Abstract
Background
The most prevalent clinical entity of extra pulmonary tuberculosis is tuberculous lymphadenitis. However, it resembles other granulomatous conditions pathologically and obtaining tissue for microbiological diagnosis is also difficult. Thus it is a challenging task for diagnosis and early initiation of management. Fine needle aspiration cytology and biopsy are the diagnostic methods generally used to obtain the lymph node samples for histopathological and microbiological diagnosis. Mycobacterium culture on Lowenstein-Jensen medium remains the gold standard for definitive diagnosis, but its major limitations is a prolonged turn-around time of 2–4 weeks. The GeneXpert Mtuberculosis/RIF assay is a novel molecular diagnostic method for rapid diagnosis of tuberculosis and rifampicin resistance in clinical specimens.
Methods
This was a cross sectional analytical study conducted on 67 cases of suspected tubercular lymphadenitis at R.L Jalappa Hospital and Research Centre, Tamaka, Kolar. The study was carried out between December 2017 to June 2019. The samples were collected using excision biopsy and subjected to GeneXpert Mtuberculosis/RIF assay and histopathology. Further, sensitivity, specificity, positive predictive value and negative predictive value was measured and compared with histopathology.
Results
The average age of the patients was 37.04 ± 19.27 and majority was males. The lymph nodes were predominantly present in cervical region. Histopathology analysis reveals 46 positive cases of tuberculosis Lymphadenitis and GeneXpert Mtuberculosis/RIF assay detects 42 cases of tuberculosis Lymphadenitis. In the present study, GeneXpert Mtuberculosis/RIF assay had a sensitivity of 82.60% and specificity of 85% when compared to histopathology. Further the PPV and NPV was found to be 92.68% and 68% respectively. GeneXpert Mtuberculosis/RIF showed 2 cases of rifampicin resistance out of 67 cases. In this study, the GeneXpert Mtuberculosis/RIF showed the results in 0.79 days.
Conclusion
The present study showed that GeneXpert Mtuberculosis/RIF is a simple and reliable technique for diagnosing tuberculosis Lymphadenitis with high specificity and sensitivity as compared histopathology. Further, the methods elicit rapid diagnosis and also detected rifampicin resistance. It is thus a reliable and useful diagnostic modality in rapid detection of the causative agent and initiation of appropriate category anti-tubercular therapy when necessary.
Keywords: Tubercular lymphadenitis, GeneXpert Mtuberculosis/RIF, Histopathology, Rifampicin resistance
1. Introduction
Extrapulmonary tuberculosis continues to be one of the leading health problem in developing countries. Lymphadenopathy is the commonest form of extrapulmonary tuberculosis. In India in general outpatients 10–20% of new tuberculosis cases have extrapulmonary manifestations, while among those suffering from HIV it could be up to 50% [1].
Extrapulmonary tuberculosis comprises of a wide gamut of diseases affecting all parts of the body excluding the lungs. Commonly affected sites include lymph nodes, pleura, urogenital tract, bones and joints, meninges, CNS, bowel and/or peritoneum, pericardium and skin. Although morbidity, mortality and adverse disease sequelae are common, it is an entity that is underplayed as it does not contribute significantly to the transmission of tuberculosis [2]. Challenges faced by treating clinicians are its myriad clinical features and the difficulty in sample collection from deep‐seated tissue. The laboratory diagnosis is an added hurdle, as a good number of specimens are paucibacillary or smear negative [3].
The investigative parameters for diagnosis of tuberculosis in lymph nodes are neither specific nor does their absence exclude the condition. Conventional Ziehl-Neelsen staining for acid-fast bacilli detection plays a key role in diagnosis and also monitoring treatment of tuberculosis but has low sensitivity ranging from 20% to 43% [2]. The gold standard method for the tuberculosis diagnosis is Mycobacterial culture, but it is time consuming and requires specialized safety precautions in laboratories [3]. Serological techniques lack sensitivity and specificity [3]. Newer molecular techniques such as PCR, although rapid, are costly to be routinely used in developing countries. In recent times, attention has been devoted to new nucleic acid amplification diagnostic technologies, owing to their rapidity, sensitivity, and specificity.
Diagnosis of extra pulmonary infection with members of the M. tuberculosis is a challenging task to establish, since the extrapulmonary specimens are have lower bacterial count as compared to pulmonary specimens. Furthermore, collection of extrapulmonary specimen for analysis requires invasive procedures.
One of the latest systems, the GeneXpert MTB/RIF assay, was evaluated only recently in a large study with pulmonary specimens [4]. The GeneXpert assay uses heminested real-time PCR to amplify an M. tuberculosis-specific gene sequence [4]. To determine rifampin resistance, the rifampin resistance-determining area i.e, “rpoB” gene is probed with molecular beacons [4]. The technique can be conducted in a nearly fully automated manner, including bacterial lysis, nucleic acid extraction and amplification, and amplicon detection [4]. The test runs on the GeneXpert platform (Cepheid, Sunnyvale, CA) using a disposable plastic cartridge with all required reagents [5]. Reports have shown that GeneXpert assay detected pulmonary tuberculosis in all tuberculosis patients, including over 90% of smear-negative patients, with a high sensitivity of over 97% [6].
The GeneXpert has been endorsed by WHO since December 2010 as a replacement for sputum smear microscopy, particularly in settings with high rates of HIV-associated tuberculosis and multidrug-resistant tuberculosis [7].
As most studies conducted have been on sputum or aspirates there are fewer evidence regarding the efficacy of this test on solid tissue samples which is the main aim of this study.
2. Materials and methods
A 18 month Cross Sectional Analytical Study was conducted between December 2017 to June 2019 on 67 patients with clinically suspected Tubercular Lymphadenitis who presented to the out-patient department of R.L Jalappa Hospital and Research Centre, Tamaka, Kolar.
Patients with clinically suspected tubercular lymphadenitis and aged above 5 years were included in the study.
Patients with lymphadenopathy who are established case of malignancy with primary or secondary deposits in the lymph nodes were excluded.
FNAC was done by inserting a thin needle into the affected lymph node and the aspirated contents subjected to microscopy. The following cytomorphological patterns can be seen in (Fig. 1).
Fig. 1.
FNAC smears from a suspected case of nodal TB [47].
-
•
Predominantly reactive picture with occasional or a few clusters of epithelioid cells.
-
•
Presence of numerous clusters of epithelioid cells with presence of multinucleate giant cells. In this pattern the diagnosis can be made with relative ease.
-
•
Mostly necrotic material, with a few epithelioid cells found on diligent search.
-
•
Mostly necrotic material with a few lymphocytes and histiocytes but no epithelioid cells.
-
•
Necrotic material only.
Excision of the affected lymph node was done under regional or general anaesthesia based on the site involved. The excised specimen was divided into two parts. One part was fixed in formalin and sent for histopathological examination. Identification of caseating granulomatous inflammation with Langhans and foreign body giant cells is suggestive of tuberculosis.
The other part was crushed and sent in special GeneXpert containers fixed in saline and subjected to GeneXpert assay. To the specimen in a centrifuge tube, double volume buffer solution is added, vortexed and left at room temperature for 15 min. Then 3 ml of the vortexed mixture was added to the sample loading area of the cartridge without air bubbles. The lid of the cartridge was closed. Then the barcode on the cartridge was scanned, patient ID entered and the instrument module opened and the cartridge was loaded. The instrument module door is closed. Once the test gets over, the door lock will be released. The results are interpreted as follows:
MTB detected: Target DNA is detected and depending on cycle threshold value, the result will be displayed as high, medium, low or very low detection.
MTB detected, RIF resistance detected: MTB target was present within the sample and mutation in rpo B gene detected.
MTB detected, RIF resistance not detected: MTB target was present within the sample and no mutation in rpo B gene detected.
MTB detected, RIF resistance indeterminate: MTB target was present within the sample and RIF resistance is not be determined due to insufficient signal detection.
MTB not detected: MTB target was not detected within the sample.
Invalid: MTB DNA cannot be determined. Repeat the test in this case.
Error: MTB cannot be determined. Sample processing control shows no result and probe check results failed. The test should be repeated.
No result: MTB cannot be determined. The test was repeated.
The duration required from time of sample collection to reporting was calculated for each of the tests – FNAC, histopathology and GeneXpert Assay.
2.1. Data analysis
The statistical analysis was carried out using SPSSv26 software.
Quantitative variables were depicted using descriptive statistics as mean, standard deviation, frequency.
Chi-Square test of independence was used to determine if there was a significant relationship between two nominal (categorical) variables.
The efficacy of GeneXpert was done by calculating the sensitivity, specificity, positive and negative predictive value.
3. Results
This study was conducted on 67 patients who were clinically suspected with tuberculous lymphadenitis at R.L Jalappa Hospital and Research Centre, Tamaka, Kolar between December 2017 to June 2019.
3.1. Demographic parameters
In this study out of 67 cases, 40 were females and 27 were males. The average age of the study subjects was 37.04 ± 19.27 years. The average BMI of the participants was found to be 23.47 ± 4.20 kg/m2. The results were displayed in Table 1
Table 1.
Demographic details of the present study.
| Demographic parameters | Values ± SD | |
|---|---|---|
| Gender |
Male | 27 (40.3%) |
| Females | 40 (59.7%) | |
| Age | 37.04 ± 19.27 Years | |
| Height | 155 ± 45.20 Cms | |
| Weight | 57.13 ± 8.50 Kgs | |
| BMI | 23.47 ± 4.20 kg/m2 | |
3.2. Clinical findings
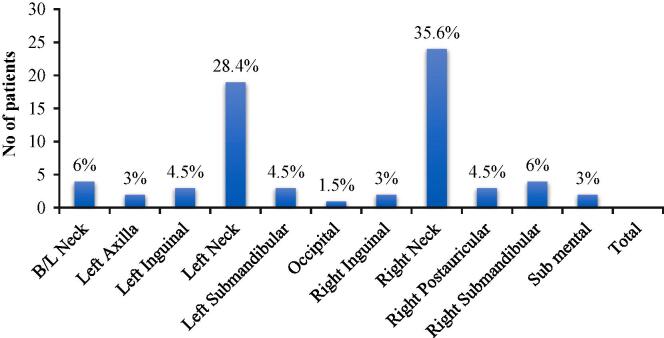
Out of 67 patients the cervical lymph nodes were most commonly involved site. The results location of involved lymph nodes were given in Chart 1.
Chart 1.
Lymph node site among the patients.
In this study based on clinical findings, all the lymph nodes were spherical in shape. Most of the patients 59 (89%) displayed well defined borders of involved lymph nodes whereas 6 cases had ill defined borders. The surface of lymph node was found to be smooth in majority of the cases i.e., 58 (86.55%) and irregular in 9 cases (13.45%). Skin overlying the affected lymph node was found to be normal in 42 patients (62.68) and erythema was visualized in 25 patients (37.32%).
Local rise of temperature was found in 37 cases (55.23%) and absent in 30 cases (44.77%). 54 cases (80.60%) had tenderness on palpation.
In our study, final diagnosis shows the occurrence of Right Cervical Lymphadenitis in majority of the patients i.e., 22 cases (32.8%) followed by Left Cervical Lymphadenitis in 18 cases (26.9%). The data were given in Chart 2
Chart 2.
Final Diagnosis.
(AT- Abdominal Tuberculosis; B/L CL - B/L Cervical Lymphadenitis; HL- Hodgkins Lymphoma; IL-Inguinal Lymphadenitis; LCL- Left Cervical Lymphadenitis; LSL- Left Submandibular Lymphadenitis; LA- Left Axillary; RCL- Right Cervical Lymphadenitis; RPL- Right Postauricular Lymphadenitis; RSL- Right Submandibular Lymphadenitis; SML- Submental Lymphadenitis)
FNAC along with ZN staining revealed the occurrence of reactive Lymphadenitis in 34 patients (50.7%), Granulomatous Lymphadenitis in 19 patients (28.4%) and 2 patients (3%) with Tubercular Lymphadenitis. The 3 above mentioned impression on FNAC were considered as suggestive of tubercular lymphadenitis. The data were given in Chart 3.
Chart 3.
FNAC Findings.
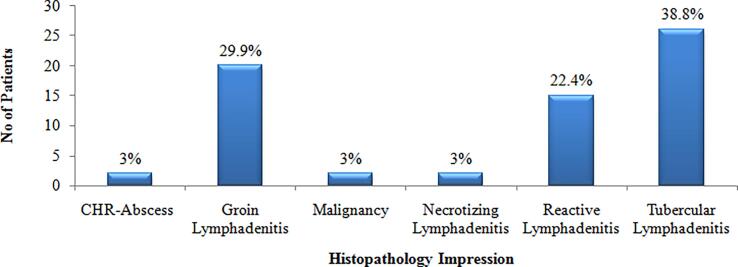
Histopathology analysis showed the occurrence of Tubercular Lymphadenitis in 26 patients (38.8%), Granulomatous Lymphadenitis in 20 patients (29.9%) and 15 patients (22.4%) with Reactive Lymphadenitis (Chart 4).
Chart 4.
Histopathology Impression.
All the samples were subjected to GeneXpert assay. Among 67 cases, 42 (62.7%) were found to be positive for M. tuberculosis and remaining 25 (37.3%) were negative. As GeneXpert assay can also tell us about the rifampicin susceptibility status (Resistant or Sensitive). Among the positive cases, only two cases were found resistant to rifampicin (4.7%) (Chart 5).
Chart 5.
Outcome of GeneXpert analysis.
In this study the GeneXpert showed sensitivity of 82.60% and specificity of 85% as compared to histopathology. Further it showed positive predictive value of 92.68% and negative predictive value of 68%. (Table 2)
Table 2.
Diagnostic Accuracy of GeneXpert Vs. Histopathology.
| Method | Histopathology Positive | Histopathology Negative |
|---|---|---|
| GeneXpert Positive | 38 | 3 |
| GeneXpert Negative | 8 | 17 |
When compared with FNAC, GeneXpert showed sensitivity of 86.36% and specificity of 48.78%. Further it showed positive predictive value of 47.50% and negative predictive value of 86.96%. (Table 3)
Table 3.
Diagnostic Accuracy of GeneXpert Vs. FNAC.
| Method | FNAC Positive | FNAC Negative |
|---|---|---|
| GeneXpert Positive | 19 | 21 |
| GeneXpert Negative | 3 | 20 |
Also, GeneXpert had displayed the results fastest in 0.79 days as compared to the FNAC 1 day and histopathology 4.46 days (Chart 6). Thus, GeneXpert was found to be a reliable and rapid method for tubercular lymphadenitis detection.
Chart 6.
Time needed for the diagnostic outcome of GeneXpert, FNAC and Histopathology.
4. Discussion
Tuberculosis is one of the primeval chronic and complex infectious diseases which is caused by a group of bacteria belonging to the genus Mycobacterium. The complex includes the human adapted species of M. tuberculosis and M. africanum, and zoonotic pathogens- M. bovis, M. caprae, M. microti and M. pinnipedii which affect cattle, goats/sheep, voles and seals/lions, respectively [8], [9].
Globally, pulmonary tuberculosis accounted for 85% whereas extrapulmonary tuberculosis accounted for the remaining 15% [10]. The most common types of extrapulmonary tuberculosis include tuberculosis of the lymphatics, pleura, bone, meninges, genitourinary tract and peritoneal tuberculosis [11], [12]. However, the incidence of extrapulmonary tuberculosis and its predominant forms differs country to country [13], [14].
Global tuberculosis control efforts have largely ignored extrapulmonary tuberculosis. This is because extrapulmonary tuberculosis is generally considered non-infectious and as such inconsequential to the global epidemic [15]. However, recent evidence from northwest England has showed the prevalence of active tuberculosis disease among household contacts of extrapulmonary tuberculosis was high (440 per 100 000 contacts screened), indicating that extrapulmonary tuberculosis cases might have substantial impact on tuberculosis transmission [16]. Moreover, it is conceivable that the slower annual decline rate of extrapulmonary tuberculosis compared to pulmonary tuberculosis could retard the progress towards the END-tuberculosis targets set by WHO [17].
Fine-needle aspiration cytology (FNAC) offers a feasible and safe option for specimen collection [18]. The use of cytology together with the confirmation of acid fastness by ZN staining and Papanicolaou stain-induced fluorescent microscopy and identification of the organism by culture offers excellent yields [19] but remains limited by the absence of species confirmation, slow turnaround times, and/or lack of drug resistance guidance. Conventional microbiological culture and drug sensitivity testing are not always available and may take 6 weeks or longer [20].
GeneXpert MTB/RIF (Cepheid, Sunnyvale, USA) is an automated, cartridge-based and isothermal nucleic acid amplification test (NAAT) for the detection of M. tuberculosis complex and rifampicin drug-resistance from sputum and other specimens, with a turnaround time of less than two hours [21], [22]. A WHO policy statement from 2013 formulated recommendations to guide the use of the test for pulmonary and extrapulmonary tuberculosis in adults and children. Based so far only on very low evidence, GeneXpert has been conditionally recommended as a replacement test for usual practice (including conventional microscopy, culture or histopathology) for testing specific non-respiratory specimens, including lymph nodes [23]. This study was carried out to evaluate the role of GeneXpert assay in rapid diagnosis of tuberculosis lymphadenitis and rifampicin sensitivity of the organism. Hence, to validate its benefits in terms of efficacy in comparison to FNAC and histopathology and low cost and time required for reporting.
In this study a total of 67 patients with suspected tuberculous lymphadenitis were subjected to FNAC, histopathology analysis and GeneXpert. Similar closely related sample size of 48 cases was reported in the previous study done by Ligthelm [24].
In our study the female predominance was noted and cervical lymphadenopathy was the most common clinical presentation.
Similarly in a study conducted by Patel, majority of patients (90%) had a symptom of neck swelling [25]. Further, in this study 40.3% had fever and 34.5% displayed weight loss as the constitutional symptoms. In a study by Patel, 22% and 24% had a symptom fever and weight loss respectively [26]. In a study done by Kant [27] et al. 73.3% of patients had fever and 66.7% reported weight loss as the symptom [27].
In this study, 73.1% patients were moderately nourished and malnutrition is very limited in this study. The association between tuberculosis and malnutrition is bi-directional. Tuberculosis predisposes the patient to malnutrition and malnutrition escalates the risk of developing active tuberculosis by 6–10 folds [28], [29].
In this study, 80.67% of patients were afebrile (no elevated body temperature) and 19.4% were febrile. In a study done by Gautam et al. 75% of tubercular lymphadenitis cases displayed fever as major symptom [30].
In our study the cervical group of lymph nodes is the predominant site of involvement with 64%. In a study done by Gautam [31]. 87.14% of lymph node was present in the neck region. Jasim [32]conducted a review of 188 cases of tuberculous lymphadenitis and reported that cervical involvement representing the most common site of involvement [65.43%] [33]. Further, Gupta and Bhake also reported that the cervical was the major site of lymph location constituting about 69.2% [34].
Cervical lymphadenitis is the consequence of lympho-hematogenous spread of pulmonary tuberculosis [35]. Further it might be also due to hyper reactivity of lymph nodes against previous pulmonary tuberculosis [36]. The major pathway of dissemination of the tubercle bacilli to the cervical lymph nodes is from lung parenchyma as the lymphatics of the right lung and the lower lobe of the left lung drain into the right supraclavicular lymph nodes and then upwards to the right lower cervical chain [36]. However, the etiology of cervical lymphadenitis without pulmonary tuberculosis cannot be explained by this theory, and also alternate routes of spread to lymph nodes, such as the tonsils and adenoids, have been proposed. Lymph node tuberculosis could be also occurring by direct exposure to infection.
In this study, FNAC was suggestive of tubercular lymphadenitis in 82.1% of the cases. In a study done by Singh et al. FNAC diagnosis of palpable lymph mass revealed 33.38% as reactive lymphadenitis, 39.77% of tubercular lymphadenitis and 7.1% of Granulomatous lymphadenitis. In a study done by Rammeh [37] FNAC analysis reveals 55.9% as tubercular lymphadenitis and 24.3% as reactive lymphadenitis [37]. In another study done by Kumar et al. FNAC analysis reveals 47.67% of tubercular lymphadenitis and 44.39% of reactive lymphadenitis [38]. Thus in our study based on the FNAC impression, the final inference shows 35.82% of cases as positive and 64.18% of cases as negative for tubercular lymphadenitis.
Our histopathology analysis suggested that 68.70% of cases are positive whereas 31.3% are negative for tubercular lymphadenitis.
The GeneXpert MTB/RIF assay shows 62.7% positive cases in the present study. In a study conducted by Bankar et al. GeneXpert MTB/RIF assay displayed 18.42% of positive cases in the detection extrapulmonary tuberculosis as compared to the culture and ZN smear microscopy [39]. In a study performed by Ghariani [40] 77% of cases were positively detected for tuberculosis among the lymph node samples [40]. In Nidhi Gupta et al. study out of 143 FNAC samples, GeneXpert Mtuberculosis/RIF assay revealed 60.1% as positive for tuberculous lymphadenitis [41].
In the present study, the sensitivity and specificity of GeneXpert MTB/RIF assay was established as 82.60% and 85% as compared to histopathological examination. Further, positive and negative predictive value was found to be 92.68% and 68% respectively.
On comparing with FNAC, sensitivity and specificity of GeneXpert MTB/RIF assay was found 86.36% and 48.78% respectively. Further, the positive and negative predictive value was found to be 47.50% and 86.96% respectively.
Multidrug resistant tuberculosis is resistant to isoniazid and rifampicin. As per WHO, globally 490,000 people are estimated to have become MDR-tuberculosis and 110,000 of rifampicin resistance in 2016. This multidrug resistance accounts for about 4.1% of new tuberculosis cases. Around 240,000 deaths were reported in 2016 due to multidrug resistant tuberculosis/ rifampicin resistant tuberculosis [40]. In this study only 2 cases (2.9%) out of 67 cases analyzed were found to be Rifampicin resistant on GeneXpert MTB/RIF. In a study by Avashia [41] 5.4% of rifampicin resistance by GeneXpert MTB/RIF were reported [41]. Similar study was also done by Gupta et al in which rifampicin resistance was detected in 5.8% of extrapulmonary tuberculosis samples [41].
Our study revealed that GeneXpert required least time (0.79 day) as compared to the FNAC (1 day) and Histopathology (4.46 days) for definitive diagnosis of tuberculosis. It also provides additional information regarding rifampicin resistance in a short duration. This is essential to overcome the delay caused in initiation of appropriate regimen of anti-tubercular therapy [42]. Thus, introducing the GeneXpert assay early in the diagnostic workflow can potentially improve detection and shorten turn-around time in the laboratory. It hence decreases unnecessary empiric treatment among smear-negative extrapulmonary tuberculosis [42].
5. Conclusion
The GeneXpert MTB/RIF assay is a cutting-edge diagnostic tool that is revolutionizing tuberculosis control by augmenting the detection of tuberculosis and drug resistance.
Major advantages of the GeneXpert MTB/RIF assay are that results are available quickly, and minimal technical training is required to run the test. Additionally, the GeneXpert can quickly identify possible multidrug-resistant tuberculosis.
From our study we conclude that GeneXpert MTB/RIF is simple and reliable technique for diagnosing extrapulmonary tuberculosis with high sensitivity and specificity as compared to the FNAC and Histopathology. Thus playing an essential role in early and accurate diagnosis of tuberculosis and prompt initiation of the appropriate treatment. It is of utmost diagnostic importance in view of the high disease burden but low socio-economic status of the population commonly affected by the disease.
References
- 1.Lalitkant Extra- pulmonary tuberculosis: Coming out of the shadows. Indian J Tuberc. 2004;51:189–190. [Google Scholar]
- 2.Balows A., Hausler W.J., Herrmann K.L., Shadomy H.J. 5th ed. American Society for Microbiology:; Washington D.C.: 1991. Manual of clinical Microbiology; pp. 308–311. [Google Scholar]
- 3.Daniel TM. “Rapid diagnosis of tuberculosis: Laboratory techniques applicable in developing countries”. Rev Infect Dis 1989; 2: S471-8. [DOI] [PubMed]
- 4.El-Hajj H.H., Marras S.A., Tyagi S., Kramer F.R., Alland D. Detection of rifampin resistance in Mycobacterium tuberculosis in a single tube with molecular beacons. J Clin Microbiol. 2001;39:4131–4137. doi: 10.1128/JCM.39.11.4131-4137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raja S. Technology for automated, rapid, and quantitative PCR or reverse transcription-PCR clinical testing. Clin Chem. 2005;51:882–890. doi: 10.1373/clinchem.2004.046474. [DOI] [PubMed] [Google Scholar]
- 6.Boehme C.C. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Tuberculosis diagnostics automated DNA test. https://www.who.int/tb/features_archive/xpert_factsheet.pdf.
- 8.Comas I, Gagneux S. A role for systems epidemiology in tuberculosis research. Trends Microbiol. 2011;19(10):492–500 pmid:21831640. [DOI] [PMC free article] [PubMed]
- 9.Smith NH, Gordon SV, de la Rua-Domenech R, Clifton-Hadley RS, Hewinson RG. Bottlenecks and broomsticks: the molecular evolution of Mycobacterium bovis. Nat Rev Microbiol. 2006;4(9):670 pmid:16912712. [DOI] [PubMed]
- 10.WHO. Global tuberculosis report 2017. Geneva: World Health Organization 2017 978-92-4-156551-6 Contract No.: WHO/HTM/tuberculosis/2017.23.
- 11.Qian X, Nguyen DT, Lyu J, Albers AE, Bi X, Graviss EA. Risk factors for extrapulmonary dissemination of tuberculosis and associated mortality during treatment for extrapulmonary tuberculosis. Emerg Microbes Infect.2018;7(1):102 pmid:29872046. [DOI] [PMC free article] [PubMed]
- 12.Alemie GA, Gebreselassie F. Common types of tuberculosis and co-infection with HIV at private health institutions in Ethiopia: A cross sectional study. BMC Public Health. 2014; 14(1) pmid: 24708793. [DOI] [PMC free article] [PubMed]
- 13.Ramirez-Lapausa M., Menendez-Saldana A., Noguerado-Asensio A. Extrapulmonary tuberculosis: an overview. Rev EspSanidPenit. 2015;17:3–11. doi: 10.4321/S1575-06202015000100002. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.Y. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc Respir Dis. 2015;78(2):47–55. doi: 10.4046/trd.2015.78.2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsnelson A. Beyond the breath: Exploring sex differences in tuberculosis outside the lungs. Nat Med. 2017;23:398 pmid:28388608. [DOI] [PubMed]
- 16.Wingfield T, MacPherson P, Cleary P, Ormerod LP. High prevalence of tuberculosis disease in contacts of adults with extra pulmonary tuberculosis. Thorax. 2017:thoraxjnl-2017-210202.
- 17.Lonnroth K, Raviglione M. The WHO’s new End tuberculosis Strategy in the post-2015 era of the Sustainable Development Goals. Trans R Soc Trop Med Hyg. 2016; 110(3):148–150 pmid:26884490. [DOI] [PMC free article] [PubMed]
- 18.Wright C.A. Fine-needle aspiration biopsy: a first-line diagnostic procedure in paediatric tuberculosis suspects with peripheral lymphadenopathy? Int J Tuberc Lung Dis. 2009;13:1373–1379. [PubMed] [Google Scholar]
- 19.Wright C.A., van Zyl Y., Burgess S.M., Blumberg L., Leiman G. Diagn; Cytopathol: 2004. Mycobacterial auto fluorescence in Papanicolaou-stained lymph node aspirates: a glimmer in the dark? [DOI] [PubMed] [Google Scholar]
- 20.WHO (2008) Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosisdrugs. WHO, Geneva, Switzerland http://whqlibdoc.who.int/hq/2008/WHO_HTM_tuberculosis_2008.392_eng.pdf. [PubMed]
- 21.Boehme C.C., Nabeta P., Hillemann D., Nicol M.P., Shenai S., Krapp F. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillemann D., Rüsch-Gerdes S., Boehme C., Richter E. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert Mtuberculosis/RIF system. J Clin Microbiol. 2011;49:1202–1205. doi: 10.1128/JCM.02268-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO|Xpert Mtuberculosis/RIF: WHO Policy update and Implementation manual [Internet]. [cited 2014 May 7]. Available from: http://www.who.int/tb/laboratory/xpert_launchupdate/en/.
- 24.Patel K. A clinical study of tuberculous cervical lymphadenopathy: surgeon’s perspectives. Int Surg J. 2019;6:581–585. [Google Scholar]
- 25.Kant K., Baveja C.P., Sarkar J., Juyal D. Microbiological evaluation of clinically suspected cases of tubercular lymphadenopathy by cytology, culture, and smear microscopy – a hospital-based study from Northern India. J Family Med Prim Care. 2019;8:828–833. doi: 10.4103/jfmpc.jfmpc_20_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhargava A., Oxlade O., Menzies D. Undernutrition and the incidence of tuberculosis in India: national and subnational estimates of the population attributable fraction related to undernutrition. Natl Med J India. 2014;27:4–9. [PubMed] [Google Scholar]
- 27.Schaible U., Kaufmann S. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gautam H., Agrawal S.K., Verma S.K., Singh U.B. Cervical tuberculous lymphadenitis: clinical profile and diagnostic modalities. Int J Mycobacteriol. 2018;7:212–216. doi: 10.4103/ijmy.ijmy_99_18. [DOI] [PubMed] [Google Scholar]
- 29.Jasim H.A., Abdullah A.A., Abdulmageed M.U. Tuberculous lymphadenitis in Baghdad city: a review of 188 cases. Int J Surgery Open. 2019;16:40–47. [Google Scholar]
- 30.Gupta V., Bhake A. Assessment of clinically suspected tubercular lymphadenopathy by real-time PCR compared to non-molecular methods on lymph node aspirates. Acta Cytol. 2018;62(1):4–11. doi: 10.1159/000480064. [DOI] [PubMed] [Google Scholar]
- 31.Kent D.C. Tuberculous lymphadenitis:not a localized disease process. Am J Med Sci. 1967;254:866–874. [PubMed] [Google Scholar]
- 32.Powell D.A. Tuberculous lymphadenitis. In: Schlossberg D., editor. Tuberculosis and nontuberculous mycobacterial infections. 4th ed. WB Saunders Company; Philadelphia: 1999. pp. 186–194. [Google Scholar]
- 33.Yew W.W., Lee J. Pathogenesis of cervical tuberculous lymphadenitis:pathways to anatomic localization. Tuber Lung Dis. 1995;76:275–276. doi: 10.1016/s0962-8479(05)80019-x. [DOI] [PubMed] [Google Scholar]
- 34.Selimoğlu E., Sütbeyaz Y., Ciftçioğlu M.A., Parlak M., Esrefoğlu M., Oztürk A. Primary tonsillar tuberculosis:a case report. J Laryngol Otol. 1995;109:880–882. doi: 10.1017/s0022215100131573. [DOI] [PubMed] [Google Scholar]
- 35.Golden M.P., Vikram H.R. Extrapulmonary tuberculosis:an overview. Am Fam Physician. 2005;72:1761–1768. [PubMed] [Google Scholar]
- 36.Singh A., Bhambani P., Nema S.K. Diagnostic accuracy of FNAC in diagnosis for causes of lymphadenopathy: a hospital based analysis. Int J Res Med Sci. 2013;1:271–277. [Google Scholar]
- 37.Rammeh S., Romdhane E., ArfaouiToumi A., Houcine Y., Lahiani R., Sassi A. Efficacy of fine-needle aspiration cytology in the diagnosis of tuberculous cervical lymphadenitis. Acta Cytol. 2018;62(2):99–103. doi: 10.1159/000487503. [DOI] [PubMed] [Google Scholar]
- 38.Kumar H., Chandanwale S.S., Gore C.R., Buch A.C., Satav V.H., Pagaro P.M. Role of fine needle aspiration cytology in assessment of cervical lymphadenopathy. Med J DY Patil Univ. 2013;6:400–404. [Google Scholar]
- 39.WHO 2016 update. WHO treatment guidelines for drug resistant tuberculosis page.23. [PubMed]
- 40.Avashia S., Bansal D., Ahuja K., Agarwal V. Comparison of conventional methods with gene xpertmtb/rif assay for rapid detection of Mycobacterium tuberculosis and rifampicin resistance in extra pulmonary samples. Int J Med Res Rev. 2016;4(2):181–185. [Google Scholar]
- 41.Gupta S., Kajal N.C., Shukla A.K., Shukla A.K., Singh A., Neki N.S. Role of Gene Xpert Mtuberculosis/RIF in diagnosis of tuberculous pus. Int J Curr Res Med Sci. 2018;4(2):81–85. [Google Scholar]
- 42.Iftikhar I., Irfan S., Farooqi J., Azizullah Z., Hasan R. Rapid detection of in vitro antituberculous drug resistance among smear-positive respiratory samples using microcolony detection-based direct drug susceptibility testing method. Int J Mycobacteriol. 2017;6:117–121. doi: 10.4103/ijmy.ijmy_41_17. [DOI] [PubMed] [Google Scholar]