaker and boiled for 10 min at 80 °C temperature. The plant extract was allowed to cool at room temperature and then filtered with whatman filter paper. The filtrate was centrifuged for 20–25 min at 10000 rpm, the supernatant was collected and stored at 4 °C. This filtrate was used as a stabilizing and reducing agents.
2.3. Preparation of 1 mM silver nitrate (AgNO3) solution
The concentration of 1 mM silver nitrate (Central Drug House Ltd.) was prepared by dissolving 0.169 g AgNO3in 1 L deionised water and stored in amber coloured bottle to prevent the self-oxidation of silver nitrate solution.
2.4. Green synthesis of silver nanoparticles
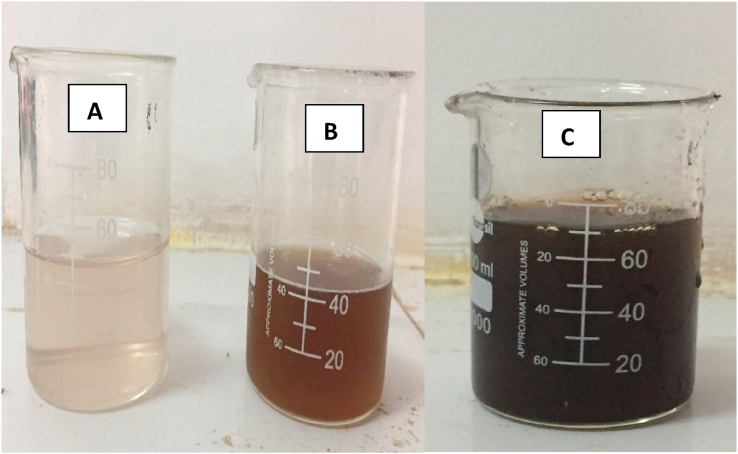
The preparation of silver nanoparticles (AgNPs) is a single step synthesis 10 ml of tulsi leaf extract, prepared as described in section 2.2 above, was added to 90 ml silver nitrate solution and the mixture was heated up at 80 °C for 15 min [27]. The colour of the solution turned from light yellow to brown (Fig. 1 A – C) indicating the formation of silver nanoparticles (AgNPs). The Ag NPs synthesized were separated by the process of centrifugation from the reaction mixture.
Fig. 1.
(A) Aqueous Silver nitrate 1 mM Solution (B) Ocimum canum Sims Leaves Extract (C) Colour Change respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.5. Purification of silver nanoparticles
In order to purify the nanoparticles prepared as described in section above, we followed the procedure suggested by Chaudhary et al. [37] The synthesized AgNPs were kept in 12 N hydrochloric acid solution for 24 h. The Ag NPs were isolated by the process of centrifugation from the mixture. The mixture was washed with distilled water till hydrochloric acid was completely removed.
2.6. Characterization of silver nanoparticles AgNPs
2.6.1. Scanning electron microscopy (SEM) study
Samples were investigated by Nova Nano FE-SEM 450 (FEI) scanning electron microscope to obtain topological, morphological and compositional information. Lens mounted DBS and LVD offer best selection of information and image optimization. Beam landing energy cam go down from 30 KeV to 50ev and resolution of 1.4 nm at 1 kV (TLD-SE) and 1 nm at 15 kV (TLD-SE). The entire sample was coated with gold before SEM analysis.
2.6.2. X- ray diffraction (XRD) analysis
XRD patterns were recorded on Philips PW 3050/10 model. The sample was recorded on a Philips X-Pert MMP diffractometer. The diffractometer was controlled and operated by a PC computer with the programs P Rofit and used a MoK (source with wavelength0.70930 A°, operating with Mo-tube radiation at 50 kV and 40 mA.
3. Result and discussion
Synthesis of silver nanoparticles by reduction of silver nitrate using aqueous leaf extract of jungli tulsi can be easily monitored during the reaction from the change in colour of reaction mixture. Silver nanoparticles bear the characteristic yellow to brown colour due to colour reaction as presented in Fig. 1 which indicated the formation of nanoparticles. This formation indicates that the Ag(I) converted into Ag (0) having the size of nanorange [38]. Studies have indicated that biomolecules like proteins, carbohydrates, flavonoids and phenols not only play a role in the capping of the nanoparticles, but also play an important role in reducing the ions to the nano size [39,40]. SEM images of silver nanoparticles have shown in Fig. 2 (A, B,C) which have indicated that morphologically the silver nanoparticles are spherical as well as rod like of various sizes (6.13–32.04 nm) and the average size of nanoparticles was calculated to be of 15.76 nm. The SEM image shows agglomeration of individual silver nanoparticles. A closer look at the agglomerated rod shape and spherical structure of silver nanoparticles shown in Fig. 2A and B. Spherical nanoparticles with the range 22.92–41.50 nm were prepared using another species of Ocimum namely Ocimum sanctum in an earlier work. [41]. In an another work [42] the average size of green synthesized silver nanoparticles using leaf extract of Ocimum sanctum was reported to be of 42 nm. Singh et al. have reported green synthesis of silver nanoparticles using sun dried tulsi leaves and its catalytic application for 4-Nitrophenol reduction [43]. Interestingly, the average particle size in the present study (15.76 nm) using Ocimum canum was found quite smaller than the size of particles synthesized by Ocimum sanctum. Similarly, the morphology is also different from nanoparticles synthesized by similar methods [38].
Fig. 2.
SEM images of silver nanoparticles (A) 100000 × (B) 25000 × (C) 10000 Magnification.
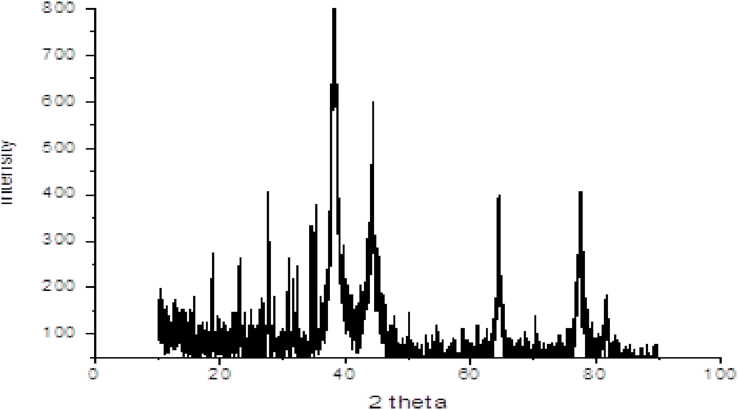
3.1. XRD analysis
From XRD it was evaluated that BM exhibits crystalline nature with prominent peaks (Fig. 3). The grain size corresponding to intense peak was determined using Scherrer's relation [44] and found that the average particles size is 15.72 nm. (See Table 1)
| (1) |
Here k is Scherrer constant having value 0.94, λ is the wavelength of X-ray, β is full width at half maxima (FWHM) which is calculated by fitting a single Gaussian curve, θ is Bragg diffraction angle and D is the particle size. Fig. 3 illustrates a typical XRD spectrum of silver nanoparticles prepared by the green method.
Fig. 3.
XRD pattern of silver nanoparticles.
Table 1.
Calculations of average particle size of silver nanoparticles from XRD data.
| 2θ | θ | in radain | sinθ | cosθ | Tanθ | β (degree) | FWHM β (radian) | 2sinθ | d(spacing) | β cosθ | D (nm) | Average Particle size |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 27.7 | 13.83 | 0.2414103 | 0.2390723 | 0.9710018 | 0.246212 | 0.26659 | 0.004653 | 0.4781446 | 0.32220379 | 0.0045185 | 32.049333 | |
| 38.1 | 19.07 | 0.3327902 | 0.3266814 | 0.9451345 | 0.345645 | 0.56094 | 0.009792 | 0.6533628 | 0.2357955 | 0.0092543 | 15.648504 | 15.725 |
| 44.2 | 22.11 | 0.3859423 | 0.3764323 | 0.9264441 | 0.40632 | 1.4605 | 0.025494 | 0.7528646 | 0.20463174 | 0.0236186 | 6.1314343 | |
| 64.5 | 32.26 | 0.5630289 | 0.53375 | 0.8456423 | 0.631177 | 0.71786 | 0.012531 | 1.0675001 | 0.14431849 | 0.0105964 | 13.666472 | |
| 77.4 | 38.71 | 0.6757046 | 0.6254472 | 0.7802665 | 0.801582 | 0.95517 | 0.016673 | 1.2508944 | 0.12315987 | 0.0130094 | 11.131642 |
3.2. Antibacterial activity
E.coli was selected to assess antimicrobial activity of silver nano particles. Pure E. coli culture was taken. Nutrient broth was prepared and a loop full of inoculum was taken from L.B. Agar plate to inoculate 10 ml of the Nutrient Broth. The broth was allowed to incubate over night. Salmonella Shigella agar was prepared by mixing 6.3 gm in 100 ml distilled water and was heated till boiling. Autoclaved petri plates were taken and S.S. agar was poured in plates and allowed to settle down. 100 μl of the broth was taken and poured on the prepared S.S. agar plate. Spread plate method was used for culture. Gel puncture was used for creating wells. Different concentration dose were prepared using standard 200 ppm solution in acetone which have been prepared by dissolving 02 g of the silver nanoparticle in 1000 ml of acetone. 20 μl nanoparticles sample of 10 ppm, 20 ppm and 30 ppm was loaded in each well. The plates were incubated for 24 h at 28 °C in incubator. Zone of inhibition was determined after overnight incubation (seeTable 2).
Table 2.
ZOI of Silver nanoparticles at Different Concentration (ppm).
| S. No. | Concentration dose of Silver Nanoparticles | Zone of Inhibition (in cm) |
||
|---|---|---|---|---|
| Replica- I | Replica- II | Mean | ||
| 1 | Control | 0.0 | 0.0 | 0.0 |
| 2 | 10 ppm | 1.9 | 1.5 | 1.7 |
| 3 | 20 ppm | 1.9 | 1.6 | 1.75 |
| 4 | 30 ppm | 2.9 | 2.0 | 2.45 |
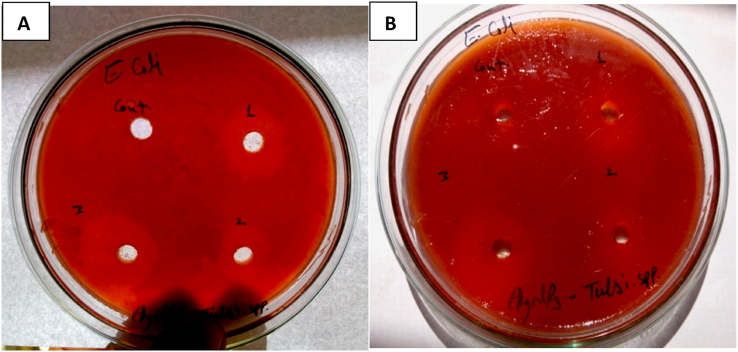
The data pertaining to the biological activity of silver nanoparticles against E. coli bacteria are presented in Table 2 and Fig. 4 which indicate that the minimum zone of inhibition of the bacterium (1.7 cm) was observed at 10 ppm concentration of silver nanoparticles while this zone was found maximum (2.45 cm) at 30 ppm concentration of AgNPs. The zone of inhibition caused by 20 ppm concentration of nanoparticles ranges in between. Thus the maximum ZOI of 2.45 cm was recorded at 30 ppm concentration (Fig. 4).
Fig. 4.
Zone of Inhibition of Ag NPs against E. coli (A:Replica I) and (B:Replica II).
4. Conclusion
To conclude, this study exhibits one step innovative green approach for the synthesis of silver nanoparticles from jungle tulsi leaf extract. The method stand out primarily due to the fact that it is eco-friendly and advantageous over the conventional physical and chemical methods. These particles are anticipated to have extensive applications in various industries. The formation of silver nanoparticles was confirmed by the colour change. The silver nanoparticles formed were quite stable in solution. The morphological studies by SEM show that the silver nanoparticles are spherical and rod shaped. The particle size and crystalline nature were determined by XRD. The carbohydrates, flavanoids and poly phenols constituents present in leaf extract act as the surface active stabilizing molecules for the synthesis of silver nanoparticles. The plant material used is of wide occurrence as a weed and as far as known used for the first time in green synthesis of nanoparticles. It is quite significant that finer particles can be prepared using Ocimum canum than the other species of this genus. The method was unique and cost effective. Still more clinical trials are needed to support its therapeutic uses.
Author contributions
GT conceived the idea and designed the experiments. BLY analyzed and interpreted the data. JC contributed materials, reagents and analysis of data and tool. MJ and CS performed the experiments. GT and BLY both the authors edited, compiled and finalized the draft. Finally, all authors were gone through and approved it for publication.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The author(s) declare(s) that there is no conflict of interest regarding the publication of this paper.
Acknowledgement
Authors (GT & BLY) are grateful to the Departments of Chemistry and Life Science, Mewar University, Gangrar, Chittorgarh, Rajasthan, India for providing laboratory facilities. Sincere thanks to MNIT- Jaipur, Rajasthan, India for providing facilities for characterization of the nanoparticles. The authors are also grateful to the Heads of the Departments of Chemistry and Department of Zoology of M.L.S University for providing necessary facilities.
References
- 1.Dijiken A.V., Meulenkamp E.A., Vanmaekelbergh D., Meijerink A. J. Lumin. 2000;90:123–128. [Google Scholar]
- 2.Singhal G., Riju B., Ashish R.S., Rajendra P.S. Adv. Sci. Eng. Med. 2012;4:62–66. [Google Scholar]
- 3.Sotiriou G.A., Pratsinis S.E. Antibacterial activity of nanosilver ions and particles. Environ. Sci. Technol. 2010;44(14):5649–5654. doi: 10.1021/es101072s. [DOI] [PubMed] [Google Scholar]
- 4.Lee J., Mahendra S., Alvarez P.J. Nanomaterials in the construction industry: are view of their applications and environmental health and safety considerations. ACS Nano. 2010;4(7):3580–3590. doi: 10.1021/nn100866w. [DOI] [PubMed] [Google Scholar]
- 5.Kim B., Park C.S., Murayama M., Hochella M.F., Jr. Discovery and characterization of silver sulfide nanoparticles in final sewage sludge products. Environ. Sci. Technol. 2010;44(19):7509–7514. doi: 10.1021/es101565j. [DOI] [PubMed] [Google Scholar]
- 6.Mehta A., Sharma M., Kumar A., Basu S. Effect of Au content on the enhanced photocatalytic efficiency of mesoporous Au/TiO2 nanocomposites in UV and sunlight. Gold Bull. 2017;50(1):33–41. [Google Scholar]
- 7.Mehta A., Mishra A., Sharma M., Singh S., Basu S. Effect of silica/titania ratio on enhanced photooxidation of industrial hazardous materials by microwave treated mesoporous SBA-15/TiO2 nanocomposites. J. Nanoparticle Res. 2016;18(7):209. [Google Scholar]
- 8.H. A. Salam, R.Sivaraj, VenckateshR ,Green synthesis and characterization of zinc oxide Nanoparticles from Ocimumbasilicum L. var. purpurascensBenth.-LAMIACEAE leaf extract,Mater. Lett., 10.1016/j.matlet.2014.05.033. [DOI]
- 9.Wu Z.G., Munoz M., Montero O. The synthesis of nickel nanoparticles by hydrazine reduction. Adv. Powder Technol. 2010;21(2):165–168. [Google Scholar]
- 10.Qin Y., Ji X., Jing J., Liu H., Wu H., Yang W. Size control over spherical silvernanoparticles by ascorbic acid reduction. Colloids Surf., A. 2010;372(1):172–176. [Google Scholar]
- 11.Bin Ahmad M., Lim J.J., Shameli K., Ibrahim N.A., Tay M.Y. Synthesis of silvernanoparticles in chitosan: gelatin and chitosan/gelatinbionanocomposites by a chemical reducing agent and their characterization. Molecules. 2011;16(9):7237–7248. doi: 10.3390/molecules16097237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vimala K., Mohan Y.M., Sivudu K.S., Varaprasad K., Ravindra S., Reddy N.N., Padma Y., Sreedhar B., MohanaRaju K. Fabrication of porous chitosan films impregnated with silver nanoparticles: a facile approach for superior antibacterial application? Colloids Surf. B Biointerfaces. 2010;76(1):248–258. doi: 10.1016/j.colsurfb.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 13.Mehta A., Basu S. Controlled photocatalytic hydrolysis of nitrites to amides bymesoporous MnO 2 nanoparticles fabricated by mixed surfactant mediated approach. J. Photochem. Photobiol. Chem. 2017;343:1–6. [Google Scholar]
- 14.Klaus T., Joerger R., Olsson E., Granqvist C.G. Silver based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. U. S. A. 1999;96(24):13611–13614. doi: 10.1073/pnas.96.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konishi Y., Ohno K., Saitoh N. Bioreductive deposition of platinum nanoparticles on the bacterium Shewanellaalgae. J. Biotechnol. 2007;128(3):648–653. doi: 10.1016/j.jbiotec.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 16.Nair B., Pradeep T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus. Cryst. Growth Des. 2002;2(4):293–298. [Google Scholar]
- 17.Willner I., Baron R., Willner B. Growing metal nanoparticles by enzymes. Adv. Mater. 2006;18(9):1109–1120. [Google Scholar]
- 18.Kumar V., Yadav S.K. Plant mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009;(84):151–157. [Google Scholar]
- 19.Vyomparashar * Parthenium leaf extract mediated synthesis of silver nanoparticles: a novel approach towards weed utilization. Digest J. Nanomater. Biostruct. 2009;4(1):45–50. [Google Scholar]
- 20.Sathishkumara Gnanasekar, Gobinatha Chandrakasan. Phyto-synthesis of silver nanoscale particles using Morindacitrifolia L. and its inhibitory activity against human pathogens. Colloids Surf. B Biointerfaces. 2012;(95):235–240. doi: 10.1016/j.colsurfb.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Rout Yogeswari, Behera Sikha, Kumar Ojha Akshya, Nayak* P.L. Green synthesis of silver nanoparticles using Ocimum sanctum (Tulashi) and study of their antibacterial and antifungal activities”. J. Microbiol. Antimicrob. 2012;4(6):103–109. [Google Scholar]
- 22.Ponarulselvam S., Panneerselvam Murugan K., Aarthi N., Kalimuthu K., Thangamani Synthesis of silver nanoparticles using leaves of Catharanthusroseus(Linn.) G. Don and their antiplasmodial activities. Asian Pacific J. Trop. Biomed. 2012:574–580. doi: 10.1016/S2221-1691(12)60100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayaseelan Chidambaram. Acaricidal efficacy of synthesized silver nanoparticles using aqueous leaf extract of Ocimumcanum against Hyalommaanatolicumanatolicum and Hyalommamarginatumisaaci(Acari:Ixodidae) Parasitol. Res. 2012;11(3):1369–1378. doi: 10.1007/s00436-011-2559-1. [DOI] [PubMed] [Google Scholar]
- 24.Mehta A., Basu S. Controlled photocatalytic hydrolysis of nitriles to amides bymesoporous MnO 2 nanoparticles fabricated by mixed surfactant mediated approach. J. Photochem. Photobiol. Chem. 2017;343:1–6. [Google Scholar]
- 25.Nakkala J.R., Mata R., Gupta A.K., Sadras S.R. Biological activities of green silvernanoparticles synthesized with Acorouscalamus rhizome extract. Eur. J. Med. Chem. 2014;85:784–794. doi: 10.1016/j.ejmech.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Sathishkumar P., Vennila K., Jayakumar R., Yusoff A.R.M., Hadibarata T., Palvannan T. Phyto-synthesis of silver nanoparticles using Alternantheratenellaleaf extract: an effective inhibitor for the migration of human breast adenocarcinoma(MCF-7) cells. Bioproc. Biosyst. Eng. 2016;39(4):651–659. doi: 10.1007/s00449-016-1546-4. [DOI] [PubMed] [Google Scholar]
- 27.Singhal G., Bhavesh R., Kasariya K., Sharma A.R., Singh R.P. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J. Nanoparticle Res. 2011;13(7):2981–2988. [Google Scholar]
- 28.Ahmed S., Ahmad M., Swami B.L., Ikram S. Green synthesis of silver nanoparticles using Azadirachtaindica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016;9(1):1–7. [Google Scholar]
- 29.Narayanan K.B., Park H.H. Antifungal activity of silver nanoparticles synthesized using turnip leaf extract (Brassica rapa L.) against wood rotting pathogens. Eur. J. Plant Pathol. 2014;140(2):185–192. [Google Scholar]
- 30.Zargar M., Shameli K., Najafi G.R., Farahani F. Plant mediated green biosynthesis of silver nanoparticles using Vitexnegundo L. extract. J. Ind. Eng. Chem. 2014;20(6):4169–4175. [Google Scholar]
- 31.Kathiravan V., Ravi S., Ashokkumar S. Synthesis of silver nanoparticles from Meliadubia leaf extract and their in vitro anticancer activity. Spectrochim. Acta, Part A. 2014;A130:116–121. doi: 10.1016/j.saa.2014.03.107. [DOI] [PubMed] [Google Scholar]
- 32.Bhandari M.M. 1978. Flora of Indian Desert MPS Repros, Jodhpur. [Google Scholar]
- 33.Yadav B.L., Meena K.L. Scientific Publishers; Jodhpur: 2011. Flora of South Central Rajasthan. [Google Scholar]
- 34.Joshi P. 1995. Ethnobotany of the primitive tribes in Rajasthan. Printwell Jaipur. [Google Scholar]
- 35.Katewa S.S., Jain A. Apex Publishing House; Udaipur: 2006. Traditional Folk Herbal Medicines. [Google Scholar]
- 36.Akono Ntonga P., Baldovini N., Mouray E., Mambu L., Belong P., Grellier P. 2014. Activity of Ocimum Basilicum, Ocimum canum and Cymbopogon citrates Essential Oils against Plasmodium falciparum and Mature Stage Larvae of Anopheles Funestus s.S. Parasite21; p. 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhary J., Tailor G., Kumar D. Res. J. Chem. Environ. 2019;23(3):10–14. [Google Scholar]
- 38.Ramteke C., Chakrabarti T., Sarangi B.K., Pandey R.A. Synthesis of silver nanoparticles from the aqueous extract of Leaves of Ocimum sanctum for enhanced antibacterial activity. J. Chem. 2013:7. doi: 10.1155/2013/278925. Article ID 278925. [DOI] [Google Scholar]
- 39.Vedpriya A. Living Systems:eco-friendly nanofactories. Digest J. Nanomater. Biostruct. 2010;5(1):9–21. [Google Scholar]
- 40.Collera-Z uniga O., Garcıa Jimenez F., Melendez Gordillo R. Comparative study of carotenoid composition in three mexican varieties of Capsicum annuumL. Food Chem. 2005;90(1–2):109–114. [Google Scholar]
- 41.Anuradha G., SyamaSundar B., Sreekanthkumar J., Ramana M.V. Synthesis and characterization of SilverNanoparticles from Ocimumbasilicum L. var.thyrsiflorum. Eur. J. Acad. Essays. 2014;1(5):5–9. [Google Scholar]
- 42.Subba Rao Y., Kotakadi Venkata S., Prasad T.N.V.K.V., Reddy A.V., Sai Gopal D.V.R. Green synthesis and spectral characterization of silver nanoparticles from Lakshmi tulasi (Ocimum sanctum) leaf extract. Spectrochim. Acta Mol. Biomol. Spectrosc. 2013;103:156–159. doi: 10.1016/j.saa.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 43.Singh J., Mehta A., Rawat M., Basu S. Green synthesis of silver nanoparticles using sun dried tulsi leaves and its catalytic application for 4-Nitrophenol reduction. J. Environ. Chem. Eng. 2018;6:1468–1474. [Google Scholar]
- 44.S.Chuhadiya, R. Sharma, Himanshu, S.L. Patel, S. Chander, M.D.Kannan, M.S. Dhaka, Thermal annealing induced physical properties of ZnSe thin film for buffer layer in solar cells, Phys. E Low-dimens. Syst. Nanostruct., 117, 1.