Highlights
-
•
P. gingivalis can be colonized in lung cancer tissues.
-
•
The microenvironment of cancerous tissues is more conducive to the survival of P. gingivalis than adjacent lung tissues.
-
•
Long term smoking and alcohol will more likely lead to P. gingivalis infections.
-
•
Effective clearance of P. gingivalis may prolong the survival time of lung cancer patients.
Keywords: Porphyromonas gingivalis, Infection, Lung cancer, Prognosis
Abstract
A variety of pathogenic microorganisms can promote the occurrence and development of malignant tumors by colonizing in the body. It has been shown that Porphyromonas gingivalis (P. gingivalis) can be colonized for a long time in upper gastrointestinal tumors and is closely related to the occurrence and development of esophageal cancer in previous studies of our team. Because the esophagus and trachea are closely adjacent and P. gingivalis can instantly enter and colonize in cells, we speculate that P. gingivalis may be colonized in lung cancer cells through oral or blood, promoting the malignant progression of lung cancer. In this study, we investigated P. gingivalis infection in lung carcinoma tissues and adjacent lung tissues, and found that the colonization rate of P. gingivalis in carcinoma tissues was significantly higher than that in adjacent lung tissues. Therefore, we propose that the microenvironment of cancer cells is more conducive to the survival of P. gingivalis. Then, we analyzed the correlation between P. gingivalis infection and clinicopathological features and survival prognosis of patients with lung cancer. It was found that P. gingivalis infection was closely related to smoking, drinking, lymph node metastasis and clinical stage. Moreover, the survival rate and median survival time of patients with P. gingivalis infection were significantly shortened. Therefore, we put forward the view that long term smoking and drinking will cause a bad oral environment, increasing the risk of P. gingivalis infection, then P. gingivalis infection will promote the malignant progression of lung cancer.
Introduction
Lung cancer is a malignant tumor that poses a great threat to human life and health, and its 5-year survival rate is less than 15% [1]. It mainly includes two major pathological types, one is small cell lung cancer, accounting for about 20%, and the other is non-small cell lung cancer, accounting for about 80%. Non-small cell lung cancer mainly includes squamous cell carcinoma and adenocarcinoma. Lung cancer has no specific clinical manifestations in the early stage, and only has the common symptoms of general respiratory diseases, so the diagnosis is difficult. Once there are chest pain, shortness of breath, pleural effusion and other clinical symptoms, the lesion has reached an advanced stage, and the prognosis is very poor [2]. Up to now, the etiology of lung cancer is not completely clear. The main risk factors include smoking, occupational and environmental exposure, past chronic lung infection, genetic susceptibility and decreased immune function. With the continuous development and progress of medical research, genetic changes and environmental exposure constitute the etiology of lung cancer. Moreover, the infection factor is the key to environmental exposure [3]. At present, a large number of studies have shown that a variety of pathogenic microorganisms can promote the occurrence and development of malignant tumors through long term colonization in the body [4–7].
Porphyromonas gingivalis (P. gingivalis), as the dominant bacteria in oral cavity, is one of the most virulent pathogens [8]. Oral and maxillofacial lack of venous valves, and rich blood supply, pathogenic bacteria can easily spread to the whole body with blood circulation. Therefore, the special anatomical structure and environment of oral cavity endow P. gingivalis with important pathophysiological significance. Extensive hematogenous invasion can promote the participation of P. gingivalis in a variety of systemic disease processes [9]. More seriously, P. gingivalis infection is closely related to the occurrence and development of a variety of tumors [[10], [11], [12], [13], [14]–15]. We had found that the detection rate of P. gingivalis in cancer tissues of patients with esophageal cancer was as high as 42%. It had been shown that a high and low distribution in upper digestive tract tumors was significantly associated with clinicopathological features and 5-year survival [12–15]. Therefore, P. gingivalis is closely related to the occurrence and development of esophageal cancer. Because the esophagus and trachea are closely adjacent to each other, and the process of P. gingivalis entering the cells is instantaneous and can be colonized in the cells for a long time, we speculate that P. gingivalis may be colonized in lung cancer cells through oral or blood, promoting the malignant progression of lung cancer.
In this study, IHC method was used to detect P. gingivalis infection in carcinoma tissues and adjacent lung tissues of patients with lung cancer. Then the correlation between P. gingivalis infection , clinicopathological features and 5-year survival time of patients with lung cancer was analyzed. The result of this study provides an effective treatment for improving the survival and prognosis of patients with lung cancer.
Materials and methods
Study subjects
From 2010 to 2013, a multicenter research of lung cancer drew subjects primarily from Henan Province, including the First Affiliated Hospital of Henan University of Science and Technology (HUST; Luoyang, Henan, China), the First Affiliated Hospital of Zhengzhou University (HZU; Zhengzhou, Henan, China) and Anyang Tumor Hospital (ATH; Anyang, Henan, China). Carcinoma tissues were surgically resected from patients with lung cancer. Adjacent lung tissues were obtained 5 cm distant to carcinoma tissues. The institutional review boards at HUST, HZU and ATH reviewed and approved this study, and all participants signed written sheets of informed consent. No restrictions regarding age, sex, or disease stage were set. Patients who had received any preoperative radiotherapy, chemotherapy or immunotherapy therapy before recruitment or any blood transfusion in the preceding 6 months or antibiotics consumption in the preceding 6 days were excluded. All examined lung cancers were small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma. The clinical stage and histological tumor type of lung cancer were determined according to the UICC/AJCC TNM Classification of 2009 (seventh edition). Patient's clinical information was collected from the medical records of the patients and stored in a database, which was updated every 3 months by telephone follow-up. The postoperative follow-up period was 60 months (5 years).The specimens were collected and treated promptly after surgery. Each specimen was sufficient to be fixed in 10% formaldehyde for making paraffin embedded blocks.
Immunohistochemistry (IHC)
Tissues were fixed in formalin and then embedded in paraffin. Serial sections of 4 mm thickness were prepared and dissolved at 60 ℃ for 1.5 h, then deparaffinized with xylene for 15 min and gradually be hydrated by submersion in three separate concentrations of ethanol (100, 95, and 70%), and rinsing continuously in distilled water for 5 min. Antigen retrieval was performed by incubating slides in antigen retrieval Citra plus solution (BioGenex, San Ramon, USA), according to the manufacturer's instructions. Slides were blocked 1.5% normal goat serum (Vector Laboratories, Burlingame, USA) for 30 min. Polyclonal rabbit anti-P. gingivalis 33277 [16] was utilized for the detection of P. gingivalis. Pre-immune rabbit IgG and normal mouse IgG was used as a negative control. Primary antibodies were incubated with tissue sections (1:1000 dilution) for 24 h, 4 ℃, followed by biotin-conjugated secondary antibody for 1 h at room temperature, streptavidin-peroxidase for 15 min at room temperature, and enzyme substrate (3,30-Diaminobenzidine, Dako, Denmark) for 10 min at room temperature. As an additional control, sections were also incubated with phosphate buffered saline (PBS) only, followed by incubation with biotin-conjugated secondary antibody, streptavidin-peroxidase, and enzyme substrate. PBS washes (3 times, 5 min each) were performed during each incubation step. Sections were counterstained with hematoxylin and visualized by light microscopy (E100+ISH500, Nikon, Japan). Every tissue section was evaluated by two senior pathologists. Staining intensity was classified using a numerical scale; grade 0 (none, ≥0 and <10% staining); grade 1 (weak, ≥10 and <30%); grade 2 (moderate, ≥30 and <60%), and grade 3 (strong, ≥60%), with a score of ≥2 considered positive of staining with P. gingivalis [15].
Statistical analysis
All statistical analyses were performed by SPSS statistical package, version 23.0 (SPSS Inc., Chicago, IL, USA). Correlations between the presence of P. gingivalis in the lung carcinoma tissues and adjacent lung tissues were analyzed by Chisquare test; Correlations between P. gingivalis and clinicopathological features of lung cancer were analyzed by Chisquare test; Survival curve was drawn by Kaplan–Meier survival analysis, and the difference of survival time was analyzed by Log-rank test; The postoperative follow-up time of patients with lung cancer was 60 months (5 years), and the survival time was from admission to the date of the last follow-up or death. Censored data were patients who were still alive after follow-up to 60 months, and the undeleted data were patients with death caused by lung cancer including small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma. P values of <0.05 were considered to be statistically significant.
Results
General characteristics of clinicopathological data of patients with lung cancer
This study included 100 patients with small cell lung cancer, 119 patients with lung adenocarcinoma and 100 patients with lung squamous cell carcinoma. The proportion of male and smoking patients with lung squamous cell carcinoma was higher than that of patients with small cell lung cancer and lung adenocarcinoma, as presented in Table 1.
Table 1.
This study included the general characteristics of clinicopathological factors of patients with lung cancer.
| Factors | No. (%) of small cell lung cancer patients | No. (%) of lung adenocarcinoma patients | No. (%) of lung squamous carcinoma patients |
|---|---|---|---|
| Sex | |||
| Male | 164(64.00) | 269(57.98) | 390(90.00) |
| Female | 36(36.00) | 50(42.02) | 10(10.00) |
| Age (years) | |||
| ≤60 | 51(51.00) | 53(44.54) | 32(32.00) |
| >60 | 49(49.00) | 66(55.46) | 68(68.00) |
| Smoking | |||
| Positive | 445(45.00) | 545(37.82) | 664(64.00) |
| Negative | 55(55.00) | 74(62.18) | 36(36.00) |
| Alcohol | |||
| Positive | 31(31.00) | 22(18.49) | 34(34.00) |
| Negative | 69(69.00) | 97(81.51) | 66(66.00) |
| Differentiation type | |||
| Poorly differentiated | 100(100.00) | 24(20.17) | 51(51.00) |
| Moderately differentiated | 0(0.00) | 65(54.62) | 37(37.00) |
| Well differentiated | 0(0.00) | 30(25.21) | 12(12.00) |
| Lymph node metastasis | |||
| Positive | 47(47.00) | 45(37.82) | 34(34.00) |
| Negative | 53(53.00) | 74(62.18) | 66(66.00) |
| Clinical stages | |||
| I/II | 46(46.00) | 59(49.58) | 61(61.00) |
| III/IV | 54(54.00) | 60(50.42) | 39(39.00) |
1,2: χ2 =0.825, P=0.364; 2,3: χ2 =28.003, P<0.001; 1,3: χ2 =19.085, P<0.001.
4,5: χ2 =1.159, P=0.282; 5,6: χ2 =14.903, P<0.001; 4,6: χ2 =7.279, P=0.007.
P. gingivalis was detected in lung carcinoma tissues and adjacent lung tissues
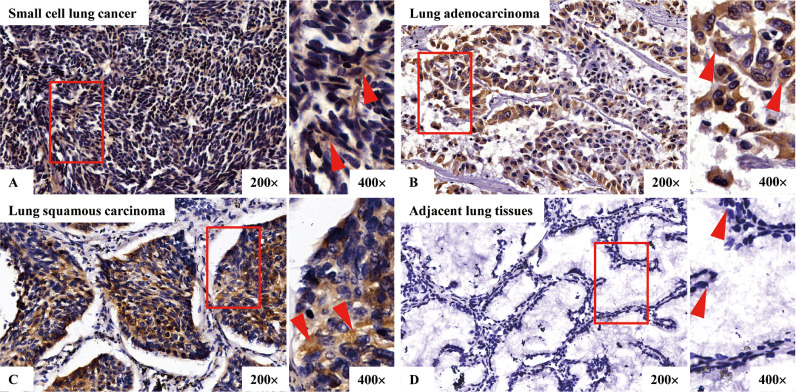
We conducted IHC to investigate the presence of P. gingivalis in paraffin embedded samples of carcinoma tissues and adjacent lung tissues from small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma. Fig. 1 indicated that, P. gingivalis staining in carcinoma tissues exhibiting florid cytoplasmic staining of malignant epithelial cells. In addition, staining was not uniformly expressed in a few adjacent lung tissues, and most of them were devoid of any staining. Moreover, it was found that the positive rates of P. gingivalis staining in carcinoma tissues of patients with small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma were 35.00%, 26.89% and 39.00%, respectively, which were significantly higher than those in the adjacent lung tissues, as presented in Table 2 and Fig. 1.
Fig. 1.
Immunohistochemical detection of P. gingivalis in carcinoma and adjacent lung tissues. A, B, and C are representative images of P. gingivalis in cancerous tissues of small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma, respectively; D is representative image of P. gingivalis in adjacent lung tissues. Shown are low-magnification (200×) (left) and high-magnification (400×) (right) micrographs of the tissues.
Table 2.
Presence of P. gingivalis in small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma tissues.
| Pathological type | n | No.(%) of P. gingivalis positive samples | |
|---|---|---|---|
| Carcinoma tissues | Adjacent lung tissues | ||
| Small cell lung cancer | 100 | 135(35.00) | 23(3.00) |
| Lung adenocarcinoma | 119 | 332(26.89) | 43(2.52) |
| Lung squamous carcinoma | 100 | 539(39.00) | 64(4.00) |
IHC: 1,2: χ2 =33.268, P<0.001; 3,4: χ2 =28.171, P<0.001; 5,6: χ2 =36.291, P<0.001; 1,3: χ2 =1.683, P=0.195; 1,5: χ2 =0.343, P=0.558; 3,5: χ2 =3.637, P=0.057.
Correlation between P. gingivalis and clinicopathological characteristics of patients with lung cancer
P. gingivalis was associated with smoking, alcohol, lymph node metastasis and clinical stages in patients with small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma (P <0.05), but not with sex, age and degree of differentiation, as presented in Table 3.
Table 3.
Expression of P. gingivalis and its association with the clinicopathological factors of lung cancer patients.
| Factors | No. (%) of small cell lung cancer patients |
No. (%) of lung adenocarcinoma patients |
No. (%) of lung squamous carcinoma patients |
|||
|---|---|---|---|---|---|---|
| P. gingivalis positive | P. gingivalis negative | P. gingivalis positive | P. gingivalis negative | P. gingivalis positive | P. gingivalis negative | |
| Sex | ||||||
| Male | 26(40.63) | 38(59.37) | 22(31.88) | 47(68.12) | 37(41.11) | 53(58.89) |
| Female | 9(25.00) | 27(75.00) | 10(20.00) | 40(80.00) | 2 (20.00) | 8(80.00) |
| Age (years) | ||||||
| ≤60 | 14(27.45) | 37(72.55) | 15(28.30) | 38(71.70) | 10(31.25) | 22(68.75) |
| >60 | 21(42.86) | 28(57.14) | 17(25.76) | 49(74.24) | 29(42.65) | 39(57.35) |
| Smoking | ||||||
| Positive | *24(53.33) | *21(46.67) | *17(37.78) | *28(62.22) | *32(50.00) | *32(50.00) |
| Negative | 11(20.00) | 44(80.00) | 15(20.27) | 59(79.73) | 7(19.44) | 29(80.56) |
| Alcohol | ||||||
| Positive | *20(64.52) | *11(34.48) | *13(59.09) | *9(40.91) | *22(64.71) | *12(35.29) |
| Negative | 15(21.74) | 54(78.26) | 19(19.59) | 78(80.41) | 17(25.76) | 49(74.24) |
| Differentiation type | ||||||
| Poorly differentiated | 35(35.00) | 65(65.00) | 9(37.50) | 15(62.50) | 21(41.18) | 30(58.82) |
| Moderately differentiated | 0(0.00) | 0(0.00) | 17(26.15) | 48(73.85) | 14(37.84) | 23(62,16) |
| Well differentiated | 0(0.00) | 0(0.00) | 6(20.0) | 24(80.00) | 4(33.33) | 8(66.67) |
| Lymph node metastasis | ||||||
| Positive | *31(65.96) | *16(34.04) | *20(44.44) | *25(55.56) | *23(67.65) | *11(32.35) |
| Negative | 4 (7.55) | 49(92.45) | 12(16.22) | 62(83.78) | 16(24.24) | 50(75.76) |
| Clinical stages | ||||||
| I/II | 5(10.87) | 41(89.13) | 4(6.78) | 55(93.22) | 13(21.31) | 48(78.69) |
| III/IV | *30(55.56) | *24(44.44) | *28(46.67) | *32(53.33) | *26(66.67) | *13(33.33) |
*The correlation between the positive and negative expression of P. gingivalis was analyzed by Chisquare test: P<0.05.
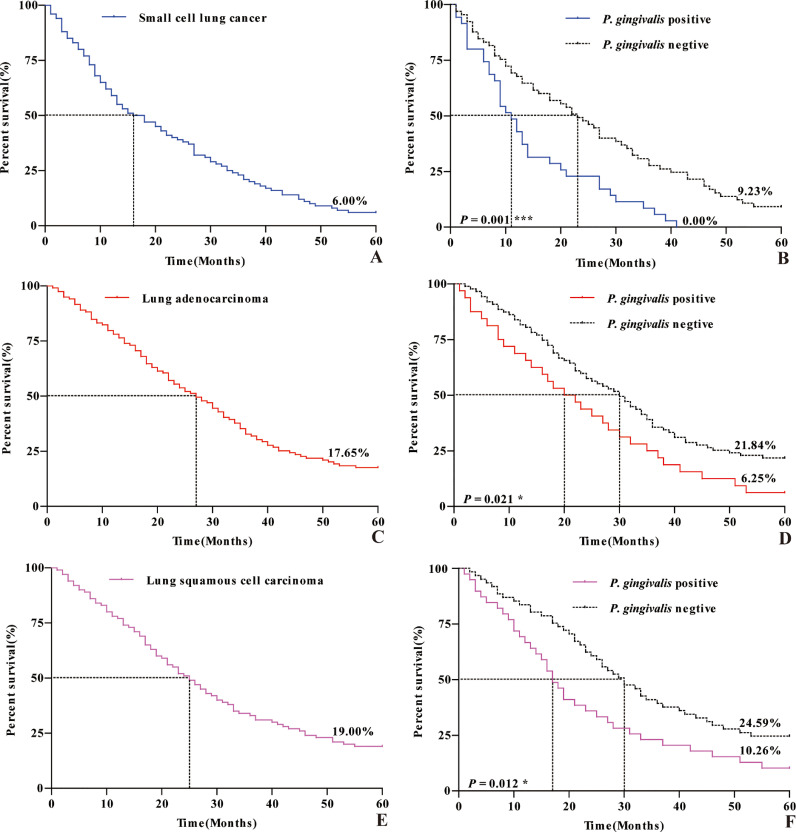
Correlation between P. gingivalis and 5-year survival prognosis of patients with lung cancer
The 5-year overall survival rate and median survival time of patients with small cell lung cancer were 6.00% and (16.00±3.50) months, respectively. The 5-year survival rate and median survival time of the P. gingivalis positive group were 0.00% and (11.00±1.48) months, while those of the P. gingivalis negative group were 9.23% and (23.00±3.46) months, respectively. The 5-year overall survival rate and median survival time of the patients with lung adenocarcinoma were 17.65% and (27.00±2.94) months, respectively. The 5-year survival rate and median survival time of the P. gingivalis positive group were 6.25% and (20.00±4.24) months, while those of the P. gingivalis negative group were 21.84% and (30.00±3.11) months, respectively. The 5-year overall survival rate and median survival time of the patients with lung squamous cell carcinoma were 19.00% and (25.00±2.50) months, respectively. The 5-year survival rate and median survival time of the P. gingivalis positive group were 10.26% and (17.00±1.78) months, while those of the P. gingivalis negative group were 24.59% and (30.00±3.41) months, respectively. In patients with these three types of lung cancer, the 5-year survival rate and median survival time in P. gingivalis positive group were significantly lower than those in P. gingivalis negative group, as presented in Table 4 and Fig. 2.
Table 4.
Means and medians for the survival time (months) of lung cancer patients with the positive or negative expression of P. gingivalis
| Pathological type | P. gingivalis group | Meana |
Mediana |
x2 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Est. | Std. error | 95% Confidence interval |
Est. | Std. error | 95% Confidence interval |
||||||
| Lower bound | Upper bound | Lower bound | Upper bound | ||||||||
| Small cell lung cancer | Positive | 14.543 | 1.965 | 10.691 | 18.395 | 11.000 | 1.478 | 8.102 | 13.898 | 13.184 | 0.001 |
| Negative | 25.985 | 2.288 | 21.501 | 30.468 | 23.000 | 3.455 | 16.229 | 29.771 | |||
| Overall | 21.980 | 1.723 | 18.603 | 25.357 | 16.000 | 3.500 | 9.140 | 22.860 | |||
| Lung adenocarcinoma | Positive | 23.781 | 3.026 | 17.849 | 29.713 | 20.000 | 4.243 | 11.684 | 28.316 | 5.336 | 0.021 |
| Negative | 32.034 | 2.029 | 28.058 | 36.011 | 30.000 | 3.109 | 23.907 | 36.093 | |||
| Overall | 29.815 | 1.725 | 26.434 | 33.196 | 27.000 | 2.937 | 21.244 | 32.756 | |||
| Lung squamous carcinoma | Positive | 23.282 | 2.894 | 17.609 | 28.955 | 17.000 | 1.784 | 13.504 | 20.496 | 6.365 | 0.012 |
| Negative | 33.148 | 2.476 | 28.294 | 38.001 | 30.000 | 3.413 | 23.311 | 36.689 | |||
| Overall | 29.300 | 1.946 | 25.486 | 33.114 | 25.000 | 2.499 | 20.101 | 29.899 | |||
Estimation is limited to the largest survival time; “Est.” and “Std.” are the abbreviations of “estimated” and “standard” respectively.
Fig. 2.
Kaplan-meier survival curve at 5 years after surgery for lung cancer patients. A, C, and E are survival curve at 5 years after surgery for patients of small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma, respectively; B, D, and F are survival curve at 5 years after surgery for patients with the positive and negative expression of P. gingivalis of small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma, respectively.
Discussion
Lung cancer is a common malignant tumor in clinic, and its morbidity and mortality are increasing year after year. Although there are surgery, chemotherapy, radiotherapy, immunotherapy and other means for the treatment of lung cancer, the therapeutic effect is still not satisfactory [17]. Therefore, it is of great significance to find the factors related to the occurrence, development and metastasis of lung cancer in order to predict its survival and prognosis and provide possible means for treatment. For a long time, the research on lung cancer has mostly focused on the cancer cells themselves, mainly due to changes in gene level [[18], [19]–20]. In contrast, there is little discussion about the chronic infection of pathogenic microorganisms. In fact, there is an important pathological relationship between long-term colonization of pathogenic microorganisms , tumorigenesis and development. The mechanism of tumorigenesis and development is not only the endogenous changes of cancer cells, but also the changes given by the microenvironment of the tumor.
Tumor microenvironment is a special environment for the growth of tumor cells, which is formed by the interaction between tumor cells and extracellular stroma [21]. It have been shown that a variety of pathogenic microorganisms can weaken the lethality of immune cells in the host microenvironment, induce immune tolerance and even functional exhaustion, and eventually lead to tumor immune escape and long-term colonization in tumor cells, promoting the occurrence and development of malignant tumors [22]. Such as Helicobacter pylori and gastric cancer, human papillomavirus and cervical cancer, chlamydia pneumoniae and lung cancer, Escherichia coli and colon cancer, these pathogenic microorganisms can regulate the tumor microenvironment through long-term colonization in the body, and finally promote the occurrence and development of malignant tumor [[4], [5], [6]–7].
P. gingivalis is an anaerobic gram-negative, rod-shaped bacterium, and much of its pathogenicity is a result of overall immunosuppression of the host cells [8,9]. This pathogenic mechanism may stem from its abilities to manipulate complement–Toll-like receptor cross-talk [23], myeloid-derived suppressor cell expansion [24], Th17/regulatory T-cell (Treg) imbalance [25], macrophage responsiveness [26], microbiota dysbiosis [27], and IFNg-inducible chemokines release [28] in a manner that promotes periodontitis and related systemic diseases such as atherosclerosis, insulin resistance, and Alzheimer disease [9,27]. Our previous study found that P. gingivalis infection could cause the overexpression of immune checkpoint B7-H4 in esophageal cancer cells. Overexpression of B7-H4 attenuates tumor immunogenicity by inhibiting T-cell expansion, division, and development, thus assisting immune escape. Meanwhile, P. gingivalis infection could cause the overexpression of lysine demethylase 5B in esophageal cancer cells, while the overexpression of KDM5B can "hypnotize" T-cells in the tumor by inhibiting the attraction and aggregation of lymphocytes in the tumor bed, thus inhibiting the immune system and assisting tumor cells to evade immune surveillance [12]. Therefore, P. gingivalis can indeed promote the occurrence and development of malignant tumors by remodeling the host immune microenvironment.
In this study, the presence of P. gingivalis infection was detected in lung carcinoma tissues and adjacent lung tissues by IHC. We found that, the positive rates of P. gingivalis staining in carcinoma tissues of patients with small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma were 35.00%, 26.89% and 39.00%, respectively, and the intensity and frequency of the sections stained with P. gingivalis were significantly enhanced in cancerous tissues of these three types of lung cancer compared to those in adjacent lung tissues, as presented in Table 2 and Fig. 1. It was not only shown for the first time that P. gingivalis could be colonized in lung cancer tissues, but also suggested that the microenvironment of cancer cells was more conducive to the survival of P. gingivalis. We speculated that P. gingivalis could evade host immune surveillance and thus be colonized for a long time in lung cancer tissues of different pathological types. Meanwhile, we found that the proportion of male and smoking patients with lung squamous cell carcinoma was higher than that of patients with small cell lung cancer and lung adenocarcinoma, as presented in Table 1. And the positive rate of P. gingivalis infection in patients with lung squamous cell carcinoma was higher than the other two types, as presented in Table 2. This may be related to the fact that smoking is a bad habit of most male patients with lung squamous cell carcinoma. Since long term heavy smoking can severely damage the immune function of the body [29], P. gingivalis may be more likely to sneak in. Then, the correlations between P. gingivalis infection and clinicopathological characteristics of patients with lung cancer were analyzed by Chi-square test. It was shown that P. gingivalis infection was related to smoking, alcohol, lymph node metastasis and clinical stages in patients with lung cancer including small cell lung cancer, lung adenocarcinoma and lung squamous cell carcinoma, as presented in Table 3. It was suggested that long term smoking and alcohol could lead to worse immune microenvironment [29,30], creating a better "hotbed" for P. gingivalis, and P. gingivalis was more likely to be infected and colonized in this environment. In this case, P. gingivalis may further induce host immunosuppression and assist the immune escape of tumor cells, thus promoting the invasion, proliferation and metastasis of lung cancer cells. In this study we also found that the 5-year survival rate and median survival time of these three types of lung cancer patients in the P. gingivalis positive group were significantly lower than those in the P. gingivalis negative group, as presented in Table 2 and Fig. 1. It was suggested that P. gingivalis infection could be closely related to survival and prognosis of lung cancer patients. P. gingivalis may assist malignant invasion, proliferation and distant metastasis of tumor cells, eventually leading to significantly reduced survival rate and median survival time of lung cancer patients. Due to the diversity and complexity of diseases, the specific pathogenic mechanism of P. gingivalis needs to be further discussed. But breaking the current situation of persistent colonization of P. gingivalis in host is of great significance to actively and effectively delay the malignant progression of lung cancer and prolong the survival time of patients.
In summary, P. gingivalis could promote the metastasis and malignant progression of lung cancer through long term colonization of lung cancer cells. Effective clearance of P. gingivalis may prolong the survival time of patients with lung cancer, which has very important scientific and theoretical significance and wide application prospects in the clinical treatment of lung cancer.
Authors' contributions
Yiwen Liu: Conceptualization, Data Curation, Writing – Original Draft, Writing – Review and Editing
Xiang Yuan: Methodology, Validation
Kuisheng Chen: Resources
Fuyou Zhou: Resources
Haijun Yang: Resources
Hong Yang: Methodology, Investigation
Yijun Qi: Methodology, Validation
Jinyu Kong: Methodology, Data Curation
Wei Sun: Methodology, Data Curation
Shegan Gao: Conceptualization, Supervision, Resources
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This work was supported in part by grants from the National Natural Science Foundation of China (81472234 for Shegan Gao), the National Natural Science Foundation of China (81702820 for Xiang Yuan), Major project of Science and Technology Department in Henan Province (161100311200 for Shegan Gao), Project of Science and Technology in Henan Province (202102310321 for Hong Yang), Project of Science and Technology in Henan Province (202102310129 for Jinyu Kong), Joint Construction Project of Medical Science and Technology Research Program in Henan Province (2018020273 for Yiwen Liu), Project of Science and Technology Plan Medical and Health in Luoyang (1813003A for Yiwen Liu).
Acknowledgments
We wish to thank Shegan Gao in the First Affiliated Hospital of Henan University of Science and Technology, Kuisheng Chen in the First Affiliated Hospital of Zhengzhou University, Fuyou Zhou and Haijun Yang in Anyang Tumor Hospital for sufficient patient specimens, related pathological information and follow-up data. We thank Professor Lamont RJ and Professor Wang HZ in University of Louisville for generously providing us with the specificial antibody of P. gingivalis strain ATCC 33277.
References
- 1.Nasim F., Sabath B.F, Eapen GA. Lung cancer. Med. Clin. N. Am. 2019;103(3):463–473. doi: 10.1016/j.mcna.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen M.M, Silverstein S.C, Quinn M. Timeliness of access to lung cancer diagnosis and treatment: a scoping literature review. Lung Cancer. 2017;112:156–164. doi: 10.1016/j.lungcan.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Bade B.C, Cruz C.S.D. Lung cancer 2020: epidemiology, etiology, and prevention. Clin. Chest Med. 2020;41(1):1–24. doi: 10.1016/j.ccm.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Bakhti S.Z., Latifi-Navid S., Safaralizadeh R. Helicobacter pylori-related risk predictors of gastric cancer: the latest models, challenges, and future prospects. Cancer Med. 2020;9(13):4808–4822. doi: 10.1002/cam4.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukri A., Hanafiah A., Zin N.M. Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. APMIS. 2020;128(2):150–161. doi: 10.1111/apm.13034. [DOI] [PubMed] [Google Scholar]
- 6.Shen M., Cai L., Jiang K. The therapeutic role of inhibition of miR-328 on pulmonary carcinoma induced by chlamydia pneumoniae through targeting histone H2AX. Cancer Biomark. 2018:1–8. doi: 10.3233/CBM-181999. [DOI] [PubMed] [Google Scholar]
- 7.Iyadorai T., Mariappan V., Vellasamy K.M. Prevalence and association of pks+ Escherichia coli with colorectal cancer in patients at the University Malaya Medical Centre, Malaysia. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0228217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hajishengallis G., Lamont R.J. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur. J. Immunol. 2014;44(2):328–338. doi: 10.1002/eji.201344202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsen I, Taubman MA, Singhrao SK. Porphyromonas gingivalis suppresses adaptive immunity in periodontitis, atherosclerosis, and Alzheimer's disease. J. Oral Microbiol. 2016;8:33029. doi: 10.3402/jom.v8.33029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utispan K., Pugdee K., Koontongkaew S. Porphyromonas gingivalis lipopolysaccharide-induced macrophages modulate proliferation and invasion of head and neck cancer cell lines. Biomed. Pharmacother. 2018;101:988–995. doi: 10.1016/j.biopha.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 11.de Mendoza I.L.I., Mendia X.M., de la Fuente A.M.G. Role of Porphyromonas gingivalis in oral squamous cell carcinoma development: a systematic review. J. Periodontal Res. 2020;55(1):13–22. doi: 10.1111/jre.12691. [DOI] [PubMed] [Google Scholar]
- 12.Yuan X., Liu Y., Li G. Blockade of immune checkpoint B7-H4 and lysine demethylase 5B in esophageal squamous cell carcinoma confers protective immunity against P. gingivalis infection. Cancer Immunol. Res. 2019;7(9):1440–1456. doi: 10.1158/2326-6066.CIR-18-0709. [DOI] [PubMed] [Google Scholar]
- 13.Gao S., Yang J., Ma Z. Preoperative serum immunoglobulin G and A antibodies to Porphyromonas gingivalis are potential serum biomarkers for the diagnosis and prognosis of esophageal squamous cell carcinoma. BMC Cancer. 2018;18(1):17. doi: 10.1186/s12885-017-3905-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao S., Li S., Ma Z. Presence of Porphyromonas gingivalis in esophagus and its association with the clinicopathological characteristics and survival in patients with esophageal cancer. Infect. Agents Cancer. 2016;11(1):3. doi: 10.1186/s13027-016-0049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan X., Liu Y., Kong J. Different frequencies of Porphyromonas gingivalis infection in cancers of the upper digestive tract. Cancer Lett. 2017;404:1–7. doi: 10.1016/j.canlet.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz Ö., Young P.A., Lamont R.J. Gingival epithelial cell signalling and cytoskeletal responses to Porphyromonas gingivalis invasion. Microbiology. 2003;149(9):2417–2426. doi: 10.1099/mic.0.26483-0. [DOI] [PubMed] [Google Scholar]
- 17.Arrieta O., Lazcano E. Lung cancer. Epidemiology, diagnosis and treatment. Salud Publ. Mex. 2019;61(3):217–218. doi: 10.21149/10660. [DOI] [PubMed] [Google Scholar]
- 18.Du X., Zhang J., Wang J. Role of miRNA in lung cancer-potential biomarkers and therapies. Curr. Pharm Des. 2018;23(39):5997–6010. doi: 10.2174/1381612823666170714150118. [DOI] [PubMed] [Google Scholar]
- 19.Chu G.C.W., Lazare K., Sullivan F. Serum and blood based biomarkers for lung cancer screening: a systematic review. BMC Cancer. 2018;18(1):181. doi: 10.1186/s12885-018-4024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong J, Chen X, Wang J. Genetic polymorphisms in the vitamin D pathway and non-small cell lung cancer survival. Pathol. Oncol. Res. 2020;26(3):1709–1715. doi: 10.1007/s12253-019-00702-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui L., Chen Y. Tumor microenvironment: sanctuary of the devil. Cancer Lett. 2015;368(1):7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 22.Wu T., Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017;387:61–68. doi: 10.1016/j.canlet.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 23.Olsen I, Lambris JD, Hajishengallis G. Porphyromonas gingivalis disturbs host-commensal homeostasis by changing complement function. J. Oral Microbiol. 2017;9(1) doi: 10.1080/20002297.2017.1340085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su L, Xu Q, Zhang P. Phenotype and function of myeloid-derived suppressor cells induced by Porphyromonas gingivalis infection. Infect. Immun. 2017;85(8) doi: 10.1128/IAI.00213-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai Y, Kobayashi R, Hashizume-Takizawa T. Porphyromonas gingivalis infection enhances Th17 responses for development of atherosclerosis. Arch. Oral Biol. 2014;59(11):1183–1191. doi: 10.1016/j.archoralbio.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Wilensky A, Tzach-Nahman R, Potempa J. Porphyromonas gingivalis gingipains selectively reduce CD14 expression, leading to macrophage hyporesponsiveness to bacterial infection. J. Innate Immun. 2014;7(2):127–135. doi: 10.1159/000365970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blasco-Baque V, Garidou L, Pomie C. Periodontitis induced by Porphyromonas gingivalis drives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut. 2017;66:872–885. doi: 10.1136/gutjnl-2015-309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jauregui CE, Wang Q, Wright CJ. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect. Immun. 2013;81(7):2288–2295. doi: 10.1128/IAI.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alrouji M, Manouchehrinia A, Gran B. Effects of cigarette smoke on immunity, neuroinflammation and multiple sclerosis. J. Neuroimmunol. 2019;329:24–34. doi: 10.1016/j.jneuroim.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Barr T, Helms C, Grant K. Opposing effects of alcohol on the immune system. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;65:242–251. doi: 10.1016/j.pnpbp.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]